6-Methoxymellein Isolated from Carrot (Daucus carota L.) Targets Breast Cancer Stem Cells by Regulating NF-κB Signaling
Abstract
1. Introduction
2. Results
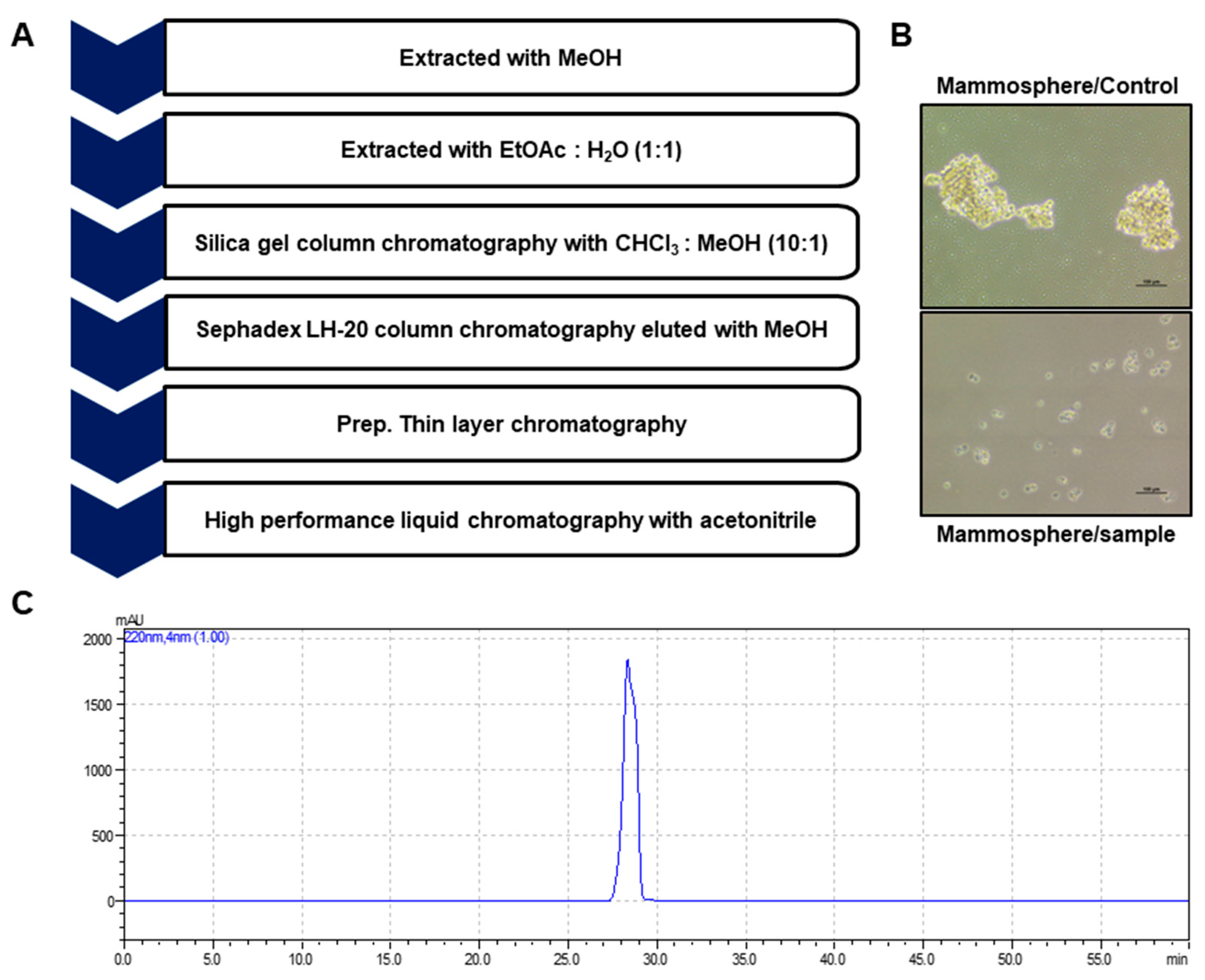
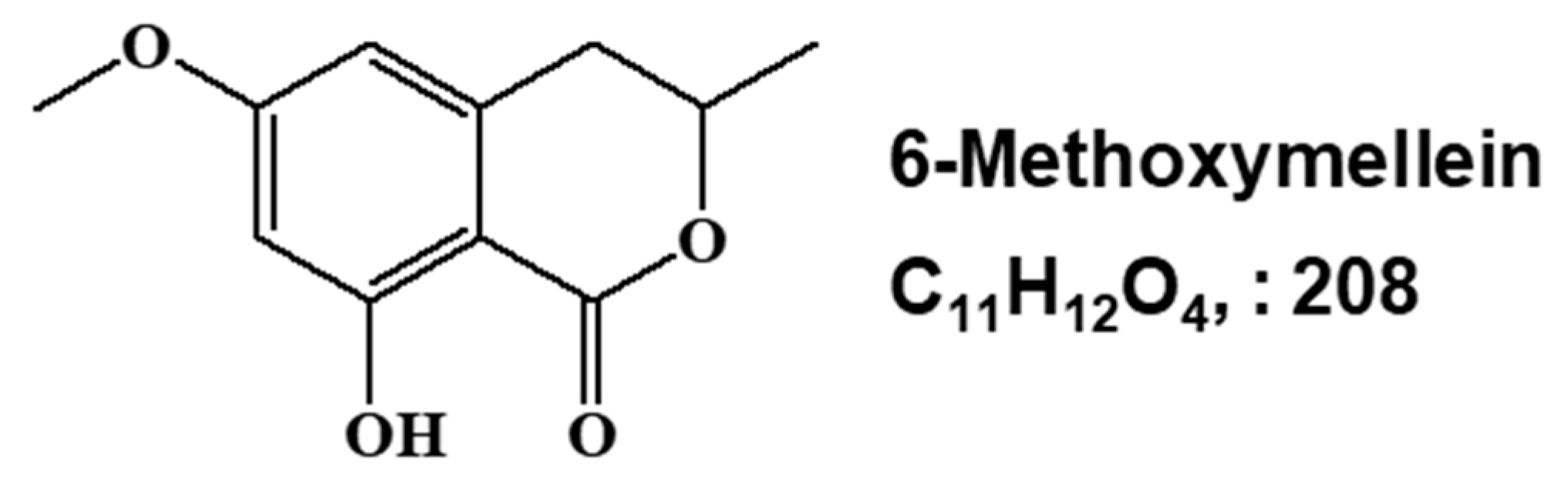
2.1. Isolation and Identification of a Breast CSC Inhibitor Derived from D. carota L.
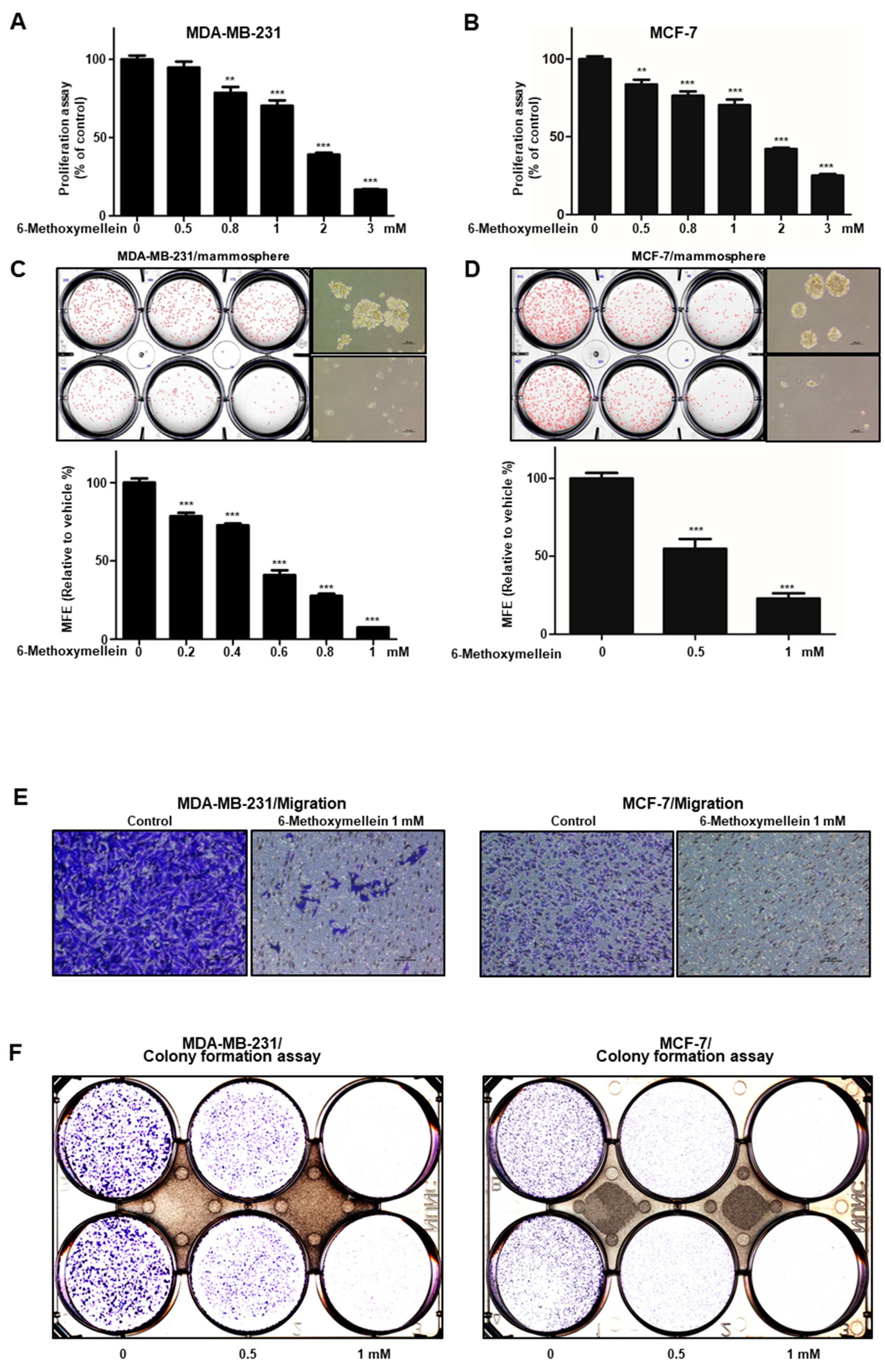
2.2. 6-Methoxymellein Suppresses the Growth of Breast Cancer Cells and Mammospheres
2.3. 6-Methoxymellein Reduces the Proportion of CD44+/CD24−-Expressing Breast Cancer Cells
2.4. 6-Methoxymellein Inhibits the Protein Expression of Cancer Stem Cell-Specific Markers and Inhibits Mammosphere Growth
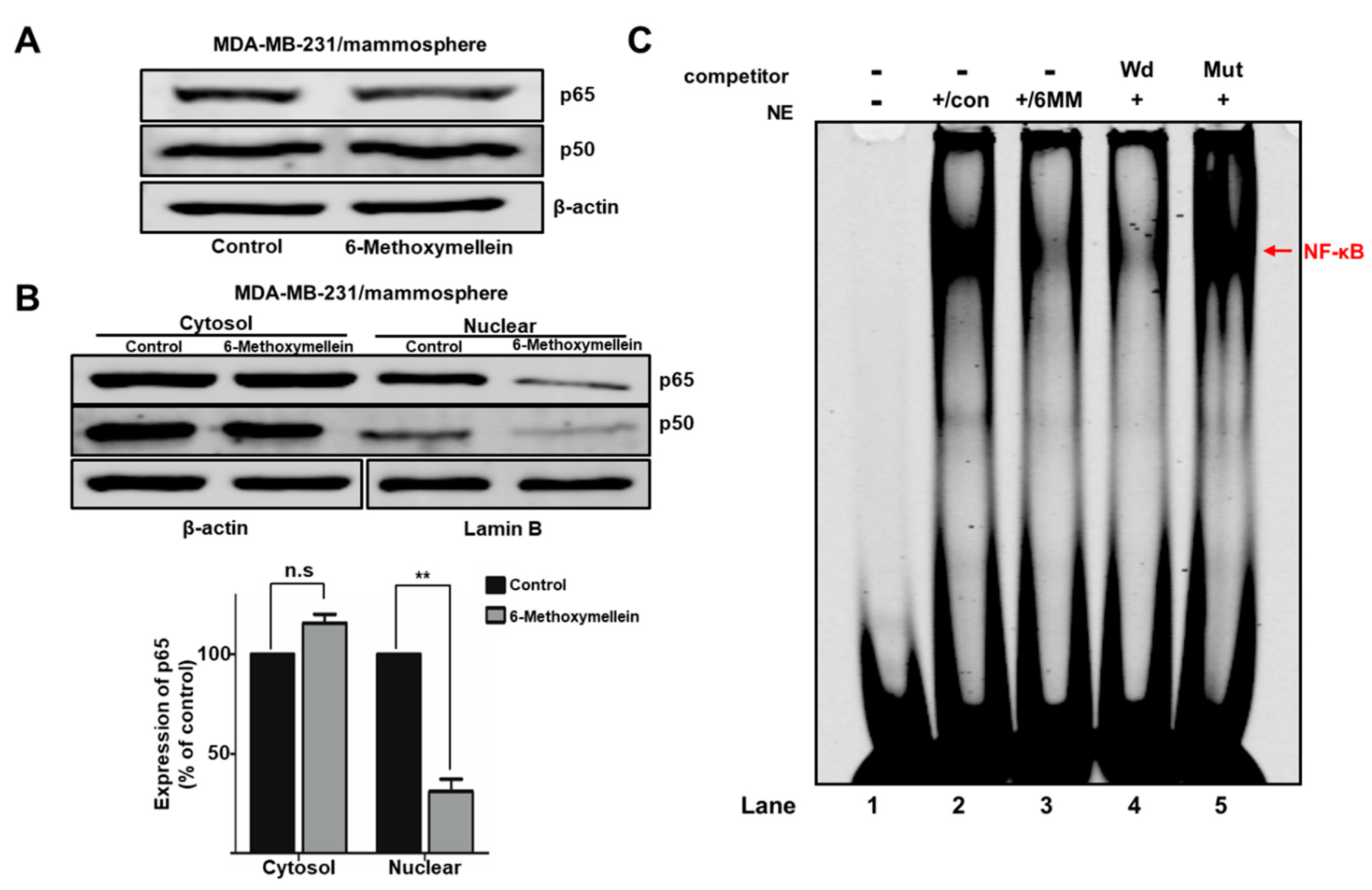
2.5. 6-Methoxymellein Suppresses the Nuclear Localization of NF-κB p65 and NF-κB p50 in BCSCs
2.6. 6-Methoxymellein Decreases the mRNA and Protein Levels of Secretory IL-6 and IL-8 in Mammospheres
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Isolation of Mammosphere Formation Inhibitor from Carrots
4.4. Structure Analysis of the Isolated Component
4.5. Cell Line and Culture of Mammospheres
4.6. Cell Proliferation
4.7. Colony Formation Assay
4.8. Flow Cytometric Assay of CD44+/CD24− Expression
4.9. Migration
4.10. RT-qPCR
4.11. Western Blotting
4.12. EMSA
4.13. Quantification of Extracellular Human IL-6 and IL-8 Cytokines Using the Cytometric Bead Array (CBA) Human Inflammatory Cytokine Assay Kit
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dawid, C.; Dunemann, F.; Schwab, W.; Nothnagel, T.; Hofmann, T. Bioactive c(1)(7)-polyacetylenes in carrots (Daucus carota L.): Current knowledge and future perspectives. J Agric. Food Chem. 2015, 63, 9211–9222. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.; Kaminska, I.; Kramer, M.; Maksylewicz-Kaul, A.; Kammerer, D.; Carle, R.; Baranski, R. The content of phenolic compounds and radical scavenging activity varies with carrot origin and root color. Plant Foods Hum. Nutr. 2013, 68, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Garba, U.; Kaur, S.; Gurumayum, S.; Rasane, P. Effect of hot water blanching time and drying temperature on the thin layer drying kinetics of and anthocyanin degradation in black carrot (Daucus carota l.) shreds. Food Technol. Biotechnol. 