Investigating the Aging Effects of Biochar on Soil C and Si Dissolution and the Interactive Impact on Copper Immobilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Soils and Biochar
2.2. Experimental Design
2.3. Chemical Analysis
2.4. Data and Statistical Analysis
3. Results and Discussion
3.1. Soil Properties of Soil and BC
3.2. Sorption Behavior of Cu by BCs
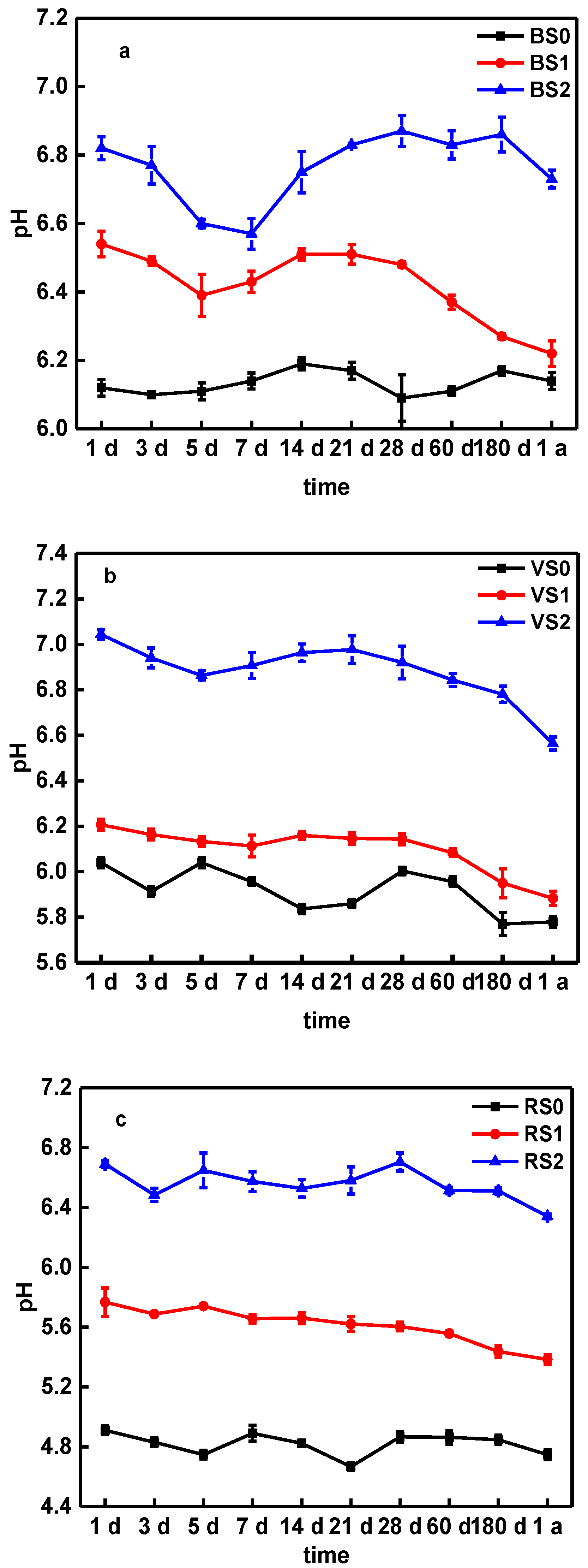
3.3. Effects of BC Amendment on Soil pH, DOC, and SOC
