Phenolic Profile of Herbal Infusion and Polyphenol-Rich Extract from Leaves of the Medicinal Plant Antirhea borbonica: Toxicity Assay Determination in Zebrafish Embryos and Larvae
Abstract
1. Introduction
2. Results
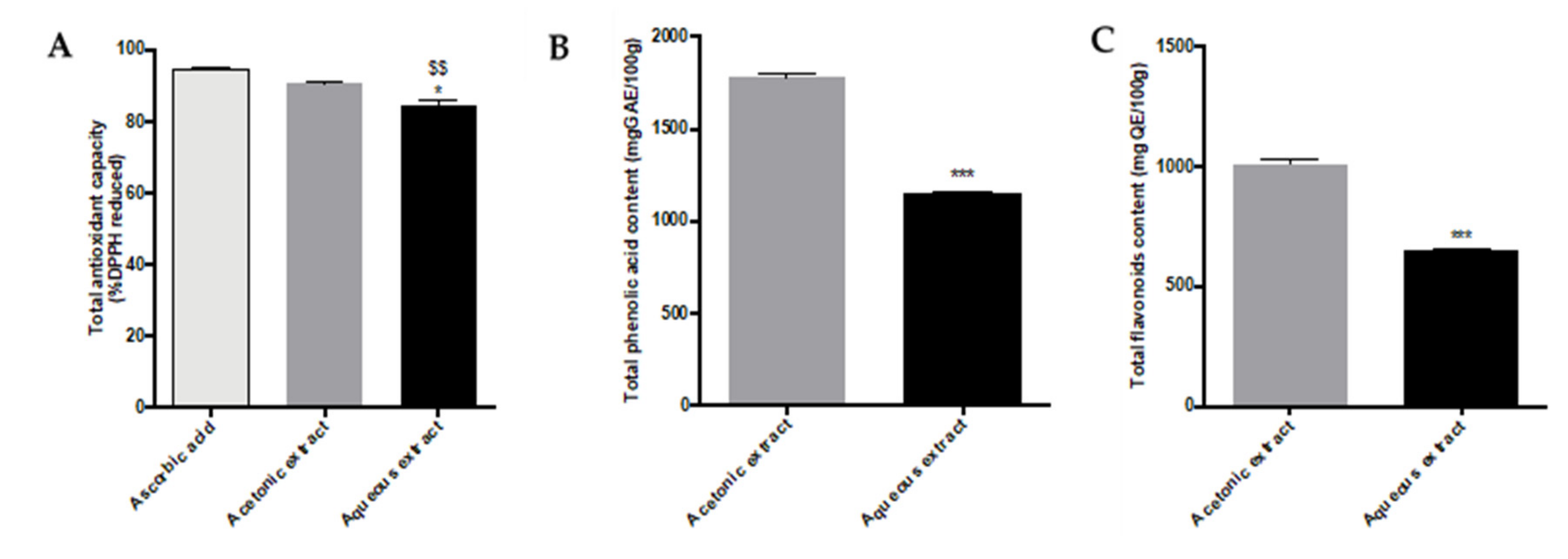
2.1. Determination of Total Antioxidant Activity and Phenolic/Flavonoid Contents of Aqueous and Acetonic Extracts
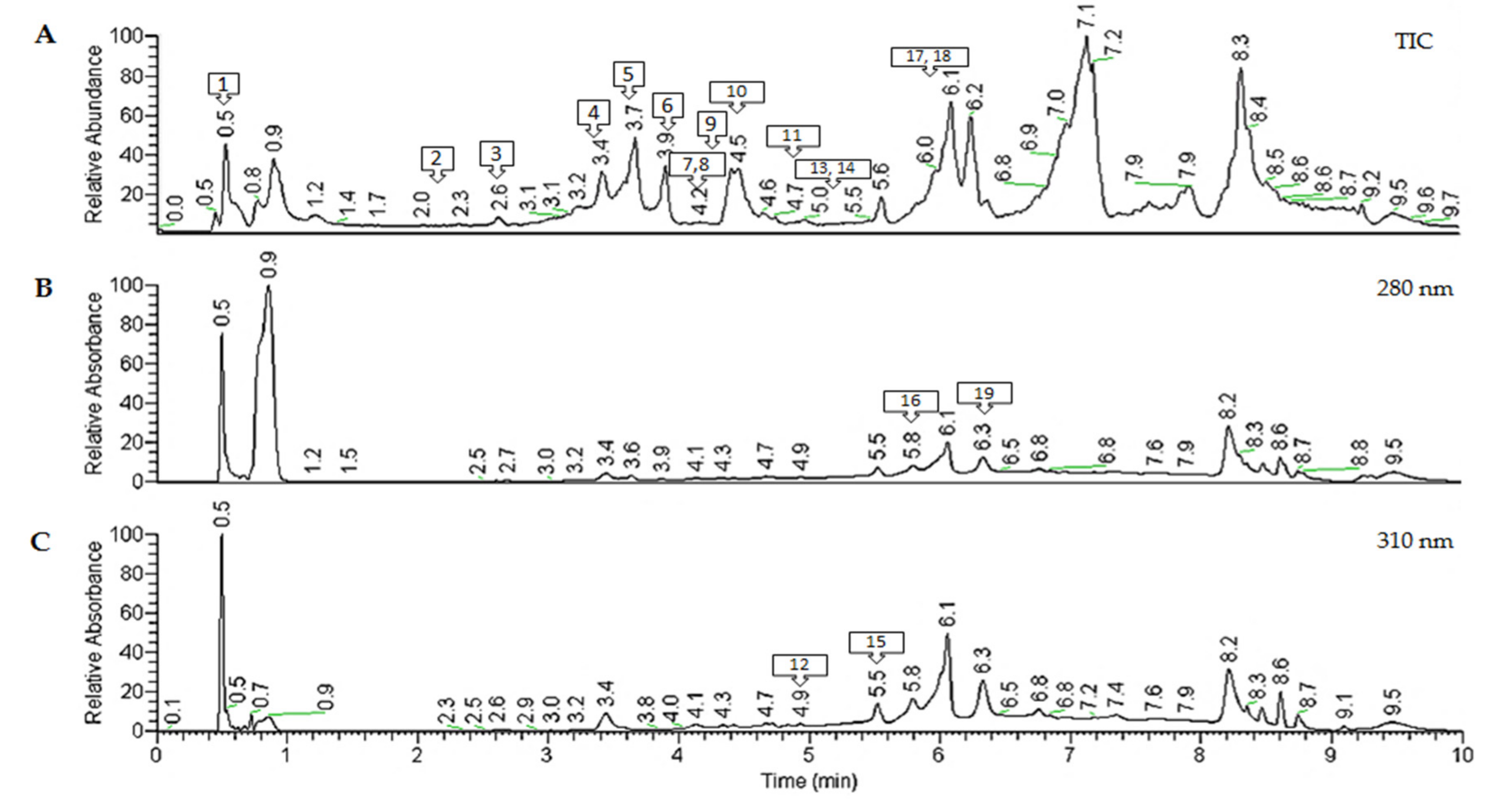
2.2. Characterization of Polyphenols from Antirhea borbonica Acetonic Extract
Phenolic Acids Quantification by UHPLC-HESI-MS
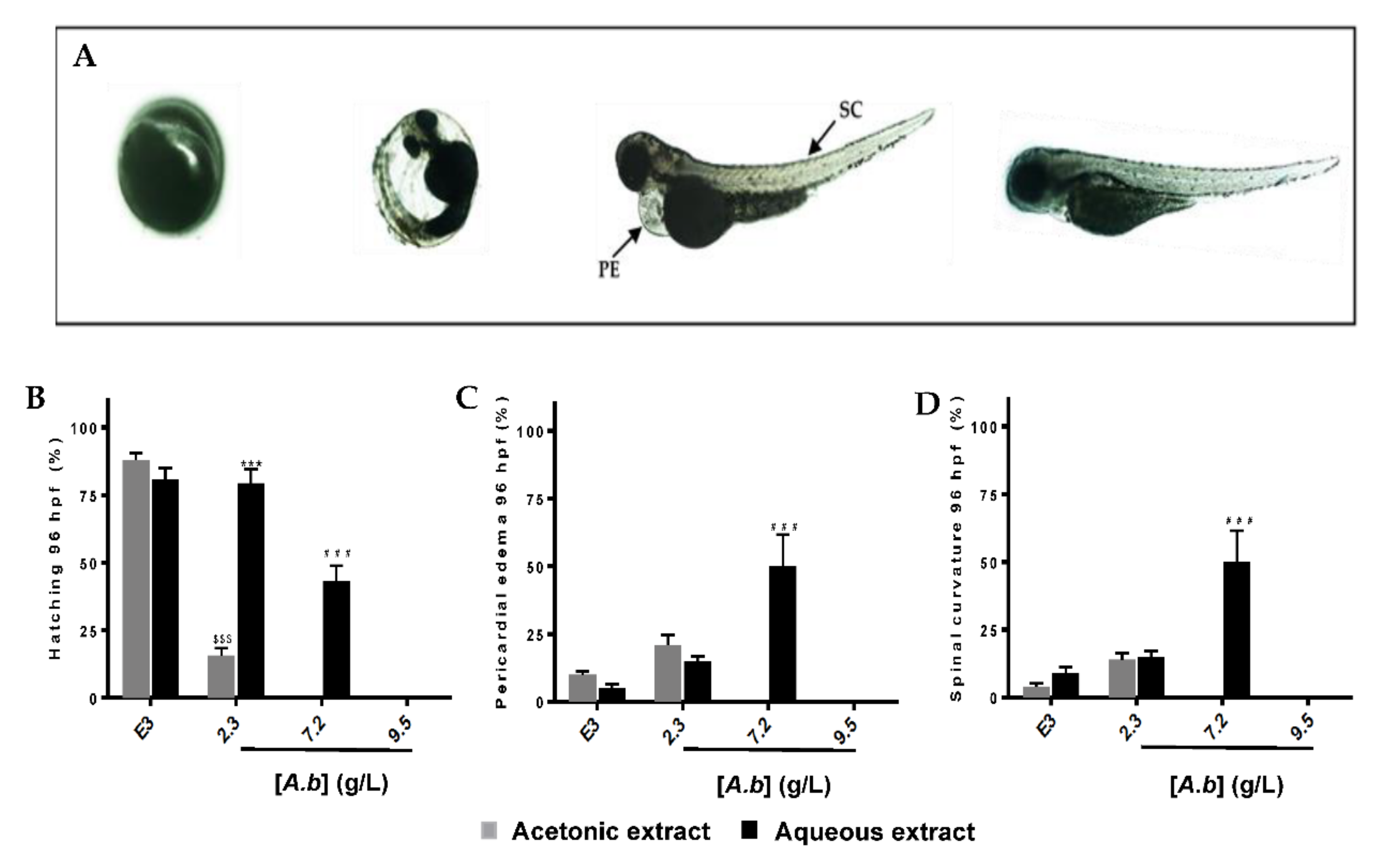
2.3. Zebrafish Embryo and Larvae Acute Toxicity Test
Survival and Lethality Curves on Zebrafish Embryos
3. Discussion
3.1. Polyphenol Content of Aqueous and Acetonic Extracts
3.2. Aqueous and Acetonic Extracts of Antirhea borbonica Exhibit Developmental and Toxicity at High Concentrations
4. Materials and Methods
4.1. Reagents/Standards
4.2. Plant Material
4.3. Preparation of the Plant Extracts
4.4. Measurement of the Total Antioxidant Capacity of Polyphenol-Rich Plant Extracts
4.5. Determination of Phenolic Acid Content
4.6. Determination of Flavonoid Content
4.7. Polyphenolic Compounds Identification and Quantification LC-UV-HESI-MS/MS
Preparation of Standard Solution, Calibration Curves, and Method Validation
4.8. Zebrafish Husbandry
4.9. Developmental Toxicity Test (Zebrafish Embryos)
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- McDougall, I.; Chamalaun, F.H. Isotopic Dating and Geomagnetic Polarity Studies on Volcanic Rocks from Mauritius, Indian Ocean. Geol. Soc. Am. Bull. 1969, 80, 1419. [Google Scholar] [CrossRef]
- Agnarsson, I.; Kuntner, M. The Generation of a Biodiversity Hotspot: Biogeography and Phylogeography of the Western Indian Ocean Islands. In Current Topics in Phylogenetics and Phylogeography of Terrestrial and Aquatic Systems; Anamthawat-Jnsson, K., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0217-5. [Google Scholar]
- Lavergne, R. Tisaneurs et Plantes Médicinales Indigènes à la Réunion; Orphie: Livry Gargan, France, 2016; ISBN 979-10-298-0073-3. [Google Scholar]
- Adsersen, A.; Adsersen, H. Plants from Réunion Island with alleged antihypertensive and diuretic effects—An experimental and ethnobotanical evaluation. J. Ethnopharmacol. 1997, 58, 189–206. [Google Scholar] [CrossRef]
