Dendrimers as Modulators of Brain Cells
Abstract
1. Introduction
2. Neurons and Glial Cells in the Brain
2.1. Neurons
2.2. Astrocytes
2.3. Microglia
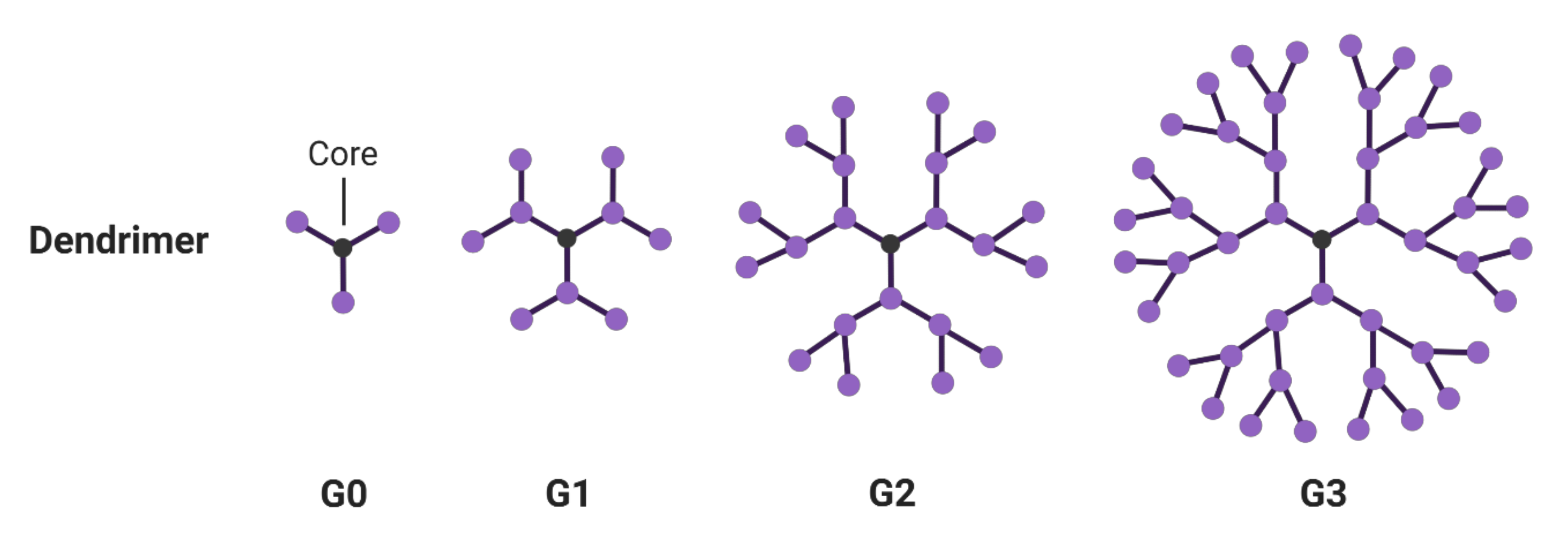
3. Dendrimers
3.1. Hyperbranched Macromolecules and Their Structure Modification
3.2. Dendrimers to Deliver Agents to Brain Tumors
| Drug or Genetic Cargo | Ligand Modification and Targeted Receptors | Other Main Features | Reference |
|---|---|---|---|
| Poly(amidoamine) (PAMAM)-Based Dendrimers | |||
| Doxorubicin | Cationic bovine serum albumin targets negatively charged endothelial cell membranes. | Protonation of free amine groups on dendrimer surface in the acidic environment of tumor tissue. | [105] |
| Nimesulide | - | PAMAM G3 dendrimer, modified with glycidol, and mixed with G0 PAMAM, reduces systemic cytotoxicity. | [111] |
| Apoptin | - | Short peptide chains on dendrimers hydrolyzed by peptidase, facilitate the release of positively charged ions and molecules, disrupt the membrane, resulting in endosomal escape via proton sponge effect. | [112] |
| Celecoxib, Fmoc-L-leucine | Biotin targets cancer cells overexpressing biotin receptors. | - | [106,107] |
| - | Intrinsic targeting ability to activated microglia/macrophages in CNS by hydroxyl-terminated G4 dendrimers. | - | [113] |
| Epirubicin, Let-7 miRNA | - | Positively charged surface with Gd and nanographene oxide used for loading drugs through adsorption and electrostatic interactions for combination therapy. | [114] |
| microRNA 21 (miR-21) inhibitor | - | miR-21 inhibitor loaded dendrimers enhance chemosensitivity of glioblastoma cells to paclitaxel through EGFR/STAT3 signaling. | [115] |
| Polyethylene Glycol (PEG)-Based Dendrimers | |||
| Bortezomib | Cyclo (Arg-Gly-Asp-D-Tyr-Lys) peptide selectively binds the integrin αvβ3 on cell membrane, resulting in integrin-mediated endocytosis. | Sustained drug release by weakening conjugation between bortezomib and dopamine upon acidic stimuli. | [116] |
| Quercetin, acetazolamide, lipoic acid | - | Telodendeimer micelles with covalently linked and physically entrapped drugs for combination therapy. Loading efficiency dependent on the physical fit between the drug and micelle core structure. | [83] |
| pDNA, RNAi | Peptide T7 (His-Ala-Ile-Tyr-Pro-Arg-His) specifically targets brain endothelial and cancer cells overexpressing transferrin (Tf) receptors. | - | [101] |
| PEGylated PAMAM-Based Dendrimers | |||
| Doxorubicin | Angiopep-2 binds low-density lipoprotein receptor-relative protein-1 (LRP1) on the endothelial cells of BBB. EP-1 peptide screened to target epidermal growth factor receptors (EGFRs). | - | [104] |
| Mesenchymal-epithelial transition (MET)-targeting cMBP peptide | Aberrant MET activation targeted which normally associates with invasiveness and drug resistance of gliomas. | - | [117] |
| Cytotoxic peptide KLAK | Dissociation of the matrix metalloproteinase 2 (MMP2)-sensitive peptide triggers PEG deshielding, and leads to exposure of the cell-penetrating peptide. | - | [118] |
| - | Glioma homing peptides (Pep-1) specifically bind the overexpressed interleukin-13 receptors α2 (IL-13Rα2) on glioma cells. | - | [109] |
| Doxorubicin | Tripeptide Arg-Gly-Asp (RGD) can identify and bind the integrin αvβ3 on cell membrane. | - | [108] |
| Dendritic Polyglycerols (dPGS) | |||
| Paclitaxel | Neural cell adhesion molecule (NCAM) overexpression has been found in many tumor cells and correlates with metastasis. | Dendrimer conjugated with NCAM-targeted peptide (NTP) efficiently inhibits endothelial cell migration and offers anti-angiogenesis potential. | [119] |
3.3. Dendrimers to Deliver Anti-Inflammatory Agents
| Modification | Disease Models | Other Main Features | Reference |
|---|---|---|---|
| Poly(amidoamine) (PAMAM)-BASED Dendrimers | |||
| Surface-modified anionic G4.5-COOH and neutral G5-OH. | Mouse model of acute pancreatitis | Inhibition of macrophage infiltration and suppression of pro-inflammatory cytokine expression. | [148] |
| PAMAM or poly(ethylenimine) dendrimers immobilized onto PSMA/polystyrene microfiber meshes, generating nucleic acid-binding polymers. | Human cancer and mouse macrophage cell lines | Inhibition of DAMP-mediated TLR stimulation and thrombosis by scavenging exDNA and HMGB1. Attenuation of inflammatory responses and coagulation induced by traumatic injury. | [149] |
| Simple surface modification of PAMAM dendrimers with -NH2, -OH, and -COOH. | Rat models of inflammation | The first study on intrinsic anti-inflammatory activity of PAMAM dendrimers. | [79] |
| Polyethylene Glycol (PEG)-Based Dendrimers | |||
| Highly dense surface hydroxyl terminals. | Models: Rabbit, cerebral palsy; Murine, glioblastoma; rat, age-related macular degeneration | Ability to cross CNS barriers, including BBB, blood–retinal barrier (BRB), and blood–brain-tumor barrier (BBTB). Selectively targets activated microglia/macrophages in CNS in vivo upon systemic administration. Intrinsic anti-oxidant and anti-inflammatory activities in vitro. | [147] |
| Polyglycerol-Based Dendrimers | |||
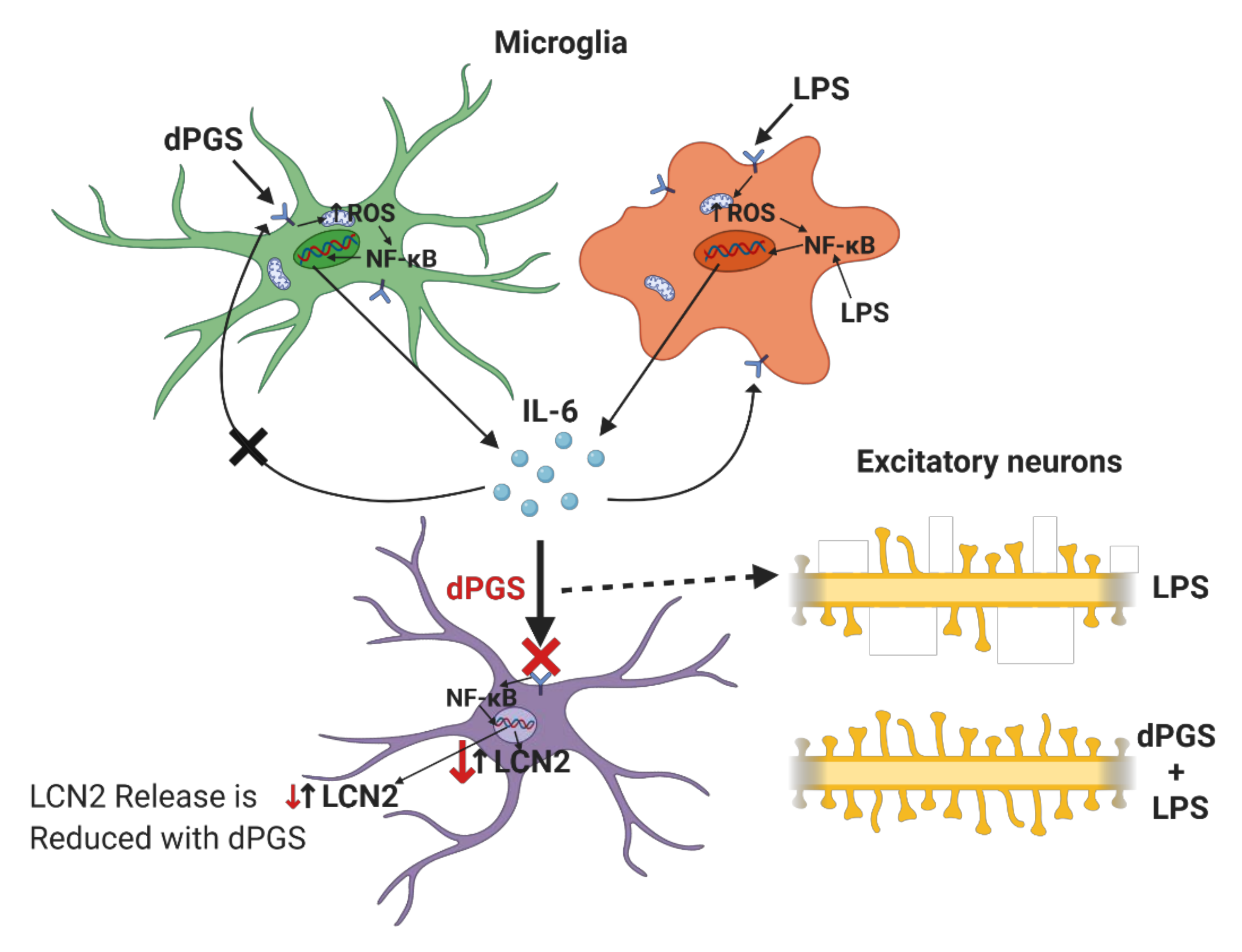
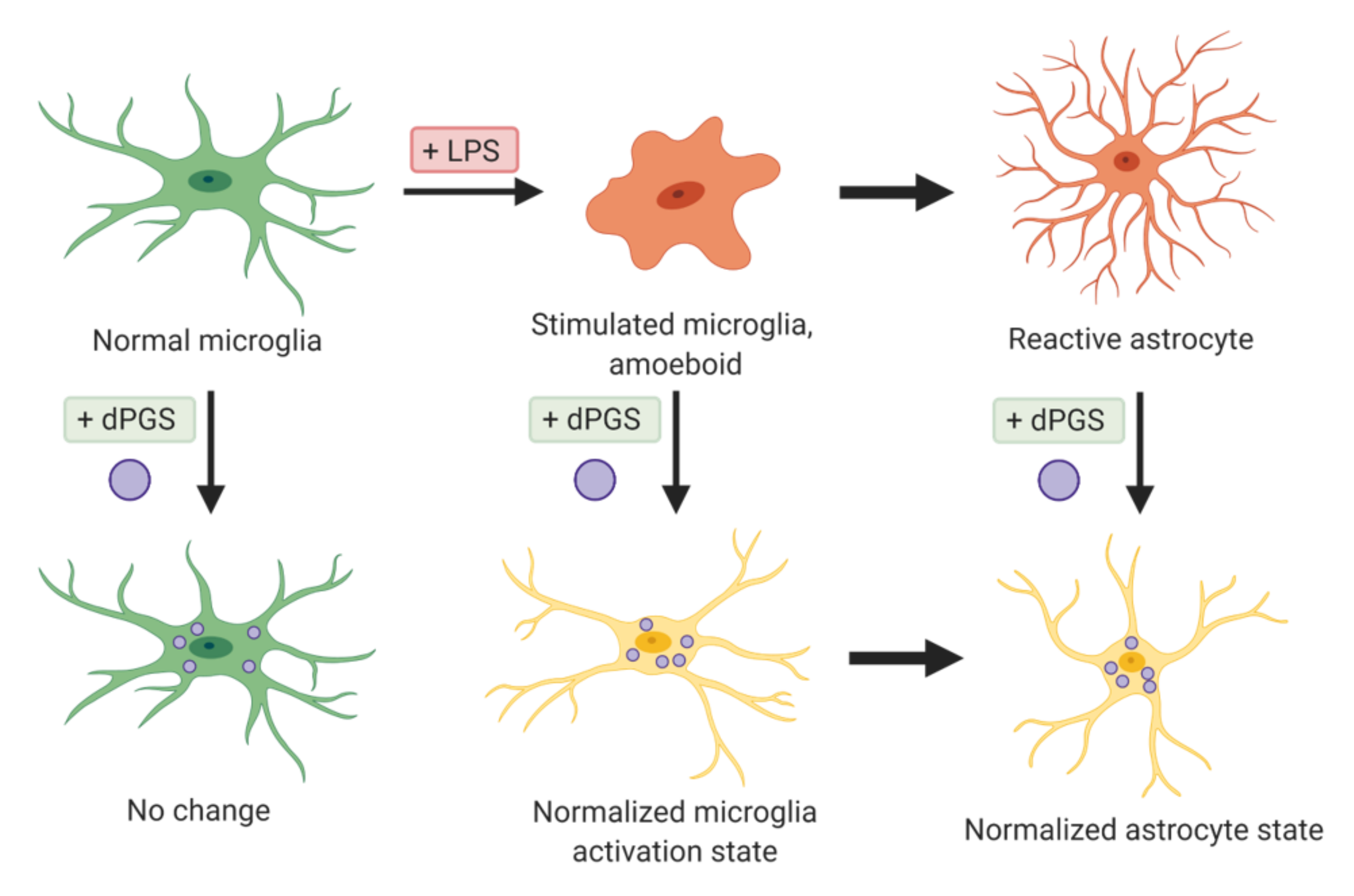
| Dendritic polyglycerols were either terminated with hydroxyl groups (dPG) or sulfate groups (dPGS). | Mouse primary cortical cultures; mouse model of microglial cell activation | dPGS alleviated LPS-induced microglia activation, reduction in LCN-2 production mainly in astrocytes. dPGS directly bound to IL-6 and LCN-2, attenuating astrocyte stimulation. | [31] |
| G3.5 dPGS | Organotypic hippocampal slice cultures | dPGS treatment in Alzheimer disease models prevents Aβ fibril formation by directly interacting with the Aβ42 peptide, and attenuating Aβ-induced neuroinflammation. | [22] |
| Sulfated polyglycerols (dPGS) and non-sulfated analogs (dPG). | Organotypic hippocampal slice cultures | dPGS reduces pro-inflammatory cytokine production from M1 microglia phenotype, and normalizes LPS-induced morphology of the hippocampal dendritic spines. | [32] |
| Anionic dPGS moieties interact with the ligand binding sites of P- and L-selectin through electrostatic interactions. | Mouse model of contact dermatitis and complement activation | The first report about the anti-inflammatory activity of dPGS. | [80] |
| Phosphorus-Based Dendrimers | |||
