Abstract
The first total synthesis of novel skeleton natural compounds kinkeloids A and B, a group of newly discovered flavan alkaloids isolated from the African plant Combretum micranthum, are described in this study. The key and final step are achieved by Mannich reaction, through which the piperidine moiety couples to the flavan moiety. The identities of synthesized kinkeloids were further confirmed through a comparison with the ones in the plant leaves extract using LC/MS.
1. Introduction
Kinkeloids are a series of newly discovered natural compounds with a novel skeleton from the leaves of the shrub plant Combretum micranthum, which is indigenous to Senegal and West Africa and commonly called kinkeliba. The leaves of kinkeliba are widely consumed as an herbal tea in West Africa and for the treatment of gastrointestinal problems, colic and vomiting [1,2]. The beverage brewed from the dried leaves is generally used as a panacea for possible weight loss and has shown antihyperglycemic activity in vivo [3]. Kinkeloids belong to the family of flavonoid alkaloids, which are chemically interesting as the skeletons can serve as a platform for targeted drug design due to their amphoteric structure with phenolic acids and basic nitrogenous moieties. The flavonoid alkaloids family has been shown to exhibit a wide range of biological activities including antiviral, anticancer, and anti-inflammatory activities [4]. For example, flavone alkaloids O-demethylbuchenavianine from Buchenavia capitata have shown HIV-inhibitory activity with an EC50 of 0.26 µM [5]. Flavopiridol derived from natural chrome alkaloid rohitukine that was extracted from Dysoxylum binectariferum can inhibit the activity of cyclin-dependent kinases (CDKs), and is being developed in clinical trial for cancer treatment [6] (Figure 1).
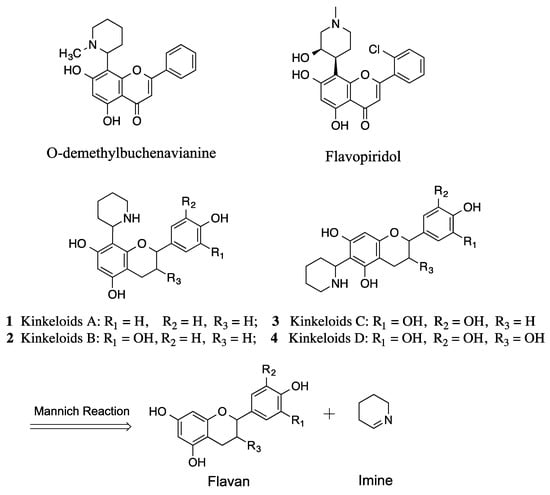
Figure 1.
Kinkeloids and retrosynthetic analysis.
Kinkeloids were first discovered by our group [1]. Their structures are mainly made up of a flavan moiety with a piperidine ring attached at either the 6 or 8 position. Kinkeloids A, B, C, and D differ from each other only by the number of hydroxyl groups attached, resulting in consecutive mass unit difference in mass spectra (Figure 1). Since kinkeloids have been identified as the major component contained in C. micranthum, which has traditionally been used in herbal tea, it was of interest to explore some of their biological activities. Due to the close physiochemical properties between theses kinkeloids in the plant extract, direct separation and accumulation using chromatography is very challenging and time-consuming, especially for large-scale production during pharmacological research.
2. Results and Discussion
Here, we report on two synthetic methods that would be used for the preparation of kinkeloids. Both synthetic routes are based on the Mannich reaction, which links the flavan moiety and the piperidine moiety (Figure 1). To the best of our knowledge, only flavone and flavanone classes have been reported from the family of flavonoids in the synthesis of their corresponding flavonoid alkaloids through a reaction with cyclic imine [7,8,9]. The chemical properties between different classes of flavonoids vary greatly and affect reaction results. In our study, the Mannich reaction between a flavan molecule and cyclic imine was explored for the first time. As reported, the other flavonoid alkaloids usually have low solubility in alcohol and even in DMSO, and the 8-substitued groups generally are less soluble than the 6-substitued ones. In contrast with other flavonoid alkaloids, the synthesized flavan alkaloids are very soluble in alcohol, including methanol and ethanol. Therefore, the solvent selected to perform the flavan Mannich reaction was 100% methanol. Due to the comparatively higher flexibility of flavan molecules, the substitution on the 6-,8-positions had a lack of selectivity, even when different solvent systems were tried including MeOH, EtOH, H2O/MeOH, H2O/THF. Yet, this substitution selectivity was observed and reported in flavonone and flavone groups, indicating that carbonyl groups at position 4 can impact the nucleophilic activities of positions 6 and 8 [9,10].
The synthesis of cyclic imine started from commercially available compound piperidine, which was converted to N-chloropiperidine 7 through the addition of N-chlorosuccinimide (NCS). Treatment of the N-chloropiperidine formed with potassium hydroxide in ethanol provided the corresponding synthetic intermediate Δ1-piperidein 8. In this step, low yields were observed when excessive heating was used for solvent removal because of the low boiling point of compound 8. Δ1-piperidein was then crystalized out in its trimeric form as tripiperidein 9 in cold acetone (Scheme 1) [11].