- Marimoutou, M.; Le Sage, F.; Smadja, J.; Lefebvre d’Hellencourt, C.; Gonthier, M.-P.; Robert-Da Silva, C. Antioxidant polyphenol-rich extracts from the medicinal plants Antirhea borbonica, Doratoxylon apetalum and Gouania mauritiana protect 3T3-L1 preadipocytes against H2O2, TNFα and LPS inflammatory mediators by regulating the expression of superoxide dismutase and NF-κB genes. J. Inflamm. (London) 2015, 12, 10. [Google Scholar] [CrossRef]
- Fortin, H.; Vigor, C.; Lohézic-Le Dévéhat, F.; Robin, V.; Le Bossé, B.; Boustie, J.; Amoros, M. In vitro antiviral activity of thirty-six plants from La Réunion Island. Fitoterapia 2002, 73, 346–350. [Google Scholar] [CrossRef]
- Ledoux, A.; Cao, M.; Jansen, O.; Mamede, L.; Campos, P.-E.; Payet, B.; Clerc, P.; Grondin, I.; Girard-Valenciennes, E.; Hermann, T.; et al. Antiplasmodial, anti-chikungunya virus and antioxidant activities of 64 endemic plants from the Mascarene Islands. Int. J. Antimicrob. Agents 2018, 52, 622–628. [Google Scholar] [CrossRef]
- Haddad, J.G.; Koishi, A.C.; Gaudry, A.; Nunes Duarte Dos Santos, C.; Viranaicken, W.; Desprès, P.; El Kalamouni, C. Doratoxylon apetalum, an Indigenous Medicinal Plant from Mascarene Islands, Is a Potent Inhibitor of Zika and Dengue Virus Infection in Human Cells. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Giraud-Techer, S. Plantes médicinales de La Réunion inscrites à la Pharmacopée française. Ethnopharmacologia 2016, 7–33. [Google Scholar]
- Poullain, C.; Girard-Valenciennes, E.; Smadja, J. Plants from reunion island: Evaluation of their free radical scavenging and antioxidant activities. J. Ethnopharmacol. 2004, 95, 19–26. [Google Scholar] [CrossRef]
- Taïlé, J.; Arcambal, A.; Clerc, P.; Gauvin-Bialecki, A.; Gonthier, M.-P. Medicinal Plant Polyphenols Attenuate Oxidative Stress and Improve Inflammatory and Vasoactive Markers in Cerebral Endothelial Cells during Hyperglycemic Condition. Antioxidants 2020, 9, 573. [Google Scholar] [CrossRef]
- Delveaux, J.; Turpin, C.; Veeren, B.; Diotel, N.; Bravo, S.B.; Begue, F.; Álvarez, E.; Meilhac, O.; Bourdon, E.; Rondeau, P. Antirhea borbonica Aqueous Extract Protects Albumin and Erythrocytes from Glycoxidative Damages. Antioxidants 2020, 9, 415. [Google Scholar] [CrossRef]