2015, 53, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Cawood, M.; Iqbal, Q.; Arino, A.; Batool, A.; Tariq, R.M.S.; Azam, M.; Akhtar, S. Phytochemicals in Daucus carota and their health benefits-review article. Foods 2019, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Que, F.; Hou, X.L.; Wang, G.L.; Xu, Z.S.; Tan, G.F.; Li, T.; Wang, Y.H.; Khadr, A.; Xiong, A.S. Advances in research on the carrot, an important root vegetable in the apiaceae family. Hortic. Res. 2019, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Aksak Karamese, S.; Toktay, E.; Unal, D.; Selli, J.; Karamese, M.; Malkoc, I. The protective effects of beta-carotene against ischemia/reperfusion injury in rat ovarian tissue. Acta Histochem. 2015, 117, 790–797. [Google Scholar] [CrossRef]
- Kim, S.R.; Nakanishi, K.; Itagaki, Y.; Sparrow, J.R. Photooxidation of a2-pe, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp. Eye Res. 2006, 82, 828–839. [Google Scholar] [CrossRef]
- Chung, R.W.S.; Leanderson, P.; Lundberg, A.K.; Jonasson, L. Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis 2017, 262, 87–93. [Google Scholar] [CrossRef]
- Akolkar, G.; da Silva Dias, D.; Ayyappan, P.; Bagchi, A.K.; Jassal, D.S.; Salemi, V.M.C.; Irigoyen, M.C.; De Angelis, K.; Singal, P.K. Vitamin c mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H795–H809. [Google Scholar] [CrossRef]
- Zecchinati, F.; Barranco, M.M.; Arana, M.R.; Tocchetti, G.N.; Dominguez, C.J.; Perdomo, V.G.; Ruiz, M.L.; Mottino, A.D.; Garcia, F.; Villanueva, S.S.M. Reversion of down-regulation of intestinal multidrug resistance-associated protein 2 in fructose-fed rats by geraniol and vitamin c: Potential role of inflammatory response and oxidative stress. J. Nutr. Biochem. 2019, 68, 7–15. [Google Scholar] [CrossRef]
- Stefanson, A.L.; Bakovic, M. Falcarinol is a potent inducer of heme oxygenase-1 and was more effective than sulforaphane in attenuating intestinal inflammation at diet-achievable doses. Oxid. Med. Cell. Longev. 2018, 2018, 3153527. [Google Scholar] [CrossRef] [PubMed]