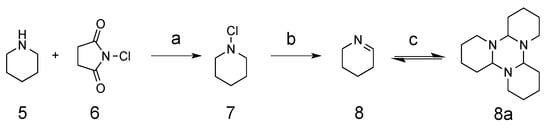
Scheme 1.
The synthesis of Δ1-piperidein intermediate. Reaction conditions and reagents: (a) ether, RT; (b) 20% KOH, ethanol, 0 °C to RT; (c) 80% acetone, −20 °C.
The two synthetic methods presented here differ from each other in the synthesis of flavan moiety [12,13]. Synthetic method I was applied for the synthesis of the kinkeloid B series (Scheme 2), while method II was used for the A series (Scheme 3). Synthetic method I started from the commercially available compounds 3,4-dihydroxybenzaldehyde and 2,4,6-trihydroxyacetophone, which were protected with MOM groups to provide compounds 11 and 12. MOM groups are an ideal selection due to its stability in the basic condition and its facile removal in an acidic condition. The ethanol mixture of 11 and 12 was then basified with 40% KOH solution in an ice bath and gradually warmed up to room temperature to provide a 62% yield of fully protected chalcone 13 [14]. De-protection of MOM groups using 10% HCl in methanol under an elevated temperature of 50 °C yielded exclusively the compound eriodictyol 14, which was cyclized from de-protected chalcone intermediate. The phenolic acid groups were then fully protected with trimethylsilyl (TMS) groups before the step of reductive de-oxygenation using mild reducing reagent NaBH3CN. NaBH3CN, as a reducing agent, has remarkable stability in acidic environments, whose hydrolysis rate is 10−8 that of sodium borohydride [15]. A wide range of acids including BF3·OEt2, ZnCl2, and concentrated HCl, were compared in the reaction and it was found that 10% HCl delivered the best conversion (60% yield) without opening the A ring at the 2 position [16,17]. The TMS protecting groups were also removed in the reaction by hydrolysis. Flavan 16 reacted with Δ1-piperidein and yielded the kinkeloid B series with a 20% yield. Low yields may have been due to the formation of a byproduct, 6,8-di-piperidine-substituted flavan, and a low conversion percentage.
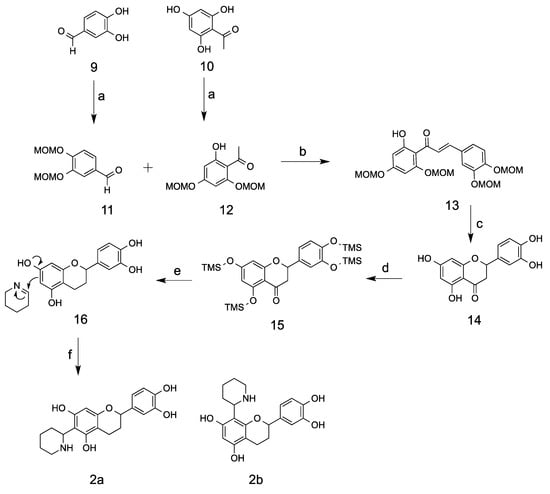
Scheme 2.
Route one for the synthesis of kinkeloids B. Reaction conditions and reagents: (a) DIPEA, MOMCl, DMAP, CH2Cl2, 0 °C to RT (88% for 11, 74% for 12); (b) 40% KOH, ethanol, 0 °C to RT (62%); (c) 10% HCl, methanol, reflux (60%); (d) TMSCl, HMDS, pyridine (e) LiBH4, NaBH3CN, THF, 10% HCl, RT (65%); (f) Δ1-piperideine, methanol, 70 °C (20%).
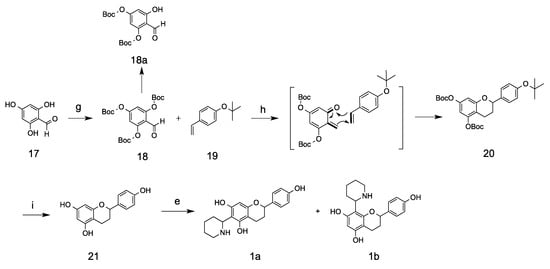
Scheme 3.
Route two for the synthesis of kinkeloids A. Reaction conditions and reagents: (g) Boc2O, DIPEA, DMAP, CH2Cl2, RT, overnight (46.5%); (h) MgBr2·OEt2, LiAlH4, ether, −78 °C to RT; (i) ZnBr2, CH2Cl2, (9.7%); (e) Δ1-piperideines, methanol, 70 °C (25%).
