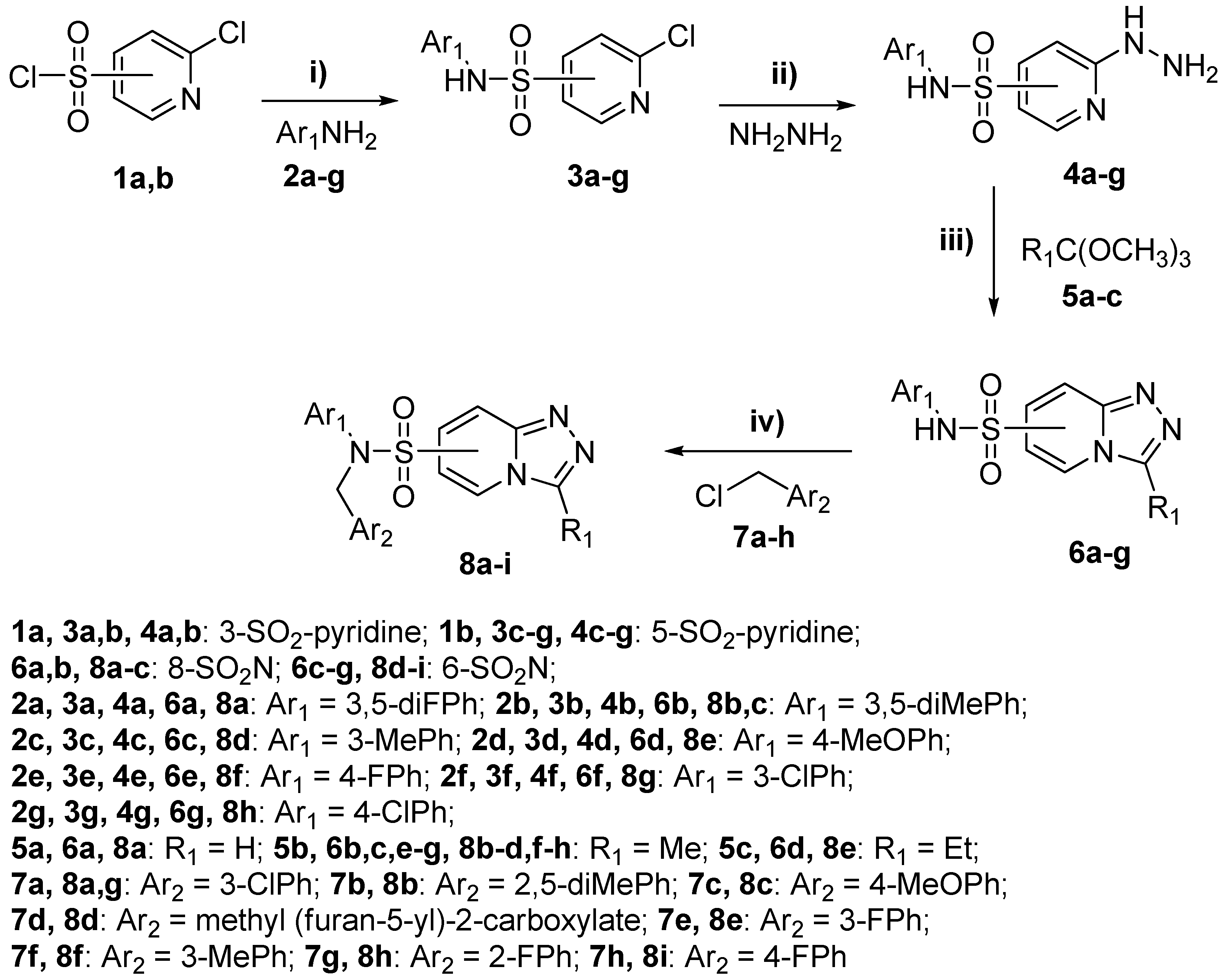3.3. General Procedures for the Synthesis
3.3.1. Synthesis of 2-Chloro-N-(aryl)Pyridinesulfonamides 3a–g. General Procedure A
Primary aniline 2a–g (0.12 mol) and triethylamine (15 mL, 0.12 mol) were added to a stirred solution of sulfonyl chloride 1a,b (21.2 g, 0.1 mol) in dry dioxane (100 mL) for 5 min at room temperature. The reaction mixture was heated at 60 °C for 3 h. After cooling, the reaction mixture was diluted with water (500 mL). The precipitate that formed was filtered and recrystallized from i-propanol. Yields of 2-chloro-N-(aryl)pyridinesulfonamides 3a–g were 70–82%.
2-Chloro-N-(3,5-difluorophenyl)pyridine-3-sulfonamide (3a). According to General Procedure A, 2-chloropyridine-3-sulfonyl chloride 1a (21.2 g, 0.1 mol) and 3,5-difluoroaniline 2a (15.5 g, 0.12 mol) yielded 2-chloro-N-(3,5-difluorophenyl)pyridine-3-sulfonamide 3a (21.9 g, 72%), as a white solid, m.p. 196–198 °C. 1H-NMR spectrum δ, ppm (J, Hz): 6.31–6.37 (m, 1H, Ph H-4), 6.55 (dd, J = 11.4, J = 4.6, 2H, Ph H-2,6), 7.63 (dd, J = 8.4, J = 6.8, 1H, H-5), 8.04 (dd, J = 8.4, J = 1.5, 1H, H-6), 8.73 (dd, J = 6.8, J = 1.5, 1H, H-4), 10.20 (s, 1H, NH). Anal. calcd. for C11H7ClF2N2O2S %: C 43.36; H 2.32; N 9.19; S 10.52. Found, %: C 43.22; H 2.31; N 9.23; S 10.49.
2-Chloro-N-(3,5-dimethylphenyl)pyridine-3-sulfonamide (3b). According to General Procedure A, 2-chloropyridine-3-sulfonyl chloride 1a (21.2 g, 0.1 mol) and 3,5-dimethylaniline 2b (14.5 g, 0.12 mol) yielded 2-chloro-N-(3,5-dimethylphenyl)pyridine-3-sulfonamide 3b (24.0 g, 81%), as a white solid, m.p. 174–176 °C. 1H-NMR spectrum δ, ppm (J, Hz): 2.24 (s, 6H, 2 CH3), 6.50 (s, 1H, Ph H-4), 6.71 (s, 2H, Ph H-2,6), 7.64 (dd, J = 8.4, J = 6.8, 1H, H-5), 8.04 (dd, J = 8.4, J = 1.5, 1H, H-6), 8.73 (dd, J = 6.8, J = 1.5, 1H, H-4), 10.20 (s, 1H, NH). Anal. calcd. for C13H13ClN2O2S %: C 52.61; H 4.42; N 9.44; S 10.80. Found, %: C 52.78; H 4.41; N 9.47; S 10.76.
2-Chloro-N-(3-methylphenyl)pyridine-5-sulfonamide (3c). According to General Procedure A, 2-chloropyridine-5-sulfonyl chloride 1b (21.2 g, 0.1 mol) and 3-methylaniline 2c (12.8 g, 0.12 mol) yielded 2-chloro-N-(3-methylphenyl)pyridine-5-sulfonamide 3c (21.5 g, 76%), as a white solid, m.p. 171–173 °C. 1H-NMR spectrum δ, ppm (J, Hz): 2.20 (s, 3H, CH3), 6.82–6.98 (m, 3H, Ar H), 7.12 (t, J = 7.6, 1H, Ph H-5), 7.71 (d, J = 8.0, 1H, H-3), 8.11 (dd, J = 8.0, J = 2.2, 1H, H-4), 8.70 (d, J = 2.2, 1H, H-6), 10.42 (s, 1H, NH). Anal. calcd. for C12H11ClN2O2S %: C 50.98; H 3.92; N 9.91; S 11.34. Found, %: C 51.16; H 3.93; N 9.88; S 11.29.
2-Chloro-N-(4-methoxyphenyl)pyridine-5-sulfonamide (3d). According to General Procedure A, 2-chloropyridine-5-sulfonyl chloride 1b (21.2 g, 0.1 mol) and 4-methoxyaniline 2d (14.8 g, 0.12 mol) yielded 2-chloro-N-(4-methoxyphenyl)pyridine-5-sulfonamide 3d (24.5 g, 82%), as a white solid, m.p. 182–183 °C. 1H-NMR spectrum δ, ppm (J, Hz): 3.67 (s, 3H, OCH3), 6.80 (d, J = 8.0, 2H, Ph H-3,5), 6.98 (d, J = 8.0, 2H, Ph H-2,6), 7.70 (d, J = 8.0, 1H, H-3), 8.11 (dd, J = 8.0, J = 2.2, 1H, H-4), 8.54 (d, J = 2.2, 1H, H-6), 10.19 (s, 1H, NH). Anal. calcd. for C12H11ClN2O3S %: C 48.25; H 3.71; N 9.38; S 10.73. Found, %: C 48.06; H 3.73; N 9.41; S 10.69.
2-Chloro-N-(4-fluorophenyl)pyridine-5-sulfonamide (3e). According to General Procedure A, 2-chloropyridine-5-sulfonyl chloride 1b (21.2 g, 0.1 mol) and 4-fluoroaniline 2e (13.3 g, 0.12 mol) yielded 2-chloro-N-(4-fluorophenyl)pyridine-5-sulfonamide 3e (20.9 g, 73%), as a white solid, m.p. 202–203 °C. 1H-NMR spectrum δ, ppm (J, Hz): 7.02–7.18 (m, 4H, Ar H), 7.72 (d, J = 8.0, 1H, H-3), 8.07 (dd, J = 8.0, J = 2.2, 1H, H-4), 8.63 (d, J = 2.2, 1H, H-6), 10.48 (br. s, 1H, NH). Anal. calcd. for C11H8ClFN2O2S %: C 46.08; H 2.81; N 9.77; S 11.18. Found, %: C 45.92; H 2.82; N 9.80; S 11.22.
2-Chloro-N-(3-chlorophenyl)pyridine-5-sulfonamide (3f). According to General Procedure A, 2-chloropyridine-5-sulfonyl chloride 1b (21.2 g, 0.1 mol) and 3-chloroaniline 2f (15.3 g, 0.12 mol) yielded 2-chloro-N-(3-chlorophenyl)pyridine-5-sulfonamide 3f (21.2 g, 70%), as a white solid, m.p. 191–193 °C. 1H-NMR spectrum δ, ppm (J, Hz): 7.02–7.18 (m, 3H, Ar H), 7.28 (t, J = 7.6, 1H, Ph H-5), 7.72 (d, J = 8.0, 1H, H-3), 8.12 (dd, J = 8.0, J = 2.2, 1H, H-4), 8.74 (d, J = 2.2, 1H, H-6), 10.80 (br. s, 1H, NH). Anal. calcd. for C11H8Cl2N2O2S %: C 43.58; H 2.66; N 9.24; S 10.58. Found, %: C 43.42; H 2.65; N 9.20; S 10.62.
2-Chloro-N-(4-chlorophenyl)pyridine-5-sulfonamide (3g). According to General Procedure A, 2-chloropyridine-5-sulfonyl chloride 1b (21.2 g, 0.1 mol) and 4-chloroaniline 2g (15.3 g, 0.12 mol) yielded 2-chloro-N-(4-chlorophenyl)pyridine-5-sulfonamide 3g (21.2 g, 70%), as a white solid, m.p. 207–209 °C. 1H-NMR spectrum δ, ppm (J, Hz): 7.11 (d, J = 8.0, 2H, Ph H-3,5), 7.32 (d, J = 8.0, 2H, Ph H-2,6), 7.71 (d, J = 8.0, 1H, H-3), 8.09 (dd, J = 8.0, J = 2.2, 1H, H-4), 8.70 (d, J = 2.2, 1H, H-6), 10.70 (s, 1H, NH). Anal. calcd. for C11H8Cl2N2O2S %: C 43.58; H 2.66; N 9.24; S 10.58. Found, %: C 43.65; H 2.67; N 9.22; S 10.56.
3.3.2. Synthesis of N-(aryl)-2-Hydrazinylpyridinesulfonamides 4a–g. General Procedure B
The corresponding 2-chloro-N-(aryl)pyridinesulfonamide 3a–g (50 mmol) was added to a solution of hydrazine hydrate (13 mL, 200 mmol) in i-propanol (50 mL). The reaction mixture was refluxed for 4 h. After cooling, the reaction mixture was diluted with water (200 mL). The precipitate that formed was filtered and recrystallized from i-propanol. Yields of N-(aryl)-2-hydrazinylpyridinesulfonamides 4a–g were 84–91%.