| Fluorescent phosphorus dendrimers. | Murine macrophages (M1 and M2 phenotypes) | Generation of the phenotype-dependent blue spectral shift upon macrophage polarization, potential use of biosensor identifying macrophages and their phenotypes. | [150] |
| Polyphosphorhydrazone (PPH)-Based Dendrimers | |||
| Azabisphosphonated (ABP) surface modification imparts anti-inflammatory activity to the dendrimer. | Mouse model of experimental autoimmune encephalomyelitis and arthritis. Human peripheral blood mononuclear cell line. | Attenuation of the pathological symptoms and mediation of the inflammatory response through regulating immune cells and decreased cytokine release. | [137,138,151] |
| Drug or Genetic Cargos | Modification | In Vitro or In Vivo | Reference |
|---|---|---|---|
| Nitric oxide (NO) | Dendrimer surface modified with 18 NO-releasing moieties. | In vitro | [152] |
| N-Acetyl-L-cysteine (NAC) | Triphenyl-phosphonium ligand modification enables mitochondrial targeting delivery of NAC. | In vitro and in vivo | [142,144] |
| - | Surface decoration with carbohydrate-based targeting moieties contributes to the macrophage-targeting ability of nanoparticles. | In vitro | [153] |
| - | Increased cellular uptake of mannose-conjugated dendrimers preferentially by injured microglia through mannose receptor-mediated endocytosis. | In vitro and in vivo | [154] |
| N-acetyl-L-cysteine (NAC) | The penetration enhancer Capmul MCM (glycerol monocaprylate) benefited in designing oral formulations of NAC. | In vitro and in vivo | [155] |
| Dexamethasone | Hyaluronic acid-conjugated dendrimers were synthesized as a subconjunctival injectable gel. | In vitro and in vivo | [146] |
| N-acetyl-L-cysteine | Positive therapeutic effects in the fetus and the newborn upon intra-amniotic administration. | In vivo | [143] |
| Triamcinolone acetonide | Inhibition of nerve injury-induced microglial activation and reduced neuropathic pain upon intrathecal administration. | In vitro and in vivo | [156] |
| - | Intravenous or intravitreally administered dendrimers could be a safer drug delivery approach compared to the current therapy, which requires direct injection in the eye. | In vivo | [157] |
| PEGylated PAMAM-Based Dendrimers | |||
| Scutellarin | Dual targetability owing to angiopepsin-2 and N-acetylated proline-glycine-proline (PGP), which selectively bind with LRP in BBB endothelial cells and CXCR2 in infiltrating neutrophils respectively. | In vitro and in vivo | [158] |
| - | Folate-conjugated dendrimers target the folate-receptor positive macrophages which play a significant role in mediating inflammatory response. | In vitro and in vivo | [159] |
| Phosphorus-Based Dendrimers | |||
| TNF-α siRNA | The cationic phosphorus dendrimers were modified with either pyrrolidinium or morpholinium terminal groups in order to improve biocompatibility of dendrimers and complexation with siRNA. | In vitro and in vivo | [141] |
3.4. Applications of Dendrimers Aside from the Delivery of Therapeutic Agents
4. Conclusions and Future Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [PubMed]
- Van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart Cancer Nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.J.; Cotter, J.D.; Knight, B.E.; Sevick-Muraca, E.M.; Sandberg, D.I.; Sirianni, R.W. Intrathecal Drug Delivery in the Era of Nanomedicine. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Majoral, J.P.; Mignani, S.M.; Shi, X.; Rodrigues, J.M.; Muñoz-Fernández, M.Á.; Ceña, V.; Roy, R. Dendrimers towards Translational Nanotherapeutics: Concise Key Step Analysis. Bioconjug. Chem. 2020, 31, 2060–2071. [Google Scholar] [CrossRef]
- John, G.; Nagarajan, S.; Vemula, P.K.; Silverman, J.R.; Pillai, C.K.S. Natural Monomers: A Mine for Functional and Sustainable Materials—Occurrence, Chemical Modification and Polymerization. Prog. Polym. Sci. 2019, 92, 158–209. [Google Scholar] [CrossRef]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging Concepts in Dendrimer-Based Nanomedicine: From Design Principles to Clinical Applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef]
- Jansens, R.J.J.; Tishchenko, A.; Favoreel, H.W. Bridging the Gap: Virus Long-Distance Spread via Tunneling Nanotubes. J. Virol. 2020, 94, e02120-19. [Google Scholar] [CrossRef]
- Pinto, G.; Brou, C.; Zurzolo, C. Tunneling Nanotubes: The Fuel of Tumor Progression? Trends Cancer 2020, 6, 874–888. [Google Scholar] [CrossRef]
- Korenkova, O.; Pepe, A.; Zurzolo, C. Fine Intercellular Connections in Development: TNTs, Cytonemes, or Intercellular Bridges? Cell Stress. 2020, 4, 30–43. [Google Scholar] [CrossRef]
- Alarcon-Martinez, L.; Villafranca-Baughman, D.; Quintero, H.; Kacerovsky, J.B.; Dotigny, F.; Murai, K.K.; Prat, A.; Drapeau, P.; Di Polo, A. Interpericyte Tunnelling Nanotubes Regulate Neurovascular Coupling. Nature 2020, 585, 91–95. [Google Scholar] [CrossRef]
- Gamache, T.R.; Araki, Y.; Huganir, R.L. Twenty Years of SynGAP Research: From Synapses to Cognition. J. Neurosci. 2020, 40, 1596–1605. [Google Scholar] [CrossRef]
- Groc, L.; Choquet, D. Linking Glutamate Receptor Movements and Synapse Function. Science 2020, 368, eaay4631. [Google Scholar] [CrossRef]
- Magee, J.C.; Grienberger, C. Synaptic Plasticity Forms and Functions. Annu. Rev. Neurosci. 2020, 43, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Yuste, R.; Majewska, A.; Holthoff, K. From Form to Function: Calcium Compartmentalization in Dendritic Spines. Nat. Neurosci. 2000, 3, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Basnayake, K.; Mazaud, D.; Bemelmans, A.; Rouach, N.; Korkotian, E.; Holcman, D. Fast Calcium Transients in Dendritic Spines Driven by Extreme Statistics. PLoS Biol. 2019, 17, e2006202. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Aparicio, P.; Rodríguez-Moreno, A. Calcium Dynamics and Synaptic Plasticity. Adv. Exp. Med. Biol. 2020, 1131, 965–984. [Google Scholar] [CrossRef] [PubMed]
- Dolphin, A.C.; Lee, A. Presynaptic Calcium Channels: Specialized Control of Synaptic Neurotransmitter Release. Nat. Rev. Neurosci. 2020, 21, 213–229. [Google Scholar] [CrossRef]
- Yasuda, R. Biophysics of Biochemical Signaling in Dendritic Spines: Implications in Synaptic Plasticity. Biophys. J. 2017, 113, 2152–2159. [Google Scholar] [CrossRef]
- Jaworski, J.; Kapitein, L.C.; Gouveia, S.M.; Dortland, B.R.; Wulf, P.S.; Grigoriev, I.; Camera, P.; Spangler, S.A.; Di Stefano, P.; Demmers, J.; et al. Dynamic Microtubules Regulate Dendritic Spine Morphology and Synaptic Plasticity. Neuron 2009, 61, 85–100. [Google Scholar] [CrossRef]
