Abstract
Organophosphates (OPs) are esters of substituted phosphates, phosphonates or phosphoramidates that react with acetylcholinesterase (AChE) by initially transferring the organophosphityl group to a serine residue in the enzyme active site, concomitant with loss of an alcohol or halide leaving group. With substituted phosphates, this transfer is followed by relatively slow hydrolysis of the organophosphoryl AChE, or dephosphorylation, that is often accompanied by an aging reaction that renders the enzyme irreversibly inactivated. Aging is a dealkylation that converts the phosphate triester to a diester. OPs are very effective AChE inhibitors and have been developed as insecticides and chemical warfare agents. We examined three reactions of two organophosphoryl AChEs, dimethyl- and diethylphosphorylated AChE, by comparing rate constants and solvent deuterium oxide isotope effects for hydrolysis, aging and oxime reactivation with pralidoxime (2-PAM). Our study was motivated (1) by a published x-ray crystal structure of diethylphosphorylated AChE, which showed severe distortion of the active site that was restored by the binding of pralidoxime, and (2) by published isotope effects for decarbamoylation that decreased from 2.8 for N-monomethylcarbamoyl AChE to 1.1 for N,N-diethylcarbamoyl AChE. We previously reconciled these results by proposing a shift in the rate-limiting step from proton transfer for the small carbamoyl group to a likely conformational change in the distorted active site of the large carbamoyl enzyme. This proposal was tested but was not supported in this report. The smaller dimethylphosphoryl AChE and the larger diethylphosphoryl AChE gave similar isotope effects for both oxime reactivation and hydrolysis, and the isotope effect values of about two indicated that proton transfer was rate limiting for both reactions.
1. Introduction
Acetylcholinesterase (AChE) catalyzes the hydrolysis of the neurotransmitter acetylcholine, and rapid hydrolysis of this ester is essential for normal cholinergic synaptic transmission. Acetylcholine hydrolysis proceeds by transfer of the acetyl group to the active site serine of AChE followed by hydrolysis, or deacetylation, of the acetyl enzyme, and both steps occur on a timescale of microseconds [1]. Other ester substrates of AChE proceed through a similar two-step catalytic pathway (Scheme 1). However, the pathways for the esters in Scheme 1 differ from that of acetylcholine in that these esters initially form a detectable reversible equilibrium complex with AChE with a dissociation constant, KD, before transfer of their carbamoyl or organophosphoryl groups (here referred to as acyl groups) to the active site serine to give carbamoyl or phosphoryl intermediates. This complex is an intermediate with acetylthiocholine but is not detectable, as explained in Equation (1) below. While the hydrolysis of the carbamoyl or organophosphoryl intermediates involves transfer of their acyl group from serine to water, their deacylation rate constants (k3 or kH) are orders of magnitude smaller than that of acetylated AChE [2,3]. Their slow deacylation makes them potent inhibitors of AChE, and some carbamates and organophosphates (also denoted OPs) have been widely used as insecticides [4].
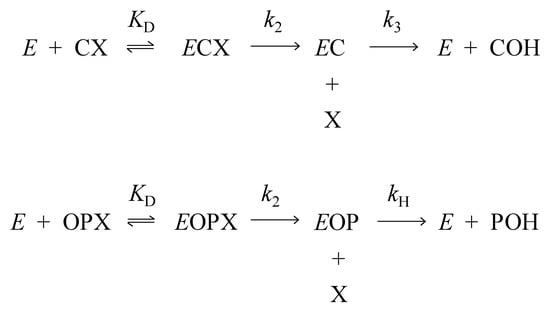
Scheme 1.
Two-step catalytic pathway for carbamates (CX; [5,6]) and organophosphates (OPX; [7]). Products are the alcohol leaving group X and the carbamic acid (COH) or phosphate diester (POH).
One noteworthy feature of AChE intermediates with carbamoyl and phosphoryl acyl groups (Figure 1) is that their relative deacylation rate constants k3 or kH decrease dramatically with an increase in acyl group size. This decrease may result in part from substantial distortion of the active site arising from the bulk of large acyl groups [8]. For example, an X-ray crystal structure of diethylphosphorylated hAChE generated from paraoxon [9] is shown in Figure 2A. In this structure, the diethylphosphoryl group is attached to Ser203. One ethoxy group faces Trp86 and the other faces Phe295, Phe297 and Phe338 in an acyl pocket. Hydrogen bonding within the catalytic triad (Ser203-His447-Glu334) remains intact but the adjacent acyl pocket residues and acyl loop (residue positions 280–297) are significantly perturbed relative to their positions in the ligand-free hAChE structure. Movements in residue backbone positions are necessary to avert steric clash, and the Cα and the sidechain of Arg296 shift by 4.9 A° and 14.9 A°, respectively. Large acyl loop backbone rearrangements have also been seen in mAChE and TcAChE inhibited by the OP diisopropylfluorophosphate [10,11]. However, stereoselective inhibition of AChE by OP nerve agents that place a smaller methyl group into the acyl pocket does not affect the acyl loop in the crystal structure in the same manner [9,10,12].
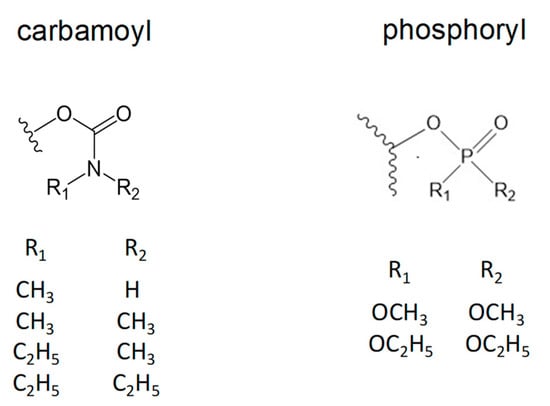
Figure 1.