- Fikry, E.M.; Gad, A.M.; Eid, A.H.; Arab, H.H. Caffeic acid and ellagic acid ameliorate adjuvant-induced arthritis in rats via targeting inflammatory signals, chitinase-3-like protein-1 and angiogenesis. Biomed. Pharmacother. 2019, 110, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xu, J.; Tang, L.; Guan, X. Breast cancer stem cell: The roles and therapeutic implications. Cell. Mol. Life Sci. 2017, 74, 951–966. [Google Scholar] [CrossRef]
- Tong, C.W.S.; Wu, M.; Cho, W.C.S.; To, K.K.W. Recent advances in the treatment of breast cancer. Front. Oncol. 2018, 8, 227. [Google Scholar] [CrossRef]
- Yeo, S.K.; Wen, J.; Chen, S.; Guan, J.L. Autophagy differentially regulates distinct breast cancer stem-like cells in murine models via egfr/stat3 and tgfbeta/smad signaling. Cancer Res. 2016, 76, 3397–3410. [Google Scholar] [CrossRef]
- Liu, S.; Cong, Y.; Wang, D.; Sun, Y.; Deng, L.; Liu, Y.; Martin-Trevino, R.; Shang, L.; McDermott, S.P.; Landis, M.D.; et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2014, 2, 78–91. [Google Scholar] [CrossRef]
- Bai, X.; Ni, J.; Beretov, J.; Graham, P.; Li, Y. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat. Rev. 2018, 69, 152–163. [Google Scholar] [CrossRef]
- Cheng, C.C.; Shi, L.H.; Wang, X.J.; Wang, S.X.; Wan, X.Q.; Liu, S.R.; Wang, Y.F.; Lu, Z.; Wang, L.H.; Ding, Y. Stat3/oct-4/c-myc signal circuit for regulating stemness-mediated doxorubicin resistance of triple-negative breast cancer cells and inhibitory effects of wp1066. Int. J. Oncol. 2018, 53, 339–348. [Google Scholar] [CrossRef]
- Bak, M.J.; Furmanski, P.; Shan, N.L.; Lee, H.J.; Bao, C.; Lin, Y.; Shih, W.J.; Yang, C.S.; Suh, N. Tocopherols inhibit estrogen-induced cancer stemness and oct4 signaling in breast cancer. Carcinogenesis 2018, 39, 1045–1055. [Google Scholar] [CrossRef]
- Chew, J.L.; Loh, Y.H.; Zhang, W.; Chen, X.; Tam, W.L.; Yeap, L.S.; Li, P.; Ang, Y.S.; Lim, B.; Robson, P.; et al. Reciprocal transcriptional regulation of pou5f1 and sox2 via the oct4/sox2 complex in embryonic stem cells. Mol. Cell. Biol. 2005, 25, 6031–6046. [Google Scholar] [CrossRef] [PubMed]