- Meyer, D.; Bonhoeffer, T.; Scheuss, V. Balance and Stability of Synaptic Structures during Synaptic Plasticity. Neuron 2014, 82, 430–443. [Google Scholar] [CrossRef]
- Okabe, S. Recent Advances in Computational Methods for Measurement of Dendritic Spines Imaged by Light Microscopy. Microscopy 2020, 69, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Maysinger, D.; Ji, J.; Moquin, A.; Hossain, S.; Hancock, M.A.; Zhang, I.; Chang, P.K.Y.; Rigby, M.; Anthonisen, M.; Grütter, P.; et al. Dendritic Polyglycerol Sulfates in the Prevention of Synaptic Loss and Mechanism of Action on Glia. ACS Chem. Neurosci. 2018, 9, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, E.S.; Huang, A.Y.-S.; Deneen, B. Astrocytogenesis: Where, When, and How. F1000Research 2020, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.C.; Morrison, E.H. Histology, Astrocytes; StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545142/ (accessed on 30 June 2020).
- Yu, X.; Nagai, J.; Khakh, B.S. Improved Tools to Study Astrocytes. Nat. Rev. Neurosci. 2020, 21, 121–138. [Google Scholar] [CrossRef]
- Upadhya, R.; Zingg, W.; Shetty, S.; Shetty, A.K. Astrocyte-Derived Extracellular Vesicles: Neuroreparative Properties and Role in the Pathogenesis of Neurodegenerative Disorders. J. Control. Release 2020, 323, 225–239. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.; Cui, B.; Liu, Z.; Shen, H. Novel Insights into Astrocyte-Mediated Signaling of Proliferation, Invasion and Tumor Immune Microenvironment in Glioblastoma. Biomed. Pharmacother. 2020, 126, 110086. [Google Scholar] [CrossRef]
- Henrik Heiland, D.; Ravi, V.M.; Behringer, S.P.; Frenking, J.H.; Wurm, J.; Joseph, K.; Garrelfs, N.W.C.; Strähle, J.; Heynckes, S.; Grauvogel, J.; et al. Tumor-Associated Reactive Astrocytes Aid the Evolution of Immunosuppressive Environment in Glioblastoma. Nat. Commun. 2019, 10, 2541. [Google Scholar] [CrossRef]
- Nutma, E.; van Gent, D.; Amor, S.; Peferoen, L.A.N. Astrocyte and Oligodendrocyte Cross-Talk in the Central Nervous System. Cells 2020, 9, 600. [Google Scholar] [CrossRef]
- Sung, K.; Jimenez-Sanchez, M. Autophagy in Astrocytes and Its Implications in Neurodegeneration. J. Mol. Biol. 2020, 432, 2605–2621. [Google Scholar] [CrossRef]
- Maysinger, D.; Lalancette-Hébert, M.; Ji, J.; Jabbour, K.; Dernedde, J.; Silberreis, K.; Haag, R.; Kriz, J. Dendritic Polyglycerols Are Modulators of Microglia-Astrocyte Crosstalk. Future Neurol. 2019, 14, FNL31. [Google Scholar] [CrossRef]
- Maysinger, D.; Gröger, D.; Lake, A.; Licha, K.; Weinhart, M.; Chang, P.K.-Y.; Mulvey, R.; Haag, R.; McKinney, R.A. Dendritic Polyglycerol Sulfate Inhibits Microglial Activation and Reduces Hippocampal CA1 Dendritic Spine Morphology Deficits. Biomacromolecules 2015, 16, 3073–3082. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Navath, R.S.; Balakrishnan, B.; Guru, B.R.; Mishra, M.K.; Romero, R.; Kannan, R.M.; Kannan, S. Intrinsic Targeting of Inflammatory Cells in the Brain by Polyamidoamine Dendrimers upon Subarachnoid Administration. Nanomedicine 2010, 5, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Nance, E.; Zhang, F.; Mishra, M.K.; Zhang, Z.; Kambhampati, S.P.; Kannan, R.M.; Kannan, S. Nanoscale Effects in Dendrimer-Mediated Targeting of Neuroinflammation. Biomaterials 2016, 101, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Aamir, K.; Shaikh, M. Impact of HMGB1, RAGE, and TLR4 in Alzheimer’s Disease (AD): From Risk Factors to Therapeutic Targeting. Cells 2020, 9, 383. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Shaikh, M. HMGB1-Mediated Neuroinflammatory Responses in Brain Injuries: Potential Mechanisms and Therapeutic Opportunities. IJMS 2020, 21, 4609. [Google Scholar] [CrossRef]
- Kang, R.; Chen, R.; Zhang, Q.; Hou, W.; Wu, S.; Cao, L.; Huang, J.; Yu, Y.; Fan, X.-G.; Yan, Z.; et al. HMGB1 in Health and Disease. Mol. Asp. Med. 2014, 40, 1–116. [Google Scholar] [CrossRef]
- Sims, G.P.; Rowe, D.C.; Rietdijk, S.T.; Herbst, R.; Coyle, A.J. HMGB1 and RAGE in Inflammation and Cancer. Annu. Rev. Immunol. 2010, 28, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Yang, H.; Harris, H. High-Mobility Group Box 1 Protein (HMGB1) Operates as an Alarmin Outside as Well as inside Cells. Semin. Immunol. 2018, 38, 40–48. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Andersson, U. Targeting Inflammation Driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef]
- Ugrinova, I.; Pasheva, E. HMGB1 Protein: A Therapeutic Target Inside and Outside the Cell. Adv. Protein Chem. Struct. Biol. 2017, 107, 37–76. [Google Scholar] [CrossRef]
- Kim, D.E.; Davalos, A.R. Alarmin Detection in Senescent Cells. Methods Mol. Biol. 2019, 1896, 71–81. [Google Scholar] [CrossRef]
- Kwak, M.S.; Kim, H.S.; Lee, B.; Kim, Y.H.; Son, M.; Shin, J.-S. Immunological Significance of HMGB1 Post-Translational Modification and Redox Biology. Front. Immunol. 2020, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- Venereau, E.; Casalgrandi, M.; Schiraldi, M.; Antoine, D.J.; Cattaneo, A.; De Marchis, F.; Liu, J.; Antonelli, A.; Preti, A.; Raeli, L.; et al. Mutually Exclusive Redox Forms of HMGB1 Promote Cell Recruitment or Proinflammatory Cytokine Release. J. Exp. Med. 2012, 209, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Janko, C.; Filipović, M.; Munoz, L.E.; Schorn, C.; Schett, G.; Ivanović-Burmazović, I.; Herrmann, M. Redox Modulation of HMGB1-Related Signaling. Antioxid. Redox Signal. 2014, 20, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Achazi, K.; Haag, R.; Ballauff, M.; Dernedde, J.; Kizhakkedathu, J.N.; Maysinger, D.; Multhaup, G. Understanding the Interaction of Polyelectrolyte Architectures with Proteins and Biosystems. Angew. Chem. Int. Ed. 2020, 38, anie-202006457. [Google Scholar] [CrossRef]
- Ori, A.; Wilkinson, M.C.; Fernig, D.G. A Systems Biology Approach for the Investigation of the Heparin/Heparan Sulfate Interactome. J. Biol. Chem. 2011, 286, 19892–19904. [Google Scholar] [CrossRef] [PubMed]
- Vainchtein, I.D.; Molofsky, A.V. Astrocytes and Microglia: In Sickness and in Health. Trends Neurosci. 2020, 43, 144–154. [Google Scholar] [CrossRef]
- Hasselmann, J.; Blurton-Jones, M. Human IPSC-derived Microglia: A Growing Toolset to Study the Brain’s Innate Immune Cells. Glia 2020, 68, 721–739. [Google Scholar] [CrossRef]