Molecular structures of carbamoyl and phosphoryl groups attached to human acetylcholinesterase (AChE; squiggled line) compared in this report. Alkyl groups R1 and R2 are attached to the carbamoyl nitrogen atom and alkoxy groups R1 and R2 are attached to the phosphoryl phosphorus atom.
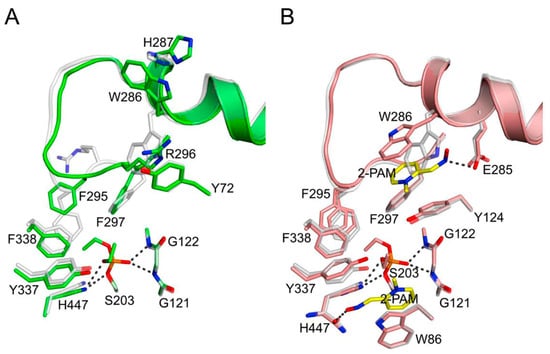
Figure 2.
Conformational changes in hAChE induced by paraoxon inhibition [9]. Structural perturbations seen in diethylphosphorylated hAChE without (A) and with bound 2-PAM (B) are visualized by superimposition with ligand-free hAChE. Carbon atoms of the oximes are colored yellow, and carbon atoms of protein residues are colored by complex: green in (A) and salmon in (B); and ligand-free hAChE, semitransparent gray. Nitrogen, oxygen, and phosphorus atoms are colored dark blue, red and orange, respectively. Residues of the helix preceding the acyl loop and of the acyl loop up to position 298 are drawn as ribbons. Atoms of select residues, reactivators and phosphate adducts are drawn as sticks and hydrogen bonds are depicted by dashed gray lines.
These shifts in the acyl pocket residues and acyl loop in diethylphosphorylated hAChE can be largely reversed by cationic ligand binding to the active site [9]. The interaction of cationic oximes with organophosphorylated AChEs is of intense interest because oximes have been shown to reactivate these AChEs by displacing the serine hydroxyl and releasing the organophosphorylated oxime [7,13]. The structure in which 50 mM 2-PAM was diffused into the crystals to form a complex of diethylphosphorylated hAChE with the cationic oxime 2-PAM reveals two bound 2-PAM molecules (Figure 2B), one in the peripheral site and the other stacked against the sidechain of Trp86. In this ternary complex, the backbone conformation of the acyl loop resembles that of the ligand-free state and appears to be stabilized by oxime binding. Therefore, diffusion of these oximes into the crystals of diethylphosphorylated hAChE promoted a restoration of the acyl pocket residues and acyl loop to their locations in the unmodified hAChE structure.
The catalytic effects of active site distortion in diethylphosphorylated AChE on the deacylation pathway can be examined by determining D2O isotope effects on the deacylation reactions. Here we investigate the reaction rate constants and D2O isotope effects for three reactions of diethylphosphorylated AChE, which shows crystallographic evidence of active site distortion, and of dimethylphosphorylated AChE, which does not [9,10,12].
2. Results
The primary objectives of this paper are to obtain the solvent D2O isotope effects for three of four reactions that involve organophosphorylated AChEs. The four reactions are outlined in Scheme 2, and the isotope effects are shown in Table 1 below. Because the determination of solvent D2O isotope effects requires accuracy and precision, an emphasis is placed on protocols for rate constant measurement. The isotope effects obtained are then compared to those previously measured for carbamoylated AChEs, and differences among them are interpreted in the context of X-ray crystal structures determined for organophosphorylated AChEs like those shown in Figure 2.
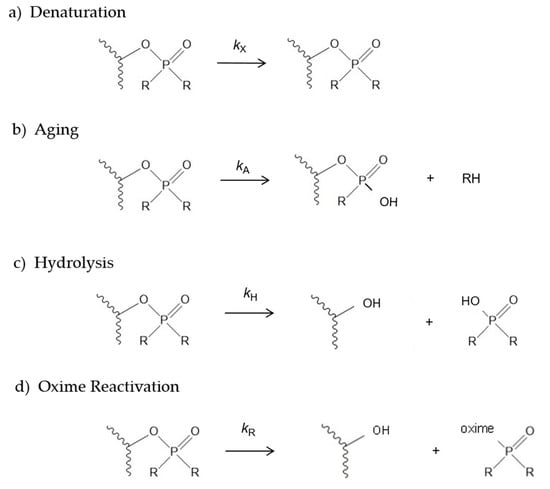
Scheme 2.
Four reactions of organophosphorylated AChE (a–d). The alcohol RH, the phosphate diester R2P(O)OH, and the phosphate triester R2(oxime)P(O) are the products released during the aging, hydrolysis and reactivation reactions, respectively.

Table 1.
Rate constants for reactions of organophosphorylated AChE and their solvent D2O isotope effects.
Protocols to measure the rate constants of the reactions of organophosphorylated AChEs in Scheme 2, in both H2O and D2O, are outlined in the Materials and Methods, and representative reactions are illustrated in Figure 3, Figure 4 and Figure 5 below.
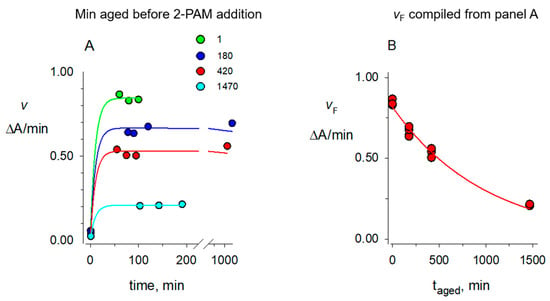
Figure 3.