Synthetic method II involved the application of o-quinone methide and an inverse electron-demand Diels–Alder reaction. The starting material 2,4,6-trihydroxybenzaldehyde was reacted with di-tert-butyl dicarbonate to form the ortho o-boc compound 18, which is thermally unstable on silica column and can decompose to the di-boc-substituted compound 18a at room temperature. The use of a cold solvent system (ethyl acetate/hexane) from a −20 °C freezer for chromatographic separation prevents its decomposition and keeps its tri-substituted status. Compound 18 was then used to generate o-quinone methides in combination with magnesium bromide and lithium aluminum hydride [18]. The generation of o-quinone methides through Grignard regents requires extreme dryness. Subsequently, the 1,4-conjugate addition of o-quinone methide with electron-rich olefins through an inverse Diels–Alder reaction yielded the protected flavan products. A low yield was observed in our experiment, which we attributed to the product’s insufficient dryness during the process. Higher yields of aldehydes, alkenes, and other materials have been reported when kept extremely dry, using sodium through Kugelrohr distillation [19], and this inverse Diels–Alder reaction has previously been applied to the synthesis of modified flavonoid molecules [19]. The t-butyl and Boc groups were subsequently removed using ZnBr2 at room temperature, and 4′,5,7-trihydroxyl flavan was achieved [20]. Kinkeloids A was synthesized through a reaction between flavan 21 and Δ1-piperidein, with a yield of 25%. Compared with the kinkeloid B series, the A series compounds are comparatively less stable and could be easily oxidized to purple compound when exposed to ambient air within just a few hours. This phenomenon correlates very well with the relative amount found in the leaf extract, where the kinkeloid B series is very abundant, while the kinkeloid A series is at trace levels. Both synthesized kinkeloids A and B were further positively confirmed through a comparison with compounds contained in kinkeliba leaf extract on LC/UV/MS, and it was found that the synthesized compounds shared identical UV profiles, mass spectrums and retention times with the ones extracted from the kinkeliba leaves.
In summary, this study presents the first synthesis of kinkeloid molecules, which are a newly discovered flavonoid alkaloids. The synthetic approach in the preparation of such flavan alkaloids allows for the procurement of large quantities that are needed for in vivo efficacy and toxicity studies. Such synthetic derivatives also contribute to the in vivo disposition, metabolism and pharmacokinetic studies through the instillation of stable radioisotopes.
3. Materials and Methods
3.1. General Experimental Procedures
1H and 13C-NMR spectra (Supplementary Materials) were performed on a Bruker Avance 400 MHz spectrometer (Billerica, MA, USA). Analytical LC-MS was performed on a Hewlett-Packard Agilent 1100 series HPLC-MSD (Agilent Technologies, Waldbronn, Germany) equipped with a quaternary pump system, a degasser, an auto-sampler, a DAD detector, an MSD trap with an electrospray ion source (ESI). Column chromatography was performed using silica gel (230–400 mesh; Selecto Scientific, Suwanee, GA, USA) and Sephadex LH-20 (25–100 μ, Sigma-Aldrich, St. Louis, MO, USA). All synthetic materials including the solvents were purchased from Sigma Aldrich.
3.2. Δ1-Piperidein
To a suspension solution of N-Chlorosuccinimide 6 (5.9 g, 45.0 mmol) in ether (50 mL), piperidine 5 (2.5 mL, 25.25 mmol) was added drop-wise over 30 min. After being stirred for 3 h at room temperature, the suspension was filtered and washed with ether (20 mL). The combined organic solvent was washed with water (20 mL), brine (20 mL), then dried using anhydrous sodium sulfate. The solvent was removed under reduced pressure, yielding intermediate N-chloropiperidine 7, which was added drop-wise into a solution of potassium hydroxide (3 g) in ethanol (15 mL) followed by stirring for another 5 h at room temperature. After the reaction, diethyl ether (25 mL) was added to the solution, which was washed with water and brine, then dried using anhydrous sodium sulfate. Solvent ether was then removed under reduced pressure without heating, which yielded the product Δ1-piperidein (1.30 g, 62%). A low yield was observed if the solvent was removed under heating. Δ1-piperidein was crystalized as tripiperidein in cold acetone at −20 °C. 1H-NMR (400 MHz CDCl3), a mixture of monomeric and trimeric forms. Monomeric form (some peaks overlapped with the trimeric form): δ 7.77–7.86 (m, 1H, N=C-H), 3.52–3.60 (m, 2H, -CH2-), 2.33 (m, 2H, -CH2-), 2.13 (m, 2H, -CH2-). Trimeric form: 3.07–3.11 (m, 1H, -CH-), 2.76–2.78 (m, 1H, -CH2-), 1.95–1.99 (m, 1H, -CH2-), 1.65–1.69 (m, 3H, 2 × -CH2-), 1.52–1.54 (m, 2H, -CH2-), 1.12–1.29 (m, 2H, -CH2-). 13C-NMR (100 MHz CDCl3) monomeric form (some peaks overlapped with the trimeric form): δ 163.2 (CH, -N=CH-), 49.35 (CH2, -CH2-N=), 28.85 (CH2, -CH2-CH=), 18.80 (CH2, -CH2-); trimeric forms: 82.05 (CH, -N-CH (CH2)-N-); 46.48 (CH2, -CH2-N-), 29.27 (CH2, -CH2-), 25.87 (CH2, -CH2-), 22.41 (CH2, -CH2-).