- Xu, R.; Li, X.; Boreland, A.J.; Posyton, A.; Kwan, K.; Hart, R.P.; Jiang, P. Human IPSC-Derived Mature Microglia Retain Their Identity and Functionally Integrate in the Chimeric Mouse Brain. Nat. Commun. 2020, 11, 1577. [Google Scholar] [CrossRef]
- Majo, M.; Koontz, M.; Rowitch, D.; Ullian, E.M. An Update on Human Astrocytes and Their Role in Development and Disease. Glia 2020, 68, 685–704. [Google Scholar] [CrossRef]
- Hirbec, H.; Déglon, N.; Foo, L.C.; Goshen, I.; Grutzendler, J.; Hangen, E.; Kreisel, T.; Linck, N.; Muffat, J.; Regio, S.; et al. Emerging Technologies to Study Glial Cells. Glia 2020, 68, 1692–1728. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.T.; Stephan, S.B.; Moffett, H.F.; McKnight, L.E.; Ji, W.; Reiman, D.; Bonagofski, E.; Wohlfahrt, M.E.; Pillai, S.P.S.; Stephan, M.T. In Situ Programming of Leukaemia-Specific T Cells Using Synthetic DNA Nanocarriers. Nat. Nanotechnol. 2017, 12, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Devanney, N.A.; Stewart, A.N.; Gensel, J.C. Microglia and Macrophage Metabolism in CNS Injury and Disease: The Role of Immunometabolism in Neurodegeneration and Neurotrauma. Exp. Neurol. 2020, 329, 113310. [Google Scholar] [CrossRef]
- Gopinath, A.; Collins, A.; Khoshbouei, H.; Streit, W. Microglia and Other Myeloid Cells in CNS Health and Disease. J. Pharmacol. Exp. Ther. 2020, 375, 154–160. [Google Scholar] [CrossRef]
- Greenhalgh, A.D.; David, S.; Bennett, F.C. Immune Cell Regulation of Glia during CNS Injury and Disease. Nat. Rev. Neurosci. 2020, 21, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Lauro, C.; Limatola, C. Metabolic Reprograming of Microglia in the Regulation of the Innate Inflammatory Response. Front. Immunol. 2020, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Prinz, M. Microglia Heterogeneity in the Single-Cell Era. Cell Rep. 2020, 30, 1271–1281. [Google Scholar] [CrossRef]
- Gerrits, E.; Heng, Y.; Boddeke, E.W.G.M.; Eggen, B.J.L. Transcriptional Profiling of Microglia; Current State of the Art and Future Perspectives. Glia 2020, 68, 740–755. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, S.; Shi, S.; Zhang, T.; Ma, Q.; Tian, T.; Zhou, T.; Cai, X.; Lin, Y. Anti-Inflammatory and Antioxidative Effects of Tetrahedral DNA Nanostructures via the Modulation of Macrophage Responses. ACS Appl. Mater. Interfaces 2018, 10, 3421–3430. [Google Scholar] [CrossRef]
- Gong, Y.; Winnik, F.M. Strategies in Biomimetic Surface Engineering of Nanoparticles for Biomedical Applications. Nanoscale 2012, 4, 360–368. [Google Scholar] [CrossRef]
- Hu, C.M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte Membrane-Camouflaged Polymeric Nanoparticles as a Biomimetic Delivery Platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed]
- Harkness, K.M.; Turner, B.N.; Agrawal, A.C.; Zhang, Y.; McLean, J.A.; Cliffel, D.E. Biomimetic Monolayer-Protected Gold Nanoparticles for Immunorecognition. Nanoscale 2012, 4, 3843. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.; Qi, Z.; Liang, K.; Bai, X.; Xu, J.; Liu, J.; Shen, J. An Artificial Supramolecular Nanozyme Based on β-Cyclodextrin-Modified Gold Nanoparticles. Catal. Lett. 2008, 124, 413–417. [Google Scholar] [CrossRef]
- Riccardi, L.; Gabrielli, L.; Sun, X.; De Biasi, F.; Rastrelli, F.; Mancin, F.; De Vivo, M. Nanoparticle-Based Receptors Mimic Protein-Ligand Recognition. Chemistry 2017, 3, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, A. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Cserép, C.; Pósfai, B.; Lénárt, N.; Fekete, R.; László, Z.I.; Lele, Z.; Orsolits, B.; Molnár, G.; Heindl, S.; Schwarcz, A.D.; et al. Microglia Monitor and Protect Neuronal Function through Specialized Somatic Purinergic Junctions. Science 2020, 367, 528–537. [Google Scholar] [CrossRef]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, W.; Vögtle, F. “Cascade”- and “nonskid-chain-like” synthesis of molecular cavity topologies. Synthesis 1978, 9, 155–158. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Grayson, S.M.; Fréchet, J.M.J. Convergent Dendrons and Dendrimers: From Synthesis to Applications. Chem. Rev. 2001, 101, 3819–3868. [Google Scholar] [CrossRef]
- Fana, M.; Gallien, J.; Srinageshwar, B.; Dunbar, G.L.; Rossignol, J. PAMAM Dendrimer Nanomolecules Utilized as Drug Delivery Systems for Potential Treatment of Glioblastoma: A Systematic Review. IJN 2020, 15, 2789–2808. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Jain, K.; Mehra, N.K.; Kesharwani, P.; Jain, N.K. A Review on Comparative Study of PPI and PAMAM Dendrimers. J. Nanopart. Res. 2016, 18, 146. [Google Scholar] [CrossRef]
- Tang, Z.; Kong, N.; Ouyang, J.; Feng, C.; Kim, N.Y.; Ji, X.; Wang, C.; Farokhzad, O.C.; Zhang, H.; Tao, W. Phosphorus Science-Oriented Design and Synthesis of Multifunctional Nanomaterials for Biomedical Applications. Matter 2020, 2, 297–322. [Google Scholar] [CrossRef]
- Gillies, E.R.; Fréchet, J.M.J. Designing Macromolecules for Therapeutic Applications: Polyester Dendrimer-Poly(ethylene oxide) “Bow-Tie” Hybrids with Tunable Molecular Weight and Architecture. J. Am. Chem. Soc. 2002, 124, 14137–14146. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Turrin, C.-O.; Majoral, J.-P. Biological Properties of Phosphorus Dendrimers. New J. Chem. 2010, 34, 1512. [Google Scholar] [CrossRef]
- Liaw, K.; Zhang, F.; Mangraviti, A.; Kannan, S.; Tyler, B.; Kannan, R.M. Dendrimer Size Effects on the Selective Brain Tumor Targeting in Orthotopic Tumor Models upon Systemic Administration. Bioeng. Transl. Med. 2020, 5, 154–160. [Google Scholar] [CrossRef]
- Stenström, P.; Manzanares, D.; Zhang, Y.; Ceña, V.; Malkoch, M. Evaluation of Amino-Functional Polyester Dendrimers Based on Bis-MPA as Nonviral Vectors for SiRNA Delivery. Molecules 2018, 23, 2028. [Google Scholar] [CrossRef]
- Chauhan, A.S.; Diwan, P.V.; Jain, N.K.; Tomalia, D.A. Unexpected In Vivo Anti-Inflammatory Activity Observed for Simple, Surface Functionalized Poly(Amidoamine) Dendrimers. Biomacromolecules 2009, 10, 1195–1202. [Google Scholar] [CrossRef]
- Dernedde, J.; Rausch, A.; Weinhart, M.; Enders, S.; Tauber, R.; Licha, K.; Schirner, M.; Zugel, U.; von Bonin, A.; Haag, R. Dendritic Polyglycerol Sulfates as Multivalent Inhibitors of Inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 19679–19684. [Google Scholar] [CrossRef]
- Neibert, K.; Gosein, V.; Sharma, A.; Khan, M.; Whitehead, M.A.; Maysinger, D.; Kakkar, A. “Click” Dendrimers as Anti-Inflammatory Agents: With Insights into Their Binding from Molecular Modeling Studies. Mol. Pharm. 2013, 10, 2502–2508. [Google Scholar] [CrossRef]
