A Colorimetric Assay for the Detection of Glucose and H2O2 Based on Cu-Ag/g-C3N4/ZIF Hybrids with Superior Peroxidase Mimetic Activity
Abstract
1. Introduction
2. Results and Discussion
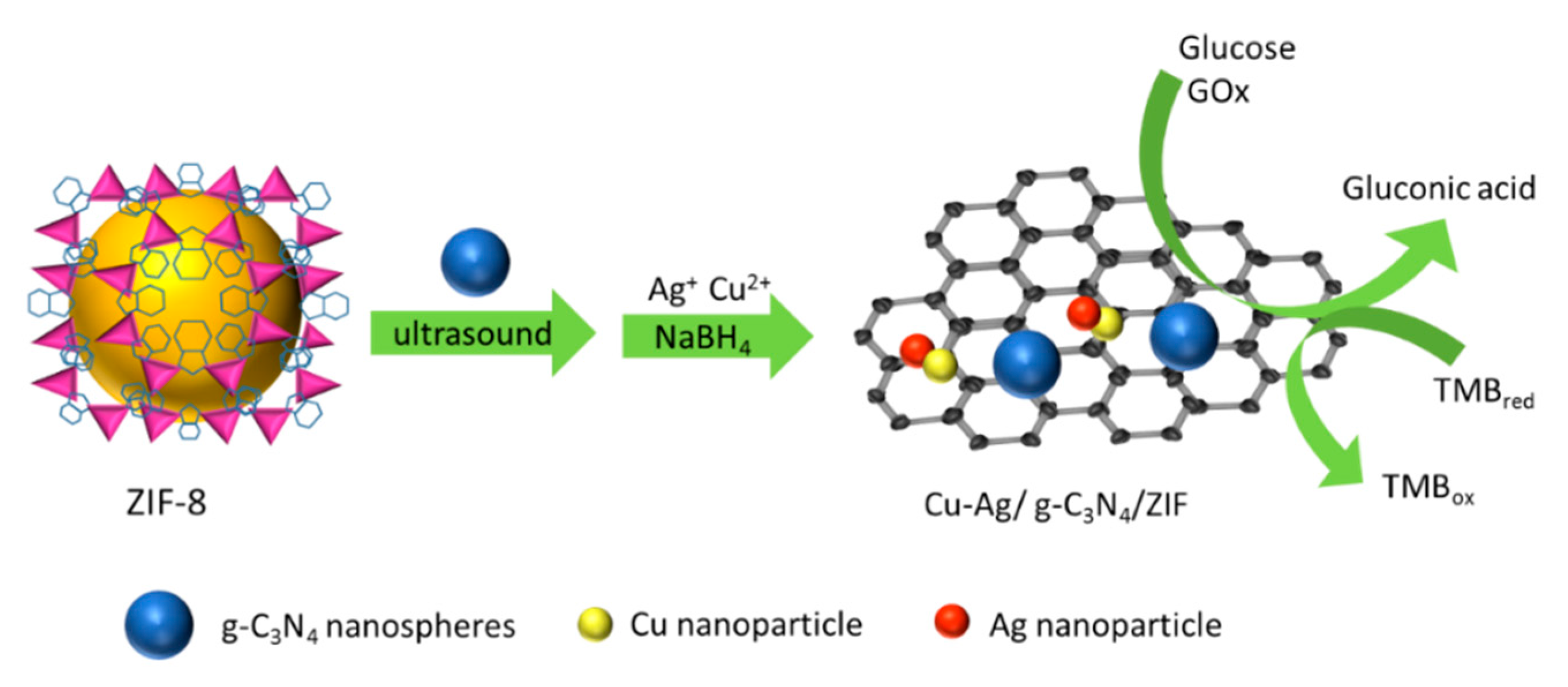
2.1. Characterizations of Cu-Ag/g-C3N4/ZIF Hybrid
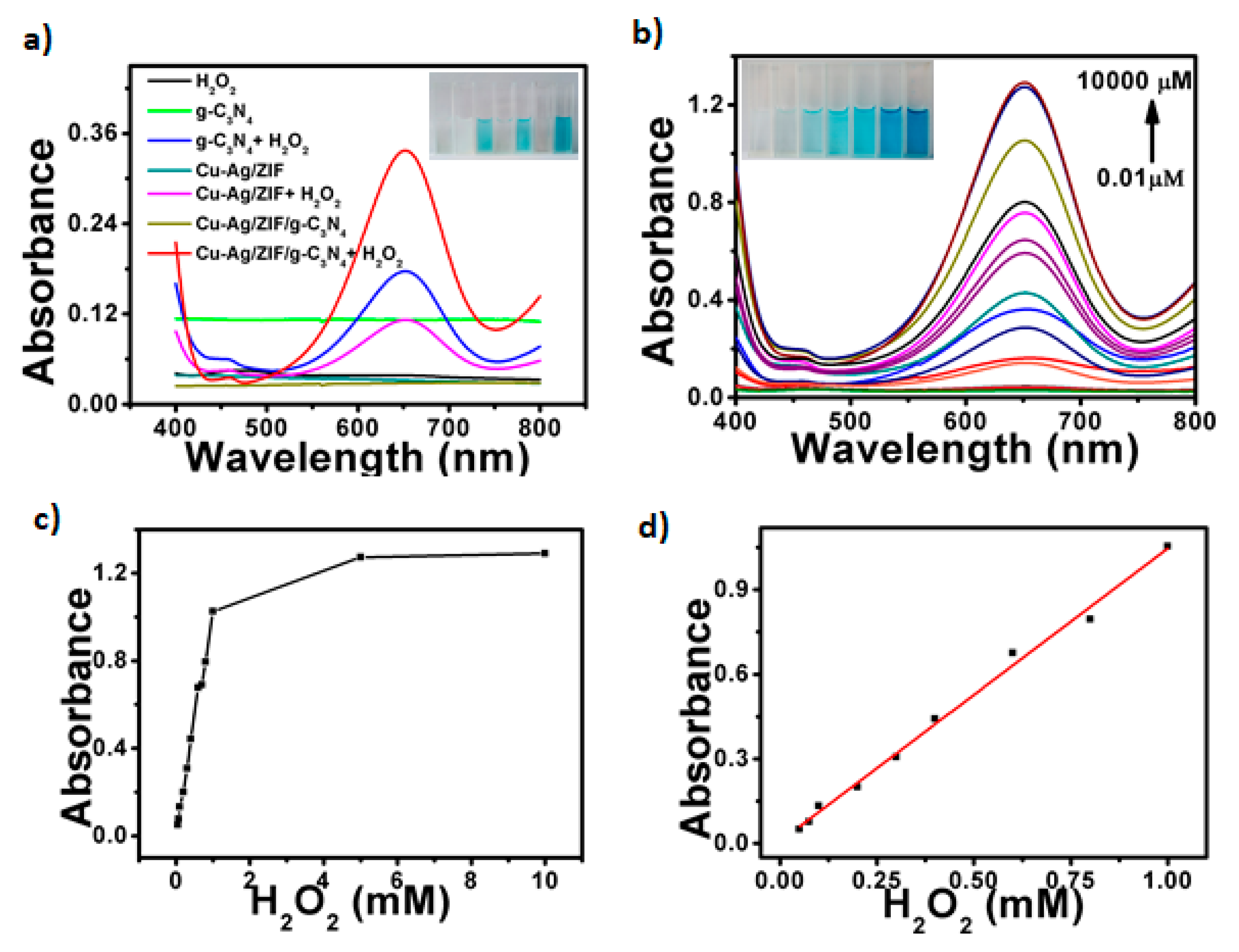
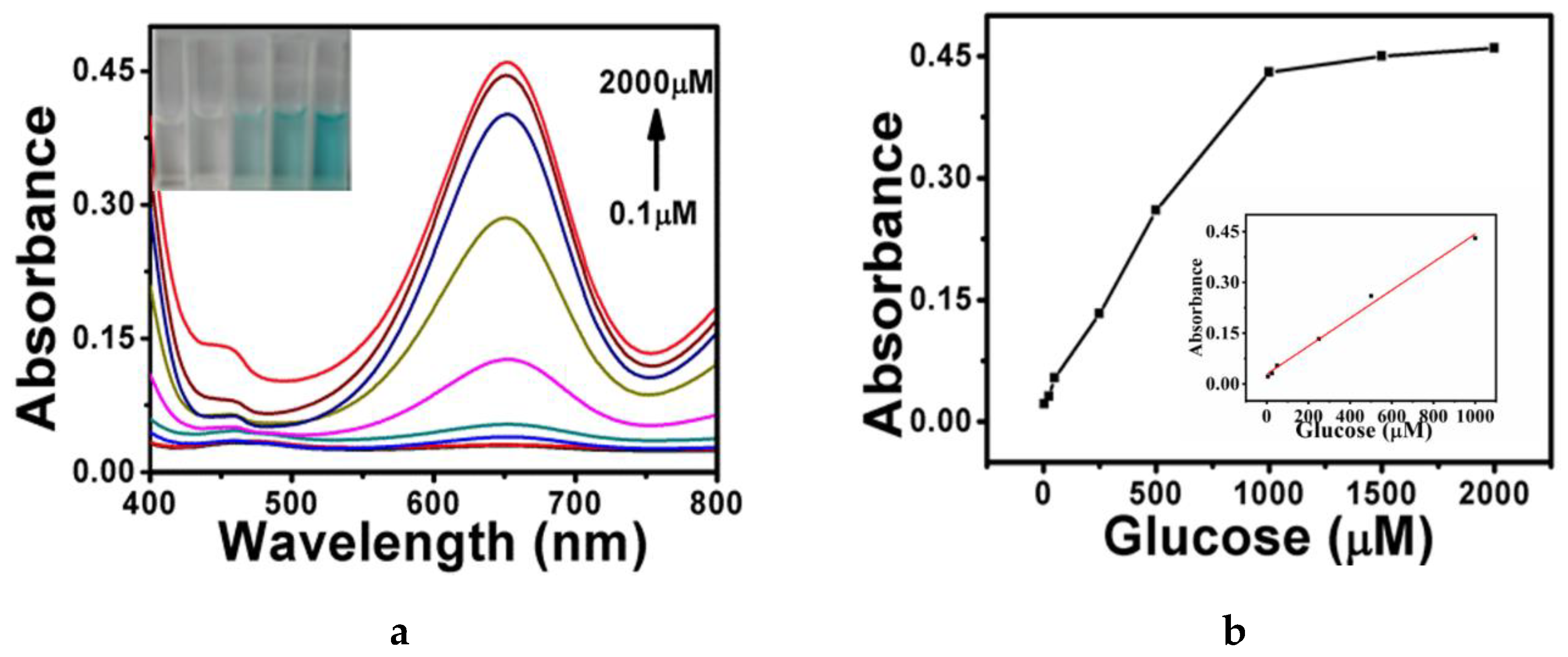
2.2. Cu-Ag/g-C3N4/ZIF Hybrid for the Detection of H2O2 and Glucose
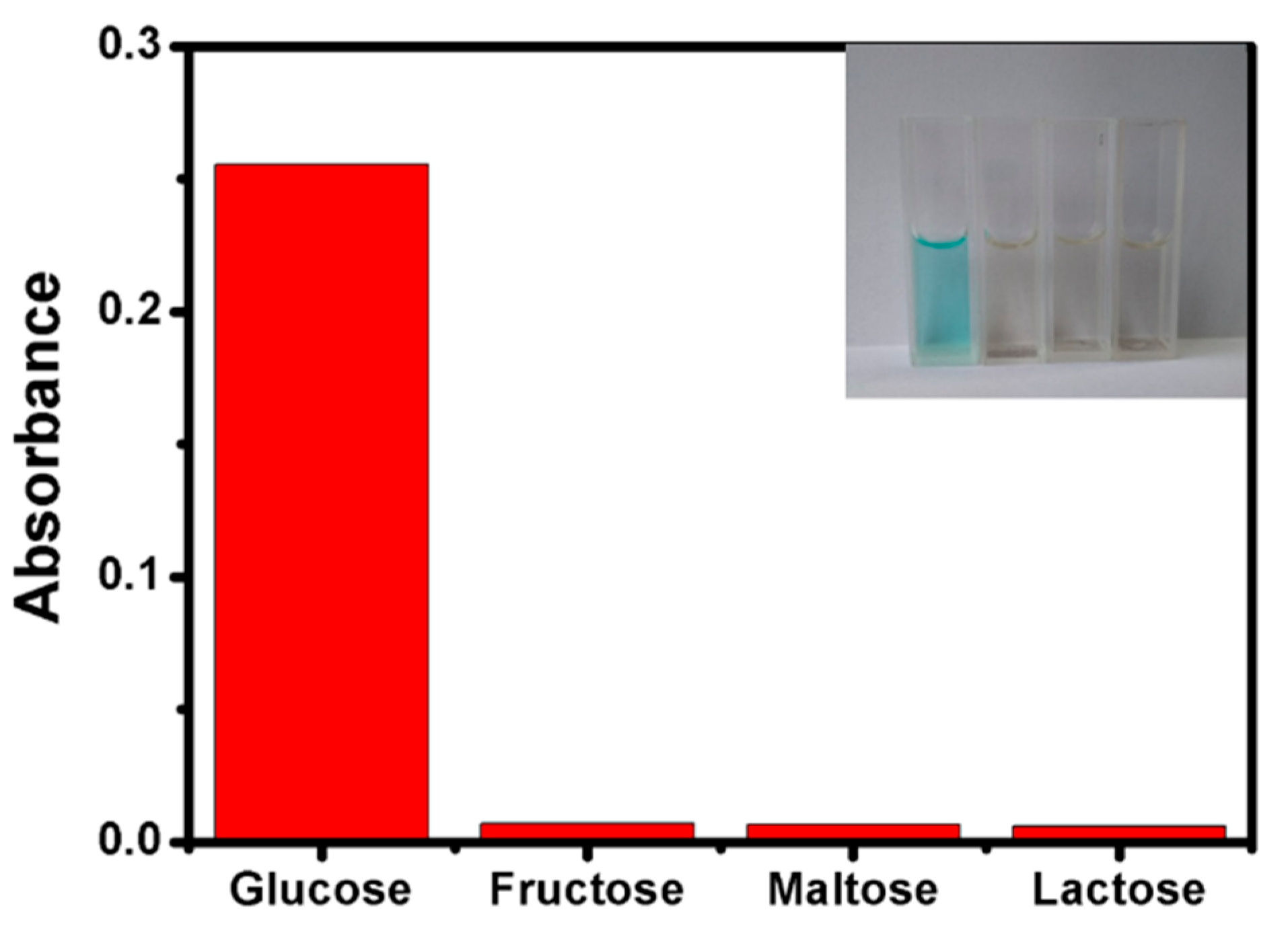
2.3. The Selectivity of Cu-Ag/g-C3N4/ZIF Hybrid to Glucose Detection
2.4. Colorimetric Detection of Glucose in Real Serum Samples
3. Experimental
3.1. Materials and Apparatus
3.2. One-Step Method to Synthesize Cu-Ag/g-C3N4/ZIF Hybrid
3.3. Cu-Ag/g-C3N4/ZIF Hybrid for Colorimetric Detection of H2O2 and Glucose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darabdhara, G.; Bordoloi, J.; Manna, P.; Das, M.R. Biocompatible bimetallic Au-Ni doped graphitic carbon nitride sheets: A novel peroxidase-mimicking artificial enzyme for rapid and highly sensitive colorimetric detection of glucose. Sens. Actuators B Chem. 2019, 285, 277–290. [Google Scholar] [CrossRef]
- Wu, N.; Wang, Y.T.; Wang, X.Y.; Guo, F.N.; Wen, H.; Yang, T.; Wang, J.H. Enhanced peroxidase-like activity of AuNPs loaded graphitic carbon nitride nanosheets for colorimetric biosensing. Anal. Chim. Acta 2019, 1091, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, N.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon nanozymes: Enzymatic properties, catalytic mechanism, and applications. Angew. Chem. Int. Ed. 2018, 57, 9224–9237. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Wei, C. Using G-Rich sequence to enhance the peroxidase-mimicking activity of DNA-Cu/Ag nanoclusters for rapid colorimetric detection of hydrogen peroxide and glucose. Chem. Select 2020, 5, 5166–5171. [Google Scholar] [CrossRef]
- Mirhosseiniab, M.; Farab, A.S.; Hakimianbc, F.; Haghiralsadat, B.F.; Fatemi, S.K.; Dashtestanib, F. Core-shell Au@Co-Fe hybrid nanoparticles as peroxidase mimetic nanozyme for antibacterial application. Process. Biochem. 2020, 95, 131–138. [Google Scholar] [CrossRef]
- Lian, J.; Liu, P.; Jin, C.; Shi, Z.; Luo, X.; Liu, Q. Perylene diimide-functionalized CeO2 nanocomposite as a peroxidase mimic for colorimetric determination of hydrogen peroxide and glutathione. Microchim. Acta 2019, 186, 332. [Google Scholar] [CrossRef]
- Zhang, B.; Huyan, Y.; Wang, J.; Wang, W.; Zhang, Q.; Zhang, H. Synthesis of CeO2 nanoparticles with different morphologies and their properties as peroxidase mimic. J. Am. Ceram. Soc. 2019, 102, 2218–2227. [Google Scholar] [CrossRef]
- Yang, W.N.; Li, J.; Yang, J.; Liu, Y.; Xu, Z.P.; Sun, X.F.; Wang, F.D. Biomass-derived hierarchically porous CoFe-LDH/CeO2 hybrid with peroxidase-like activity for colorimetric sensing of H2O2 and glucose. J. Alloys Compd. 2020, 815, 152276. [Google Scholar]