N-(3,5-difluorophenyl)-2-hydrazinylpyridine-3-sulfonamide (4a). According to General Procedure B, 2-chloro-N-(3,5-difluorophenyl)pyridine-3-sulfonamide 3a (15.2 g, 50 mmol) yielded N-(3,5-difluorophenyl)-2-hydrazinylpyridine-3-sulfonamide 4a (13.1 g, 87%), as a white solid, m.p. 214–216 °C. 1H-NMR spectrum δ, ppm (J, Hz): 4.49 (br. s, 2H, NH2), 6.31–6.37 (m, 1H, Ph H-4), 7.10 (d, J = 6.6, 2H, Ph H-2,6), 7.40 (dd, J = 8.4, J = 6.8, 1H, H-5), 7.95 (dd, J = 8.4, J = 1.5, 1H, H-6), 8.25 (dd, J = 6.8, J = 1.5, 1H, H-4), 8.80 (br. s, 1H, NH), 10.20 (s, 1H, SO2NH). Anal. calcd. for C11H10F2N4O2S %: C 44.00; H 3.36; N 18.66; S 10.68. Found, %: C 43.87; H 3.37; N 18.73; S 10.65.
N-(3,5-dimethylphenyl)-2-hydrazinylpyridine-3-sulfonamide (4b). According to General Procedure B, 2-chloro-N-(3,5-dimethylphenyl)pyridine-3-sulfonamide 3b (14.8 g, 50 mmol) yielded N-(3,5-dimethylphenyl)-2-hydrazinylpyridine-3-sulfonamide 4b (12.4 g, 85%), as a white solid, m.p. 201–203 °C. 1H-NMR spectrum δ, ppm (J, Hz): 2.24 (s, 6H, 2 CH3), 4.59 (br. s, 2H, NH2), 6.50 (s, 1H, Ph H-4), 6.66 (dd, J = 8.4, J = 6.8, 1H, H-5), 6.71 (s, 2H, Ph H-2,6), 7.50 (dd, J = 6.8, J = 1.5, 1H, H-4), 7.87 (dd, J = 8.4, J = 1.5, 1H, H-6), 8.46 (br. s, 1H, NH), 9.75 (s, 1H, SO2NH). Anal. calcd. for C13H16N4O2S %: C 53.41; H 5.52; N 19.16; S 10.97. Found, %: C 53.26; H 5.54; N 19.24; S 11.01.
2-Hydrazinyl-N-(3-methylphenyl)pyridine-5-sulfonamide (4c). According to General Procedure B, 2-chloro-N-(3-methylphenyl)pyridine-5-sulfonamide 3c (14.1 g, 50 mmol) yielded 2-hydrazinyl-N-(3-methylphenyl)pyridine-5-sulfonamide 4c (11.7 g, 84%), as a white solid, m.p. 187–189 °C. 1H-NMR spectrum δ, ppm (J, Hz): 2.18 (s, 3H, CH3), 4.35 (br. s, 2H, NH2), 6.67 (d, J = 8.0, 1H, H-3), 6.78–6.90 (m, 3H, Ar H), 7.09 (t, J = 7.6, 1H, Ph H-5), 7.62 (dd, J = 8.0, J = 2.2, 1H, H-4), 8.21 (s, 1H, H-6), 8.41 (br. s, 1H, NH), 9.90 (br. s, 1H, SO2NH). Anal. calcd. for C12H14N4O2S %: C 51.78; H 5.07; N 20.13; S 11.52. Found, %: C 51.96; H 5.05; N 20.19; S 11.47.
2-Hydrazinyl-N-(4-methoxyphenyl)pyridine-5-sulfonamide (4d). According to General Procedure B, 2-chloro-N-(4-methoxyphenyl)pyridine-5-sulfonamide 3d (14.9 g, 50 mmol) yielded 2-hydrazinyl-N-(4-methoxyphenyl)pyridine-5-sulfonamide 4d (13.1 g, 89%), as a white solid, m.p. 198–200 °C. 1H-NMR spectrum δ, ppm (J, Hz): 3.67 (s, 3H, OCH3), 4.35 (br. s, 2H, NH2), 6.67 (d, J = 8.0, 1H, H-3), 6.79 (d, J = 8.0, 2H, Ph H-3,5), 6.97 (d, J = 8.0, 2H, Ph H-2,6), 7.57 (dd, J = 8.0, J = 2.2, 1H, H-4), 8.09 (d, J = 2.2, 1H, H-6), 8.36 (br. s, 1H, NH), 9.54 (br. s, 1H, SO2NH). Anal. calcd. for C12H14N4O3S %: C 48.97; H 4.79; N 19.04; S 10.89. Found, %: C 49.14; H 4.80; N 18.98; S 10.93.
N-(4-fluorophenyl)-2-hydrazinylpyridine-5-sulfonamide (4e). According to General Procedure B, 2-chloro-N-(4-fluorophenyl)pyridine-5-sulfonamide 3e (14.3 g, 50 mmol) yielded N-(4-fluorophenyl)-2-hydrazinylpyridine-5-sulfonamide 4e (12.8 g, 91%), as a white solid, m.p. 219–221 °C. 1H-NMR spectrum δ, ppm (J, Hz): 4.35 (br. s, 2H, NH2), 6.67 (d, J = 8.0, 1H, H-3), 7.02–7.12 (m, 4H, Ar H), 7.57 (dd, J = 8.0, J = 2.2, 1H, H-4), 8.17 (d, J = 2.2, 1H, H-6), 8.43 (br. s, 1H, NH), 9.86 (br. s, 1H, SO2NH). Anal. calcd. for C11H11FN4O2S %: C 46.80; H 3.93; N 19.85; S 11.36. Found, %: C 46.62; H 3.92; N 19.91; S 11.32.
N-(3-chlorophenyl)-2-hydrazinylpyridine-5-sulfonamide (4f). According to General Procedure B, 2-chloro-N-(3-chlorophenyl)pyridine-5-sulfonamide 3f (15.2 g, 50 mmol) yielded N-(3-chlorophenyl)-2-hydrazinylpyridine-5-sulfonamide 4f (13.3 g, 89%), as a white solid, m.p. 212–214 °C. 1H-NMR spectrum δ, ppm (J, Hz): 4.38 (br. s, 2H, NH2), 6.68 (d, J = 8.0, 1H, H-3), 7.00–7.10 (m, 3H, Ar H), 7.28 (t, J = 7.6, 1H, Ph H-5), 7.64 (dd, J = 8.0, J = 2.2, 1H, H-4), 8.26 (d, J = 2.2, 1H, H-6), 8.49 (s, 1H, NH), 10.20 (br. s, 1H, SO2NH). Anal. calcd. for C11H11ClN4O2S %: C 44.22; H 3.71; N 18.75; S 10.73. Found, %: C 44.07; H 3.72; N 18.78; S 10.76.
N-(4-chlorophenyl)-2-hydrazinylpyridine-5-sulfonamide (4g). According to General Procedure B, 2-chloro-N-(4-chlorophenyl)pyridine-5-sulfonamide 3g (15.2 g, 50 mmol) yielded N-(4-chlorophenyl)-2-hydrazinylpyridine-5-sulfonamide 4g (13.6 g, 91%), as a white solid, m.p. 224–226 °C. 1H-NMR spectrum δ, ppm (J, Hz): 4.38 (br. s, 2H, NH2), 6.68 (d, J = 8.0, 1H, H-3), 7.09 (d, J = 8.0, 2H, Ph H-3,5), 7.28 (d, J = 8.0, 2H, Ph H-2,6), 7.61 (dd, J = 8.0, J = 2.2, 1H, H-4), 8.21 (d, J = 2.2, 1H, H-6), 8.50 (s, 1H, NH), 10.12 (br. s, 1H, SO2NH). Anal. calcd. for C11H11ClN4O2S %: C 44.22; H 3.71; N 18.75; S 10.73. Found, %: C 44.34; H 3.69; N 18.70; S 10.69.
3.3.3. Synthesis of N-(aryl)-[1,2,4]Triazolo[4,3-a]Pyridinesulfonamides 6a–g. General Procedure C
A corresponding methyl ortho-ester 5a–c (25 mmol) was added to a stirred solution of corresponding N-(aryl)-2-hydrazinylpyridinesulfonamide 4a–g (20 mmol) in anhydrous DMF (20 mL). The reaction mixture was refluxed for 16 h. After cooling, the reaction mixture was diluted with water (100 mL). The precipitate that formed was filtered and recrystallized from a mixture of DMF (10 mL) and i-propanol (20 mL). Yields of N-(aryl)-[1,2,4]triazolo[4,3-a]pyridinesulfonamides 6a–g were 68–82%.
N-(3,5-difluorophenyl)-[1,2,4]triazolo[4,3-a]pyridine-8-sulfonamide (6a). According to General Procedure C, N-(3,5-difluorophenyl)-2-hydrazinylpyridine-3-sulfonamide 4a (6.0 g, 20 mmol) and methyl ortho-formate 5a (2.74 mL, 25 mmol) yielded N-(3,5-difluorophenyl)-[1,2,4]triazolo[4,3-a]pyridine-8-sulfonamide 6a (5.09 g, 82%), as a cream solid, m.p. 184–186 °C. 1H-NMR spectrum δ, ppm (J, Hz): 6.70–6.88 (m, 3H, Ar H), 7.11 (t, J = 7.6, 1H, H-6), 8.10 (d, J = 7.6, 1H, H-7), 8.79 (d, J = 7.6, 1H, H-5), 9.40 (s, 1H, H-3), 11.44 (br. s, 1H, SO2NH). Anal. calcd. for C12H8F2N4O2S %: C 46.45; H 2.60; N 18.06; S 10.33. Found, %: C 46.29; H 2.61; N 17.99; S 10.38.
N-(3,5-dimethylphenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-8-sulfonamide(6b). According to General Procedure C, N-(3,5-dimethylphenyl)-2-hydrazinylpyridine-3-sulfonamide 4b (5.85 g, 20 mmol) and methyl ortho-acetate 5b (3.18 mL, 25 mmol) yielded N-(3,5-dimethylphenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-8-sulfonamide 6b (4.81 g, 76%), as a cream solid, m.p. 176–178 °C. 1H-NMR spectrum δ, ppm (J, Hz): 2.06 (s, 6H, 2 CH3), 2.68 (s, 3H, 3-CH3), 6.57 (s, 1H, Ph H-4), 6.70 (s, 2H, Ph H-2,6), 7.04 (t, J = 7.6, 1H, H-6), 7.91 (d, J = 7.6, 1H, H-7), 8.53 (d, J = 7.6, 1H, H-5), 10.00 (br. s, 1H, SO2NH). Anal. calcd. for C15H16N4O2S %: C 56.95; H 5.10; N 17.71; S 10.13. Found, %: C 57.12; H 5.08; N 17.66; S 10.09.
3-Methyl-N-(3-methylphenyl)-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide (6c). According to General Procedure C, N-(3-methylphenyl)-2-hydrazinylpyridine-5-sulfonamide 4c (5.57 g, 20 mmol) and methyl ortho-acetate 5b (3.18 mL, 25 mmol) yielded 3-methyl-N-(3-methylphenyl)-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide 6c (4.23 g, 70%), as a cream solid, m.p. 164–166 °C. 1H-NMR spectrum δ, ppm (J, Hz): 2.20 (s, 3H, Ph CH3), 2.71 (s, 3H, 3-CH3), 6.88 (d, J = 7.6, 1H, Ar H), 6.91–6.98 (m, 2H, Ar H), 7.11 (t, J = 7.6, 1H, Ph H-5), 7.46 (d, J = 7.6, 1H, H-8), 7.85 (d, J = 7.6, 1H, H-7), 8.76 (s, 1H, H-5), 10.45 (br. s, 1H, SO2NH). Anal. calcd. for C14H14N4O2S %: C 55.62; H 4.67; N 18.53; S 10.60. Found, %: C 55.48; H 4.68; N 18.60; S 10.57.
3-Ethyl-N-(4-methoxyphenyl)-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide (6d). According to General Procedure C, 2-hydrazinyl-N-(4-methoxyphenyl)pyridine-5-sulfonamide 4d (5.88 g, 20 mmol) and methyl ortho-propionate 5c (3.55 mL, 25 mmol) yielded 3-ethyl-N-(4-methoxyphenyl)-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide 6d (4.52 g, 68%), as a cream solid, m.p. 161–163 °C. 1H-NMR spectrum δ, ppm (J, Hz): 1.28 (t, J = 7.0, 3H, CH2CH3), 3.09 (q, J = 7.0, 2H, CH2CH3), 3.66 (s, 3H, OCH3), 6.80 (d, J = 7.6, 2H, Ph H-3,5), 7.00 (d, J = 7.6, 2H, Ph H-2,6), 7.44 (d, J = 7.6, 1H, H-8), 7.88 (d, J = 7.6, 1H, H-7), 8.59 (s, 1H, H-5), 10.22 (s, 1H, SO2NH). Anal. calcd. for C15H16N4O3S %: C 54.20; H 4.85; N 16.86; S 9.65. Found, %: C 54.03; H 4.87; N 16.90; S 9.67.