- Franc, G.; Kakkar, A.K. “Click” Methodologies: Efficient, Simple and Greener Routes to Design Dendrimers. Chem. Soc. Rev. 2010, 39, 1536. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Moquin, A.; Bomal, E.; Na, L.; Maysinger, D.; Kakkar, A. Telodendrimers for Physical Encapsulation and Covalent Linking of Individual or Combined Therapeutics. Mol. Pharm. 2017, 14, 2607–2615. [Google Scholar] [CrossRef]
- Zhong, Y.; Dimde, M.; Stöbener, D.; Meng, F.; Deng, C.; Zhong, Z.; Haag, R. Micelles with Sheddable Dendritic Polyglycerol Sulfate Shells Show Extraordinary Tumor Targetability and Chemotherapy in Vivo. ACS Appl. Mater. Inter. 2016, 8, 27530–27538. [Google Scholar] [CrossRef] [PubMed]
- Sadekar, S.; Ghandehari, H. Transepithelial Transport and Toxicity of PAMAM Dendrimers: Implications for Oral Drug Delivery. Adv. Drug Deliv. Rev. 2012, 64, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-T.; Neerman, M.F.; Parrish, A.R.; Simanek, E.E. Cytotoxicity, Hemolysis, and Acute in Vivo Toxicity of Dendrimers Based on Melamine, Candidate Vehicles for Drug Delivery. J. Am. Chem. Soc. 2004, 126, 10044–10048. [Google Scholar] [CrossRef] [PubMed]
- Kolhatkar, R.B.; Kitchens, K.M.; Swaan, P.W.; Ghandehari, H. Surface Acetylation of Polyamidoamine (PAMAM) Dendrimers Decreases Cytotoxicity While Maintaining Membrane Permeability. Bioconjug. Chem. 2007, 18, 2054–2060. [Google Scholar] [CrossRef]
- Barraza, L.F.; Jiménez, V.A.; Alderete, J.B. Effect of PEGylation on the Structure and Drug Loading Capacity of PAMAM-G4 Dendrimers: A Molecular Modeling Approach on the Complexation of 5-Fluorouracil with Native and PEGylated PAMAM-G4. Macromol. Chem. Phys. 2015, 216, 1689–1701. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Mohd Amin, M.C.I.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM Dendrimers: Enhancing Efficacy and Mitigating Toxicity for Effective Anticancer Drug and Gene Delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef]
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in Drug Delivery and Targeting: Drug-Dendrimer Interactions and Toxicity Issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150. [Google Scholar] [CrossRef]
- Tiwari, A.; Srivastava, S.; Pant, M. Brain Tumor Segmentation and Classification from Magnetic Resonance Images: Review of Selected Methods from 2014 to 2019. Pattern Recognit. Lett. 2020, 131, 244–260. [Google Scholar] [CrossRef]
- Karimi, S.; Zuccato, J.A.; Mamatjan, Y.; Mansouri, S.; Suppiah, S.; Nassiri, F.; Diamandis, P.; Munoz, D.G.; Aldape, K.D.; Zadeh, G. The Central Nervous System Tumor Methylation Classifier Changes Neuro-Oncology Practice for Challenging Brain Tumor Diagnoses and Directly Impacts Patient Care. Clin. Epigenetics 2019, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Neftel, C.; Laffy, J.; Filbin, M.G.; Hara, T.; Shore, M.E.; Rahme, G.J.; Richman, A.R.; Silverbush, D.; Shaw, M.L.; Hebert, C.M.; et al. An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 2019, 178, 835–849. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Abelseth, E.; de la Vega, L.; Willerth, S.M. Bioprinting a Novel Glioblastoma Tumor Model Using a Fibrin-Based Bioink for Drug Screening. Mater. Today Chem. 2019, 12, 78–84. [Google Scholar] [CrossRef]
- Heinrich, M.A.; Bansal, R.; Lammers, T.; Zhang, Y.S.; Michel Schiffelers, R.; Prakash, J. 3D-Bioprinted Mini-Brain: A Glioblastoma Model to Study Cellular Interactions and Therapeutics. Adv. Mater. 2019, 31, 1806590. [Google Scholar] [CrossRef] [PubMed]
- Lara-Velazquez, M.; Al-Kharboosh, R.; Jeanneret, S.; Vazquez-Ramos, C.; Mahato, D.; Tavanaiepour, D.; Rahmathulla, G.; Quinones-Hinojosa, A. Advances in Brain Tumor Surgery for Glioblastoma in Adults. Brain Sci. 2017, 7, 166. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, R.; Liu, M.; Feng, J.; Chen, J.; Hu, K. Remodeling the Blood–Brain Barrier Microenvironment by Natural Products for Brain Tumor Therapy. Acta Pharm. Sin. B 2017, 7, 541–553. [Google Scholar] [CrossRef]
- Fertil, B.; Malaise, E.-P. Inherent Cellular Radiosensitivity as a Basic Concept for Human Tumor Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1981, 7, 621–629. [Google Scholar] [CrossRef]
- Tang, W.; Fan, W.; Lau, J.; Deng, L.; Shen, Z.; Chen, X. Emerging Blood–Brain-Barrier-Crossing Nanotechnology for Brain Cancer Theranostics. Chem. Soc. Rev. 2019, 48, 2967–3014. [Google Scholar] [CrossRef]
- Dhaliwal, H.K.; Fan, Y.; Kim, J.; Amiji, M.M. Intranasal Delivery and Transfection of MRNA Therapeutics in the Brain Using Cationic Liposomes. Mol. Pharm. 2020, 17, 1996–2005. [Google Scholar] [CrossRef]
- Kuang, Y.; An, S.; Guo, Y.; Huang, S.; Shao, K.; Liu, Y.; Li, J.; Ma, H.; Jiang, C. T7 Peptide-Functionalized Nanoparticles Utilizing RNA Interference for Glioma Dual Targeting. Int. J. Pharm. 2013, 454, 11–20. [Google Scholar] [CrossRef]
- Dumitru, A.C.; Herruzo, E.T.; Rausell, E.; Ceña, V.; Garcia, R. Unbinding Forces and Energies between a SiRNA Molecule and a Dendrimer Measured by Force Spectroscopy. Nanoscale 2015, 7, 20267–20276. [Google Scholar] [CrossRef] [PubMed]
- Scaggiante, B.; Dapas, B.; Farra, R.; Grassi, M.; Pozzato, G.; Giansante, C.; Grassi, G. Improving SiRNA Bio-Distribution and Minimizing Side Effects. CDM 2011, 12, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, Z.; Gao, H.; Rostami, I.; You, Q.; Jia, X.; Wang, C.; Zhu, L.; Yang, Y. Enhanced Blood-Brain-Barrier Penetrability and Tumor-Targeting Efficiency by Peptide-Functionalized Poly(Amidoamine) Dendrimer for the Therapy of Gliomas. Nanotheranostics 2019, 3, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Muniswamy, V.J.; Raval, N.; Gondaliya, P.; Tambe, V.; Kalia, K.; Tekade, R.K. ‘Dendrimer-Cationized-Albumin’ Encrusted Polymeric Nanoparticle Improves BBB Penetration and Anticancer Activity of Doxorubicin. Int. J. Pharm. 2019, 555, 77–99. [Google Scholar] [CrossRef]
- Uram, Ł.; Filipowicz, A.; Misiorek, M.; Pie, N.; Markowicz, J. Biotinylated PAMAM G3 Dendrimer Conjugated with Celecoxib and / or Fmoc-L-Leucine and Its Cytotoxicity for Normal and Cancer Human Cell Lines. Eur. J. Pharm. Sci. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Uram, Ł.; Misiorek, M.; Pichla, M.; Filipowicz-Rachwał, A.; Markowicz, J.; Wołowiec, S.; Wałajtys-Rode, E. The Effect of Biotinylated PAMAM G3 Dendrimers Conjugated with COX-2 Inhibitor (Celecoxib) and PPARγ Agonist (Fmoc-L-Leucine) on Human Normal Fibroblasts, Immortalized Keratinocytes and Glioma Cells in Vitro. Molecules 2019, 24, 3801. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, S.; Qian, L.; Pei, Y.; Qiu, Y.; Jiang, Y. RGD-Modified PEG-PAMAM-DOX Conjugates: In Vitro and in Vivo Studies for Glioma. Eur. J. Pharm. Biopharm. 2011, 79, 232–240. [Google Scholar] [CrossRef]