Aging of dimethylphosphorylated AChE in H2O. A mixture of 2.4 μM paraoxon methyl (OP) and 0.5 μM AChE in 100 mM sodium phosphate and 0.1% BSA (pH 8.0) was incubated for 60 min at 25 °C. The incubation was then aged for the various times indicated before addition of 2-PAM, with the earliest addition of 2-PAM designated as taged = 0. To ensure that the OP remained in excess during aging, additional OP was added (1.7 μM at taged 5 and 185 min and 3.6 μM at taged 425 min). At the indicated taged of 1, 180, 420 and 1470 min, portions of the aged reaction were diluted 24-fold into 1.0 mM 2-PAM. (A) Aliquots (30 μL) of each dilution were assayed as indicated in the Materials and Methods at the times indicated on the x-axis. Assay values v were fit to Equation (2), with the indicated taged fixed. Fixed initial estimates (min−1 for k values) of kX = 2.5 × 10−5, kH = 6.5 × 10−3, kr = 9 × 10−2 and kA = 2 × 10−3 gave fitted Etot = 0.86 ΔA/min (green line); this Etot was then fixed along with the same values of kX, kH, and kr to fit kA = 1–2 × 10−3 and E0 = 0.01–0.02 ΔA/min (blue, red, and cyan lines). Values for times after the initial time point were observed to correspond to the final value of v, denoted vF, in this equation. (B) Assay values corresponding to vF in panel A were analyzed with Equation (3) with a fixed kX = 2.5 × 10−5 min−1 to give Etot = 0.82 ΔA/min and a kA value of (980 + 60) × 10−6 min−1 included in Table 1.
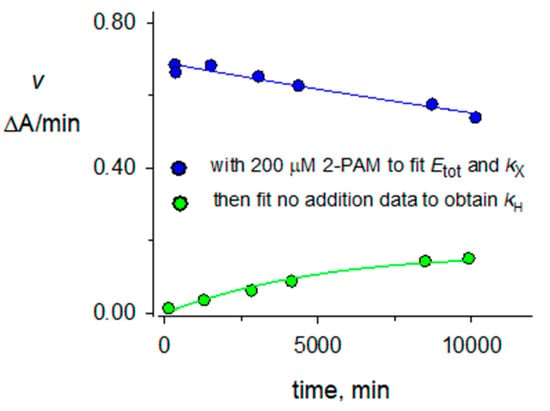
Figure 4.
Denaturation and hydrolysis of diethylphosphorylated AChE in D2O. A mixture of 2.0 μM paraoxon and 1.4 μM AChE in 100 mM sodium phosphate and 0.1% BSA (buffer) in D2O (pH 8.0) was incubated for 70 min at 25 °C. A 4-fold excess of buffer in D2O was added, and 200 μL was applied to a 0.5 mL Sephadex G50 spin column (Fisher) that had been prewashed four times in buffer in D2O and centrifuged at 1000× g for 1 min. The eluent was cooled to 4 °C, and 60 μL was added either to 640 μL of buffer in D2O at 25 °C or 640 μL of the same buffer with 2-PAM (to 200 μM) at 25 °C. Aliquots (30 μL) were assayed as in Figure 3 at the times indicated on the x-axis. Assay values v for the solution containing 2-PAM (blue points that correspond to enzyme denaturation) were first fit to Equation (2) with taged set to 0 and fixed initial estimates (min−1 for k values) of kA = 4 × 10−5, kH = 4 × 10−5, kr = 2 × 10−2 and E0 = 0.01 ΔA/min to obtain kX = 2.2 × 10−5 and Etot =0.69 ΔA/min. These values of taged, kA, and Etot along with kX = 4 × 10−5 and kr = 0 were then fixed into Equation (2) to fit the assay values v for the solution without 2-PAM (green points that reflect enzyme denaturation and diethylphosphorylated enzyme hydrolysis). This fitting gave E0 = 0.003 ΔA/min and a kH value of (47 + 3) × 10−6 min−1 included in Table 1.
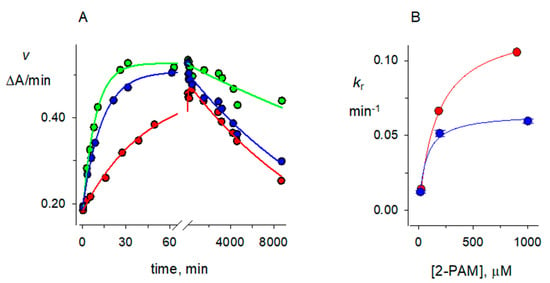
Figure 5.
2-PAM reactivation of dimethylphosphorylated AChE in H2O. A mixture of 1.6 μM paraoxon methyl and 1.5 μM AChE in 100 mM sodium phosphate and 0.1% BSA (buffer) in H2O (pH 8.0) was incubated for 30 min at 25 °C. A 2.5-fold excess of buffer in H2O was added, and 100 μL was applied to a 0.5 mL spin column that had been prewashed four times in buffer in H2O and centrifuged at 1000× g for 1 min. The eluent was added to 215 μL of buffer in H2O and cooled to 4 °C, and 100 μL aliquots were added at time zero to 700 μL of buffer in H2O containing final concentrations of 905, 185, or 24 μM 2-PAM at 25 °C. (A) Aliquots (30 μL) were assayed as indicated in Figure 3 at the times indicated on the x-axis. Assay values v for the solutions containing 905 μM (green points), 185 μM (blue points) or 24 μM 2-PAM (red points) were analyzed with Equation (2) in two steps, with taged set to 0 and kA and kH fixed at 2 × 10−3 min−1 and 6 × 10−3 min−1, respectively, and kr, kX, Etot, and E0 as fitted variables. In the first step, points over the entire 8800-min time course were fitted to obtain kX (in min−1) = 2.5 × 10−5 (green points), 6.3 × 10−5 (blue points), and 7.0 × 10−5 (red points). These kX were then fixed along with kA and kH in the second step in which time courses of 250 to 450 min were fitted to give more precise values of kr. (B) Values of kr from the second fitting step in panel A were then fitted to Equation (4) to obtain values of kR (12.5 + 0.2) × 10−2 min−1 included in Table 1. The corresponding fit of kr values obtained in D2O is also shown. Fitted values of KP were 170 μM in H2O and 90 μM in D2O. Error values typical of kr for both H2O and D2O data sets are shown with the D2O data points.