- Xing, Y.; Si, H.; Sun, D.; Hou, X. Magnetic Fe3O4@NH2-MIL-101(Fe) nanocomposites with peroxidase-like activity for colorimetric detection of glucose. Microchem. J. 2020, 156, 104929. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Algara-Siller, G.; Severin, N.; Chong, S.Y.; Björkman, T.; Palgrave, R.G.; Laybourn, A.; Antonietti, M.; Khimyak, Y.Z.; Krasheninnikov, A.V.; Rabe, J.P.; et al. Triazine-based graphitic carbon nitride: A two-dimensional semiconductor. Angew. Chem. 2014, 126, 7580–7585. [Google Scholar] [CrossRef]
- Cao, S.W.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, C.; Luo, J.; Yang, M. C3N4 nanosheet-supported Prussian Blue nanoparticles as a peroxidase mimic: Colorimetric enzymatic determination of lactate. Microchim. Acta 2019, 186, 735. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, R.; Lin, J.; Zhu, Y. Enhancement of photocurrent and photocatalytic activity of ZnO hybridized with graphite-like C3N4. Energy Environ. Sci. 2011, 4, 2922–2929. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Zhu, Y. Visible photocatalytic activity enhancement of ZnWO4 by graphene hybridization. Adv. Funct. Mater. 2012, 22, 1518–1524. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Shanker, V. Surface plasmon resonance-induced photocatalysis by Au nanoparticles decorated mesoporous g-C3N4 nanosheets under direct sunlight irradiation. Mater. Res. Bull. 2016, 75, 51–58. [Google Scholar] [CrossRef]
- Fu, Y.; Huang, T.; Jia, B.; Zhu, J.; Wang, X. Reduction of nitrophenols to aminophenols under concerted catalysis by Au/g-C3N4 contact system. Appl. Catal. B Environ. 2017, 202, 430–437. [Google Scholar] [CrossRef]