- Poli, V.; Fagnocchi, L.; Fasciani, A.; Cherubini, A.; Mazzoleni, S.; Ferrillo, S.; Miluzio, A.; Gaudioso, G.; Vaira, V.; Turdo, A.; et al. Myc-driven epigenetic reprogramming favors the onset of tumorigenesis by inducing a stem cell-like state. Nat. Commun. 2018, 9, 1024. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Li, Z.; Wu, Q.; Lathia, J.D.; McLendon, R.E.; Hjelmeland, A.B.; Rich, J.N. C-myc is required for maintenance of glioma cancer stem cells. PLoS ONE 2008, 3, e3769. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xie, Y.; Tran, L.; Lan, J.; Yang, Y.; Murugan, N.L.; Wang, R.; Wang, Y.J.; Semenza, G.L. Chemotherapy-induced s100a10 recruits kdm6a to facilitate oct4-mediated breast cancer stemness. J. Clin. Investig. 2020, 130, 4607–4623. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.A.; Mannoor, K.; Cao, J.; Qadri, F.; Song, X. Sox2 in cancer stemness: Tumor malignancy and therapeutic potentials. J. Mol. Cell Biol. 2020, 12, 85–98. [Google Scholar] [CrossRef]
- Prager, B.C.; Xie, Q.; Bao, S.; Rich, J.N. Cancer stem cells: The architects of the tumor ecosystem. Cell Stem Cell 2019, 24, 41–53. [Google Scholar] [CrossRef]
- Bocci, F.; Gearhart-Serna, L.; Boareto, M.; Ribeiro, M.; Ben-Jacob, E.; Devi, G.R.; Levine, H.; Onuchic, J.N.; Jolly, M.K. Toward understanding cancer stem cell heterogeneity in the tumor microenvironment. Proc. Natl. Acad. Sci. USA 2019, 116, 148–157. [Google Scholar] [CrossRef]
- Bhat, V.; Allan, A.L.; Raouf, A. Role of the microenvironment in regulating normal and cancer stem cell activity: Implications for breast cancer progression and therapy response. Cancers 2019, 11, 1240. [Google Scholar] [CrossRef]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a key event in cancer development. Mol. Cancer Res. 2006, 4, 221–233. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Hirsch, H.A.; Wang, G.; Struhl, K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via il6 secretion. Proc. Natl. Acad. Sci. USA 2011, 108, 1397–1402. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kang, J.W.; Song, X.; Kim, B.K.; Yoo, Y.D.; Kwon, Y.T.; Lee, Y.J. Role of the il-6-jak1-stat3-oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell. Signal. 2013, 25, 961–969. [Google Scholar] [PubMed]