2.1. Denaturation Rate Constants kX for Organophosphorylated AChE
Before the rate constants for reactions b, c and d in Scheme 2 can be determined, the loss of AChE activity arising from enzyme denaturation must be taken into consideration. The reaction denoted denaturation in Scheme 2a was detected with control AChE and with fully reactivated AChE in the absence of OPs. Bovine serum albumen (BSA) was added to the assay solution to stabilize the AChE, but the enzyme still showed a slow loss of activity with a rate constant kX that (1) in some cases was close enough to the aging rate constant kA and the hydrolysis rate constant kH to require inclusion in the analytical equations and (2) was stabilized by 2-PAM and thus depended on the concentration of 2-PAM. Individual measurements of kX ranged from 2–7 × 10−5 min−1 and are illustrated in Figure 4 and Figure 5A below. The same denaturation constant kX was assumed to apply to free and organophosphorylated AChE.
2.2. Aging Rate Constants kA for Organophosphorylated AChE
The fact that organophosphorylated AChE can undergo multiple reactions (Scheme 2) required a sequential approach to obtaining the rate constants for these reactions. Fitting some of the data here to equations required preliminary estimates of some rate constants that were refined in the fitting of later data. The aging reaction in Scheme 2b is the loss of an alkoxy group attached to the phosphorus atom [14]. The resulting phosphate diester that remains covalently linked to AChE is an extremely stable inactive enzyme form that cannot be reactivated by oxime (Figure 3). The protocol we used to measure the aging rate constants kA is indicated in the Figure 3 legend. We followed this protocol to obtain our best estimates of kA in both H2O and D2O. AChE was maintained in a fully organophosphorylated form for various times taged, after which it was diluted into a high concentration of 2-PAM to rapidly reactivate all of the enzyme that had not been aged. Figure 3A demonstrates that, with reasonable initial estimates of kX, kA, kH, and kr, 2-PAM reactivation proceeds rapidly. Fitting confirmed that all values of v measured after the initial value near t = 0 essentially corresponded to the final value of v, denoted vF. Plots of these vF against taged could be analyzed with Equation (3) as shown in Figure 3B to give values of kA presented in Table 1.
2.3. Hydrolysis Rate Constants kH for Organophosphorylated AChE
The hydrolysis rate constants kH were measured in H2O and D2O with the protocol in the Figure 4 legend. AChE was organophosphorylated and diluted with or without 200 μM 2-PAM. Assay values v from the dilution with 2-PAM rapidly reached maximums and were analyzed with Equation (2) to determine Etot. This value of Etot was then fixed in Equation (2) to fit the values of v from the dilution without 2-PAM to obtain kH (Figure 4).
In contrast to the protocol for the aging reaction in Figure 3A, where a slight excess of OP during preincubation was rapidly turned over by dilution into a high concentration of 2-PAM, the hydrolysis reaction was defined in the absence of 2-PAM and its time course was sensitive to any excess of OP. An excess was indicated by an E0 close to zero and by a lag in the increase in v with time. Centrifugation through a spin column was employed to minimize unreacted OP but, in addition, the stoichiometry of AChE and OP was set close to one and the incubation time was limited to insure that, in most cases, E0 was significantly greater than zero. However, this was not the case in Figure 4, where E0 approached zero.
2.4. 2-PAM Reactivation Rate Constants kR for Organophosphorylated AChE
The reactivation rate constants kr depend on the concentration of 2-PAM, but at the 2-PAM concentrations employed here they were considerably larger than rate constants for denaturation, aging and hydrolysis described above. Nevertheless, the determination of accurate solvent D2O isotope effects required inclusion of these smaller rate constants in Equation (2). The protocol for obtaining values of kr is shown in Figure 5A. A rapid increase in assay values v was observed on adding organophosphorylated AChE to 2-PAM, followed by a much slower decrease in v that we attributed to enzyme denaturation. The much longer slow decrease dominated the fitting procedure when the entire time course was analyzed, so fitting was divided into two steps. In the first step only the denaturation rate constant kX was retained, and it showed a clear decrease in value at higher 2-PAM concentrations (Figure 5A) as noted above. The second fitting step retained the value of kX from the first step as an additional fixed variable, and kr was fitted from the initial increase in v (Figure 5A). Values of kr obtained at different 2-PAM concentrations were then analyzed according to Equation (4) to obtain the maximum reactivation rate constant kR at concentrations of 2-PAM that saturated the organophosphorylated enzyme (Figure 5B).
2.5. Accuracy of Rate Constants for Reactions of Organophosphorylated hAChE
In addition to the rate constants given in Table 1, we also determined kX values in the absence of 2-PAM in H2O ((53 ± 7) × 10−6 min−1, n = 7) and in D2O (41 ± 14) × 10−6 min−1, n = 3). These values are significant because, in some cases, several measured rate constants are close in value, rendering the accuracy of one rate constant highly dependent upon another. For example, values of kH, kA and kX for diethylphosphorylated AChE are almost identical in D2O. The rate constants most sensitive to these equivalencies are the kA values in Table 1. These values were obtained by fitting Equation (3), where the exponential rate constant is kA + kX. Therefore, from the 40 × 10−6 min−1 value of kA in Table 1, a 2-fold increase in kX renders kA close to zero. The value of kX also plays an important, although lesser, role in the determination of kH for diethylphosphorylated AChE in D2O (Table 1 and Figure 4): a 2-fold increase in the fixed value of kX in Equation (2) increases kH by 45%, whereas a 2-fold decrease in kX decreases kH by 20%. Values of kH, kA, and kX for dimethylphosphorylated AChE diverge to a much greater extent (see Table 1), making their accuracy largely independent of each other. In view of these differences in the rate constant interdependence of kA and kH, the similarities in the solvent D2O isotope effects for dimethyl- and diethylphosphorylated AChE in Table 1 are reassuring but possibly serendipitous.