- Fu, J.; Chang, B.B.; Tian, Y.L.; Xi, F.N.; Dong, X.P. Novel C3N4 -CdS composite photocatalysts with organic-inorganic heterojunctions: In suit synthesis, exceptional activity, high stability and photocatalytic mechanism. J. Mater. Chem. A. 2013, 1, 3083–3090. [Google Scholar] [CrossRef]
- Wang, J.; Guo, P.; Guo, Q.S.; Jonsson, P.G.; Zhao, Z. Fabrication of novel g-C3N4/nanocage ZnS composites with enhanced photocatalytic activities under visible light irradiation. Cryst. Eng. Comm. 2014, 16, 4485–4492. [Google Scholar] [CrossRef]
- Li, Y.B.; Zhang, H.M.; Liu, P.R.; Wang, D.; Li, Y.; Zhao, H.J. Cross-linked g-C3N4/rGO nanocomposites with tunable band structure and enhanced visible light photocatalytic activity. Small 2013, 9, 3336–3344. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.G.; Zhao, X.S. Nitrogen-doped titanate-anatase core-shell nanobelts with exposed {101} anatase facets and enhanced visible light photocatalytic activity. J. Am. Chem. Soc. 2012, 134, 5754–5757. [Google Scholar] [CrossRef]
- Darabdhara, G.; Sharma, B.; Dasa, M.R.; Boukherrou, R.; Szunerits, S. Cu-Ag bimetallic nanoparticles on reduced graphene oxidenanosheets as peroxidase mimic for glucose and ascorbic aciddetection. Sens. Actuators B Chem. 2017, 238, 842–851. [Google Scholar] [CrossRef]
- Song, X.; Sun, H.; Cao, X.; Wang, Z.; Zhao, D.; Sun, J.; Zhang, H.; Li, X. Hierarchically porous ternary Au/ZnO@ZIF-8 nanocomposite: Spatial in situ Au encapsulation and catalytic activity for the reduction of p-nitrophenol. RSC Adv. 2016, 6, 112451–112454. [Google Scholar] [CrossRef]