N-(4-fluorophenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide (6e). According to General Procedure C, N-(4-fluorophenyl)-2-hydrazinylpyridine-5-sulfonamide 4e (5.97 g, 20 mmol) and methyl ortho-acetate 5b (3.18 mL, 25 mmol) yielded N-(4-fluorophenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide 6e (4.90 g, 80%), as a cream solid, m.p. 176–177°C. 1H-NMR spectrum δ, ppm (J, Hz): 2.69 (s, 3H, 3-CH3), 7.00–7.18 (m, 4H, Ar H), 7.40 (d, J = 7.6, 1H, H-8), 7.87 (d, J = 7.6, 1H, H-7), 8.68 (s, 1H, H-5), 10.50 (br. s, 1H, SO2NH). Anal. calcd. for C13H11FN4O2S %: C 50.97; H 3.62; N 18.29; S 10.47. Found, %: C 51.14; H 3.62; N 18.35; S 10.50.
N-(3-chlorophenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide (6f). According to General Procedure C, N-(3-chlorophenyl)-2-hydrazinylpyridine-5-sulfonamide 4f (5.97 g, 20 mmol) and methyl ortho-acetate 5b (3.18 mL, 25 mmol) yielded N-(3-chlorophenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide 6f (4.97 g, 77%), as a cream solid, m.p. 179–181 °C. 1H-NMR spectrum δ, ppm (J, Hz): 2.70 (s, 3H, 3-CH3), 7.01–7.20 (m, 3H, Ar H), 7.24 (t, J = 7.6, 1H, Ph H-5), 7.44 (d, J = 7.6, 1H, H-8), 7.88 (d, J = 7.6, 1H, H-7), 8.83 (s, 1H, H-5), 10.90 (br. s, 1H, SO2NH). Anal. calcd. for C13H11ClN4O2S %: C 48.38; H 3.44; N 17.36; S 9.93. Found, %: C 48.22; H 3.42; N 17.41; S 9.90.
N-(4-chlorophenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide (6g). According to General Procedure C, N-(4-chlorophenyl)-2-hydrazinylpyridine-5-sulfonamide 4g (5.97 g, 20 mmol) and methyl ortho-acetate 5b (3.18 mL, 25 mmol) yielded N-(4-chlorophenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide 6g (5.23 g, 81%), as a cream solid, m.p. 206–208 °C. 1H-NMR spectrum δ, ppm (J, Hz): 2.70 (s, 3H, 3-CH3), 7.15 (d, J = 8.0, 2H, Ph H-3,5), 7.28 (d, J = 8.0, 2H, Ph H-2,6), 7.42 (d, J = 7.6, 1H, H-8), 7.87 (d, J = 7.6, 1H, H-7), 8.78 (s, 1H, H-5), 10.75 (br. s, 1H, SO2NH). Anal. calcd. for C13H11ClN4O2S %: C 48.38; H 3.44; N 17.36; S 9.93. Found, %: C 48.47; H 3.46; N 17.30; S 9.97.
3.3.4. Synthesis of N,N-Disubstituted [1,2,4]Triazolo[4,3-a]Pyridinesulfonamides 8a–i. General Procedure D
A powder of dry K2CO3 (0.42 g, 3 mmol) was added to a stirred solution of corresponding N-(aryl)-[1,2,4]triazolo[4,3-a]pyridinesulfonamide 6a-g (1 mmol) in anhydrous DMF (5 mL). Then, appropriate benzyl chloride 7a–h (1.1 mmol) was added and the reaction mixture was heated at 90 °C for 2 h. After cooling, the reaction mixture was diluted with water (25 mL). The precipitate that formed was filtered off, washed with water (5 mL) and recrystallized from a mixture of DMF (5 mL) and i-propanol (20 mL). Yields of N,N-disubstituted [1,2,4]triazolo[4,3-a]pyridinesulfonamides 8a–i were 60–72%.
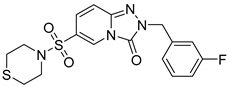
N-(3-chlorobenzyl)-N-(3,5-difluorophenyl)-[1,2,4]triazolo[4,3-a]pyridine-8-sulfonamide (8a). According to General Procedure D, N-(3,5-difluorophenyl)-[1,2,4]triazolo[4,3-a]pyridine-8-sulfonamide 6a (0.31 g, 1 mmol) and 3-chlorobenzyl chloride 7a (0.18 g, 1.1 mmol) yielded N-(3-chlorobenzyl)-N-(3,5-difluorophenyl)-[1,2,4]triazolo[4,3-a]pyridine-8-sulfonamide 8a (0.27 g, 62%), as a white solid, m.p. 160–162 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 5.25 (s, 2H, CH2), 7.00–7.18 (m, 4H, Ar H), 7.22–7.45 (m, 4H, Ar H), 7.87 (d, J = 7.6, 1H, H-7), 8.88 (d, J = 7.6, 1H, H-5), 9.51 (s, 1H, H-3). 13C-NMR spectrum, δ, ppm: 53.8 (CH2), 103.5 (t, JC-F = 25.7 Hz, Ph C-4), 111.5 (dd, JC-F = 18.1 Hz, 9.1 Hz, 2 C, Ph C-2,6), 112.5, 124.4, 126.6, 127.6, 127.7, 130.4, 130.9, 132.9, 133.1, 137.9, 138.8, 140.7 (t, JC-F = 12.8 Hz, Ph C-1), 143.6, 161.9 (dd, JC-F = 246.8 Hz, 15.1 Hz, 2 C, Ph C-3,5). LC/MS m/z (%): 435.6 [M + H]+ (100.0). Anal. calcd. for C19H13ClF2N4O2S %: C 52.48, H 3.01, N 12.88, S 7.37. Found, %: C 52.65, H 2.99, N 12.86, S 7.40.
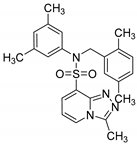
N-(2,5-dimethylbenzyl)-N-(3,5-dimethylphenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-8-sulfonamide (8b). According to General Procedure D, N-(3,5-dimethylphenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-8-sulfonamide 6b (0.31 g, 1 mmol) and 2,5-dimethylbenzyl chloride 7b (0.17 g, 1.1 mmol) yielded N-(2,5-dimethylbenzyl)-N-(3,5-dimethylphenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-8-sulfonamide 8b (0.26 g, 60%), as a white solid, m.p. 195–196 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 2.06 (s, 6H, 2 CH3), 2.15 (s, 3H, CH3), 2.18 (s, 3H, CH3), 2.78 (s, 3H, 3-CH3), 5.20 (s, 2H, CH2), 6.66 (s, 2H, Ph H-2,6), 6.80 (s, 1H, Ar H), 6.88–7.01 (m, 3H, Ar H), 7.06 (s, 1H, Ar H), 7.55 (d, J = 7.6, 1H, H-7), 8.61 (d, J = 7.6, 1H, H-5). 13C-NMR spectrum, δ, ppm: 10.0 (3-CH3), 18.3 (CH3), 20.6 (3 C, CH3), 53.9 (CH2), 111.6, 125.4, 126.0 (2 C), 128.0, 129.0, 129.4, 129.6, 130.0, 131.2, 132.9, 134.5, 134.6, 138.0 (2 C), 138.4, 144.6, 145.2. LC/MS m/z (%): 435.4 [M + H]+ (100.0). Anal. calcd. for C24H26N4O2S %: C 66.33, H 6.03, N 12.89, S 7.38. Found, %: C 66.15, H 6.05, N 12.95, S 7.41.
N-(3,5-dimethylphenyl)-N-(4-methoxybenzyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-8-sulfonamide (8c). According to General Procedure D, N-(3,5-dimethylphenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-8-sulfonamide 6b (0.31 g, 1 mmol) and 4-methoxybenzyl chloride 7c (0.17 g, 1.1 mmol) yielded N-(2,5-dimethylbenzyl)-N-(4-methoxyphenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-8-sulfonamide 8b (0.27 g, 62%), as a white solid, m.p. 168–169 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 2.04 (s, 6H, 2CH3), 2.76 (s, 3H, 3-CH3), 3.71 (s, 3H, OCH3), 5.16 (s, 2H, CH2), 6.60 (s, 2H, Ph H-2,6), 6.78–6.85 (m, 3H, Ph H-4 + Bn H-3,5), 7.00 (t, J = 7.6, 1H, H-6), 7.19 (d, J = 8.0, 2H, Bn H-2,6), 7.60 (d, J = 7.6, 1H, H-7), 8.62 (d, J = 7.6, 1H, H-5). 13C-NMR spectrum, δ, ppm: 10.0 (3-CH3), 20.7 (2C, Ph 3,5-CH3), 55.0 (CH2), 55.6 (OCH3), 111.6, 113.8 (2C), 125.7, 126.1 (2C), 129.0, 129.1, 129.3 (2C), 129.4, 131.0, 138.1 (2C), 138.3, 144.4, 145.0, 158.6 (Bn C-4). LC/MS m/z (%): 437.4 [M + H]+ (100.0). Anal. calcd. for C23H24N4O3S %: C 63.28, H 5.54, N 12.83, S 7.35. Found, %: C 63.44, H 5.55, N 12.89, S 7.32.
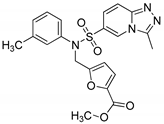
Methyl 5-{[3-methyl-N-(3-methylphenyl)-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamido]methyl}furan-2-carboxylate (8d). According to General Procedure D, 3-methyl-N-(3-methylphenyl)-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide 6c (0.30 g, 1 mmol) and methyl 5-(chloromethyl)furan-2-carboxylate 7d (0.19 g, 1.1 mmol) yielded methyl 5-{[3-methyl-N-(3-methylphenyl)-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamido]methyl}furan-2-carboxylate 8d (0.28 g, 60%), as a white solid, m.p. 152–154 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 2.24 (s, 3H, Ph CH3), 2.77 (s, 3H, 3-CH3), 3.74 (s, 3H, OCH3), 4.98 (s, 2H, CH2), 6.42 (d, J = 3.6, 1H, furan H-4), 7.00 (d, J = 7.6, 1H, Ar H), 7.06–7.18 (m, 4H, Ar H), 7.23 (t, J = 7.6, 1H, Ar H), 7.81 (d, J = 7.6, 1H, H-7), 8.81 (s, 1H, H-5). 13C-NMR spectrum, δ, ppm: 9.9 (3-CH3), 20.7 (CH3), 47.6 (CH2), 51.8 (OCH3), 111.8, 115.8, 119.0, 123.8, 125.1, 125.6, 127.3, 129.0, 129.2, 129.4, 138.2, 138.7, 143.4, 145.8, 148.4, 154.0, 158.0. LC/MS m/z (%): 441.4 [M + H]+ (100.0). Anal. calcd. for C21H20N4O5S %: C 57.26, H 4.58, N 12.72, S 7.28. Found, %: C 57.08, H 4.56, N 12.69, S 7.31.
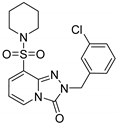
3-Ethyl-N-(3-fluorobenzyl)-N-(4-methoxyphenyl)-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide (8e). According to General Procedure D, 3-ethyl-N-(4-methoxyphenyl)-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide 6d (0.33 g, 1 mmol) and 3-fluorobenzyl chloride 7e (0.16 g, 1.1 mmol) yielded 3-ethyl-N-(3-fluorobenzyl)-N-(4-methoxyphenyl)-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide 8e (0.28 g, 64%), as a brown solid, m.p. 158–159 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 1.34 (t, J = 7.0, 3H, CH2CH3), 3.18 (q, J = 7.0, 2H, CH2CH3), 3.70 (s, 3H, OCH3), 4.83 (s, 2H, CH2), 6.83 (d, J = 7.6, 2H, Ph H-3,5), 6.98–7.30 (m, 7H, Ar H), 7.85 (d, J = 7.6, 1H, H-7), 8.78 (s, 1H, H-5). 13C NMR spectrum, δ, ppm: 10.8 (3-Et-CH3), 17.3 (3-Et-CH2), 53.5 (N-CH2), 55.3 (OCH3), 114.3 (2C), 114.6, 114.7 (d, JC-F = 27.2 Hz), 116.2, 124.1 (d, JC-F = 17.4 Hz), 124.2, 125.0, 126.9, 130.1 (2C), 130.4 (d, JC-F = 8.3 Hz), 130.6, 139.2 (d, JC-F = 7.5 Hz), 148.5, 149.9, 158.8 (Ph C-4), 162.1 (d, JC-F = 243.8 Hz, Bn C-3). LC/MS m/z (%): 441.2 [M + H]+ (100.0). Anal. calcd. for C22H21FN4O3S %: C 59.99, H 4.81, N 12.72, S 7.28. Found, %: C 59.82, H 4.84, N 12.65, S 7.33.