2.6. Solvent Deuterium Oxide Isotope Effects on Reaction Rate Constants
Analyses of isotope effects in D2O relative to H2O have been useful in clarifying details of the AChE reaction pathway with a variety of substrates [15,16], but we’ve found few reports of these isotope effects on the reactions of phosphorylated AChE. In our experiments in this report, we paired rate constant determinations in H2O and D2O by running them on the same day, starting with the same stock AChE frozen aliquot and employing the same dilution sequence. Our results are presented in Table 1, and they are examined in detail in the Discussion.
3. Discussion
3.1. Kinetics of Decarbamoylation and of the Hydrolysis of Organophosphorylated AChE
Rate constants for organophosphorylated AChE in Table 1 are in reasonable agreement with corresponding values previously reported in slightly different solvents, pH conditions and temperatures. Values of kA = 3000 × 10−6 min−1, of kH = 17,000 × 10−6 min−1, and of kR = 480,000 × 10−6 min−1 were obtained for dimethylphosphorylated human AChE [17]. For diethylphosphorylated AChE, values of kA = 170 × 10−6 min−1 and of kH = 120 × 10−6 min−1 were obtained with the mouse enzyme [18] and of kR = 60,000 × 10−6 min−1 for the human enzyme [19].
An increase in size of the carbamoyl or organophosphoryl group attached to AChE significantly reduced the value of k3 or kH for deacylation. In Table 1, the kH for diethylphosphorylated AChE is about 75-fold lower than that for dimethylphosphorylated AChE. A similar trend was seen with carbamoylated AChE, where k3 for N,N-diethylcarbamoyl AChE was about 300-fold lower than that for N,N-dimethylcarbamoyl AChE [2]. The difference for the organophosphorylated AChEs may result from distortion of the AChE active site by the larger diethylphosphoryl group as seen in Figure 2A. The organophosphoryl group size appears less important for oxime reactivation, as kR in Table 1 for 2-PAM reactivation of diethylphosphorylated AChE is only 5-fold lower than that for dimethylphosphorylated AChE. To address the consequences of active site distortion more directly, we analyzed the solvent D2O isotope effects in the following section.
3.2. Solvent Deuterium Oxide Isotope Effects on Rate Constants for Reactions of Organophosphorylated AChE
When proton transfer occurs in the rate-limiting step of a reaction, the rate constant for that reaction shows a solvent D2O isotope effect. Rate-limiting proton transfer in an acylation or deacylation reaction results in a rate constant decrease of 2 to 3-fold when D2O replaces H2O as the solvent [20]. AChE-catalyzed acetylcholine hydrolysis falls well within this range, as kcat (a combination of acylation and deacylation rate constants) has a solvent D2O isotope effect of 2.4 [15,21]. However, the isotope effect drops from 2.4 for kcat to 1.1–1.2 for the second order hydrolysis rate constant kcat/Kapp (also denoted kE) with good substrates of AChE like acetylcholine. To interpret this drop, we proposed that proton transfer in the acylation step k2 was rate-limiting for kcat but that an earlier step like substrate binding, or an induced-fit conformational change that does not involve proton transfer, was rate-limiting for the second order rate constant kcat/Kapp [15]. More explicitly, the solvent D2O isotope effect is determined by the commitment to catalysis, denoted C [16]. In the very simple case of Scheme 3 and Equation (1), acetylcholine (S) binds to enzyme E with an association rate constant of kS and a dissociation rate constant of k-S to give an ES complex.
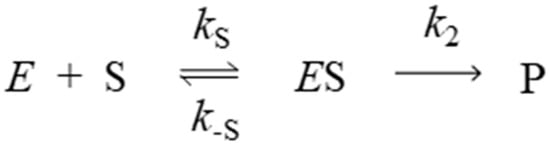
Scheme 3.
Pathway for hydrolysis of acetylthiocholine (S) by AChE (E).
Subsequent acylation with a rate constant k2 gives an acyl enzyme and choline products, here just denoted P. The observed rate constant kE is given by Equation (1). The acylation step k2 involves proton transfer and has an isotope effect of 2–3, while kS and k−S are assumed not to involve proton transfer. The commitment to catalysis is defined as C = k2/k−S. When C is small, kE = kS k2/k−S, and kE shows an isotope effect. When C is large, kE = kS and it does not.
A comparable interpretation may be applied to the aging reaction in Scheme 2. This reaction is enzyme catalyzed [22] and involves loss of an alkyl group as the organophosphoryl adduct is converted from a phosphate triester to a diester. While a proton is transferred to the alkyl leaving group in this reaction, this transfer does not appear to be rate limiting as the solvent D2O isotope effects for the aging reaction rate constant kA in Table 1 are about 1.0. We have found no other reports of isotope effects on aging of dimethyl- or diethylphosphorylated AChE, but a similar isotope effect of 1.2 has been reported for aging of 2-propoxy-methylphosphonylated AChE [23].
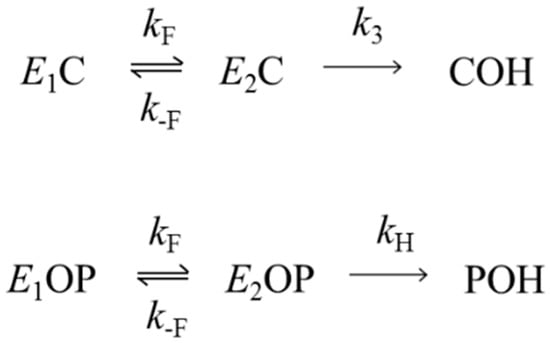
We next asked whether the hydrolysis and oxime reactivation reactions in Scheme 2 involve general acid-base catalysis with rate-limiting proton transfer. To facilitate the analysis, we consider Scheme 4, which shows a slight extension of Scheme 1 in which a second acyl enzyme species is added in conformational equilibrium with the first. In particular, Scheme 4 includes an inactive enzyme form (E1C or E1OP) with a distorted active site and an active enzyme form (E2C or E2OP) that can undergo hydrolysis with a rate constant k3 or kH. The forward and reverse rate constants for these equilibria are kF and k-F, respectively, and they are assumed not to involve proton transfer. The general solution to Scheme 4 is more complicated than that of Scheme 3, as neither E2C nor E2OP necessarily is in the steady state. However, two extreme cases may be considered in which these intermediates are in the steady state: (1) the commitment to catalysis k3/k−F or kH/k−F is small, and the deacylation rate constant k3kF/(k−F+k3) or kHkF/(k−F+kH) shows the solvent D2O isotope effect inherent in k3 or kH; (2) the commitment to catalysis k3/k−F or kH/k-F is large, the acyl intermediates are not equilibrated and the deacylation rate constant k3kF/(k−F+kH) or kHkF/(k−F+kH) shows only a fraction of the isotope effect inherent in k3 or kH and no isotope effect if k3 or kH >> k−F.