- Sansone, P.; Storci, G.; Tavolari, S.; Guarnieri, T.; Giovannini, C.; Taffurelli, M.; Ceccarelli, C.; Santini, D.; Paterini, P.; Marcu, K.B.; et al. Il-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J. Clin. Investig. 2007, 117, 3988–4002. [Google Scholar] [PubMed]
- Dominguez, C.; McCampbell, K.K.; David, J.M.; Palena, C. Neutralization of il-8 decreases tumor pmn-mdscs and reduces mesenchymalization of claudin-low triple-negative breast cancer. JCI Insight 2017, 2. [Google Scholar]
- Singh, J.K.; Farnie, G.; Bundred, N.J.; Simoes, B.M.; Shergill, A.; Landberg, G.; Howell, S.J.; Clarke, R.B. Targeting cxcr1/2 significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting her2 via her2-dependent and -independent mechanisms. Clin. Cancer Res. 2013, 19, 643–656. [Google Scholar]
- Choi, H.S.; Kim, S.L.; Kim, J.H.; Deng, H.Y.; Yun, B.S.; Lee, D.S. Triterpene acid (3-o-p-coumaroyltormentic acid) isolated from aronia extracts inhibits breast cancer stem cell formation through downregulation of c-myc protein. Int. J. Mol. Sci. 2018, 19, 2528. [Google Scholar]
- Zhen, X.; Choi, H.S.; Kim, J.H.; Kim, S.L.; Liu, R.; Yun, B.S.; Lee, D.S. Machilin d, a lignin derived from saururus chinensis, suppresses breast cancer stem cells and inhibits nf-kappab signaling. Biomolecules 2020, 10, 245. [Google Scholar]
- Nan, J.; Wang, Y.; Yang, J.; Stark, G.R. Irf9 and unphosphorylated stat2 cooperate with nf-kappab to drive il6 expression. Proc. Natl. Acad. Sci. USA 2018, 115, 3906–3911. [Google Scholar]
- Yoon, S.; Woo, S.U.; Kang, J.H.; Kim, K.; Shin, H.J.; Gwak, H.S.; Park, S.; Chwae, Y.J. Nf-kappab and stat3 cooperatively induce il6 in starved cancer cells. Oncogene 2012, 31, 3467–3481. [Google Scholar]
- Liubomirski, Y.; Lerrer, S.; Meshel, T.; Rubinstein-Achiasaf, L.; Morein, D.; Wiemann, S.; Korner, C.; Ben-Baruch, A. Tumor-stroma-inflammation networks promote pro-metastatic chemokines and aggressiveness characteristics in triple-negative breast cancer. Front. Immunol. 2019, 10, 757. [Google Scholar]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot-a review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar]
- Zaini, R.G.; Brandt, K.; Clench, M.R.; Le Maitre, C.L. Effects of bioactive compounds from carrots (Daucus carota l.), polyacetylenes, beta-carotene and lutein on human lymphoid leukaemia cells. Anticancer Agents Med. Chem. 2012, 12, 640–652. [Google Scholar] [PubMed]