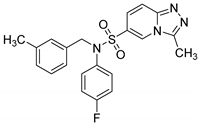
N-(4-fluorophenyl)-3-methyl-N-(3-methylbenzyl)-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide (8f). According to General Procedure D, N-(4-fluorophenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide 6e (0.31 g, 1 mmol) and 3-methylbenzyl chloride 7f (0.16 g, 1.1 mmol) yielded N-(4-fluorophenyl)-3-methyl-N-(3-methylbenzyl)-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide 8f (0.25 g, 61%), as a brown solid, m.p. 104–105 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 2.16 (s, 3H, Bn CH3), 2.77 (s, 3H, 3-CH3), 4.78 (s, 2H, CH2), 6.93–7.28 (m, 9H, Ar H), 7.82 (d, J = 7.6, 1H, H-7), 8.82 (s, 1H, H-5). 13C NMR spectrum, δ, ppm: 10.3 (3-CH3), 21.3 (Bn CH3), 54.5 (CH2), 116.3 (d, JC-F = 23.7 Hz, 2C, Ph C-3,5), 116.5, 124.1, 125.6, 125.7, 127.5, 127.6, 128.6, 128.7, 129.2, 131.5 (d, JC-F = 9.2 Hz, 2C, Ph C-2,6), 135.0, 136.1, 138.0, 146.2, 161.7 (d, JC-F = 243.4 Hz, Ph C-4). LC/MS m/z (%): 411.0 [M + H]+ (100.0). Anal. calcd. for C21H19FN4O2S %: C 61.45, H 4.67, N 13.65, S 7.81. Found, %: C 61.66, H 4.70, N 13.58, S 7.83.
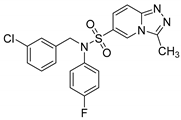
N-(3-chlorobenzyl)-N-(4-fluorophenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide (8g). According to General Procedure D, N-(4-fluorophenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide 6e (0.31 g, 1 mmol) and 3-chlorobenzyl chloride 7a (0.18 g, 1.1 mmol) yielded N-(3-chlorobenzyl)-N-(4-fluorophenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide 8g (0.27 g, 63%), as a pink solid, m.p. 107–109 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 2.77 (s, 3H, 3-CH3), 4.84 (s, 2H, CH2), 7.08–7.35 (m, 9H, Ar H), 7.83 (d, J = 7.6, 1H, H-7), 8.84 (s, 1H, H-5). 13C NMR spectrum, δ, ppm:10.3 (3-CH3), 53.8 (CH2), 116.4 (d, JC-F = 22.9 Hz, 2C, Ph C-3,5), 116.5, 124.1, 125.3, 127.3, 127.8, 128.0, 128.4, 130.8, 131.4 (d, JC-F = 9.2 Hz, 2C, Ph C-2,6), 133.5, 134.8, 138.9, 146.1, 148.9, 161.7 (d, JC-F = 247.2 Hz, Ph C-4). LC/MS m/z (%): 431.0 [M + H]+ (100.0). Anal. calcd. for C20H16ClFN4O2S %: C 55.75, H 3.74, N 13.00, S 7.44. Found, %: C 55.59, H 3.75, N 12.94, S 7.42.
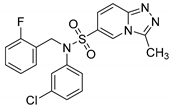
N-(3-chlorophenyl)-N-(2-fluorobenzyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide (8h). According to General Procedure D, N-(3-chlorophenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide 6f (0.32 g, 1 mmol) and 2-fluorobenzyl chloride 7g (0.16 g, 1.1 mmol) yielded N-(3-chlorophenyl)-N-(4-fluorobenzyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide 8h (0.29 g, 67%), as a pink solid, m.p. 153–155 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 2.77 (s, 3H, 3-CH3), 4.94 (s, 2H, CH2), 7.03–7.38 (m, 9H, Ar H), 7.82 (d, J = 7.6, 1H, H-7), 8.88 (s, 1H, H-5). 13C NMR spectrum, δ, ppm:10.3 (3-CH3), 48.5 (CH2), 115.7 (d, JC-F = 21.4 Hz), 116.4, 122.8 (d, JC-F = 13.8 Hz), 124.1, 124.8, 125.3, 127.9, 128.0, 128.8, 129.2, 130.5 (d, JC-F = 8.4 Hz), 130.9, 131.5, 133.5, 140.1, 146.3, 148.9, 160.8 (d, JC-F = 248.7 Hz, Bn C-2). LC/MS m/z (%): 431.4 [M + H]+ (100.0). Anal. calcd. for C20H16ClFN4O2S %: C 55.75, H 3.74, N 13.00, S 7.44. Found, %: C 55.62, H 3.73, N 12.96, S 7.48.
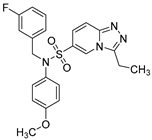
N-(4-chlorophenyl)-N-(4-fluorobenzyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide (8i). According to General Procedure D, N-(4-chlorophenyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide 6g (0.32 g, 1 mmol) and 4-fluorobenzyl chloride 7h (0.16 g, 1.1 mmol) yielded N-(4-chlorophenyl)-N-(4-fluorobenzyl)-3-methyl-[1,2,4]triazolo[4,3-a]pyridine-6-sulfonamide 8h (0.31 g, 72%), as a pink solid, m.p. 198–199 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 2.77 (s, 3H, 3-CH3), 4.81 (s, 2H, CH2), 7.00–7.38 (m, 9H, Ar H), 7.82 (d, J = 7.6, 1H, H-7), 8.88 (s, 1H, H-5). 13C NMR spectrum, δ, ppm:10.3 (3-CH3), 53.5 (CH2), 115.7 (d, JC-F = 21.4 Hz, 2C, Bn C-3,5), 116.6, 124.0, 125.4, 127.7, 129.5 (2C), 130.7 (d, JC-F = 8.4 Hz, 2C, Bn C-2,6), 131.0 (2C), 132.2, 133.1, 137.5, 146.3, 148.8, 162.0 (d, JC-F = 251.0 Hz, Bn C-4). LC/MS m/z (%): 431.0 [M + H]+ (100.0). Anal. calcd. for C20H16ClFN4O2S %: C 55.75, H 3.74, N 13.00, S 7.44. Found, %: C 55.89, H 3.76, N 12.95, S 7.40.
3.3.5. Synthesis of 2-Chloro-N-Substitutedpyridinesulfonamides 10a–g. General Procedure E
Secondary amine 9a–f (0.22 mol) was added, for 5 min at room temperature, to a stirred solution of sulfonyl chloride 1a,b (21.2 g, 0.1 mol) in dry dioxane (100 mL). The reaction mixture was stirred at 60 °C for 1 h. After cooling, the reaction mixture was diluted with water (500 mL). The precipitate that formed was filtered and recrystallized from i-propanol. Yields of 2-chloro-N-substitutedpyridinesulfonamides 10a–g were 67–92%.
2-Chloro-3-(piperidin-1-ylsulfonyl)pyridine (10a). According to General Procedure E, 2-chloropyridine-3-sulfonyl chloride 1a (21.2 g, 0.1 mol) and piperidine 9a (21.7 mL, 0.22 mol) yielded 2-chloro-3-(piperidin-1-ylsulfonyl)pyridine 10a (22.9 g, 88%), as a white solid, m.p. 116–118 °C. 1H-NMR spectrum δ, ppm (J, Hz): 1.45–1.55 (m, 6H, 3 CH2), 3.16–3.24 (m, 4H, 2 NCH2), 7.65 (dd, J = 8.0, J = 7.2, 1H, H-5), 8.35 (dd, J = 8.0, J = 1.5, 1H, H-6), 8.65 (dd, J = 7.2, J = 1.5, 1H, H-4). Anal. calcd. for C10H13ClN2O2S %: C 46.06; H 5.03; N 10.74; S 12.30. Found, %: C 45.92; H 5.01; N 10.77; S 12.25.
4-(2-Chloropyridin-3-ylsulfonyl)morpholine (
10b). According to General Procedure E, 2-chloropyridine-3-sulfonyl chloride
1a (21.2 g, 0.1 mol) and morpholine
9b (19.0 mL, 0.22 mol) yielded 4-(2-chloropyridin-3-ylsulfonyl)morpholine
10b (24.2 g, 92%), as a white solid, m.p. 127–128 °C (128–129 °C [
38]
). 1H-NMR spectrum δ, ppm (
J, Hz): 3.17–3.24 (m, 4H, 2 NCH
2), 3.55–3.62 (m, 4H, 2 OCH
2), 7.66 (dd,
J = 8.0,
J = 7.2, 1H, H-5), 8.37 (dd,
J = 8.0,
J = 1.5, 1H, H-6), 8.67 (dd,
J = 7.2,
J = 1.5, 1H, H-4). Anal. calcd. for C
9H
11ClN
2O
3S %: C 41.15; H 4.22; N 10.66; S 12.20. Found, %: C 41.02; H 4.21; N 10.70; S 12.24.
2-Chloro-5-(piperidin-1-ylsulfonyl)pyridine (10c). According to General Procedure E, 2-chloropyridine-5-sulfonyl chloride 1b (21.2 g, 0.1 mol) and piperidine 9a (21.7 mL, 0.22 mol) yielded 2-chloro-5-(piperidin-1-ylsulfonyl)pyridine 10c (24.0 g, 92%), as a white solid, m.p. 114–116 °C. 1H-NMR spectrum δ, ppm (J, Hz): 1.27–1.58 (m, 6H, 3 CH2), 2.88–2.98 (m, 4H, 2 NCH2), 7.78 (d, J = 8.0, 1H, H-3), 8.16 (dd, J = 8.0, J = 2.2, 1H, H-4), 8.73 (d, J = 2.2, 1H, H-6). Anal. calcd. for C10H13ClN2O2S %: C 46.06; H 5.03; N 10.74; S 12.30. Found, %: C 45.95; H 5.04; N 10.71; S 12.32.
2-Chloro-5-(4-methylpiperidin-1-ylsulfonyl)pyridine (10d). According to General Procedure E, 2-chloropyridine-5-sulfonyl chloride 1b (21.2 g, 0.1 mol) and 4-methylpiperidine 9c (25.4 mL, 0.22 mol) yielded 2-chloro-5-(4-methylpiperidin-1-ylsulfonyl)pyridine 10d (23.9 g, 87%), as a white solid, m.p. 105–107 °C. 1H-NMR spectrum δ, ppm (J, Hz): 0.86 (d, J = 7.0, 3H, CH3), 0.98–1.40 (m, 3H), 1.54–1.68 (m, 2H), 2.22–2.40 (m, 2H, NCH2), 3.52–3.66 (m, 2H, NCH2), 7.77 (d, J = 8.0, 1H, H-3), 8.17 (dd, J = 8.0, J = 2.2, 1H, H-4), 8.72 (d, J = 2.2, 1H, H-6). Anal. calcd. for C11H15ClN2O2S %: C 48.08; H 5.50; N 10.20; S 11.67. Found, %: C 47.93; H 5.48; N 10.17; S 11.71.
4-(6-Chloropyridin-3-ylsulfonyl)thiomorpholine (10e). According to General Procedure E, 2-chloropyridine-5-sulfonyl chloride 1b (21.2 g, 0.1 mol) and thiomorpholine 9d (22.1 mL, 0.22 mol) yielded 4-(6-chloropyridin-3-ylsulfonyl)thiomorpholine 10e (24.8 g, 89%), as a white solid, m.p. 117–119 °C. 1H-NMR spectrum δ, ppm (J, Hz): 2.60–2.68 (m, 4H, 2 SCH2), 3.20–3.32 (m, 4H, 2 NCH2), 7.79 (d, J = 8.0, 1H, H-3), 8.18 (dd, J = 8.0, J = 2.2, 1H, H-4), 8.77 (d, J = 2.2, 1H, H-6). Anal. calcd. for C9H11ClN2O2S2 %: C 38.78; H 3.98; N 10.05; S 23.00. Found, %: C 38.92; H 3.96; N 10.01; S 22.95.
2-Chloro-3-(pyrrolidine-1-ylsulfonyl)pyridine (10f). According to General Procedure E, 2-chloropyridine-3-sulfonyl chloride 1a (21.2 g, 0.1 mol) and pyrrolidine 9e (18.1 mL, 0.22 mol) yielded 2-chloro-3-(pyrrolidin-1-ylsulfonyl)pyridine 10f (21.7 g, 88%), as a white solid, m.p. 101–102 °C. 1H-NMR spectrum δ, ppm (J, Hz): 1.61–1.70 (m, 4H, 2 CH2), 3.16–3.24 (m, 4H, 2 NCH2), 7.40 (dd, J = 8.0, J = 7.2, 1H, H-5), 8.22 (dd, J = 8.0, J = 1.5, 1H, H-6), 8.52 (dd, J = 7.2, J = 1.5, 1H, H-4). Anal. calcd. for C9H11ClN2O2S %: C 43.82; H 4.49; N 11.35; S 13.00. Found, %: C 43.96; H 4.51; N 11.38; S 12.95.