3.3. 3,4-bis(methoxymethoxy)benzaldehyde (11)
To an ice-cooled suspension of 3,4-dihydroxybenzaldehyde (500 mg, 3.62 mmol) in 10 mL of dichloromethane, N,N-diisopropylethylamine (6.0 mL) and DMAP (5 crystals) were added. Then, MOMCl (chloromethyl methyl ether, 642 mg, 7.96 mmol) was added drop-wise at 0 °C, and the reaction was gradually warmed up to room temperature and stirred overnight. After the disappearance of the starting material on TLC, the reaction was poured into water (10 mL) and extracted with organic solvent dichloromethane (2 × 20 mL). The combined organic phase was washed with water, brine and dried over anhydrous sodium sulfate. The solvent was then removed under reduced pressure and the residue was further purified on silica column using hexane/ethyl acetate (9:1) to yield compound 11 as a white solid (720 mg, 88%). 1H-NMR (400 MHz CDCl3) δ 3.51 (3H, s, -OCH3), 3.52 (3H, s, -OCH3), 5.28 (2H, s, -OCH2O-), 5.32 (2H, s, -OCH2O-), 7.27 (1H, d, J = 8.4 Hz, H-5′), 7.50 (1H, dd, J = 8.4 and 1.9, H-6′), 7.67 (1H, d, J = 1.9 Hz, H-2′), 9.86 (1H, s, -CHO); 13C-NMR (100 MHz CDCl3) 56.5 (CH3, -OCH3), 56.6 (CH3, -OCH3), 95.1 (CH2, -OCH2O-), 95.5 (CH2, -OCH2O-), 115.6 (CH, C-5′), 116.1 (CH, C-2′), 126.4 (CH, C-6′), 131.3 (C, C-1′), 147.6 (C, C-3′), 152.8 (C, C-4′), 190.9 (C, CHO).
3.4. 1-(2-hydroxy-4,6-bis(methoxymethoxy)phenyl)ethan-1-one (12)
2,4,6-trihydroxyacetophone (500 mg, 2.97 mmol) was suspended in ice-old dichloromethane (10 mL) followed by the addition of N,N-diisopropylethylamine (6.0 mL) and DMAP (5 crystals). After around 10 min, MOMCl (515 mg, 6.40 mmol) was added drop-wise and the mixture was stirred overnight. After the disappearance of starting material shown on TLC, the mixture was poured into water (10 mL), and extracted with dichloromethane (2 × 20 mL) two times. The organic phase was washed with water (10 mL) and brine (10 mL), and dried using anhydrous sodium sulfate. The solvent was then removed under reduced pressure and the residue was purified on silica gel column using hexane/ethyl acetate (9:1) to yield compound 12 (560 mg, 73.4%) as a colorless oil that was crystalized as a white solid. 1H-NMR (400 MHz CDCl3) δ 2.66 (s, 3H, -COCH3), 3.47 (s, 3H, -OCH3), 3.51 (3H, s, -OCH3), 5.17 (2H, s, -OCH2O-), 5.25 (2H, s, -OCH2O-), 6.24 (1H, d, J = 2.3 Hz, Ar-H), 6.27 (1H, d, J = 2.3 Hz, Ar-H); 13C-NMR (100 MHz CDCl3) δ 33.1 (CH3, -COCH3), 56.6 (CH3, -OCH3), 56.9 (CH3, -OCH3), 94.2 (CH2, 2 × -OCH2O-), 94.7 (CH, C-5′), 97.4 (CH, C-3′), 107.1 (C, C-1′), 160.5 (C, C-6′), 163.6 (C, C-4′), 167.0 (C, C-2′), 203.4 (C=O, C-1).
3.5. 3-(3,4-bis(methoxymethoxy)phenyl)-1-(2-hydroxy-4,6-bis(methoxymethoxy)phenyl)prop-2-en-1-one (13)
Compounds 11 (160 mg, 0.71 mmol) and 12 (150 mg, 0.59 mmol) were co-suspended in an ice-cold solution of ethanol (5 mL) followed by the addition of 40% KOH solution (1 mL). The mixture was gradually warmed to room temperature and stirred overnight. After that, the reaction was poured into water (10 mL) and extracted with dichloromethane (3 × 20 mL). The combined organic phase was washed with water (10 mL) and brine (10 mL), and dried using anhydrous Na2SO4. The solvent was then purified on silica column using hexane/ethyl acetate (5:1) to obtain compound 13 (170 mg, 62.0%) as a yellow solid. 1H-NMR (400 MHz CDCl3) δ 3.48 (3H, s, -OCH3), 3.52 (3H, s, -OCH3), 3.53 (3H, s, -OCH3), 3.54 (3H, s, -OCH3), 5.18 (2H, s, -OCH2O-), 5.27 (2H, s, -OCH2O-), 5.28 (2H, s, -OCH2O-), 5.30 (2H, s, -OCH2O-), 6.27 (1H, d, J = 2.3 Hz, H-3′), 6.31 (1H, d, J = 2.3 Hz, H-5′), 7.17–7.22 (2H, m, H-5, 6), 7.51 (1H, d, J = 1.7 Hz, H-2). 7.74 (1H, d, J = 15.5, H-α), 7.86 (1H, d, J = 15.5, H-β), 13.92 (1H, s, -OH); 13C-NMR (100 MHz CDCl3) δ 56.4 (CH3, -OCH3), 56.5 (CH3, -OCH3), 56.6 (CH3, -OCH3), 57.0 (CH3, -OCH3), 94.2 (CH2, -OCH2O-), 94.9 (CH2, -OCH2O-), 95.2 (CH2, -OCH2O-), 95.3 (CH2, -OCH2O-), 95.7 (CH, C-5′), 97.7 (CH, C-3′), 107.7 (C, C-1′), 115.5 (CH, C-2), 116.4 (CH, C-5), 124.3 (CH, C-6), 126.1 (CH, C-α), 130.1 (C, C-1), 142.5 (CH, C-β), 147.7 (C, C-4), 149.3 (C, C-3), 160.1 (C, C-2′), 163.5 (C, C-6′), 167.5 (C, C-4′), 192.9 (C, C=O).