- Sevimli-Gur, C.; Cetin, B.; Akay, S.; Gulce-Iz, S.; Yesil-Celiktas, O. Extracts from black carrot tissue culture as potent anticancer agents. Plant Foods Hum. Nutr. 2013, 68, 293–298. [Google Scholar]
- Daaboul, H.E.; Daher, C.F.; Bodman-Smith, K.; Taleb, R.I.; Shebaby, W.N.; Boulos, J.; Dagher, C.; Mroueh, M.A.; El-Sibai, M. Antitumor activity of beta-2-himachalen-6-ol in colon cancer is mediated through its inhibition of the pi3k and mapk pathways. Chem. Biol. Interact. 2017, 275, 162–170. [Google Scholar] [PubMed]
- Staniszewska, M.; Kula, J.; Wieczorkiewicz, M.; Kusewicz, D. Essential oils of wild and cultivated carrots—The chemical composition and antimicrobial activity. J. Essent. Oil Res. 2005, 17, 579–583. [Google Scholar]
- Maxia, A.; Marongiu, B.; Piras, A.; Porcedda, S.; Tuveri, E.; Goncalves, M.J.; Cavaleiro, C.; Salgueiro, L. Chemical characterization and biological activity of essential oils from Daucus carota l. Subsp. Carota growing wild on the mediterranean coast and on the atlantic coast. Fitoterapia 2009, 80, 57–61. [Google Scholar]
- Shebaby, W.N.; El-Sibai, M.; Smith, K.B.; Karam, M.C.; Mroueh, M.; Daher, C.F. The antioxidant and anticancer effects of wild carrot oil extract. Phytother. Res. 2013, 27, 737–744. [Google Scholar]
- Shebaby, W.N.; Daher, C.F.; El-Sibai, M.; Bodman-Smith, K.; Mansour, A.; Karam, M.C.; Mroueh, M. Antioxidant and hepatoprotective activities of the oil fractions from wild carrot (Daucus carota ssp. Carota). Pharm. Biol. 2015, 53, 1285–1294. [Google Scholar]
- Saeed, A. Isocoumarins, miraculous natural products blessed with diverse pharmacological activities. Eur. J. Med. Chem. 2016, 116, 290–317. [Google Scholar]
- Dickschat, J.S. Fungal volatiles—A survey from edible mushrooms to moulds. Nat. Prod. Rep. 2017, 34, 310–328. [Google Scholar]
- Escriva, L.; Font, G.; Manyes, L.; Berrada, H. Studies on the presence of mycotoxins in biological samples: An overview. Toxins 2017, 9, 251. [Google Scholar]
- Condon, P.; Kuc, J. Isolation of a fungitoxic compound from carrot root tissue inoculated with cerato-cystis fimbriata. Phytopathology 1960, 50, 267–270. [Google Scholar]