- Yang, L.; Yan, D.; Liu, C. Vertically oriented reduced grapheme oxide supported dealloyed palladium–copper nanoparticles for methanol electrooxidation. J. Power Sources 2015, 278, 725–732. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Xie, A.; Qiu, L.; Li, S.; Wang, Y. Novel structure CuI/PANI nanocomposites with bifunctions: Superhydrophobicity and photocatalytic activity. J. Mater. Chem. A 2011, 21, 9641–9646. [Google Scholar] [CrossRef]
- Cash, K.J.; Clark, H.A. Nanosensors and nanomaterials for monitoring glucose indiabetes. Trends Mol. Med. 2010, 16, 584–593. [Google Scholar] [CrossRef]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene oxide: Intrinsic peroxidase cat-alytic activity and its application to glucose detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Q.; Long, Y.; Cheng, Z.; Chen, S.; Zheng, H.; Huang, Y. Carbon nanodotsas peroxidase mimetics and their applications to glucose detection. Chem. Commun. 2011, 47, 6695–6697. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Seredych, M.; Rodríguez-Castellón, E.; Bandosz, T.J. Mesoporous graphitic carbon nitride-based nanospheres as visible-light active chemical warfare agents decontaminant. ChemNanoMat 2016, 2, 268–272. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds not available from the authors. |


| Nanoenzyme | Lineary Range (μM) | Detection Limits (μM) | Ref. |
|---|---|---|---|
| Fe3O4-porphyrin compositions | 5–25 | 2.21 | [24] |
| Fe3O4 nanoparticles | 50–1000 | 30 | [28] |
| Fe3O4-GO compositions | 1–200 | 0.74 | [3] |
| Carbon nitride | 5–100 | 1 | [29] |
| Co3O4-rGO compositions | 1–100 | 1 | [30] |