- Jiang, Y.; Lv, L.; Shi, H.; Hua, Y.; Lv, W.; Wang, X.; Xin, H.; Xu, Q. PEGylated Polyamidoamine Dendrimer Conjugated with Tumor Homing Peptide as a Potential Targeted Delivery System for Glioma. Colloids Surf. B. Biointerfaces 2016, 147, 242–249. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, B.; Shen, S.; Chen, J.; Zhang, Q.; Jiang, X.; Pang, Z. CREKA Peptide-Conjugated Dendrimer Nanoparticles for Glioblastoma Multiforme Delivery. J. Colloid Interface Sci. 2015, 450, 396–403. [Google Scholar] [CrossRef]
- Zaręba, M.; Sareło, P.; Kopaczyńska, M.; Białońska, A.; Uram, Ł.; Walczak, M.; Aebisher, D.; Wołowiec, S. Mixed-Generation PAMAM G3-G0 Megamer as a Drug Delivery System for Nimesulide: Antitumor Activity of the Conjugate Against Human Squamous Carcinoma and Glioblastoma Cells. Int. J. Mol. Sci. 2019, 20, 4998. [Google Scholar] [CrossRef]
- Bae, Y.; Thuy, L.T.; Lee, Y.H.; Ko, K.S.; Han, J.; Choi, J.S. Polyplexes of Functional PAMAM Dendrimer/Apoptin Gene Induce Apoptosis of Human Primary Glioma Cells In Vitro. Polymers 2019, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Mastorakos, P.; Mishra, M.K.; Mangraviti, A.; Hwang, L.; Zhou, J.; Hanes, J.; Brem, H.; Olivi, A.; Tyler, B.; et al. Uniform Brain Tumor Distribution and Tumor Associated Macrophage Targeting of Systemically Administered Dendrimers. Biomaterials 2015, 52, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-W.; Huang, C.-Y.; Lin, C.-W.; Liu, H.-L.; Huang, C.-W.; Liao, S.-S.; Chen, P.-Y.; Lu, Y.-J.; Wei, K.-C.; Ma, C.-C.M. Gadolinium-functionalized nanographene oxide for combined drug and microRNA delivery and magnetic resonance imaging. Biomaterials 2014, 35, 6534–6542. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhou, X.; Mei, M.; Yuan, X.-B.; Han, L.; Wang, G.-X.; Jia, Z.-F.; Xu, P.; Pu, P.-Y.; Kang, C.-S. MicroRNA-21 Inhibitor Sensitizes Human Glioblastoma Cells U251 (PTEN-Mutant) and LN229 (PTEN-Wild Type) to Taxol. BMC Cancer 2010, 10, 27. [Google Scholar] [CrossRef]
- Hu, X.; Chai, Z.; Lu, L.; Ruan, H.; Wang, R.; Zhan, C.; Xie, C.; Pan, J.; Liu, M.; Wang, H.; et al. Bortezomib Dendrimer Prodrug-Based Nanoparticle System. Adv. Funct. Mater. 2019, 29, 1807941. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, Q.; Zeng, F.; Zhu, J.; Chen, J.; Fan, D.; Li, X.; Duan, W.; Guo, Q.; Cao, Z.; et al. Peptide-Functionalized Nanoinhibitor Restrains Brain Tumor Growth by Abrogating Mesenchymal-Epithelial Transition Factor (MET) Signaling. Nano Lett. 2018, 18, 5488–5498. [Google Scholar] [CrossRef]
- Liu, F.-H.; Hou, C.-Y.; Zhang, D.; Zhao, W.-J.; Cong, Y.; Duan, Z.-Y.; Qiao, Z.-Y.; Wang, H. Enzyme-Sensitive Cytotoxic Peptide–Dendrimer Conjugates Enhance Cell Apoptosis and Deep Tumor Penetration. Biomater. Sci. 2018, 6, 604–613. [Google Scholar] [CrossRef]
- Vossen, L.I.; Markovsky, E.; Eldar-Boock, A.; Tschiche, H.R.; Wedepohl, S.; Pisarevsky, E.; Satchi-Fainaro, R.; Calderón, M. PEGylated Dendritic Polyglycerol Conjugate Targeting NCAM-Expressing Neuroblastoma: Limitations and Challenges. Nanomedicine: Nanotechnology. Biol. Med. 2018, 14, 1169–1179. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Choudhary, M.; Kandasubramanian, B. Recent Advances in Dendrimer-Based Nanoplatform for Cancer Treatment: A Review. Eur. Polym. J. 2020, 126, 109546. [Google Scholar] [CrossRef]
- Igartúa, D.E.; Martinez, C.S.; Alonso, S.V.; Chiaramoni, N.S.; Prieto, M.J. Toxicity Assessment of Free and Dendrimer-Complexed Curcumin in Zebrafish Larvae. PharmaNutrition 2020, 13, 100201. [Google Scholar] [CrossRef]
- Hsu, H.-J.; Bugno, J.; Lee, S.; Hong, S. Dendrimer-Based Nanocarriers: A Versatile Platform for Drug Delivery: Dendrimer-Based Nanocarriers. Wires Nanomed. Nanobiotechnol. 2017, 9, e1409. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Narmani, A.; Jafari, S.M. Dendrimers as Efficient Nanocarriers for the Protection and Delivery of Bioactive Phytochemicals. Adv. Colloid Interface Sci. 2020, 278, 102125. [Google Scholar] [CrossRef] [PubMed]
- Parhi, P.; Mohanty, C.; Sahoo, S.K. Nanotechnology-Based Combinational Drug Delivery: An Emerging Approach for Cancer Therapy. Drug Discov. Today 2012, 17, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Lai, T.C.; Tomoda, K.; Kwon, G.S. Polymeric Micelles for Multi-Drug Delivery in Cancer. Aaps Pharmscitech. 2015, 16, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Lou, S.; Hu, Y.; Zhu, J.; Zhang, C. A Nano-in-Nano Polymer–Dendrimer Nanoparticle-Based Nanosystem for Controlled Multidrug Delivery. Mol. Pharm. 2017, 14, 2697–2710. [Google Scholar] [CrossRef]
- Mejlsøe, S.; Kakkar, A. “Telodendrimers: Promising architectural polymers for applications in biology”. Molecules 2020, 25, 3995. [Google Scholar] [CrossRef]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 Activation by Natural Phytochemicals: An Overview. Front. Pharmacol. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Lee, S.-T.; Chu, K.; Oh, H.-J.; Im, W.-S.; Lim, J.-Y.; Kim, S.-K.; Park, C.-K.; Jung, K.-H.; Lee, S.K.; Kim, M.; et al. Let-7 MicroRNA Inhibits the Proliferation of Human Glioblastoma Cells. J. Neurooncol. 2011, 102, 19–24. [Google Scholar] [CrossRef]
- He, Q.; Chen, J.; Yan, J.; Cai, S.; Xiong, H.; Liu, Y.; Peng, D.; Liu, Z.; Mo, M. Tumor Microenvironment Responsive Drug Delivery Systems. Asian J. Pharm. Sci. 2020, 15, 416–4481. [Google Scholar] [CrossRef]
- Han, M.; Huang-Fu, M.-Y.; Guo, W.-W.; Guo, N.-N.; Chen, J.; Liu, H.-N.; Xie, Z.-Q.; Lin, M.-T.; Wei, Q.-C.; Gao, J.-Q. MMP-2-Sensitive HA End-Conjugated Poly(Amidoamine) Dendrimers via Click Reaction To Enhance Drug Penetration into Solid Tumor. ACS Appl. Mater. Inter. 2017, 9, 42459–42470. [Google Scholar] [CrossRef]
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR Effect: Combined Strategies to Improve the EPR Effect in the Tumor Microenvironment. Theranostics 2019, 9, 8073–8090. [Google Scholar] [CrossRef] [PubMed]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of Enhanced Permeability and Retention Effect (EPR): Nanoparticle-Based Precision Tools for Targeting of Therapeutic and Diagnostic Agent in Cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.R.H.; Bicanic, T.; Salim, R.; Hope, W. Liposomal Amphotericin B (AmBisome®): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef]