3.6. 2-(3,4-dihydroxyphenyl)-5,7-dihydroxychroman-4-one (14)
Compound 13 (70 mg, 0.15 mmol) was suspended in methanol (5 mL) followed by the addition of 37% HCl solution (1 mL). The mixture was heated up and refluxed for 5 h. After the reaction, the solution was cooled down and poured into water, then extracted with EtOAc (3 × 10 mL). The combined organic phases were washed with water and brine, then dried using anhydrous Na2SO4. Then solvent was removed under reduced pressure. The residue was dissolved in methanol (0.5 mL) and purified over Sephadex LH-20 column using 100% methanol to yield compound 14 (30.5 mg, 71%) as a white solid. This compound could also be purified on silica gel column using solvent hexane/ethyl acetate (4:1). 1H-NMR (400 MHz CDCl3) δ 2.67 (1H, dd, J = 3.0 Hz and 17.1 Hz, H-3), 3.19 (1H, dd, J = 17.1 and 12.5 Hz, H-3), 5.37 (1H, dd, J = 12.5 and 3.0 Hz, H-2), 5.87 and 5.88 (2H, d, H-6 and 8), 6.74 (2H, s, H-5′ and 6′), 6.87 (1H, s, H-2); 13C-NMR (100 MHz CDCl3) δ 42.1 (CH, C-3), 78.4 (CH, C-2), 94.9 (CH, C-6), 95.7 (CH, C-8), 101.8 (C, C-10), 114.3 (CH, C-2′), 115.3 (CH, C-5′), 117.9 (CH, C-6′), 129.4 (C, C-1′), 145.2 (C, C-4′), 145.7 (C, C-3′),162.9 (C, C-5), 163.4 (C, C-9), 166.6 (C, C-7), 196.3 (C, C-4).
3.7. 2-(3,4-dihydroxyphenyl)chromane-5,7-diol (16)
To a solution of compound 14 (288 mg, 1.0 mmol) in dry pyridine (1 mL) was added hexamethyldisilazane (1 mL) and trimethylchlorosilane (0.6 mL). The mixture was then further stirred at room temperature for 1 h. The volatile constituents were distilled out under reduced pressure; the residue was suspended in toluene and the precipitate was then filtered out. Solvent toluene solution was then distilled out under reduced pressure and the residue was dissolved in THF (2.5 mL) followed by the addition of LiBH4 (11 mg, 0.5 mmol). The mixture was further stirred at room temperature for half an hour. Next, 0.5 mg of methyl orange and sodium cyanoborohydride (63 mg, 1.0 mmol) were added into the solution. The mixture was slowly titrated using hydrochloric acid solution (1 N) with the release of hydrogen until the color of the solution turned red. The color of the solution was controlled in red by the slow, continuous addition of hydrochloric acid. After approximately 2 h, an extra amount of hydrochloric acid (1 to 1.5 mL) was added to make the color permanent, and the reaction was stirred overnight at room temperature. After this, the solvent THF was distilled out under reduced pressure and the residue was extracted with ethyl acetate and washed with 1 N hydrochloric acid solution, distilled water, and brine. The organic solution was dried using anhydrous sodium sulfate and purified on silica column using 10–20% ethyl acetate in hexane as eluent in gradient with a 65% yield. 1H-NMR (400 MHz, MeOD-d4) δ 1.88 (1H, m, H-3), 2.08 (1H, m, H-3), 2.61 (2H, m, H-4), 4.77 (1H, dd, H-2), 5.84 (1H, d, J = 2.3 Hz, H-8), 5.90 (1H, d, J = 2.3 Hz, H-6), 6.67 (2H, m, H-5′,6′), 6.85 (1H, d, J = 1.84, H-2′); 13C-NMR (100 MHz, MeOD-d4) δ 20.35 (C-4), 30.89 (C-3), 78.848 (C-2), 96.00 (C-8), 96.08 (C-6) 102.6 (C-10), 114.47 (C-5′), 116.17 (C-2′), 118.83 (C-6′), 135.14 (C-1′), 145.93 (C-3′), 146.24 (C-4′), 157.31, 157.49, 157.97 (C-5,7,9).