We recently investigated the hydrolysis of carbamoylated AChEs for a series of carbamate esters and obtained the solvent D2O isotope effects summarized in Figure 6 [2]. The rate constant k3 for the smallest carbamoyl group, an N-monomethylcarbamoyl, was 12 × 10−3 min−1, and its isotope effect of 2.8 was consistent with rate-limiting proton transfer and a small value of C. However, as the N-alkyl groups on carbamoylated AChEs increased in size, the decarbamoylation rate constant k3 decreased to about 0.02 × 10−3 min−1 for N,N-diethylcarbamoylated AChE and the isotope effect was only slightly above 1 (Figure 6), indicating a high value of C. In that report we noted the crystallographic evidence of active site distortion in diethylphosphorylated AChE shown in Figure 2A, and we suggested that a larger size of the carbamoyl group is likely to be an important factor in a shift away from proton transfer in the rate-limiting step for k3. It may be useful to explore molecular modeling of the array of conformational variants available in AChEs with large carbamoyl groups to obtain support for this proposal.
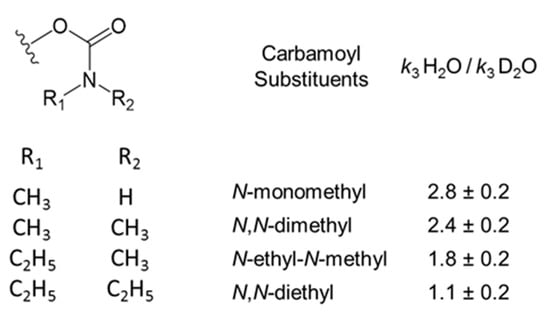
Figure 6.
Solvent D2O isotope effects on the AChE decarbamoylation rate constant k3 at 25 °C [2].
We designed the experiments here to examine whether active site distortion in diethylphosphorylated AChE in fact does result in a shift away from rate-limiting proton transfer in the hydrolysis and oxime reactivation reactions of this AChE intermediate. For comparison, we included measurements of solvent D2O isotope effects for dimethylphosphorylated AChE, an intermediate unlikely to involve active site distortion [10,12]. Oxime reactivation, like hydrolysis in Scheme 2, involves attack of a nucleophile on the tetravalent phosphorus to presumably form a pentavalent transition state [24]. This state then decomposes to regenerate active enzyme and an organophosphorylated oxime [7,25], and both the formation and decomposition of this state are likely to involve rate-limiting proton transfer. In the structure in Figure 2B, 50 mM 2-PAM was diffused into the crystal [9]. This structure suggests that 2-PAM stabilizes the active E2OP species in Scheme 4, perhaps to the exclusion of the distorted E1OP species, and kR for diethylphosphorylated AChE in Table 1 is only 5-fold lower than that for dimethylphosphorylated AChE. These observations suggest that the concentration of E1OP bound to 2-PAM is negligible and that kR will reflect the rate-limiting proton transfer inherent when both acyl groups migrate to the oxime. The solvent D2O isotope effects of 2.0 and 1.7 for the dimethyl- and diethylphosphorylated AChE are consistent with this interpretation.
Finally, we examined the rate constants kH for hydrolysis of both diethylphosphorylated AChE and dimethylphosphorylated AChE to determine if their solvent D2O isotope effects reflect the fact that only diethylphosphorylated AChE appears to form the distorted active conformation in Figure 2A. The values in Table 1 do not support this proposal, as both organophosphorylated species show isotope effects that are close to 2.0. This value, although somewhat low for typical enzyme deacylation, suggests that any distorted conformation E1OP involving diethylphosphorylated AChE equilibrates relatively rapidly with the active conformation E2OP and that the commitment to catalysis C = kH/k−F is small. These isotope effects for organophosphorylated AChE hydrolysis are significant because this reaction, of those in Scheme 2, bears the closest resemblance to the hydrolysis of the carbamoylated enzymes in Figure 6. Furthermore, the conclusion above that the commitment to catalysis C = k3/k−F is large for N,N-diethylcarbamoylated AChE while C = kH/k−F is small for diethylphosphorylated AChE is difficult to justify. Their respective rate constants, k3 = 0.02 × 10−3 min−1 for decarbamoylation and kH = 0.08 × 10−3 min−1 for dephosphorylation (Table 1 and Figure 6), are too similar to support these opposing conclusions about C if the same value of k-F holds for both acylated AChEs.
4. Materials and Methods
4.1. Reagents
Recombinant hAChE was expressed as a secreted, disulfide-linked dimer in drosophila S2 cells and purified by affinity chromatography as outlined previously [26]. Initial 0.5 mL fractions were maintained in 5 mM decamethonium bromide (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) at 4 °C. Fractions were dialyzed against 20 mM sodium phosphate and 0.02% Triton X-100 (pH 7.0) at 4 °C, and 10- or 20- μL aliquots of the dialyzates were frozen at −20 °C until use. O,O-Diethyl O-(4-nitrophenyl) phosphate (paraoxon) and O,O-dimethyl O-(4-nitrophenyl) phosphate (paraoxon methyl) were commercial samples from Sigma-Aldrich Chemical Co (St. Louis, MO, USA).