1-(2-Chloropyridin-3-ylsulfonyl)-1,2,3,4-tetrahydroquinoline (10g). According to General Procedure E, 2-chloropyridine-3-sulfonyl chloride 1a (21.2 g, 0.1 mol) and 1,2,3,4-tetrahydroquinoline 9f (27.7 mL, 0.22 mol) yielded 1-(2-chloropyridin-3-ylsulfonyl)-1,2,3,4-tetrahydroquinoline 10g (20.7 g, 67%), as a white solid, m.p. 144–146 °C. 1H-NMR spectrum δ, ppm (J, Hz): 1.82–1.94 (m, 2H, THQ 3-CH2), 2.54–2.80 (m, 2H, THQ 4-CH2), 3.37–3.47 (m, 2H, THQ 2-CH2), 6.56 (t, J = 7.6, 1H, Ar H), 6.64 (d, J = 7.6, 1H, Ar H), 7.02 (d, J = 7.6, 1H, Ar H), 7.13 (t, J = 7.6, 1H, Ar H), 7.71 (dd, J = 8.0, J = 7.2, 1H, H-5), 8.41 (dd, J = 8.0, J = 1.5, 1H, H-6), 8.68 (dd, J = 7.2, J = 1.5, 1H, H-4). Anal. calcd. for C14H13ClN2O2S %: C 54.46; H 4.24; N 9.07; S 10.38. Found, %: C 54.64; H 4.22; N 9.11; S 10.41.
3.3.6. Synthesis of 2-Hydrazinyl-Pyridinesulfonamides 11a–g. General Procedure F
Corresponding 2-chloro-N-substitutedpyridinesulfonamide 10a–g (50 mmol) was added to a solution of hydrazine hydrate (13 mL, 200 mmol) in i-propanol (50 mL). The reaction mixture was refluxed for 4 h. After cooling, the reaction mixture was diluted with water (200 mL). The precipitate that formed was filtered and recrystallized from EtOH. The yields of 2-hydrazinyl-pyridinesulfonamides 11a–g were 82–90%.
2-Hydrazinyl-3-(piperidin-1-ylsulfonyl)pyridine (11a). According to General Procedure F, 2-chloro-3-(piperidin-1-ylsulfonyl)pyridine 10a (13.0 g, 50 mmol) yielded 2-hydrazinyl-3-(piperidin-1-ylsulfonyl)pyridine 11a (11.0 g, 86%), as a white solid, m.p. 154–156 °C. 1H-NMR spectrum δ, ppm (J, Hz): 1.26–1.46 (m, 6H, 3 CH2), 3.00–3.15 (m, 4H, 2 NCH2), 4.59 (br. s, 2H, NH2), 6.72 (t, J = 8.0, 1H, H-5), 7.85 (d, J = 8.0, 1H, H-6), 8.35 (d, J = 7.2, 1H, H-4), 8.80 (br. s, 1H, NH). Anal. calcd. for C10H16N4O2S %: C 46.86; H 6.29; N 21.86; S 12.51. Found, %: C 47.02; H 6.31; N 21.79; S 12.46.
4-(2-Hydrazinylpyridin-3-ylsulfonyl)morpholine (
11b)
. According to General Procedure F, 4-(2-chloropyridin-3-ylsulfonyl)morpholine
10b (13.1 g, 50 mmol) yielded 4-(2-hydrazinylpyridin-3-ylsulfonyl)morpholine
11b (11.5 g, 89%), as a white solid, m.p. 160–162 °C (160–161 °C [
38]).
1H-NMR spectrum δ, ppm (
J, Hz): 2.93–3.09 (m, 4H, 2 NCH
2), 3.61–3.67 (m, 4H, 2 OCH
2), 4.20 (br. s, 2H, NH
2), 7.20 (dd,
J = 8.0,
J = 7.2, 1H, H-5), 8.11 (dd,
J = 8.0,
J = 1.5, 1H, H-6), 8.51 (dd,
J = 7.2,
J = 1.5, 1H, H-4), 9.00 (br. s, 1H, NH). Anal. calcd. for C
9H
14N
4O
3S %: C 41.85; H 5.46; N 21.69; S 12.41. Found, %: C 41.73; H 5.48; N 21.74; S 12.44.
2-Hydrazinyl-5-(piperidin-1-ylsulfonyl)pyridine (11c). According to General Procedure F, 2-chloro-5-(piperidin-1-ylsulfonyl)pyridine 10c (13.0 g, 50 mmol) yielded 2-hydrazinyl-5-(piperidin-1-ylsulfonyl)pyridine 11c (11.5 g, 90%), as a white solid, m.p. 158–160 °C. 1H-NMR spectrum δ, ppm (J, Hz): 1.26–1.59 (m, 6H, 3 CH2), 2.74–2.87 (m, 4H, 2 NCH2), 4.36 (br. s, 2H, NH2), 6.78 (d, J = 8.0, 1H, H-3), 7.63 (dd, J = 8.0, J = 2.2, 1H, H-4), 8.20 (d, J = 2.2, 1H, H-6), 8.44 (br. s, 1H, NH). Anal. calcd. for C10H16N4O2S %: C 46.86; H 6.29; N 21.86; S 12.51. Found, %: C 47.00; H 6.28; N 21.92; S 12.48.
2-Hydrazinyl-5-(4-methylpiperidin-1-ylsulfonyl)pyridine (11d). According to General Procedure F, 2-chloro-5-(4-methylpiperidin-1-ylsulfonyl)pyridine 10d (13.7 g, 50 mmol) yielded 2-hydrazinyl-5-(4-methylpiperidin-1-ylsulfonyl)pyridine 11d (11.6 g, 86%), as a white solid, m.p. 151–153 °C. 1H-NMR spectrum δ, ppm (J, Hz): 0.86 (d, J = 7.0, 3H, CH3), 0.98–1.36 (m, 3H), 1.54–1.68 (m, 2H), 2.07–2.22 (m, 2H, NCH2), 3.44–3.56 (m, 2H, NCH2), 4.38 (br. s, 2H, NH2), 6.78 (d, J = 8.0, 1H, H-3), 7.62 (dd, J = 8.0, J = 2.2, 1H, H-4), 8.19 (d, J = 2.2, 1H, H-6), 8.48 (br. s, 1H, NH). Anal. calcd. for C11H18N4O2S %: C 48.87; H 6.71; N 20.72; S 11.86. Found, %: C 49.04; H 6.69; N 20.66; S 11.90.
4-(6-Hydrazinylpyridin-3-ylsulfonyl)thiomorpholine (11e). According to General Procedure F, 4-(6-chloropyridin-3-ylsulfonyl)thiomorpholine 10e (13.9 g, 50 mmol) yielded 4-(6-hydrazinylpyridin-3-ylsulfonyl)thiomorpholine 11e (11.4 g, 83%), as a white solid, m.p. 147–149 °C. 1H-NMR spectrum δ, ppm (J, Hz): 2.56–2.68 (m, 4H, 2 SCH2), 3.00–3.20 (m, 4H, 2 NCH2), 4.44 (br. s, 2H, NH2), 6.78 (d, J = 8.0, 1H, H-3), 7.63 (dd, J = 8.0, J = 2.2, 1H, H-4), 8.22 (d, J = 2.2, 1H, H-6), 8.53 (br. s, 1H, NH). Anal. calcd. for C9H14N4O2S2 %: C 39.40; H 5.14; N 20.42; S 23.37. Found, %: C 39.29; H 5.13; N 20.36; S 23.44.
2-Hydrazinyl-3-(pyrrolidin-1-ylsulfonyl)pyridine (11f). According to General Procedure F, 2-chloro-3-(pyrrolidin-1-ylsulfonyl)pyridine 10f (12.3 g, 50 mmol) yielded 2-hydrazinyl-3-(pyrrolidin-1-ylsulfonyl)pyridine 11f (10.3 g, 85%), as a white solid, m.p. 142–144 °C. 1H-NMR spectrum δ, ppm (J, Hz): 1.67–1.73 (m, 4H, 2 CH2), 3.15–3.19 (m, 4H, 2 NCH2), 4.59 (br. s, 2H, NH2), 7.41 (dd, J = 8.0, J = 7.2, 1H, H-5), 8.21 (dd, J = 8.0, J = 1.5, 1H, H-6), 8.51 (dd, J = 7.2, J = 1.5, 1H, H-4), 9.10 (t, J = 4.0, 1H, NH). Anal. calcd. for C9H14N4O2S %: C 44.61; H 5.82; N 23.12; S 13.23. Found, %: C 44.49; H 5.81; N 23.07; S 13.18.
1-(2-Hydrazinylpyridin-3-ylsulfonyl)-1,2,3,4-tetrahydroquinoline (11g). According to General Procedure F, 1-(2-chloropyridin-3-ylsulfonyl)-1,2,3,4-tetrahydroquinoline 10g (15.4 g, 50 mmol) yielded 1-(2-hydrazinylpyridin-3-ylsulfonyl)-1,2,3,4-tetrahydroquinoline 11g (12.5 g, 82%), as a white solid, m.p. 167–169 °C. 1H-NMR spectrum δ, ppm (J, Hz): 1.84–1.92 (m, 2H, THQ 3-CH2), 2.49–2.76 (m, 2H, THQ 4-CH2), 3.37–3.47 (m, 2H, THQ 2-CH2), 4.59 (br. s, 2H, NH2), 6.56 (t, J = 7.6, 1H, Ar H), 6.64 (d, J = 7.6, 1H, Ar H), 7.02 (d, J = 7.6, 1H, Ar H), 7.13 (t, J = 7.6, 1H, Ar H), 7.55 (dd, J = 8.0, J = 7.2, 1H, H-5), 8.32 (dd, J = 8.0, J = 1.5, 1H, H-6), 8.70 (dd, J = 7.2, J = 1.5, 1H, H-4), 9.30 (t, J = 4.0, NH). Anal. calcd. for C14H16N4O2S %: C 55.25; H 5.30; N 18.41; S 10.53. Found, %: C 55.38; H 5.28; N 18.37; S 10.49.
3.3.7. Synthesis of Sulfonamido[1,2,4]Triazolo[4,3-a]Pyridin-3-Ones 12a–e. General Procedure G
CDI (4.86 g, 30 mmol) was added to a stirred solution of corresponding 2-hydrazinyl-pyridinesulfonamide 11a–g (20 mmol) in anhydrous dioxane (50 mL). The reaction mixture was refluxed for 8 h. After cooling, the reaction mixture was diluted with water (100 mL). The precipitate that formed was filtered and recrystallized from a mixture of DMF (10 mL) and EtOH (20 mL). Yields of sulfonamido[1,2,4]triazolo[4,3-a]pyridin-3-ones 12a–e were 80–86%.
8-(Piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one (12a). According to General Procedure G, 2-hydrazinyl-3-(piperidin-1-ylsulfonyl)pyridine 11a (5.13 g, 20 mmol) yielded 8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 12a (4.63 g, 82%), as a white solid, m.p. 284–286 °C. 1H-NMR spectrum δ, ppm (J, Hz): 1.32–1.54 (m, 6H, 3 CH2), 3.08–3.18 (m, 4H, 2 NCH2), 6.66 (t, J = 7.6, 1H, H-6), 7.67 (d, J = 7.6, 1H, H-7), 8.02 (d, J = 7.6, 1H, H-5), 12.72 (s, 1H, NH). Anal. calcd. for C11H14N4O3S %: C 46.80; H 5.00; N 19.84; S 11.36. Found, %: C 46.62; H 4.98; N 19.79; S 11.40.
8-(Morpholinosulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one (12b). According to General Procedure G, 4-(2-hydrazinylpyridin-3-ylsulfonyl)morpholine 11b (5.16 g, 20 mmol) yielded 8-(morpholinosulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 12b (4.83 g, 85%), as a white solid, m.p. 290–292 °C (160–161 °C [L]). 1H-NMR spectrum δ, ppm (J, Hz): 3.12–3.18 (m, 4H, 2 NCH2), 3.52–3.60 (m, 4H, 2 OCH2), 6.67 (t, J = 7.6, 1H, H-6), 7.68 (d, J = 7.6, 1H, H-7), 8.08 (d, J = 7.6, 1H, H-5), 12.40 (br. s, 1H, NH). Anal. calcd. for C10H12N4O4S %: C 42.25; H 4.25; N 19.71; S 11.28. Found, %: C 42.40; H 4.24; N 21.64; S 11.26.