- Arcambal, A.; Taïlé, J.; Couret, D.; Planesse, C.; Veeren, B.; Diotel, N.; Gauvin-Bialecki, A.; Meilhac, O.; Gonthier, M.-P. Protective Effects of Antioxidant Polyphenols against Hyperglycemia-Mediated Alterations in Cerebral Endothelial Cells and a Mouse Stroke Model. Mol. Nutr. Food Res. 2020, 64, e1900779. [Google Scholar] [CrossRef]
- Ghaddar, B.; Veeren, B.; Rondeau, P.; Bringart, M.; Lefebvre d’Hellencourt, C.; Meilhac, O.; Bascands, J.-L.; Diotel, N. Impaired brain homeostasis and neurogenesis in diet-induced overweight zebrafish: A preventive role from A. borbonica extract. Sci. Rep. 2020, 10, 14496. [Google Scholar] [CrossRef]
- Horzmann, K.A.; Freeman, J.L. Making Waves: New Developments in Toxicology with the Zebrafish. Toxicol. Sci. 2018, 163, 5–12. [Google Scholar] [CrossRef]
- Tal, T.; Yaghoobi, B.; Lein, P.J. Translational toxicology in zebrafish. Curr. Opin. Toxicol. 2020, 23–24, 56–66. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the Six Isomers of Dicaffeoylquinic Acid by LC-MSn. J. Agric. Food Chem 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Fang, N.; Yu, S.; Prior, R.L. LC/MS/MS characterization of phenolic constituents in dried plums. J. Agric. Food Chem. 2002, 50, 3579–3585. [Google Scholar] [CrossRef]
- Jaiswal, R.; Patras, M.A.; Eravuchira, P.J.; Kuhnert, N. Profile and characterization of the chlorogenic acids in green Robusta coffee beans by LC-MS(n): Identification of seven new classes of compounds. J. Agric. Food Chem. 2010, 58, 8722–8737. [Google Scholar] [CrossRef]
- Clifford, M.N.; Marks, S.; Knight, S.; Kuhnert, N. Characterization by LC-MS(n) of four new classes of p-coumaric acid-containing diacyl chlorogenic acids in green coffee beans. J. Agric. Food Chem. 2006, 54, 4095–4101. [Google Scholar] [CrossRef]
- Wu, S.-B.; Meyer, R.S.; Whitaker, B.D.; Litt, A.; Kennelly, E.J. A new liquid chromatography–mass spectrometry-based strategy to integrate chemistry, morphology, and evolution of eggplant (Solanum) species. J. Chromatogr. A 2013, 1314, 154–172. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Surucu, B.; Kuhnert, N. Characterization by LC-MSn of Four New Classes of Chlorogenic Acids in Green Coffee Beans: Dimethoxycinnamoylquinic Acids, Diferuloylquinic Acids, Caffeoyl-dimethoxycinnamoylquinic Acids, and Feruloyl-dimethoxycinnamoylquinic Acids. J. Agric. Food Chem. 2006, 54, 1957–1969. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Drummond, I.A.; Majumdar, A.; Hentschel, H.; Elger, M.; Solnica-Krezel, L.; Schier, A.F.; Neuhauss, S.C.; Stemple, D.L.; Zwartkruis, F.; Rangini, Z.; et al. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 1998, 125, 4655–4667. [Google Scholar] [PubMed]
- Goldstone, J.V.; McArthur, A.G.; Kubota, A.; Zanette, J.; Parente, T.; Jönsson, M.E.; Nelson, D.R.; Stegeman, J.J. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genom. 2010, 11, 643. [Google Scholar] [CrossRef] [PubMed]
- Field, H.A.; Ober, E.A.; Roeser, T.; Stainier, D.Y.R. Formation of the digestive system in zebrafish. I. Liver morphogenesis. Dev. Biol. 2003, 253, 279–290. [Google Scholar] [CrossRef]
- Flores-Pérez, A.; Marchat, L.A.; Sánchez, L.L.; Romero-Zamora, D.; Arechaga-Ocampo, E.; Ramírez-Torres, N.; Chávez, J.D.; Carlos-Reyes, Á.; Astudillo-de la Vega, H.; Ruiz-García, E.; et al. Differential proteomic analysis reveals that EGCG inhibits HDGF and activates apoptosis to increase the sensitivity of non-small cells lung cancer to chemotherapy. Prot. Clin. Appl. 2016, 10, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Touillaud, M.; Rothwell, J.A.; Romieu, I.; Scalbert, A. Measuring exposure to the polyphenol metabolome in observational epidemiologic studies: Current tools and applications and their limits. Am. J. Clin. Nutr. 2014, 100, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Modulation of signaling pathways in prostate cancer by green tea polyphenols. Biochem. Pharm. 2013, 85, 667–672. [Google Scholar] [CrossRef]
- Afshin, A.; Micha, R.; Khatibzadeh, S.; Mozaffarian, D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef]
- Rasines-Perea, Z.; Teissedre, P.-L. Grape Polyphenols’ Effects in Human Cardiovascular Diseases and Diabetes. Molecules 2017, 22, 68. [Google Scholar] [CrossRef]