| Cu-Ag/g-C3N4/ZIF hybrid | 0.1–1000 | 0.01 | This work |
| Sample | Glucometer Method (mM) | Proposed Method (mM) | Added (mM) | Total Found (mM) | Recovery (%) |
|---|---|---|---|---|---|
| 1 | 4.87 | 4.90 | 3.00 | 7.93 | 101.0% |
| 2 | 5.42 | 5.38 | 3.00 | 8.46 | 102.7% |
| 3 | 5.97 | 6.02 | 3.00 | 9.17 | 105.0% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Q.; Kong, Y.; Chen, K.; Mao, M.; Wan, X.; She, X.; Gao, Q.; He, Y.; Song, G. A Colorimetric Assay for the Detection of Glucose and H2O2 Based on Cu-Ag/g-C3N4/ZIF Hybrids with Superior Peroxidase Mimetic Activity. Molecules 2020, 25, 4432. https://doi.org/10.3390/molecules25194432
Pan Q, Kong Y, Chen K, Mao M, Wan X, She X, Gao Q, He Y, Song G. A Colorimetric Assay for the Detection of Glucose and H2O2 Based on Cu-Ag/g-C3N4/ZIF Hybrids with Superior Peroxidase Mimetic Activity. Molecules. 2020; 25(19):4432. https://doi.org/10.3390/molecules25194432
Chicago/Turabian StylePan, Quan, Yuelin Kong, Kuan Chen, Mi Mao, Xiaohui Wan, Xiaoyan She, Qingsong Gao, Yu He, and Gongwu Song. 2020. "A Colorimetric Assay for the Detection of Glucose and H2O2 Based on Cu-Ag/g-C3N4/ZIF Hybrids with Superior Peroxidase Mimetic Activity" Molecules 25, no. 19: 4432. https://doi.org/10.3390/molecules25194432
APA StylePan, Q., Kong, Y., Chen, K., Mao, M., Wan, X., She, X., Gao, Q., He, Y., & Song, G. (2020). A Colorimetric Assay for the Detection of Glucose and H2O2 Based on Cu-Ag/g-C3N4/ZIF Hybrids with Superior Peroxidase Mimetic Activity. Molecules, 25(19), 4432. https://doi.org/10.3390/molecules25194432