- Rupp, R.; Rosenthal, S.L.; Stanberry, L.R. VivaGel (SPL7013 Gel): A Candidate Dendrimer—Microbicide for the Prevention of HIV and HSV Infection. Int. J. Nanomed. 2007, 2, 561–566. [Google Scholar]
- Price, C.F.; Tyssen, D.; Sonza, S.; Davie, A.; Evans, S.; Lewis, G.R.; Xia, S.; Spelman, T.; Hodsman, P.; Moench, T.R.; et al. SPL7013 Gel (VivaGel®) Retains Potent HIV-1 and HSV-2 Inhibitory Activity Following Vaginal Administration in Humans. PLoS ONE 2011, 6, e24095. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Varilh, M.; Turrin, C.-O.; Saoudi, A.; Caminade, A.-M.; Poupot, R.; Liblau, R.S. Phosphorus-Based Dendrimer ABP Treats Neuroinflammation by Promoting IL-10-Producing CD4+ T Cells. Biomacromolecules 2015, 16, 3425–3433. [Google Scholar] [CrossRef]
- Hayder, M.; Poupot, M.; Baron, M.; Nigon, D.; Turrin, C.-O.; Caminade, A.-M.; Majoral, J.-P.; Eisenberg, R.A.; Fournie, J.-J.; Cantagrel, A.; et al. A Phosphorus-Based Dendrimer Targets Inflammation and Osteoclastogenesis in Experimental Arthritis. Sci. Transl. Med. 2011, 3, 81ra35. [Google Scholar] [CrossRef]
- Iezzi, R.; Guru, B.R.; Glybina, I.V.; Mishra, M.K.; Kennedy, A.; Kannan, R.M. Dendrimer-Based Targeted Intravitreal Therapy for Sustained Attenuation of Neuroinflammation in Retinal Degeneration. Biomaterials 2012, 33, 979–988. [Google Scholar] [CrossRef]
- Vijayaraj Kumar, P.; Agashe, H.; Dutta, T.; Jain, N. PEGylated Dendritic Architecture for Development of a Prolonged Drug Delivery System for an Antitubercular Drug. Curr. Drug Deliv. 2007, 4, 11–19. [Google Scholar] [CrossRef]
- Bohr, A.; Tsapis, N.; Andreana, I.; Chamarat, A.; Foged, C.; Delomenie, C.; Noiray, M.; El Brahmi, N.; Majoral, J.-P.; Mignani, S.; et al. Anti-Inflammatory Effect of Anti-TNF-α SiRNA Cationic Phosphorus Dendrimer Nanocomplexes Administered Intranasally in a Murine Acute Lung Injury Model. Biomacromolecules 2017, 18, 2379–2388. [Google Scholar] [CrossRef]
- Sharma, R.; Kambhampati, S.P.; Zhang, Z.; Sharma, A.; Chen, S.; Duh, E.I.; Kannan, S.; Tso, M.O.M.; Kannan, R.M. Dendrimer Mediated Targeted Delivery of Sinomenine for the Treatment of Acute Neuroinflammation in Traumatic Brain Injury. J. Control. Release 2020, 323, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Rosenzweig, J.M.; Mishra, M.K.; Alshehri, W.; Brancusi, F.; McLane, M.; Almalki, A.; Bahabry, R.; Arif, H.; Rozzah, R.; et al. Maternal Dendrimer-Based Therapy for Inflammation-Induced Preterm Birth and Perinatal Brain Injury. Sci. Rep. 2017, 7, 6106. [Google Scholar] [CrossRef]
- Sharma, A.; Liaw, K.; Sharma, R.; Zhang, Z.; Kannan, S.; Kannan, R.M. Targeting Mitochondrial Dysfunction and Oxidative Stress in Activated Microglia Using Dendrimer-Based Therapeutics. Theranostics 2018, 8, 5529–5547. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kim, S.-Y.; Sharma, A.; Zhang, Z.; Kambhampati, S.P.; Kannan, S.; Kannan, R.M. Activated Microglia Targeting Dendrimer–Minocycline Conjugate as Therapeutics for Neuroinflammation. Bioconjug. Chem. 2017, 28, 2874–2886. [Google Scholar] [CrossRef] [PubMed]
- Soiberman, U.; Kambhampati, S.P.; Wu, T.; Mishra, M.K.; Oh, Y.; Sharma, R.; Wang, J.; Al Towerki, A.E.; Yiu, S.; Stark, W.J.; et al. Subconjunctival Injectable Dendrimer-Dexamethasone Gel for the Treatment of Corneal Inflammation. Biomaterials 2017, 125, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, R.; Zhang, Z.; Liaw, K.; Kambhampati, S.P.; Porterfield, J.E.; Lin, K.C.; DeRidder, L.B.; Kannan, S.; Kannan, R.M. Dense Hydroxyl Polyethylene Glycol Dendrimer Targets Activated Glia in Multiple CNS Disorders. Sci. Adv. 2020, 6, eaay8514. [Google Scholar] [CrossRef]
- Tang, Y.; Han, Y.; Liu, L.; Shen, W.; Zhang, H.; Wang, Y.; Cui, X.; Wang, Y.; Liu, G.; Qi, R. Protective Effects and Mechanisms of G5 PAMAM Dendrimers against Acute Pancreatitis Induced by Caerulein in Mice. Biomacromolecules 2015, 16, 174–182. [Google Scholar] [CrossRef]
- Lee, J.; Jackman, J.G.; Kwun, J.; Manook, M.; Moreno, A.; Elster, E.A.; Kirk, A.D.; Leong, K.W.; Sullenger, B.A. Nucleic Acid Scavenging Microfiber Mesh Inhibits Trauma-Induced Inflammation and Thrombosis. Biomaterials 2017, 120, 94–102. [Google Scholar] [CrossRef]
- Shakhbazau, A.; Mishra, M.; Chu, T.-H.; Brideau, C.; Cummins, K.; Tsutsui, S.; Shcharbin, D.; Majoral, J.-P.; Mignani, S.; Blanchard-Desce, M.; et al. Fluorescent Phosphorus Dendrimer as a Spectral Nanosensor for Macrophage Polarization and Fate Tracking in Spinal Cord Injury: Fluorescent Phosphorus Dendrimer as a Macrophage Tracer. Macromol. Biosci. 2015, 15, 1523–1534. [Google Scholar] [CrossRef]
- Portevin, D.; Poupot, M.; Rolland, O.; Turrin, C.-O.; Fournié, J.-J.; Majoral, J.-P.; Caminade, A.-M.; Poupot, R. Regulatory Activity of Azabisphosphonate-Capped Dendrimers on Human CD4+ T Cell Proliferation Enhances Ex-Vivo Expansion of NK Cells from PBMCs for Immunotherapy. J. Transl. Med. 2009, 7, 82. [Google Scholar] [CrossRef]
- Garzón-Porras, A.M.; Bertuzzi, D.L.; Lucas, K.; da Silva, L.C.E.; de Oliveira, M.G.; Ornelas, C. Nitric Oxide Releasing Polyamide Dendrimer with Anti-Inflammatory Activity. ACS Appl. Polym. Mater. 2020, 2, 2027–2034. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, M.; Li, Z.; Xiao, Y.; Bai, X.; Boakye-Yiadom, K.O.; Xu, X.; Zhang, X.-Q. Biodegradable Nanoparticles Decorated with Different Carbohydrates for Efficient Macrophage-Targeted Gene Therapy. J. Control. Release 2020, 323, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Porterfield, J.E.; Smith, E.; Sharma, R.; Kannan, S.; Kannan, R.M. Effect of Mannose Targeting of Hydroxyl PAMAM Dendrimers on Cellular and Organ Biodistribution in a Neonatal Brain Injury Model. J. Control. Release 2018, 283, 175–189. [Google Scholar] [CrossRef]
- Kannan, S.; Dai, H.; Navath, R.S.; Balakrishnan, B.; Jyoti, A.; Janisse, J.; Romero, R.; Kannan, R.M. Dendrimer-Based Postnatal Therapy for Neuroinflammation and Cerebral Palsy in a Rabbit Model. Sci. Transl. Med. 2012, 4, 130ra46. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, B.; Lim, H.; Min, H.; Oh, J.H.; Choi, S.; Cho, J.G.; Park, J.-S.; Lee, S.J. Polyamidoamine Dendrimer-Conjugated Triamcinolone Acetonide Attenuates Nerve Injury-Induced Spinal Cord Microglia Activation and Mechanical Allodynia. Mol. Pain 2017, 13, 174480691769700. [Google Scholar] [CrossRef]