- Colizzi, C. The protective effects of polyphenols on Alzheimer’s disease: A systematic review. Alzheimers Dement. (New York) 2019, 5, 184–196. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Le Sage, F.; Meilhac, O.; Gonthier, M.-P. Anti-inflammatory and antioxidant effects of polyphenols extracted from Antirhea borbonica medicinal plant on adipocytes exposed to Porphyromonas gingivalis and Escherichia coli lipopolysaccharides. Pharm. Res. 2017, 119, 303–312. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: An application of the phenol-explorer database. J. Agric. Food Chem. 2010, 58, 4959–4969. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; Williamson, G. Methods in Polyphenol Analysis; Royal Society of Chemistry: Cambridge, UK, 2003; ISBN 978-0-85404-580-8. [Google Scholar]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef] [PubMed]
- Złotek, U.; Mikulska, S.; Nagajek, M.; Świeca, M. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J. Biol. Sci. 2016, 23, 628–633. [Google Scholar] [CrossRef]
- Liu, W.; Yin, D.; Li, N.; Hou, X.; Wang, D.; Li, D.; Liu, J. Influence of Environmental Factors on the Active Substance Production and Antioxidant Activity in Potentilla fruticosa L. and Its Quality Assessment. Sci. Rep. 2016, 6, 28591. [Google Scholar] [CrossRef]
- Regos, I.; Treutter, D. Optimization of a high-performance liquid chromatography method for the analysis of complex polyphenol mixtures and application for sainfoin extracts (Onobrychis viciifolia). J. Chromatogr. A 2010, 1217, 6169–6177. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Hatia, S.; Septembre-Malaterre, A.; Le Sage, F.; Badiou-Bénéteau, A.; Baret, P.; Payet, B.; Lefebvre d’hellencourt, C.; Gonthier, M.P. Evaluation of antioxidant properties of major dietary polyphenols and their protective effect on 3T3-L1 preadipocytes and red blood cells exposed to oxidative stress. Free Radic. Res. 2014, 48, 387–401. [Google Scholar] [CrossRef]
- Sirota, R.; Gibson, D.; Kohen, R. The timing of caffeic acid treatment with cisplatin determines sensitization or resistance of ovarian carcinoma cell lines. Redox Biol. 2017, 11, 170–175. [Google Scholar] [CrossRef]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81, 326S–329S. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Cortinovis, C.; Caloni, F. Alkaloid-Containing Plants Poisonous to Cattle and Horses in Europe. Toxins 2015, 7, 5301–5307. [Google Scholar] [CrossRef]