3.8. Kinkeloids B
The synthesized flavan compound 16 (40 mg, 0.15 mmol) was dissolved in methanol (4 mL) followed by the addition of Δ1-piperideines 8 (12.1 mg, 0.15 mmol). The mixture was stirred at 70 °C under nitrogen atmosphere for 4 h. After the reaction, the solvent was removed under reduced pressure and the residue was purified on Sephadex column LH-20 using methanol to produce a mixture of compound 2a and 2b (10 mg, 20%, the low yield was due to the formation of di-piperidine-substituted products). Compound 2a: 1H-NMR (400 MHz, DMSO-d6): δ 1.30–1.72 (6H, m, H-4″, 5″,6″), 1.72–1.88 (1H, m, H-3), 2.00 (1H, m, H-3), 2.48 (2H, m, H-4) 2.60 (1H, m, H-3″), 3.08 (1H, m, H-3″), 4.10 (1H, m, H-1″), 4.73 (1H, m, H-2), 5.78 (1H, S, H-8), 6.61–6.72 (2H, m, H-5′,6′), 6.76 (1H, d, J = 1.92, H-2′); 13C-NMR (100 MHz, DMSO-d6) δ 19.0 (C-4), 24.0, 24.5 (C-4″,5″), 29.0 (C-3), 30.3 (C-6″), 45.7 (C-3′’) 52.8 (C-1″), 76.4 (C-2), 93.9 (C-8), 101.4 (C-10), 107.1 (C-6), 113.7 (C-2′), 115.3 (C-5′), 117.1 (C-6′), 132.7 (C-1′), 144.8, 145.1 (C-3′,4′) 153.2, 154.6, 155.9 (C-5,7,9). Compound 2b: 1H-NMR (400 MHz DMSO-d6): δ 1.30–1.72 (6H, m, H-4″,5″,6″), 1.72–1.88 (1H, m, H-3), 2.00 (1H, m, H-3), 2.50 (2H, m, H-4) 2.70 (1H, m, H-3″), 3.16 (1H, m, H-3″), 4.20 (1H, m, N-H), 4.86 (1H, m, H-2), 5.97 (1H, S, H-6), 6.61–6.8 (3H, m, H-5′,6′,2′); 13C-NMR (100 MHz, DMSO-d6) δ 18.7 (C-4), 23.3 23.5 (C-4″,5″), 28.6 (C-3), 29.6 (C-6″), 45.5 (C-3″) 52.5 (C-1″), 76.4 (C-2), 95.3 (C-6), 100.2 (C-10), 104.0 (C-8), 113.4 (C-2′), 115.4 (C-5′), 116.5 (C-6′), 132.7 (C-1′), 144.8, 145.2 (C-3′,4′) 152.7, 155.3, 155.5 (C-5,7,9).
3.9. Tri-tert-butyl (2-formylbenzene-1, 3, 5-triyl) tricarbonate (18)
2,4,6-trihydroxybenzaldehyde (1.0 g, 6.50 mmol) was placed in an oven-dried flask filled with nitrogen, followed by dissolution using solvent CH2Cl2. Next, N,N-diisopropylethylamine (DIPEA) (0.7 mL), catalyst DMAP (four crystals), and Boc2O (5 mL, 21.8 mmol) were added, and the mixture was stirred at room temperature overnight. The reaction was stopped using excessive 1M NH4Cl and was extracted by dichloromethane (2 × 20 mL). The combined organic solvent was washed with water (10 mL) and brine (10 mL), then dried using anhydrous MgSO4. The solution was filtered and dried under reduced pressure. The residue was purified on silica column using refrigerated/cold hexane/ethyl acetate (95:5), yielding compound 18 (1.37 g, 46.5%) as a colorless glue. Cold solvents are necessary for purification due to the instability of tri-Boc protected material, which can decompose to di-Boc protected side product (18a) at an elevated temperature. 1H-NMR (400 MHz, CDCl3) δ 1.55 (9H, s, 3 × -CH3), 1.56 (18H, s, 6 × -CH3), 7.10 (2H, s, 2 × Ar-H), 10.20 (1H, s, -CHO).