6-(Piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one (12c). According to General Procedure G, 2-hydrazinyl-5-(piperidin-1-ylsulfonyl)pyridine 11c (5.13 g, 20 mmol) yielded 6-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 12c (4.87 g, 86%), as a white solid, m.p. 303–305 °C. 1H-NMR spectrum δ, ppm (J, Hz): 1.30–1.56 (m, 6H, 3 CH2), 2.95–3.09 (m, 4H, 2 NCH2), 7.20–7.38 (m, 2H, H-7 + H-8), 8.00 (s, 1H, H-5), 12.55 (br. s, 1H, NH). Anal. calcd. for C11H14N4O3S %: C 46.80; H 5.00; N 19.84; S 11.36. Found, %: C 46.67; H 4.99; N 19.87; S 11.31.
6-(4-Methylpiperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one (12d). According to General Procedure G, 2-hydrazinyl-5-(4-methylpiperidin-1-ylsulfonyl)pyridine 11d (5.41 g, 20 mmol) yielded 6-(4-methylpiperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 12d (4.74 g, 80%), as a white solid, m.p. 279–281 °C. 1H-NMR spectrum δ, ppm (J, Hz): 0.86 (d, J = 7.0, 3H, CH3), 0.98–1.36 (m, 3H), 1.54–1.72 (m, 2H), 2.17–2.42 (m, 2H, NCH2), 3.44–3.60 (m, 2H, NCH2), 7.20–7.40 (m, 2H, H-7 + H-8), 8.06 (s, 1H, H-5), 12.60 (br. s, 1H, NH). Anal. calcd. for C12H16N4O3S %: C 48.64; H 5.44; N 18.91; S 10.82. Found, %: C 48.81; H 5.46; N 18.86; S 10.79.
6-(Thiomorpholinosulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one (12e). According to General Procedure G, 4-(6-hydrazinylpyridin-3-ylsulfonyl)thiomorpholine 11e (5.49 g, 20 mmol) yielded 6-(thiomorpholinosulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 12e (5.05 g, 84%), as a white solid, m.p. >320 °C. 1H-NMR spectrum δ, ppm (J, Hz): 2.52–2.78 (m, 4H, 2 SCH2), 3.34–3.50 (m, 4H, 2 NCH2), 7.20–7.38 (m, 2H, H-7 + H-8), 8.07 (s, 1H, H-5), 12.75 (br. s, 1H, NH). Anal. calcd. for C10H12N4O3S2 %: C 39.99; H 4.03; N 18.65; S 21.35. Found, %: C 40.12; H 4.01; N 18.59; S 21.27.
3.3.8. Synthesis of 2-Benzyl-Sulfonamido[1,2,4]Triazolo[4,3-a]Pyridin-3-Ones 13a–j. General Procedure H
A powder of dry K2CO3 (0.42 g, 3 mmol) was added to a stirred solution of corresponding sulfonamido[1,2,4]triazolo[4,3-a]pyridin-3-one 12a–e (1 mmol) in anhydrous DMF (5 mL). Then, appropriate benzyl chloride 7a,e–g,i–n (1.1 mmol) was added and the reaction mixture was heated at 100 °C for 2 h. After cooling, the reaction mixture was diluted with water (25 mL). The precipitate that formed was filtered off, washed with water (5 mL) and recrystallized from a mixture of DMF (5 mL) and EtOH (20 mL). The yields of 2-benzyl-sulfonamido[1,2,4]triazolo[4,3-a]pyridin-3-ones 13a–j were 60–65%.
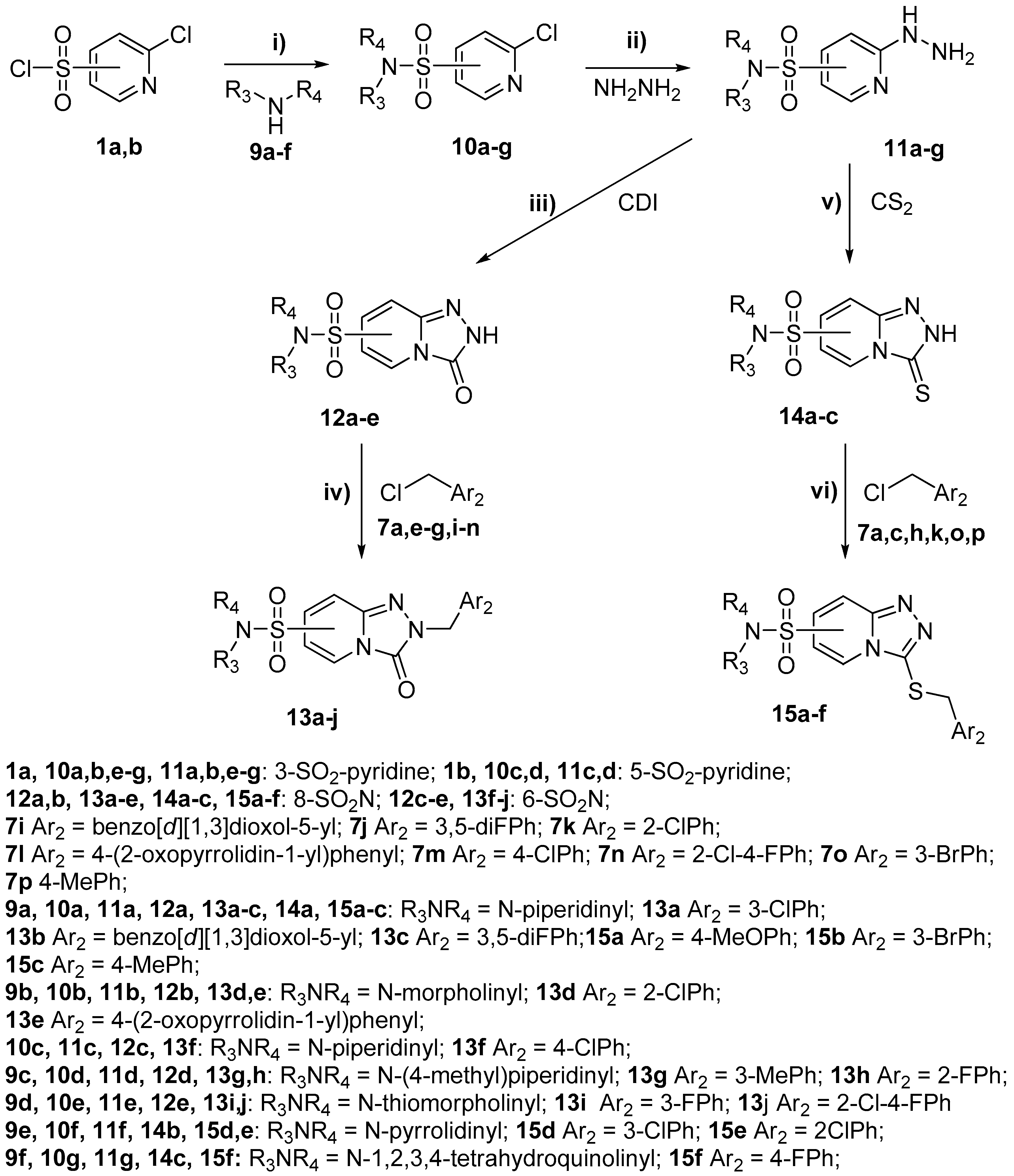
2-(3-Chlorobenzyl)-8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one (13a). According to General Procedure H, 8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 12a (0.28 g, 1 mmol) and 3-chlorobenzyl chloride 7a (0.18 g, 1.1 mmol) yielded 2-(3-chlorobenzyl)-8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 13a (0.25 g 62%), as a yellow solid, m.p. 176–177 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 1.29–1.47 (m, 6H, 3 CH2), 3.04–3.15 (m, 4H, 2 NCH2), 5.17 (s, 2H, CH2), 6.72 (t, J = 7.6, 1H, H-6), 7.27–7.40 (m, 4H, Ar H), 7.71 (d, J = 7.6, 1H, H-7), 8.12 (d, J = 7.6, 1H, H-5). 13C-NMR spectrum, δ, ppm: 22.9 (piperidine 4-CH2), 25.1 (2C, piperidine 3,5-CH2), 46.3 (2C, piperidine 2,6-CH2), 48.1 (Bn CH2), 109.5, 124.7, 126.6, 127.6, 127.7, 129.0, 130.3, 133.2, 134.8, 136.8, 138.7, 147.8 (C=O). LC/MS m/z (%): 407.3 [M + H]+ (100.0). Anal. calcd. for C18H19ClN4O3S %: C 53.13, H 4.71, N 13.77, S 7.88. Found, %: C 52.96, H 4.73, N 13.84, S 7.82.
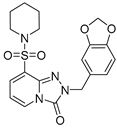
2-(Benzo[d][1,3]dioxol-5-ylmethyl)-8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one (13b). According to General Procedure H, 8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 12a (0.28 g, 1 mmol) and 5-(chloromethyl)benzo[d][1,3]dioxole 7i (0.19 g, 1.1 mmol) yielded 2-(benzo[d][1,3]dioxol-5-ylmethyl)-8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 13b (0.25 g, 60%), as a yellow solid, m.p. 160–161°C, 1H-NMR spectrum, δ, ppm (J, Hz): 1.38–1.52 (m, 6H, 3 CH2), 3.06–3.18 (m, 4H, 2 NCH2), 5.17 (s, 2H, CH2), 5.96 (s, 2H, OCH2O), 6.70 (t, J = 7.6, 1H, H-6), 6.79–6.86 (m, 2H, Ph H-3,4), 6.88 (s, 1H, Ph H-6), 7.68 (d, J = 7.6, 1H, H-7), 8.06 (d, J = 7.6, 1H, H-5). 13C-NMR spectrum, δ, ppm: 23.4 (piperidine 4-CH2), 25.5 (2C, piperidine 3,5-CH2), 46.7 (2C, piperidine 2,6-CH2), 49.2 (Bn CH2), 101.5 (OCH2O), 108.5, 108.9, 109.8, 122.0, 129.1, 130.4, 134.9, 135.0, 136.9, 147.2, 147.8, 148.1 (C=O). LC/MS m/z (%): 417.2 [M + H]+ (100.0). Anal. calcd. for C19H20N4O5S %: C 54.80, H 4.84, N 13.45, S 7.70. Found, %: C 54.97, H 4.87, N 13.48, S 7.75.
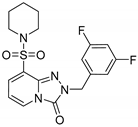
2-(3,5-Difluorobenzyl)-8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one (13c). According to General Procedure H, 8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 12a (0.28 g, 1 mmol) and 3,5-difluorobenzyl chloride 7j (0.18 g, 1.1 mmol) yielded 2-(3,5-difluorobenzyl)-8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 13c (0.26 g, 64%), as a yellow solid, m.p. 221–222 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 1.30–1.48 (m, 6H, 3 CH2), 3.09–3.17 (m, 4H, 2 NCH2), 5.18 (s, 2H, CH2), 6.73 (t, J = 7.6, 1H, H-6), 7.05 (d, J = 9.2, 2H, Ph H-2,6), 7.09 (t, J = 9.6, 1H, Ph H-4), 7.73 (d, J = 7.6, 1H, H-7), 8.11 (d, J = 7.6, 1H, H-5). 13C-NMR spectrum, δ, ppm: 22.9 (piperidine 4-CH2), 25.0 (2C, piperidine 3,5-CH2), 46.3 (2C, piperidine 2,6-CH2), 47.8 (Bn CH2), 103.0 (t, JC-F = 25.7 Hz, Ph C-4), 109.4, 110.9 (dd, JC-F = 22.6 Hz, 7.9 Hz, 2C, Ph C-2,6), 124.6, 129.1, 135.0, 137.0, 140.8 (t, JC-F = 9.4 Hz, Ph C-1), 147.9 (C=O), 162.4 (dd, JC-F = 246.0 Hz, JC-F = 13.2 Hz, 2C, Ph C-3,5). LC/MS m/z (%): 409.5 [M + H]+ (100.0). Anal. calcd. for C18H18F2N4O3S %: C 52.93, H 4.44, N 13.72, S 7.85. Found, %: C 53.07, H 4.46, N 13.76, S 7.90.