- Cheeke, P.R. Toxicity and metabolism of pyrrolizidine alkaloids. J. Anim. Sci. 1988, 66, 2343–2350. [Google Scholar] [CrossRef]
- Diaz, G.J. Toxicosis by Plant Alkaloids in Humans and Animals in Colombia. Toxins 2015, 7, 5408–5416. [Google Scholar] [CrossRef]
- Sano, K.; Inohaya, K.; Kawaguchi, M.; Yoshizaki, N.; Iuchi, I.; Yasumasu, S. Purification and characterization of zebrafish hatching enzyme—An evolutionary aspect of the mechanism of egg envelope digestion. FEBS J. 2008, 275, 5934–5946. [Google Scholar] [CrossRef]
- Muraina, I.A.; Maret, W.; Bury, N.R.; Hogstrand, C. Hatching gland development and hatching in zebrafish embryos: A role for zinc and its transporters Zip10 and Znt1a. Biochem. Biophys. Res. Commun. 2020, 528, 698–705. [Google Scholar] [CrossRef]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [PubMed]
- McCampbell, K.K.; Wingert, R.A. New tides: Using zebrafish to study renal regeneration. Transl. Res. 2014, 163, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Hanke, N.; Staggs, L.; Schroder, P.; Litteral, J.; Fleig, S.; Kaufeld, J.; Pauli, C.; Haller, H.; Schiffer, M. “Zebrafishing” for novel genes relevant to the glomerular filtration barrier. BioMed Res. Int. 2013, 2013, 658270. [Google Scholar] [CrossRef]
- Morello, J.; Derks, R.J.E.; Lopes, S.S.; Steenvoorden, E.; Monteiro, E.C.; Mayboroda, O.A.; Pereira, S.A. Zebrafish Larvae Are a Suitable Model to Investigate the Metabolic Phenotype of Drug-Induced Renal Tubular Injury. Front. Pharm. 2018, 9, 1193. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, R.C.; Krekels, E.H.J.; Hankemeier, T.; Spaink, H.P.; van der Graaf, P.H. Systems pharmacology of hepatic metabolism in zebrafish larvae. Drug Discov. Today Dis. Models 2016, 22, 27–34. [Google Scholar] [CrossRef]
- Singleton, V.L. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
Sample Availability: Crushed leaves of A. borbonica available from the authors. |

| IC50 (g/L) | ||
|---|---|---|
| Ascorbic Acid | Acetonic Extract | Aqueous Extract |
| 2.8 ± 0.1 | 3.1 ± 0.3 | 3.3 ± 0.3 |
| Peak Number | RT (min) | Compound | Molecular Formula | Mass Error (ppm) | [M − H]− | MS/MS Fragments | mzCloud Best Match (%) |
|---|---|---|---|---|---|---|---|