3.10. 2-(4-hydroxyphenyl)chromane-5,7-diol (21)
The synthesized intermediate compound 18 (100 mg, 0.22 mmol) was dissolved in anhydrous ether and added to a flask containing 1-tert-butoxy-4-vinylbenzene (4 mL, 21 mmol) and magnesium bromide ethyl etherate MgBr2·OEt2 (58 mg, 0.22 mmol) at −78 °C with the protection of argon. The mixture was added to LiAlH4 (9.0 mg, 0.24 mmol) solution in anhydrous ether through a cannula. The reaction was gradually warmed to room temperature and stirred for another 3 h at room temperature. The reaction was quenched using 1M NaHCO3 and extracted using ether (3 × 10 mL). The combined organic phase was washed with water (5 mL) and brine (5 mL), then dried using anhydrous Na2SO4. The solvent was removed and the residue was re-dissolved in the DCM (5 mL) followed by the addition of ZnBr2 (250 mg, 2.2 mmol). The mixture was stirred at room temperature overnight. The reaction was quenched with 1M HCl solution followed by extraction with EtOAc (3 × 10 mL). The combined organic phase was dried under reduced pressure and purified on silica column with hexane/ethyl acetate (3:1) to yield compound 16 (5.5 mg, 9.7%) as a white solid. Due to the reaction’s extreme water sensitivity, a low yield was observed. 1H-NMR (400 MHz, MeOD-d4) δ 7.22 (2H, d, J = 8.6 Hz, H-3′,5′), 6.77 (2H, d, J = 8.6 Hz, H-2′,6′), 5.90 (1H, d, J = 2.3, H-6), 5.83 (1H, d, J = 2.3, H-8), 4.82 (1H, br, d, H-2), 2.54–2.71 (2H, m, H-4), 1.87–2.13 (2H, m, H-3). 13C-NMR (100 MHz, MeOD-d4) δ 158.09, 157.99, 157.49, 157.33 (C-5,7,9,4′), 134.34 (C-1′), 128.47 (C-3′,5′), 116.05 (C-2′,6′), 96.02 (C-6), 95.94 (C-8), 78.84 (C-2), 30.88 (C-3), 20.43 (C-4).
3.11. Kinkeloids A
The synthesized flavan compound 21 (200 mg, 0.78 mmol) was dissolved in methanol followed by the addition of Δ1-piperideines (77 mg, 0.93 mmol). The mixture was stirred at 70 °C under nitrogen atmosphere for 5 h. After the reaction, the solvent was removed under reduced pressure and the residue was purified on silica column using methanol/dichloromethane (15:85) to yield a mixture of compound 1a and 1b. Further purification on silica gel with methanol/dichloromethae (1:5) and Sephadex column LH-20 with methanol yielded compound 1a and 1b as a white solid (25%). It was observed that compounds 1a and 1b would be oxidized to purple compounds after a lengthy exposure to the air. Compound 1a: 1H-NMR (400 MHz, DMSO-d6) δ 1.60 (2H, m, H-5″ and H-4″), 1.88 (4H, m, H-3,4″,5″,6″), 2.09 (2H m, H-3,6″), 2.62 (2H, m, H-4), 2.90 (1H, m, H-3″), 3.30 (1H, d, H-3′’), 4.42 (1H, m, H-1″), 4.88 (1H, m, H-2), 6.03 (1H, S, H-8), 6.80 (2H, d, H-2′,6′), 7.20 (2H, d, H-3′,5′); 13C-NMR (400 MHz, DMSO-d6) δ 19.58 (C-4), 21.8, 22.75 (C-4″,5″) 28.56 (C-6″), 28.54 (C-3), 45.40 (C-3″) 57.65 (C-1″), 76.30 (C-2), 95.35 (C-6), 101.78 (C-10), 104.85 (C-8), 114.98 (C-2′,6′), 127.20 (C-3′,5′), 157.50, 155.53, 154.10, 153.90 (C-5,7,9,4′). Compound 1b: 1H-NMR (400 MHz, DMSO-d6) δ 1.60 (2H, m, H-5″ and H-4″), 1.88 (4H, m, H-3,4″,5″,6″), 2.09 (2H m, H-3,6″), 2.62 (2H, m, H-4), 2.90 (1H, m, H-3″), 3.00 (1H, m, N-H), 4.32 (1H, m, H-1″), 5.00 (1H, m, H-2), 6.25 (1H, s, H-6), 6.80 (2H, d, H-2′,6′), 7.20 (2H, d, H-3′,5′), 13C-NMR (100 MHz, DMSO-d6) δ 19.58 (C-4), 21.8, 22.75 (C-4″,5″) 28.56 (C-6′’), 28.54 (C-3), 45.40 (C-3″) 57.65 (C-1′″), 76.30 (C-2), 95.35 (C-6), 101.78 (C-10), 104.80 (C-6), 114.98 (C-2′,6′), 127.20 (C-3′,5′), 157.50, 155.53, 154.10, 153.90 (C-5,7,9,4′).
Supplementary Materials
The following are available online, for related NMR spectra.