4.2. Assay of Substrate Hydrolysis
Hydrolysis rates v for the substrate acetylthiocholine were measured in a coupled Ellman reaction in which thiocholine generated in the presence of the indicated concentration of DTNB was determined by the formation of the thiolate dianion of DTNB at 412 nm (Δε412 nm = 14,150 M−1cm−1) [27]. Total AChE concentrations (Etot) could be calculated assuming 450 units/nmol [28]. One unit of AChE activity corresponds to 1 μmol of acetylthiocholine hydrolyzed/min under standard pH-stat assay conditions at pH 8 [28,29]. Our conventional spectrophotometric assay at 412 nm was conducted in pH 7.0 buffer. With wild type hAChE and 0.5 mM acetylthiocholine, this assay resulted in 4.8 ΔA412 nm/min with 1 nM AChE or about 76% of the pH stat assay standard, but here Etot was expressed simply in terms of ΔA412/min. The assay mixture here contained final concentrations of acetylthiocholine and DTNB of 1.0 mM and 0.3 mM, respectively, in 100 mM sodium phosphate and 0.1% BSA at pH 8.0. Aliquots of AChE were added to a final volume was 3.0 mL, and the assay was conducted at 25 °C. Absorbance at 412 nm was recorded with time on a Varian Cary 3A spectrophotometer.
4.3. Reactions of Organophosphorylated hAChE
We generated organophosphorylated AChE (EOP in Scheme 1) from either paraoxon (to give diethylphosphorylated AChE) or paraoxon methyl (to give dimethylphosphorylated AChE) as outlined in the legends to Figure 3, Figure 4 and Figure 5. Experimental traces of organophosphorylated AChE reactions measured with acetylthiocholine activity assays (v) were obtained and the equation for the general solution used to fit these traces to Scheme 2 is given in Equation (2). Data were fitted to Equation (2) by unweighted nonlinear regression with SigmaPlot (version 12.0, Systat Software Inc., Chicago, IL, USA). When kr >> kH + kA + kX and t = taged >> kr−1, Equation (2) reduces to Equation (3).
In Equations (2) and (3), taged is the time that enzyme and OP were incubated prior to the start of the measured reaction, t is the duration in min of the measured reaction and E0 is the initial enzyme concentration (ΔA412/min at t = 0).
Values of the second order reactivation rate constants kr were obtained at various 2-PAM concentrations that were run in parallel, and these values were analyzed with Equation (4) to obtain the maximal first order reactivation rate constant kR at saturating concentrations of 2-PAM and the dissociation constant KP for 2-PAM binding to organophosphorylated AChE.
4.4. Solvent Deuterium Oxide Isotope Effects
Reactions of organophosphorylated AChE were conducted in 100 mM sodium phosphate and 0.1% BSA at pH 8.0. The pH was the uncorrected value read by the pH meter for both H2O and D2O buffers, and the pH of 8.0 (rather than more typical pH values of 7.0–7.4 for AChE studies) was selected to minimize enzyme pKa differences [3] between H2O and D2O. Organophosphorylated AChE reactions in D2O were paired with reactions in H2O to maximize precision in comparing rates. While frozen stocks of AChE were in H2O, all subsequent dilutions were identical in either H2O or D2O. The percentage H2O in an assayed D2O aging reaction mixture was 3%, and the maximum percentage in hydrolysis or oxime reactivation mixtures was 1%.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no competing financial interests.
Abbreviations
AChE: acetylcholinesterase; BSA, bovine serum albumin; hAChE, human acetylcholinesterase; mAChE, mouse acetylcholinesterase; TcAChE, Torpedo californica acetylcholinesterase; DTNB, 5,5’-dithiobis-(2-nitrobenzoic acid); 2-PAM, 2-pyridinealdoxime methiodide.
References
- Rosenberry, T.L. Quantitative simulation of endplate currents at neuro-muscular junctions based on the reactions of acetylcholine with acetylcholine receptor and acetylcholinesterase. Biophys. J. 1979, 26, 263–290. [Google Scholar] [CrossRef]
- Venkatasubban, K.S.; Johnson, J.L.; Thomas, J.L.; Fauq, A.; Cusack, B.; Rosenberry, T.L. Decarbamoylation of acetylcholinesterases is markedly slowed as carbamoyl groups increase in size. Arch. Biochem. Biophys. 2018, 655, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Reiner, E.; Aldridge, W.N. Effect of pH on inhibition and spontaneous reactivation of acetylcholinesterase treated with esters of phosphorus acids and of carbamic acids. Biochem. J. 1967, 105, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Fukuto, T.R. Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Perspect. 1990, 87, 245–254. [Google Scholar] [CrossRef]
- Myers, D.K. Studies on cholinesterase. 10. Return of cholinesterase activity in the rat after inhibition by carbamoyl fluorides. Biochem. J. 1956, 62, 556–563. [Google Scholar]
- Froede, H.C.; Wilson, I.B. Acetylcholinesterase. In The Enzymes, 3rd ed.; Boyer, P.D., Ed.; Academic Press: New York, NY, USA, 1971; pp. 87–114. [Google Scholar]
- Wilson, I.B. Acetylcholinesterase. XI. Reversibility of tetraethyl pyrophosphate inhibiton. J. Biol. Chem. 1951, 190, 111–117. [Google Scholar]
- Rosenberry, T.L.; Cheung, J. Rate-limiting step in the decarbamoylation of acetylcholinesterases with large carbamoyl groups. Chem. Biol. Interact. 2019, 308, 392–395. [Google Scholar] [CrossRef]