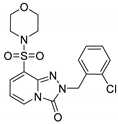
2-(2-Chlorobenzyl)-8-(morpholinosulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one (13d). According to General Procedure H, 8-(morpholinosulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 12b (0.28 g, 1 mmol) and 2-chlorobenzyl chloride 7k (0.18 g, 1.1 mmol) yielded 2-(3-chlorobenzyl)-8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 13a (0.25 g, 62%), as a yellow solid, m.p. 226–227 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 3.08–3.12 (m, 4H, 2 NCH2), 3.46–3.50 (m, 4H, 2 OCH2), 5.20 (s, 2H, Bn CH2), 6.74 (t, J = 7.6, 1H, H-6), 7.28–7.35 (m, 3H, Ar H), 7.45 (d, J = 7.6, 1H, Ar H), 7.72 (d, J = 7.6, 1H, H-7), 8.12 (d, J = 7.6, 1H, H-5). 13C NMR spectrum, δ, ppm: 46.0 (2C, 2 NCH2), 47.2 (Bn CH2), 66.1 (2C, 2 OCH2), 109.9, 124.8, 127.7, 129.6, 129.8, 130.2, 131.1, 132.9, 133.8, 135.6, 137.1, 148.2 (C=O). LC/MS m/z (%): 409.1 [M + H]+ (100.0). Anal. calcd. for C17H17ClN4O4S %: C 49.94, H 4.19, N 13.70, S 7.84. Found, %: C 50.09, H 4.17, N 13.76, S 7.78.
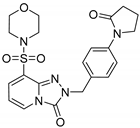
8-(Morpholinosulfonyl)-2-[4-(2-oxopyrrolidin-1-yl)benzyl]-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one (13e). According to General Procedure H, 8-(morpholinosulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 12b (0.28 g, 1 mmol) and 1-(4-(chloromethyl)phenyl)pyrrolidin-2-one 7l (0.23 g, 1.1 mmol) yielded 8-(morpholinosulfonyl)-2-[4-(2-oxopyrrolidin-1-yl)benzyl]-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 13e (0.28 g, 61%), as a yellow solid, m.p. 258–260 °C (dec.), 1H-NMR spectrum, δ, ppm (J, Hz): 2.10 (qn, J = 7.0, 2H, pyrrolidine 4-CH2), 2.42–2.48 (m, 2H, pyrrolidine 3-CH2), 3.12–3.18 (m, 4H, 2 NCH2), 3.46–3.52 (m, 4H, 2 OCH2), 3.82 (t, J = 7.0, 2H, pyrrolidine 5-CH2), 5.08 (s, 2H, Bn CH2), 6.72 (t, J = 7.6, 1H, H-6), 7.34 (d, J = 7.6, 2H, Bn H-3,5), 7.61 (d, J = 7.6, 2H, Bn H-2,6), 7.71 (d, J = 7.6, 1H, H-7), 8.09 (d, J = 7.6, 1H, H-5). 13C NMR spectrum, δ, ppm: 17.8 (pyrrolidine 4-CH2), 32.7 (pyrrolidine 3-CH2), 46.1 (2C, 2 NCH2), 48.5 (pyrrolidine 5-CH2), 49.0 (Bn CH2), 66.1 (2C, 2 OCH2), 109.8, 119.9 (2C), 128.8 (2C), 129.4, 129.5, 131.9, 135.4, 136.8, 136.9, 139.6 (C=O), 174.2 (pyrrolidone C=O). LC/MS m/z (%): 458.1 [M + H]+ (100.0). Anal. calcd. for C21H23N5O5S %: C 55.13, H 5.07, N 15.31, S 7.01. Found, %: C 52.98, H 5.10, N 15.26, S 6.98.
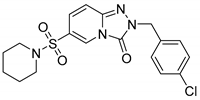
2-(4-Chlorobenzyl)-6-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one (13f). According to General Procedure H, 6-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 12c (0.28 g, 1 mmol) and 4-chlorobenzyl chloride 7m (0.18 g, 1.1 mmol) yielded 2-(4-chlorobenzyl)-6-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 13f (0.26 g, 64%), as a white solid, m.p. 173–174 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 1.30–1.52 (m, 6H, 3 CH2), 2.95–3.09 (m, 4H, 2 NCH2), 5.15 (s, 2H, CH2), 7.28–7.35 (m, 6H, Ar H), 8.07 (s, 1H, H-5). 13C NMR spectrum, δ, ppm: 23.3 (piperidine 4-CH2), 25.3 (2C, piperidine 3,5-CH2), 46.7 (2C, piperidine 2,6-CH2), 48.8 (Bn CH2), 116.8, 122.2, 127.3, 127.4, 128.9 (2C), 130.3 (2C), 132.9, 135.5, 140.7, 148.4 (C=O). LC/MS m/z (%): 407.1 [M + H]+ (100.0). Anal. calcd. for C18H19ClN4O3S %: C 53.13, H 4.71, N 13.77, S 7.88. Found, %: C 52.98, H 4.70, N 13.81, S 7.83.
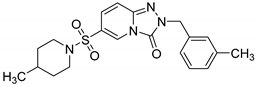
2-(3-Methylbenzyl)-6-(4-methylpiperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one (13g). According to General Procedure H, 6-(4-methylpiperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 12d (0.30 g, 1 mmol) and 3-methylbenzyl chloride 7f (0.15 g, 1.1 mmol) yielded 2-(3-methylbenzyl)-6-(4-methylpiperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 13g (0.24 g, 60%), as a beige solid, m.p. 145–146 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 0.86 (d, J = 7.0, 3H, piperidine 4-CH3), 1.00–1.70 (m, 5H), 2.35 (s, 3H, Ph CH3), 3.52–3.67 (m, 4H, 2 NCH2), 5.09 (s, 2H, CH2), 7.15–7.40 (m, 6H, Ar H), 8.06 (s, 1H, H-5). 13C NMR spectrum, δ, ppm:19.2 (CH3), 21.6 (piperidine 4-CH3), 29.6 (piperidine 4-CH), 33.4(2C, piperidine 3,5-CH2), 46.2 (2C, piperidine 2,6-CH2), 47.5 (Bn CH2), 116.8, 122.1, 126.3, 127.1, 127.4, 128.3, 129.5, 130.6, 134.5, 136.6, 140.6, 148.3 (C=O). LC/MS m/z (%): 401.3 [M + H]+ (100.0). Anal. calcd. for C20H24N4O3S %: C 59.98, H 6.04, N 13.99, S 8.01. Found, %: C 60.11, H 6.05, N 13.94, S 7.98.
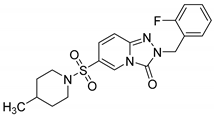
2-(2-Fluorobenzyl)-6-(4-methylpiperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one (13h). According to General Procedure H, 6-(4-methylpiperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 12d (0.30 g, 1 mmol) and 2-fluorobenzyl chloride 7g (0.16 g, 1.1 mmol) yielded 2-(2-fluorobenzyl)-6-(4-methylpiperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 13h (0.25 g, 63%), as a beige solid, m.p. 150–151 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 0.86 (d, J = 7.0, 3H, piperidine 4-CH3), 1.00–1.70 (m, 5H), 3.52–3.67 (m, 4H, 2 NCH2), 5.16 (s, 2H, Bn CH2), 7.12–7.40 (m, 6H, Ar H), 8.06 (s, 1H, H-5). 13C NMR spectrum, δ, ppm: 21.6 (piperidine 4-CH3), 29.6 (piperidine 4-CH), 33.4 (2C, piperidine 3,5-CH2), 43.4 (2C, piperidine 2,6-CH2), 46.2 (Bn CH2), 115.8 (d, JC-F = 19.8 Hz), 116.8, 122.3, 123.2 (d, JC-F = 14.6 Hz), 124.9, 127.3, 127.4, 130.7, 131.2 (d, JC-F = 8.4 Hz), 140.7, 148.3 (C=O), 160.5 (d, JC-F = 251.0 Hz, Bn C-2). LC/MS m/z (%): 405.2 [M + H]+ (100.0). Anal. calcd. for C19H21FN4O3S %: C 56.42, H 5.23, N 13.85, S 7.93. Found, %: C 56.61, H 5.25, N 13.90, S 7.88.
2-(3-Fluorobenzyl)-6-(thiomorpholinosulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one (13i). According to General Procedure H, 6-(thiomorpholinosulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 12e (0.30 g, 1 mmol) and 3-fluorobenzyl chloride 7e (0.16 g, 1.1 mmol) yielded 2-(3-fluorobenzyl)-6-(thiomorpholinosulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 13i (0.29 g, 65%), as a white solid, m.p. 154–155 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 2.60–2.72 (m, 4H, 2 SCH2), 3.36–3.50 (m, 4H, 2 NCH2), 5.15 (s, 2H, Bn CH2), 7.03–7.43 (m, 6H, Ar H), 8.11 (s, 1H, H-5). 13C NMR spectrum, δ, ppm: 27.0 (2C, 2 SCH2), 47.9 (2C, 2 NCH2), 48.8 (Bn CH2), 114.9 (d, JC-F = 21.4 Hz, Bn C-4), 115.1, 117.1, 122.5, 124.3, 126.9, 127.8, 131.0 (d, JC-F = 8.4 Hz), 139.3, 140.8, 148.5 (C=O), 162.6 (d, JC-F = 244.9 Hz, Bn C-3). LC/MS m/z (%): 409.1 [M + H]+ (100.0). Anal. calcd. for C17H17FN4O3S2 %: C 49.99, H 4.19, N 13.72, S 15.70. Found, %: C 50.08, H 4.21, N 13.78, S 15.63.
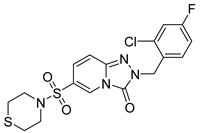
2-(2-Chloro-4-fluorobenzyl)-6-(thiomorpholinosulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one (13j). According to General Procedure H, 6-(thiomorpholinosulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 12e (0.30 g, 1 mmol) and 2-chloro-4-fluorobenzyl chloride 7n (0.20 g, 1.1 mmol) yielded 2-(2-chloro-4-fluorobenzyl)-6-(thiomorpholinosulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 13j (0.26 g, 64%), as a white solid, m.p. 190–191 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 2.60–2.72 (m, 4H, 2 SCH2), 3.46–3.50 (m, 4H, 2 NCH2), 5.20 (s, 2H, Bn CH2), 7.18–7.53 (m, 5H, Ar H), 8.07 (s, 1H, H-5). 13C NMR spectrum, δ, ppm: 27.0 (2C, 2 SCH2), 46.6 (2C, 2 NCH2), 47.9 (Bn CH2), 115.0 (d, JC-F = 20.6 Hz), 117.1, 122.6, 126.0, 127.0, 127.8, 130.2, 131.6, 132.5 (d, JC-F = 9.2 Hz), 140.9, 148.4 (C=O), 162.0 (d, JC-F = 248.0 Hz, Bn C-4). LC/MS m/z (%): 443.4 [M + H]+ (100.0). Anal. calcd. for C17H16ClFN4O3S2. Calculated, %: C 46.10, H 3.64, N 12.65, S 14.48. Found, %: C 45.94, H 3.62, N 12.59, S 14.53.
3.3.9. Synthesis of 3-Thioxo-Sulfonamido[1,2,4]Triazolo[4,3-a]Pyridines 14a–c. General Procedure I
Corresponding 2-hydrazinyl-pyridinesulfonamide 11a,f,g (20 mmol) was dissolved in DMF (50 mL). Then, triethylamine (9.76 mL, 70 mmol) and carbon disulfide (1.0 mL, 40 mmol) were added. The reaction mixture was heated at 40 °C for 2 h, and then the temperature was raised to 90 °C. The obtained solution was heated at 90 °C for 6 h. After cooling to room temperature, the reaction mixture was acidified by AcOH (4.0 mL, 70 mmol) and diluted with water (200 mL). The precipitate that formed was filtered, washed with water (10 mL) and recrystallized from a mixture of DMF (20 mL) and EtOH (20 mL). The yields of 3-thioxo-sulfonamido[1,2,4]triazolo[4,3-a]pyridines 14a–c were 67–74%.
8-(Piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-thione (14a). According to General Procedure I, 2-hydrazinyl-3-(piperidin-1-ylsulfonyl)pyridine 11a (5.13 g, 20 mmol) yielded 8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine-3(2H)-thione 14a (4.30 g, 72%), as a yellow solid, m.p. 303–305 °C. 1H-NMR spectrum δ, ppm (J, Hz): 1.46–1.54 (m, 6H, 3 CH2), 3.16–3.24 (m, 4H, 2 NCH2), 7.05 (dd, J = 8.0, J = 7.2, 1H, H-6), 7.90 (dd, J = 7.0, J = 1.5, 1H, H-7), 8.45 (dd, J = 8.0, J = 1.5, 1H, H-5), 14.85 (s, 1H, NH). Anal. calcd. for C11H14N4O2S2 %: C 44.28; H 4.73; N 18.78; S 21.49. Found, %: C 44.43; H 4.72; N 18.72; S 21.41.