Author Contributions
J.Z., Q.W. and J.E.S. conceived the need to synthesize novel compounds inspired by the natural kinkeloids A and B.J.Z. and Q.W. conceived and designed the experiment; J.Z. performed the experiments and analyzed the data; J.Z., Q.W. and J.E.S. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
We thank the New Jersey Agricultural Experiment Station, Hatch Projects 1005685 and NJ12158, the School of Environmental and Biological Sciences at Rutgers and the New Use Agriculture and National Plant Products Program for funding and support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Welch, C.R. Chemistry and pharmacology of Kinkéliba (Combretum micranthum), a west African medicinal plant. Ph.D. Thesis, Rutgers, The State University of New Jersey, New Brunswick, NJ, USA, 2010. [Google Scholar]
- Akeem, A.A.; Ejikeme, U.C.; Okarafor, E.U. Antibacterial Potentials of the Ethanolic Extract of the Stem Bark of Combretum micranthum G. Don and Its Fractions. J. Plant Stud. 2012, 1, 75. [Google Scholar]
- Ancolio, C.; Azas, N.; Mahiou, V.; Ollivier, E.; Di Giorgio, C.; Keita, A.; Timon-David, P.; Balansard, G. Antimalarial activity of extracts and alkaloids isolated from six plants used in traditional medicine in Mali and Sao Tome. Phytother. Res. 2002, 16, 646–649. [Google Scholar] [PubMed]
- Houghton, P.J. Chemistry and biological activity of natural and semi-synthetic chromone alkaloids. Stud. Nat. Prod. Chem. 2000, 21, 123–155. [Google Scholar]
- Beutler, J.A.; Cardellina, J.H.; Mcmahon, J.B.; Boyd, M.R. Anti-Hiv and Cytotoxic Alkaloids from Buchenavia-Capitata. J. Nat. Prod. 1992, 55, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.R.; Swain, S.M. Review of flavopiridol, a cyclin-dependent kinase inhibitor, as breast cancer therapy. Semin. Oncol. 2002, 29, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Leete, E. A One-Step Synthesis of Ficine and Isoficine. J. Nat. Prod. 1982, 45, 605–607. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Lozach, O.; Surpateanu, G.; Wang, Q.; Retailleau, P.; Iorga, B.I.; Meijer, L.; Gueritte, F. Synthesis, Biological Evaluation, and Molecular Modeling of Natural and Unnatural Flavonoidal Alkaloids, Inhibitors of Kinases. J. Med. Chem. 2012, 55, 2811–2819. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, T.S.; Zhu, J.; Wu, H.M.; Yao, Z.J. Application of a regioselective Mannich reaction on naringenin and its use in fluorescent labeling. Synlett 2006, 8, 1225–1229. [Google Scholar]
- Nguyen, T.B.; Wang, Q.; Gueritte, F. An Efficient One-Step Synthesis of Piperidin-2-yl and Pyrrolidin-2-yl Flavonoid Alkaloids through Phenolic Mannich Reactions. Eur. J. Org. Chem. 2011, 2011, 7076–7079. [Google Scholar] [CrossRef]
- Claxton, G.P.; Allen, L.; Grisar, J.M. 2,3,4,5-Tetrahydropyridine Trimer. Org. Synth. 1977, 56, 118. [Google Scholar]
- Rouchaud, A.; Braekman, J.C. Synthesis of New Analogues of the Tetraponerines. Eur. J. Org. Chem. 2009, 2009, 2666–2674. [Google Scholar] [CrossRef]
- Grundon, M.; Reynolds, B. 459. Δ1-piperideines. J. Chem. Soc. 1964, 2445–2450. [Google Scholar] [CrossRef]
- Urgaonkar, S.; La Pierre, H.S.; Meir, I.; Lund, H.; RayChaudhuri, D.; Shaw, J.T. Synthesis of antimicrobial natural products targeting FtsZ:(±)-dichamanetin and (±)-2″-hydroxy-5″-benzylisouvarinol-B. Org. Lett. 2005, 7, 5609–5612. [Google Scholar] [CrossRef] [PubMed]
- Clinton, F. Sodium cyanoborohydride—A highly selective reducing agent for organic functional groups. Synthesis 1975, 3, 135–146. [Google Scholar]
- Srikrishna, A.; Sattigeri, J.; Viswajanani, R.; Yelamaggad, C. A simple and convenient one step method for the reductive deoxygenation of aryl ketones to hydrocarbons. Synlett 1995, 1995, 93–94. [Google Scholar] [CrossRef]
- Kulangiappar, K.; Anbukulandainathan, M.; Raju, T. Nuclear Versus Side-Chain Bromination of 4-Methoxy Toluene by an Electrochemical Method. Synth. Commun. 2014, 1, 2494–2502. [Google Scholar] [CrossRef]
- Van De Water, R.W.; Magdziak, D.J.; Chau, J.N.; Pettus, T.R. New construction of ortho ring-alkylated phenols via generation and reaction of assorted o-quinone methides. J. Am. Chem. Soc. 2000, 122, 6502–6503. [Google Scholar] [CrossRef]
- Selenski, C. General method for the construction of type-A proanthocyanidins: Synthesis of racemic-diinsininone. Ph.D. Thesis, University of California, Santa Barbara, Santa Barbara, CA, USA, 2006. [Google Scholar]
- Selenski, C.; Pettus, T.R. Enantioselective [4+2] Cycloadditions of o-Quinone Methides: Total Synthesis of (+)-Mimosifoliol and Formal Synthesis of (+)-Tolterodine. J. Org. Chem. 2004, 69, 9196–9203. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).