- Kambhampati, S.P.; Clunies-Ross, A.J.M.; Bhutto, I.; Mishra, M.K.; Edwards, M.; McLeod, D.S.; Kannan, R.M.; Lutty, G. Systemic and Intravitreal Delivery of Dendrimers to Activated Microglia/Macrophage in Ischemia/Reperfusion Mouse Retina. Invest. Ophth. Vis. Sci. 2015, 56, 4413. [Google Scholar] [CrossRef]
- Dang, Y.; An, C.; Li, Y.; Han, D.; Liu, X.; Zhang, F.; Xu, Y.; Zhong, H.; Karim Khan, M.K.; Zou, F.; et al. Neutrophil-Mediated and Low Density Lipoprotein Receptor-Mediated Dual-Targeting Nanoformulation Enhances Brain Accumulation of Scutellarin and Exerts Neuroprotective Effects against Ischemic Stroke. RSC Adv. 2019, 9, 1299–1318. [Google Scholar] [CrossRef]
- Poh, S.; Putt, K.S.; Low, P.S. Folate-Targeted Dendrimers Selectively Accumulate at Sites of Inflammation in Mouse Models of Ulcerative Colitis and Atherosclerosis. Biomacromolecules 2017, 18, 3082–3088. [Google Scholar] [CrossRef]
- Lamy, C.M.; Sallin, O.; Loussert, C.; Chatton, J.-Y. Sodium Sensing in Neurons with a Dendrimer-Based Nanoprobe. ACS Nano 2012, 6, 1176–1187. [Google Scholar] [CrossRef]
- Jansen, J.F.G.A.; de Brabander-van den Berg, E.M.M.; Meijer, E.W. Encapsulation of Guest Molecules into a Dendritic Box. Science 1994, 266, 1226–1229. [Google Scholar] [CrossRef]
- Kline, K.K.; Morgan, E.J.; Norton, L.K.; Tucker, S.A. Encapsulation and Quantification of Multiple Dye Guests in Unmodified Poly(Amidoamine) Dendrimers as a Function of Generation. Talanta 2009, 78, 1489–1491. [Google Scholar] [CrossRef] [PubMed]
- Kreitzer, A.C.; Gee, K.R.; Archer, E.A.; Regehr, W.G. Monitoring Presynaptic Calcium Dynamics in Projection Fibers by In Vivo Loading of a Novel Calcium Indicator. Neuron 2000, 27, 25–32. [Google Scholar] [CrossRef]
- Walsh, R.; Morales, J.M.; Skipwith, C.G.; Ruckh, T.T.; Clark, H.A. Enzyme-Linked DNA Dendrimer Nanosensors for Acetylcholine. Sci. Rep. 2015, 5, 14832. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Zhao, L.; Xu, X.; Zhu, M.; Liu, C.; Sun, N.; Yang, J.; Shi, X.; Zhao, J. LyP-1-Modified Multifunctional Dendrimers for Targeted Antitumor and Antimetastasis Therapy. ACS Appl. Mater. Inter. 2020, 12, 12395–12406. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Guo, C.; Ren, D.; Zhao, Y.; Xiao, W.; Jiao, W. Aptamer-Dendrimer Bioconjugates for Targeted Delivery of MiR-34a Expressing Plasmid and Antitumor Effects in Non-Small Cell Lung Cancer Cells. PLoS ONE 2015, 10, e0139136. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, K.; Wang, Y.; Zhang, C.; Shi, M.; Wang, P.; Shen, L.; Xia, J.; Ye, L.; Shi, X.; et al. A Multifunctional Low-Generation Dendrimer-Based Nanoprobe for the Targeted Dual Mode MR/CT Imaging of Orthotopic Brain Gliomas. J. Mater. Chem. B 2019, 7, 3639–3643. [Google Scholar] [CrossRef]
- Morshed, M.N.; Behary, N.; Bouazizi, N.; Guan, J.; Chen, G.; Nierstrasz, V. Surface Modification of Polyester Fabric Using Plasma-Dendrimer for Robust Immobilization of Glucose Oxidase Enzyme. Sci. Rep. 2019, 9, 15730. [Google Scholar] [CrossRef]
- Yang, S.K.; Shi, X.; Park, S.; Ha, T.; Zimmerman, S.C. A Dendritic Single-Molecule Fluorescent Probe That Is Monovalent, Photostable and Minimally Blinking. Nat. Chem. 2013, 5, 692–697. [Google Scholar] [CrossRef]
- Darbre, T.; Reymond, J.-L. Peptide Dendrimers as Artificial Enzymes, Receptors, and Drug-Delivery Agents. Acc. Chem. Res. 2006, 39, 925–934. [Google Scholar] [CrossRef]

| Dendrimer | Application | Main Features | Reference |
|---|---|---|---|
| PAMAM | Diagnostic probe; Anti-cancer and antimetastatic agent | PAMAM dendrimers labeled with radioactive 131I and modified with LyP-1 peptides, allow conjugates to target tumors. Used in SPECT imaging, radionuclide therapy, and as an antimetastatic agent. | [165] |
| PAMAM | Drug delivery | Hyaluronic acid terminated surface deshielded through MMP-2 cleavable linkages at tumor sites. Dendrimer size shrinks from ~200 to ~10 nm, promoting EPR effect, facilitating cellular uptake. | [131] |
| PAMAM | Gene transfection | Aptamer S6 against A549 lung carcinoma screened and selected by cell-SELEX. | [166] |
| PAMAM | Nanoprobe | Highly sensitive to dynamic cellular sodium changes, and encapsulates fluorescent dyes. | [160] |
| PEGylated PAMAM | Nanoprobe | MR/CT dual-mode imaging, Dendrimers encapsulate AuNPs for CT imaging, and chelate with Mn(II) for high contrast abilities. Functionalization with RGD peptides leads to avb3 integrin targeting. | [167] |
| PEG PAMAM | Fibrous bio-catalyst | PAMAM or PEG dendrimers grafted on polyester fabrics activated through plasma treatments, followed by immobilization of glucose oxidase enzyme. | [168] |
| dPGS PCL | Drug delivery | Sheddable dPGS shell selectively binds L-selection. Intrinsic targeting ability to inflammatory and tumor sites. GSH-cleavable disulfide bonds provide controlled drug release. The first study showing that dPGS could be a potential alternative to PEG. | [84] |
| Polyglycerol | Fluorescent probe | Polyglycerol dendrimers were conjugated to a BODIPY core for single-molecule imaging. | [169] |
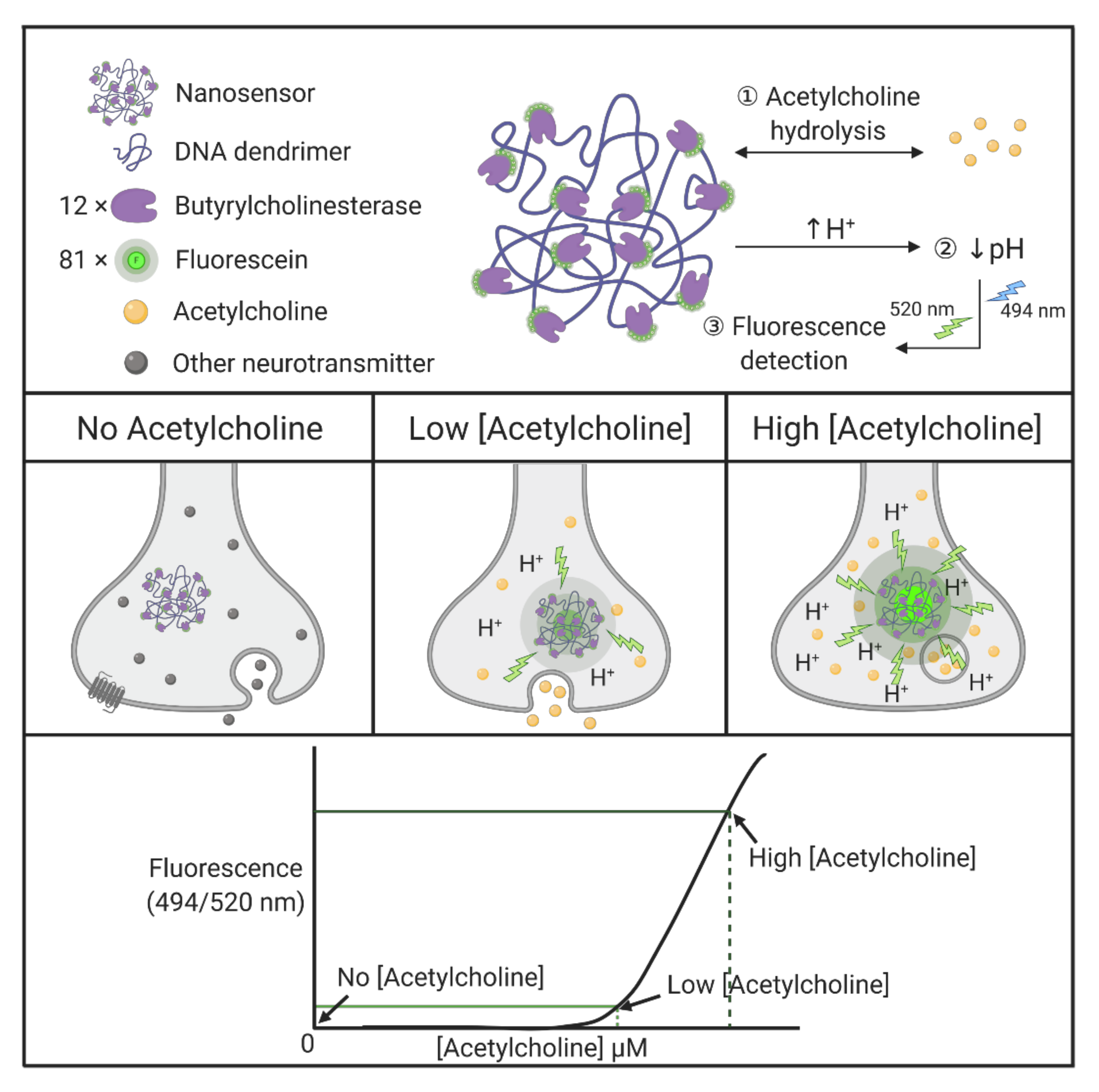
| DNA | Nanosensor | Butyrylcholinesterase and fluorescein-conjugated with DNA dendrimers, generating dendritic scaffolds, act as selective nanosensors for acetylcholine. Butyrylcholinesterase enzymes selectively hydrolyze acetylcholine and lower local pH, followed by detection of the pH-sensitive fluorescent indicator in a single synapse. | [164] |
| Amino acid | Enzyme model; Drug delivery | Dendritic peptides used as models of natural enzymes. The globular shape mimics protein structure and shows catalytic activity. | [170] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maysinger, D.; Zhang, Q.; Kakkar, A. Dendrimers as Modulators of Brain Cells. Molecules 2020, 25, 4489. https://doi.org/10.3390/molecules25194489
Maysinger D, Zhang Q, Kakkar A. Dendrimers as Modulators of Brain Cells. Molecules. 2020; 25(19):4489. https://doi.org/10.3390/molecules25194489
Chicago/Turabian StyleMaysinger, Dusica, Qiaochu Zhang, and Ashok Kakkar. 2020. "Dendrimers as Modulators of Brain Cells" Molecules 25, no. 19: 4489. https://doi.org/10.3390/molecules25194489
APA StyleMaysinger, D., Zhang, Q., & Kakkar, A. (2020). Dendrimers as Modulators of Brain Cells. Molecules, 25(19), 4489. https://doi.org/10.3390/molecules25194489