| 1 | 0.52 | d-Quinic acid | C7H12O6 | 0.4 | 191.0554 | 111.0076 | 85.5 |
| 2 | 2.17 | Protocatechuic acid | C7H6O4 | 0.13 | 153.0184 | 109.0283 | 82.7 |
| 3 | 2.63 | 3-Caffeoylquinic acid | C16H18O9 | 1.03 | 353.0877 | 191.0554, 179.0343, 173.0447, 135.0441 | 85 |
| 4 | 3.47 | 5-Caffeoylquinic acid | C16H18O9 | 1.03 | 353.0877 | 191.0554, 179.0343, 173.0447, 135.0441 | 88.3 |
| 5 | 3.68 | Caffeic acid | C9 H8 O4 | 0.2 | 179.0341 | 135.0441 | 80.2 |
| 6 | 4.09 | p-Coumaroyl quinic acid isomer | C16H18O8 | 1.3 | 337.0931 | 191.0550, 173.0446, 163.0392 | 84.6 |
| 7 | 4.18 | p-Coumaroyl quinic acid isomer | C16H18O8 | 1.3 | 337.0931 | 191.0550, 173.0446, 163.0392 | 84.6 |
| 8 | 4.2 | o/m-Coumaric acid | C9H8O3 | 0.2 | 163.0391 | 119.049 | 81.2 |
| 9 | 4.36 | Feruloylquinic acid | C17H20O9 | 0.5 | 367.1035 | 191.0550, 173.0446 | _ |
| 10 | 4.43 | p-Coumaric acid | C9H8O3 | 0.1 | 163.0391 | 119.049 | 81.2 |
| 11 | 4.74 | Quercetin-3-O-rutinoside (Rutin) | C27H30O16 | 1.6 | 609.1466 | 300.0274 | 94.8 |
| 12 | 4.94 | Quercetin-3-O-galactoside | C21H20O12 | 1.33 | 463.0884 | 300.0274 | 90.9 |
| 13 | 5.01 | Quercetin-3-O-glucoside | C21H20O12 | 1.33 | 463.0884 | 300.0274 | 90.9 |
| 14 | 5.26 | Kaempferol-O-hexoside | C21H20O11 | 1.35 | 447.0935 | 284.0326 | 83.7 |
| 15 | 5.45 | Kaempferol-O-hexoside | C21H20O11 | 1.35 | 447.0935 | 284.0326 | 83.7 |
| 16 | 5.82 | 3,5-Dicaffeoylquinic acid | C25H24O12 | 1.04 | 515.1196 | 353.0878, 191.0554, 179.0343, 173.0447, 135.0441 | 83.6 |
| 17 | 6.02 | 3,4-Dicaffeoylquinic acid | C25H24O12 | 1.04 | 515.1195 | 353.0878, 173.0447, 191.0554, 179.0343, 135.0441 | 88.1 |
| 18 | 6.2 | 4-Caffeoylquinic acid | C16H18O9 | 1.03 | 353.0877 | 173.0447, 191.0554, 179.0343, 173.0447, 135.0441 | 86.3 |
| 19 | 6.36 | 1,4/4,5-Dicaffeoylquinic acid | C25H24O12 | 1.04 | 515.1194 | 353.0878, 173.0447, 191.0554, 179.0343, 135.0441 | 89.1 |
| Concentration in Acetonic Extract (mg/mL) | Concentration in Aqueous Extract (mg/mL) | ||
|---|---|---|---|
| Peak | Phenolic Acids | ||
| 5 | Caffeic acid | 0.002162 ± 0.000066 | 0.000703 ± 0.000039 *** |
| 10 | p-Coumaric acid | 0.002755 ± 0.000728 | 0.001768 ± 0.000176 * |
| 8 | m/o-Coumaric acid | 0.000470 ± 0.000003 | 0.000208 ± 0.000004 |
| 4 | 5-CQA | 0.004718 ± 0.000279 | 0.008558 ± 0.000477 *** |