8-(Pyrrolidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-thione (14b). According to General Procedure I, 2-hydrazinyl-3-(pyrrolidin-1-ylsulfonyl)pyridine 11f (4.85 g, 20 mmol) yielded 8-(pyrrolidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine-3(2H)-thione 14b (4.21 g, 74%), as a yellow solid, m.p. 303–305 °C. 1H-NMR spectrum δ, ppm (J, Hz): 1.62–1.78 (m, 4H, 2 CH2), 3.32–3.40 (m, 4H, 2 NCH2), 7.06 (t, J = 7.2, 1H, H-6), 7.93 (d, J = 7.2, 1H, H-7), 8.44 (d, J = 7.6, 1H, H-5), 14.70 (s, 1H, NH). Anal. calcd. for C10H12N4O2S2 %: C 42.24; H 4.25; N 19.70; S 22.55. Found, %: C 42.36; H 4.27; N 19.63; S 22.49.
8-(3,4-Dihydroquinolin-1(2H)-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-thione (14c). According to General Procedure I, 1-(2-hydrazinylpyridin-3-ylsulfonyl)-1,2,3,4-tetrahydroquinoline 11g (6.09 g, 20 mmol) yielded 8-(3,4-dihydroquinolin-1(2H)-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine-3(2H)-thione 14c (4.64 g, 67%), as a yellow solid, m.p. >320 °C. 1H-NMR spectrum δ, ppm (J, Hz): 1.82–1.94 (m, 2H, THQ 3-CH2), 2.49–2.79 (m, 2H, THQ 4-CH2), 3.37–3.47 (m, 2H, THQ 2-CH2), 6.56 (t, J = 7.6, 1H, Ar H), 6.64 (d, J = 7.6, 1H, Ar H), 7.02 (d, J = 7.6, 1H, Ar H), 7.08–7.16 (m, 2H, H-6 + Ar H), 7.93 (d, J = 7.2, 1H, H-7), 8.51 (d, J = 7.6, 1H, H-5), 14.95 (s, 1H, NH). Anal. calcd. for C15H14N4O2S2 %: C 52.01; H 4.07; N 16.17; S 18.51. Found, %: C 51.84; H 4.09; N 16.23; S 18.45.
3.3.10. Synthesis of 3-Thio-Sulfonamido[1,2,4]Triazolo[4,3-a]Pyridines 15a–f. General Procedure J
The corresponding benzyl chloride 7a,c,h,k,o,p (1.1 mmol) was added to the stirred solution of 3-thioxo-sulfonamido[1,2,4]triazolopyridine 14a–c (1 mmol) and triethylamine (0.17 mL, 1.2 mmol) in anhydrous DMF (5 mL). The reaction mixture was heated at 80 °C for 2 h. After cooling, the reaction mixture was diluted with water (25 mL). The precipitate that formed was filtered off, washed with water (5 mL) and recrystallized from a mixture of DMF (5 mL) and EtOH (20 mL). The yields of 3-thio-sulfonamido[1,2,4]triazolo[4,3-a]pyridines 15a–f were 81–94%.
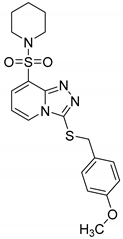
3-(4-Methoxybenzylthio)-8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine (15a). According to General Procedure H, 8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine-3(2H)-thione 14a (0.30 g, 1 mmol) and 4-methoxybenzyl chloride 7c (0.17 g, 1.1 mmol) yielded 3-(4-methoxybenzylthio)-8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine 15a (0.34 g, 81%), as a cream solid, m.p. 169–170 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 1.30–1.52 (m, 6H, 3 CH2), 3.16–3.25 (m, 4H, 2 NCH2), 3.66 (s, 3H, OCH3), 4.28 (s, 2H, Bn CH2), 6.72 (d, J = 8.0, 2H, Bn H-3,5), 7.02–7.12 (m, 3H, H-6 + Bn H-2,6), 7.85 (d, J = 7.2, 1H, H-7), 8.38 (d, J = 7.2, 1H, H-5). 13C-NMR spectrum, δ, ppm: 23.0 (piperidine 4-CH2), 25.1 (2C, piperidine 3,5-CH2), 38.2 (Bn CH2), 46.6 (2C, piperidine 2,6-CH2), 55.1 (OCH3), 113.0, 113.8 (2C), 125.4, 128.3, 128.6, 130.1 (2C), 131.5, 141.5, 145.9, 158.7 (Bn C-4). LC/MS m/z (%): 419.4 [M + H]+ (100.0). Anal. calcd. for C19H22N4O3S2 %: C 54.53, H 5.30, N 13.39, S 15.32. Found, %: C 54.36, H 5.29, N 13.42, S 15.38.
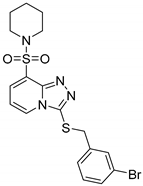
3-(3-Bromobenzylthio)-8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine (15b). According to General Procedure H, 8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine-3(2H)-thione 14a (0.30 g, 1 mmol) and 3-bromobenzyl chloride 7o (0.23 g, 1.1 mmol) yielded 3-(3-bromobenzylthio)-8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine 15b (0.44 g, 94%), as a cream solid, m.p. 160–162 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 1.32–1.52 (m, 6H, 3 CH2), 3.16–3.25 (m, 4H, 2 NCH2), 4.30 (s, 2H, Bn CH2), 7.05–7.23 (m, 3H, H-6 + Ar H), 7.05–7.23 (m, 2H, Ar H), 7.86 (d, J = 7.2, 1H, H-7), 8.40 (d, J = 7.2, 1H, H-5). 13C-NMR spectrum, δ, ppm: 23.0 (piperidine 4-CH2), 25.1 (2C, piperidine 3,5-CH2), 37.6 (Bn CH2), 46.6 (2C, piperidine 2,6-CH2), 113.1, 121.4, 125.4, 128.0, 128.2, 130.3, 130.6, 131.5, 131.6, 139.9, 141.1, 146.1. LC/MS m/z (%): 469.1 [M + H]+ (100.0). Anal. calcd. for C18H19BrN4O2S2 %: C 46.26, H 4.10, N 11.99, S 13.72. Found, %: C 46.11, H 4.09, N 12.04, S 13.68.
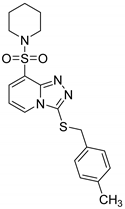
3-(4-Methylbenzylthio)-8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine (15c). According to General Procedure H, 8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine-3(2H)-thione 14a (0.30 g, 1 mmol) and 4-methylbenzyl chloride 7p (0.15 g, 1.1 mmol) yielded 3-(4-methylbenzylthio)-8-(piperidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine 15c (0.37 g, 92%), as a cream solid, m.p. 155–156 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 1.30–1.50 (m, 6H, 3 CH2), 2.18 (s, 3H, CH3), 3.16–3.25 (m, 4H, 2 NCH2), 4.28 (s, 2H, Bn CH2), 6.93–7.09 (m, 5H, H-6 + Ar H), 7.86 (d, J = 7.2, 1H, H-7), 8.38 (d, J = 7.2, 1H, H-5). 13C-NMR spectrum, δ, ppm: 20.6 (CH3), 23.0 (piperidine 4-CH2), 25.1 (piperidine CH2), 25.5 (piperidine CH2), 38.3 (Bn CH2), 46.6 (2C, piperidine 2,6-CH2), 112.9, 125.4, 128.3, 128.8 (2 C), 129.0 (2 C), 131.5, 133.7, 136.8, 141.4, 145.9. LC/MS m/z (%): 403.4 [M + H]+ (100.0). Anal. calcd. for C19H22N4O2S2 %: C 56.69, H 5.51, N 13.92, S 15.93. Found, %: C 56.86, H 5.49, N 13.87, S 15.88.
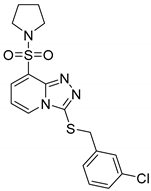
3-(3-Chlorobenzylthio)-8-(pyrrolidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine (15d). According to General Procedure H, 8-(pyrrolidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine-3(2H)-thione 14b (0.28 g, 1 mmol) and 3-chlorobenzyl chloride 7a (0.18 g, 1.1 mmol) yielded 3-(3-chlorobenzylthio)-8-(pyrrolidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine 15d (0.38 g, 93%), as a cream solid, m.p. 143–145 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 1.59–1.74 (m, 4H, 2 CH2), 3.40–3.52 (m, 4H, 2 NCH2), 4.31 (s, 2H, Bn CH2), 7.02–7.22 (m, 5H, H-6 + Ar H), 7.91 (d, J = 7.2, 1H, H-7), 8.40 (d, J = 7.2, 1H, H-5). 13C-NMR spectrum, δ, ppm: 25.3 (2C, pyrrolidine 3,4-CH2), 37.7 (Bn CH2), 47.9 (2C, pyrrolidine 2,5-CH2), 113.1, 125.3, 127.4, 127.6, 128.2, 128.7, 130.2, 132.4, 132.9, 139.7, 141.1, 146.2. LC/MS m/z (%): 409.4 [M + H]+ (100.0). Anal. calcd. for C17H17ClN4O2S2 %: C 49.93, H 4.19, N 13.70, S 15.68. Found, %: C 50.11, H 4.18, N 13.65, S 15.72.
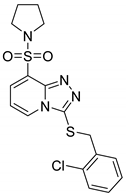
3-(2-Chlorobenzylthio)-8-(pyrrolidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine (15e). According to General Procedure H, 8-(pyrrolidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine-3(2H)-thione 14b (0.28 g, 1 mmol) and 2-chlorobenzyl chloride 7k (0.18 g, 1.1 mmol) yielded 3-(2-chlorobenzylthio)-8-(pyrrolidin-1-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine 15e (0.36 g, 88%), as a cream solid, m.p. 164–166 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 1.59–1.74 (m, 4H, 2 CH2), 3.40–3.52 (m, 4H, 2 NCH2), 4.34 (s, 2H, Bn CH2), 7.06–7.26 (m, 4H, H-6 + Ar H), 7.32 (d, J = 7.6, 1H, Ar H), 7.91 (d, J = 7.2, 1H, H-7), 8.38 (d, J = 7.2, 1H, H-5). 13C-NMR spectrum, δ, ppm: 25.3 (2C, pyrrolidine 3,4-CH2), 36.4 (Bn CH2), 47.9 (2C, pyrrolidine 2,5-CH2), 113.2, 125.3, 127.3, 128.0, 129.4, 129.6, 131.4, 132.4, 132.9, 134.6, 140.7, 146.3. LC/MS m/z (%): 409.4 [M + H]+ (100.0). Anal. calcd. for C17H17ClN4O2S2 %: C 49.93, H 4.19, N 13.70, S 15.68. Found, %: C 50.07, H 4.21, N 13.68, S 15.73.
1-(3-(4-Fluorobenzylthio)-[1,2,4]triazolo[4,3-a]pyridin-8-ylsulfonyl)-1,2,3,4-tetrahydroquinoline (15f). According to General Procedure H, 8-(3,4-dihydroquinolin-1(2H)-ylsulfonyl)-[1,2,4]triazolo[4,3-a]pyridine-3(2H)-thione 14c (0.35 g, 1 mmol) and 4-fluorobenzyl chloride 7h (0.16 g, 1.1 mmol) yielded 1-(3-(4-fluorobenzylthio)-[1,2,4]triazolo[4,3-a]pyridin-8-ylsulfonyl)-1,2,3,4-tetrahydroquinoline 15f (0.42 g, 92%), as a cream solid, m.p. 113–115 °C, 1H-NMR spectrum, δ, ppm (J, Hz): 2.75–2.85 (m, 2H, THQ 3-CH2), 3.60–3.70 (m, 2H, CH2), 4.29 (s, 2H, Bn CH2), 4.46–4.53 (m, 2H, CH2), 6.90 (t, J = 7.6, 2H, Bn H-3,5), 7.02–7.12 (m, 5H, H-6 + Ar H), 7.18 (t, J = 7.6, 2H, Bn H-2,6), 7.96 (d, J = 7.2, 1H, H-7), 8.38 (d, J = 7.2, 1H, H-5). 13C-NMR spectrum, δ, ppm: 28.3 (THQ 3-CH2), 37.6 (Bn CH2), 43.6 (THQ CH2), 46.7 (THQ CH2), 113.1, 115.2 (d, JCF = 21.9 Hz, 2C, Bn C-3,5), 125.4, 126.1, 126.2, 126.6, 128.4, 128.6, 130.9 (d, JCF = 8.3 Hz, 2C, Bn C-2,6), 131.8, 132.0, 133.3, 133.4, 141.3, 145.9, 161.5 (d, JCF = 244 Hz, Bn C-4). LC/MS m/z (%): 455.3 [M + H]+ (100.0). Anal. calcd. for C22H19FN4O2S2 %: C 58.13, H 4.21, N 12.33, S 14.11. Found, %: C 57.98, H 4.19, N 12.38, S 14.14.