- Franklin, M.C.; Rudolph, M.J.; Ginter, C.; Cassidy, M.S.; Cheung, J. Structures of paraoxon-inhibited human acetylcholinesterase reveal perturbations of the acyl loop and the dimer interface. Proteins 2016, 84, 1246–1256. [Google Scholar] [CrossRef]
- Hörnberg, A.; Tunemalm, A.K.; Ekström, F. Crystal structures of acetylcholinesterase in complex with organophosphorus compounds suggest that the acyl pocket modulates the aging reaction by precluding the formation of the trigonal bipyramidal transition state. Biochemistry 2007, 46, 4815–4825. [Google Scholar] [CrossRef]
- Millard, C.B.; Kryger, G.; Ordentlich, A.; Greenblatt, H.M.; Harel, M.; Raves, M.L.; Segall, Y.; Barak, D.; Shafferman, A.; Silman, I.; et al. Crystal structures of aged phosphonylated acetylcholinesterase: Nerve agent reaction products at the atomic level. Biochemistry 1999, 38, 7032–7039. [Google Scholar] [CrossRef]
- Millard, C.B.; Koellner, G.; Ordentlich, A.; Shafferman, A.; Silman, I.; Sussman, J.L. Reaction products of acetylcholinesterase and VX reveal a mobile histidine in the catalytic triad. J. Am. Chem. Soc. 1999, 121, 9883–9884. [Google Scholar] [CrossRef]
- Kovalevsky, A.; Blumenthal, D.K.; Cheng, X.; Taylor, P.; Radic, Z. Limitations in current acetylcholinesterase structure–based design of oxime antidotes for organophosphate poisoning. Ann. N. Y. Acad. Sci. 2016, 1378, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Michel, H.O.; Hackley, B.E.; Berkowitz, L.; List, G.; Hackley, E.B.; Gillilan, W.; Pankau, M. Ageing and dealkylation of soman (pinacolylmethylphosphonofluoridate)-Inactivated eel cholinesterase. Arch. Biochem. Biophys. 1967, 121, 29–34. [Google Scholar] [CrossRef]
- Rosenberry, T.L. Catalysis by acetylcholinesterase. Evidence that the rate-limiting step for acylation with certain substrates precedes general acid-base catalysis. Proc. Natl. Acad. Sci. USA 1975, 72, 3834–3838. [Google Scholar]
- Quinn, D.M. Acetylcholinesterase: Enzyme structure, reaction dynamics, and virtual transition states. Chem. Rev. 1987, 87, 955–979. [Google Scholar] [CrossRef]
- Worek, F.; Diepold, C.; Eyer, P. Dimethylphosphoryl-inhibited human cholinesterases: Inhibition, reactivation, and aging kinetics. Arch. Toxicol. 1999, 73, 7–14. [Google Scholar] [CrossRef]
- Jennings, L.L.; Malecki, M.; Komives, E.A.; Taylor, P. Direct analysis of the kinetic profiles of organophosphate-acetylcholinesterase adducts by MALDI-TOF mass spectrometry. Biochemistry 2003, 42, 11083–11091. [Google Scholar] [CrossRef]
- Grosfeld, H.; Barak, D.; Ordentlich, A.; Velan, B.; Shafferman, A. Interactions of oxime reactivators with diethylphosphoryl adducts of human acetylcholinesterase and its mutant derivatives. Mol. Pharmacol. 1996, 50, 639–649. [Google Scholar]
- Bender, M.L.; Hamilton, G.A. Kinetic isotope effects of deuterium oxide on several a-chymotrypsin-catalyzed reactions. J. Am. Chem. Soc. 1962, 84, 2570–2576. [Google Scholar] [CrossRef]
- Bender, M.L.; Clement, G.E.; Kezdy, F.J.; Heck, H.D.A. The correlation of the pH (pD) dependence and the stepwise mechanism of α-chymotrypsin-catalyzed reactions. J. Am. Chem. Soc. 1964, 86, 3680–3690. [Google Scholar] [CrossRef]
- Qian, N.; Kovach, I.M. Key active site residues in the inhibition of acetylcholinesterases by soman. FEBS Lett. 1993, 336, 263–266. [Google Scholar] [CrossRef]
- Kovach, I.M.; Bennet, A.J. Comparative study of nucleophilic am) enzymic reactions of 2-propyl methylphosphonate derivatives. Phosphorus Sulfur Silicon Relat. Elem. 1990, 51, 51–56. [Google Scholar] [CrossRef]
- Wong, L.; Radic, Z.; Bruggemann, R.J.M.; Hosea, N.; Berman, H.A.; Taylor, P. Mechanism of oxime reactivation of acetylcholinesterase analyzed by chirality and mutagenesis. Biochemistry 2000, 39, 5750–5757. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Saxena, A.; Smith, M.; Garcia, G.; Radic, Z.; Taylor, P.; Doctor, B.P. Phosphoryl oxime inhibition of acetylcholinesterase during oxime reactivation is prevented by edrophonium. Biochemistry 1999, 38, 9937–9947. [Google Scholar] [CrossRef] [PubMed]
- Mallender, W.D.; Szegletes, T.; Rosenberry, T.L. Organophosphorylation of acetylcholinesterase in the presence of peripheral site ligands: Distinct effects of propidium and fasciculin. J. Biol. Chem. 1999, 274, 8491–8499. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Cusack, B.; Davies, M.P.; Fauq, A.; Rosenberry, T.L. Unmasking tandem site interaction in human acetylcholinesterase. Substrate activation with a cationic acetanilide substrate. Biochemistry 2003, 42, 5438–5452. [Google Scholar] [PubMed]
- De Ferrari, G.V.; Mallender, W.D.; Inestrosa, N.C.; Rosenberry, T.L. Thioflavin T is a fluorescent probe of the acetylcholinesterase peripheral site that reveals conformational interactions between the peripheral and acylation sites. J. Biol. Chem. 2001, 276, 23282–23287. [Google Scholar] [CrossRef]
- Rosenberry, T.L.; Scoggin, D.M. Structure of human erythrocyte acetylcholinesterase. Characterization of intersubunit disulfide bonding and detergent interaction. J. Biol. Chem. 1984, 259, 5643–5652. [Google Scholar]
Sample Availability: Not available. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).