- Condon, P.; Kuc, J.; Draudt, H. Production of 3-methyl-6-methoxy-8-hydroxy-3, 4-dihydroisocoumarin by carrot root tissue. Phytopathology 1963, 53, 1244. [Google Scholar]
- Kuc, J. Resistance of plants to infectious agents. Annu. Rev. Microbiol. 1966, 20, 337–370. [Google Scholar] [PubMed]
- Harding, V.; Heale, J. Isolation and identification of the antifungal compounds accumulating in the induced resistance response of carrot root slices to botrytis cinerea. Physiol. Plant Pathol. 1980, 17, 277–289. [Google Scholar]
- Geng, S.Q.; Alexandrou, A.T.; Li, J.J. Breast cancer stem cells: Multiple capacities in tumor metastasis. Cancer Lett. 2014, 349, 1–7. [Google Scholar]
- Park, S.Y.; Choi, J.H.; Nam, J.S. Targeting cancer stem cells in triple-negative breast cancer. Cancers 2019, 11, 965. [Google Scholar]
- Dittmer, J. Breast cancer stem cells: Features, key drivers and treatment options. Semin. Cancer Biol. 2018, 53, 59–74. [Google Scholar]
- Kise, K.; Kinugasa-Katayama, Y.; Takakura, N. Tumor microenvironment for cancer stem cells. Adv. Drug Deliv. Rev. 2016, 99, 197–205. [Google Scholar]
- Lin, C.; Wang, L.; Wang, H.; Yang, L.; Guo, H.; Wang, X. Tanshinone iia inhibits breast cancer stem cells growth in vitro and in vivo through attenuation of il-6/stat3/nf-kb signaling pathways. J. Cell. Biochem. 2013, 114, 2061–2070. [Google Scholar]
- Chen, W.; Qin, Y.; Liu, S. Cytokines, breast cancer stem cells (bcscs) and chemoresistance. Clin. Transl. Med. 2018, 7, 27. [Google Scholar]
- Liu, S.; Ginestier, C.; Ou, S.J.; Clouthier, S.G.; Patel, S.H.; Monville, F.; Korkaya, H.; Heath, A.; Dutcher, J.; Kleer, C.G.; et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011, 71, 614–624. [Google Scholar] [PubMed]
- Korkaya, H.; Liu, S.; Wicha, M.S. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J. Clin. Investig. 2011, 121, 3804–3809. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.L.; Burton, R.L.; Hill, A.N.; Litorja, M.; Nahm, M.H.; Hwang, J. Low-cost, high-throughput, automated counting of bacterial colonies. Cytometry A 2010, 77, 790–797. [Google Scholar] [PubMed]