| 3 | 3-CQA | 0.000840 ± 0.000093 | 0.001604 ± 0.000157 *** |
| 17 | 3,4-diCQA | 0.004704 ± 0.000326 | 0.000503 ± 0.000034 *** |
| 19 | 1,4/4,5-diCQA | 0.000262 ± 0.000020 | 0.000090 ± 0.000003 ** |
| 16 | 3,5-diCQA | 0.002629 ± 0.000161 | 0.000842 ± 0.000029 *** |
| 2 | Protocatechuic acid | 0.004070 ± 0.000250 | 0.002415 ± 0.000387 *** |
| Total | 0.023061 | 0.016693 | |
| Flavonols | |||
| 11 | Quercetin-3-O-rutinoside | 0.011933 ± 0.002018 | 0.003977 ± 0.000473 *** |
| 12 | Quercetin-3-O-galactoside | 0.001791 ± 0.000204 | 0.000591 ± 0.000033 *** |
| 14/15 | Kaempferol hexosides | 0.000216 ± 0.000054 | 0.000044 ± 0.000005 ** |
| Total | 0.013941 | 0.004612 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veeren, B.; Ghaddar, B.; Bringart, M.; Khazaal, S.; Gonthier, M.-P.; Meilhac, O.; Diotel, N.; Bascands, J.-L. Phenolic Profile of Herbal Infusion and Polyphenol-Rich Extract from Leaves of the Medicinal Plant Antirhea borbonica: Toxicity Assay Determination in Zebrafish Embryos and Larvae. Molecules 2020, 25, 4482. https://doi.org/10.3390/molecules25194482
Veeren B, Ghaddar B, Bringart M, Khazaal S, Gonthier M-P, Meilhac O, Diotel N, Bascands J-L. Phenolic Profile of Herbal Infusion and Polyphenol-Rich Extract from Leaves of the Medicinal Plant Antirhea borbonica: Toxicity Assay Determination in Zebrafish Embryos and Larvae. Molecules. 2020; 25(19):4482. https://doi.org/10.3390/molecules25194482
Chicago/Turabian StyleVeeren, Bryan, Batoul Ghaddar, Matthieu Bringart, Shaymaa Khazaal, Marie-Paule Gonthier, Olivier Meilhac, Nicolas Diotel, and Jean-Loup Bascands. 2020. "Phenolic Profile of Herbal Infusion and Polyphenol-Rich Extract from Leaves of the Medicinal Plant Antirhea borbonica: Toxicity Assay Determination in Zebrafish Embryos and Larvae" Molecules 25, no. 19: 4482. https://doi.org/10.3390/molecules25194482
APA StyleVeeren, B., Ghaddar, B., Bringart, M., Khazaal, S., Gonthier, M.-P., Meilhac, O., Diotel, N., & Bascands, J.-L. (2020). Phenolic Profile of Herbal Infusion and Polyphenol-Rich Extract from Leaves of the Medicinal Plant Antirhea borbonica: Toxicity Assay Determination in Zebrafish Embryos and Larvae. Molecules, 25(19), 4482. https://doi.org/10.3390/molecules25194482