- Choi, H.S.; Kim, D.A.; Chung, H.; Park, I.H.; Kim, B.H.; Oh, E.S.; Kang, D.H. Screening of breast cancer stem cell inhibitors using a protein kinase inhibitor library. Cancer Cell Int. 2017, 17, 25. [Google Scholar]
- Choi, H.S.; Kim, J.H.; Kim, S.L.; Deng, H.Y.; Lee, D.; Kim, C.S.; Yun, B.S.; Lee, D.S. Catechol derived from aronia juice through lactic acid bacteria fermentation inhibits breast cancer stem cell formation via modulation stat3/il-6 signaling pathway. Mol. Carcinog. 2018, 57, 1467–1479. [Google Scholar]
- Zhen, X.; Sun, H.N.; Liu, R.; Choi, H.S.; Lee, D.S. Non-thermal plasma-activated medium induces apoptosis of aspc1 cells through the ros-dependent autophagy pathway. In Vivo 2020, 34, 143–153. [Google Scholar] [CrossRef]
- Choi, H.S.; Hwang, C.K.; Kim, C.S.; Song, K.Y.; Law, P.Y.; Wei, L.N.; Loh, H.H. Transcriptional regulation of mouse mu opioid receptor gene: Sp3 isoforms (m1, m2) function as repressors in neuronal cells to regulate the mu opioid receptor gene. Mol. Pharmacol. 2005, 67, 1674–1683. [Google Scholar]
Sample Availability: Samples of the 6-methoxymellein are available from the authors. |



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Choi, H.S.; Kim, S.-L.; Kim, J.-H.; Yun, B.-S.; Lee, D.-S. 6-Methoxymellein Isolated from Carrot (Daucus carota L.) Targets Breast Cancer Stem Cells by Regulating NF-κB Signaling. Molecules 2020, 25, 4374. https://doi.org/10.3390/molecules25194374
Liu R, Choi HS, Kim S-L, Kim J-H, Yun B-S, Lee D-S. 6-Methoxymellein Isolated from Carrot (Daucus carota L.) Targets Breast Cancer Stem Cells by Regulating NF-κB Signaling. Molecules. 2020; 25(19):4374. https://doi.org/10.3390/molecules25194374
Chicago/Turabian StyleLiu, Ren, Hack Sun Choi, Su-Lim Kim, Ji-Hyang Kim, Bong-Sik Yun, and Dong-Sun Lee. 2020. "6-Methoxymellein Isolated from Carrot (Daucus carota L.) Targets Breast Cancer Stem Cells by Regulating NF-κB Signaling" Molecules 25, no. 19: 4374. https://doi.org/10.3390/molecules25194374
APA StyleLiu, R., Choi, H. S., Kim, S.-L., Kim, J.-H., Yun, B.-S., & Lee, D.-S. (2020). 6-Methoxymellein Isolated from Carrot (Daucus carota L.) Targets Breast Cancer Stem Cells by Regulating NF-κB Signaling. Molecules, 25(19), 4374. https://doi.org/10.3390/molecules25194374