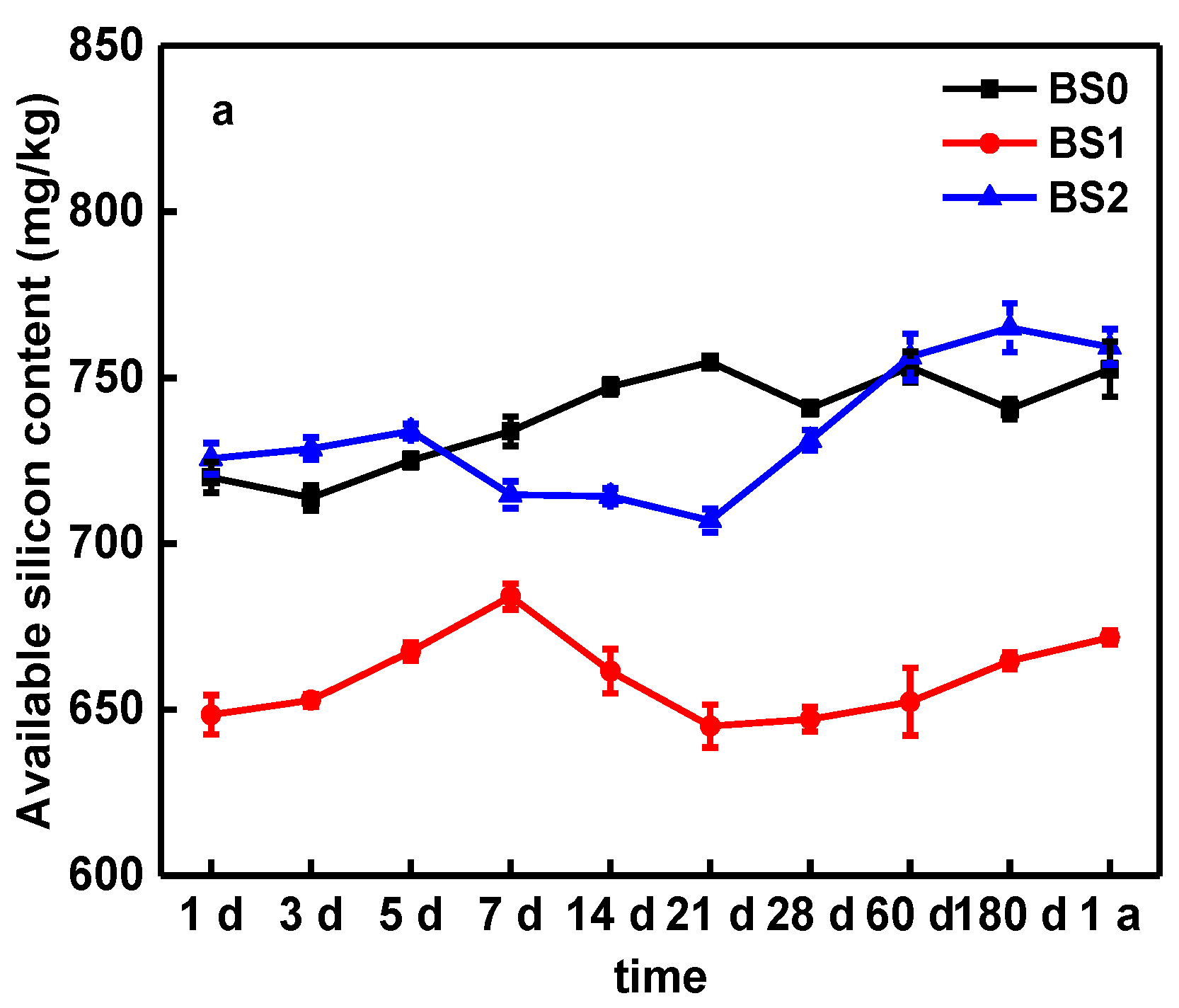
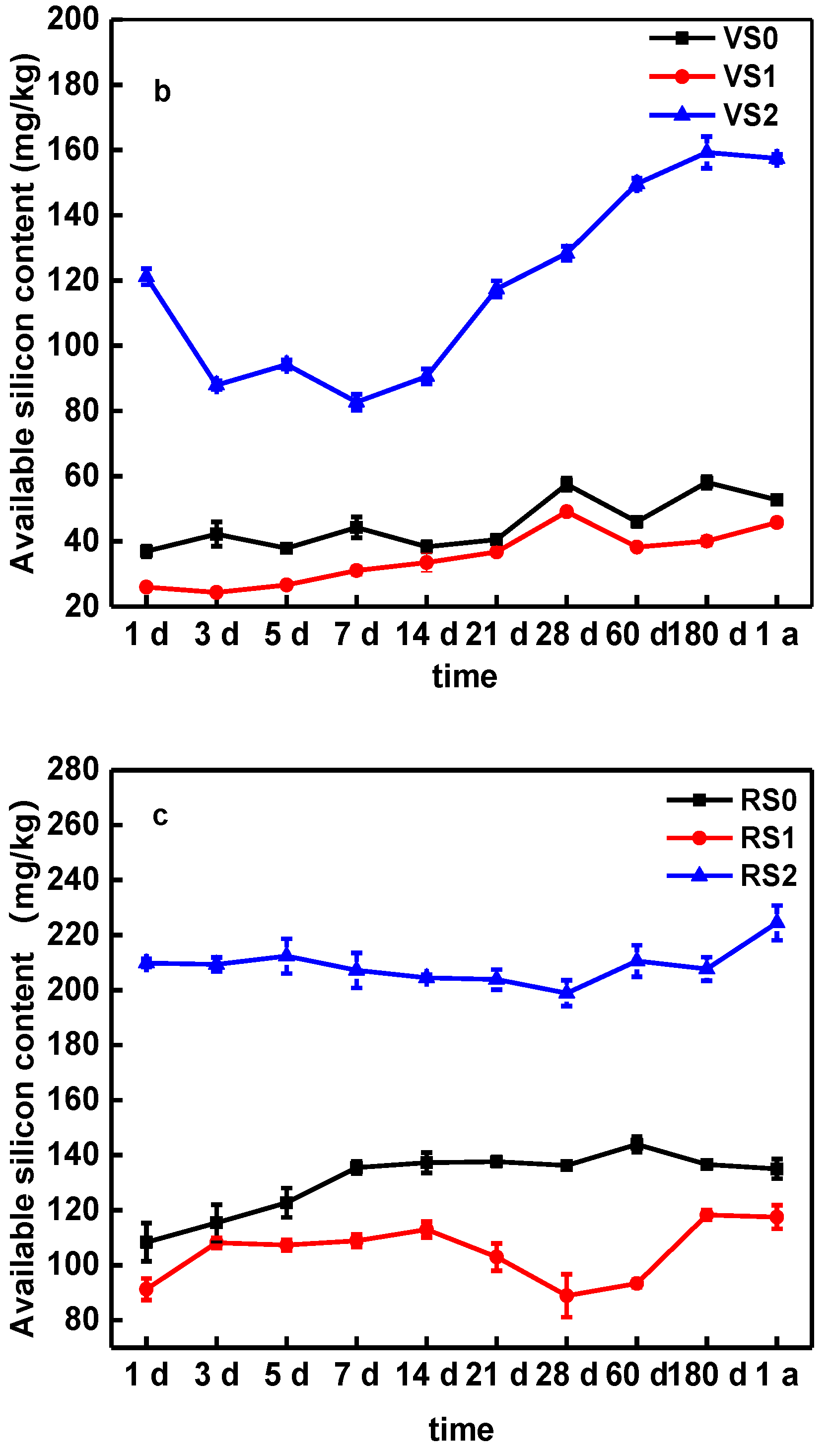
3.4. Effect of Biochar on Soil Available Silicon
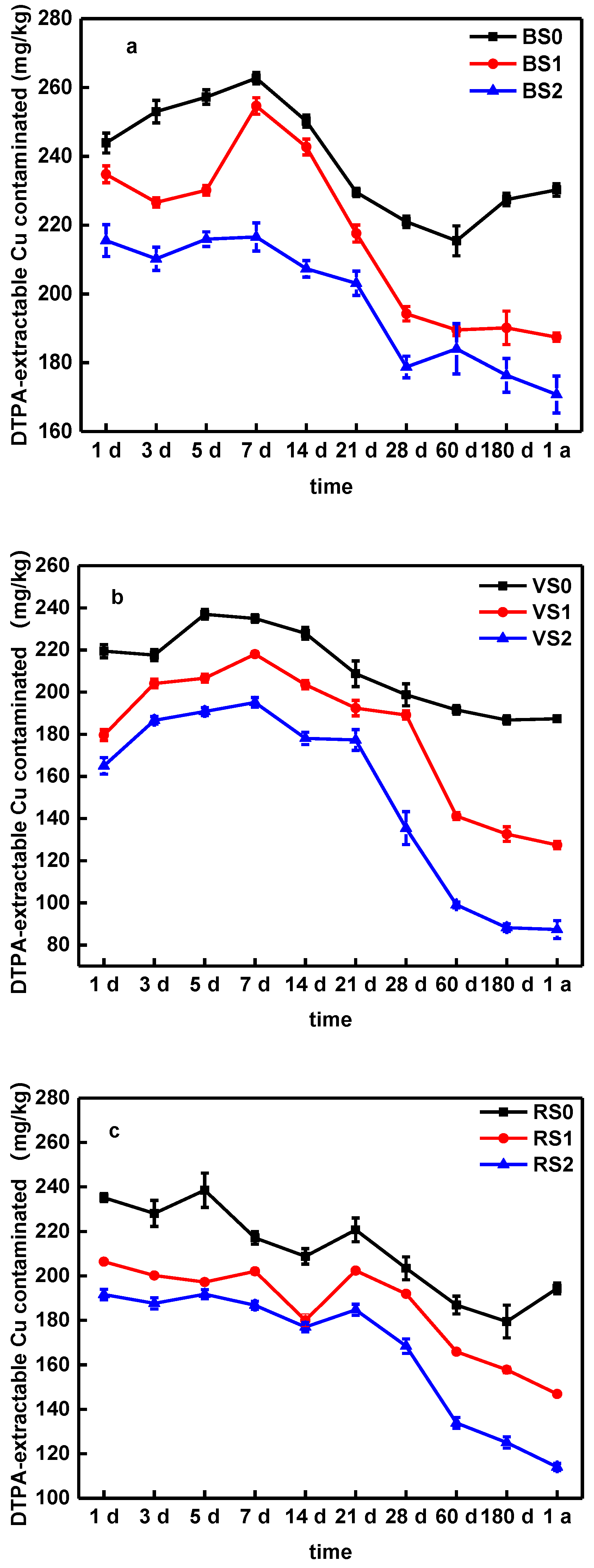
3.5. DTPA-Extractable Cu in the Soil
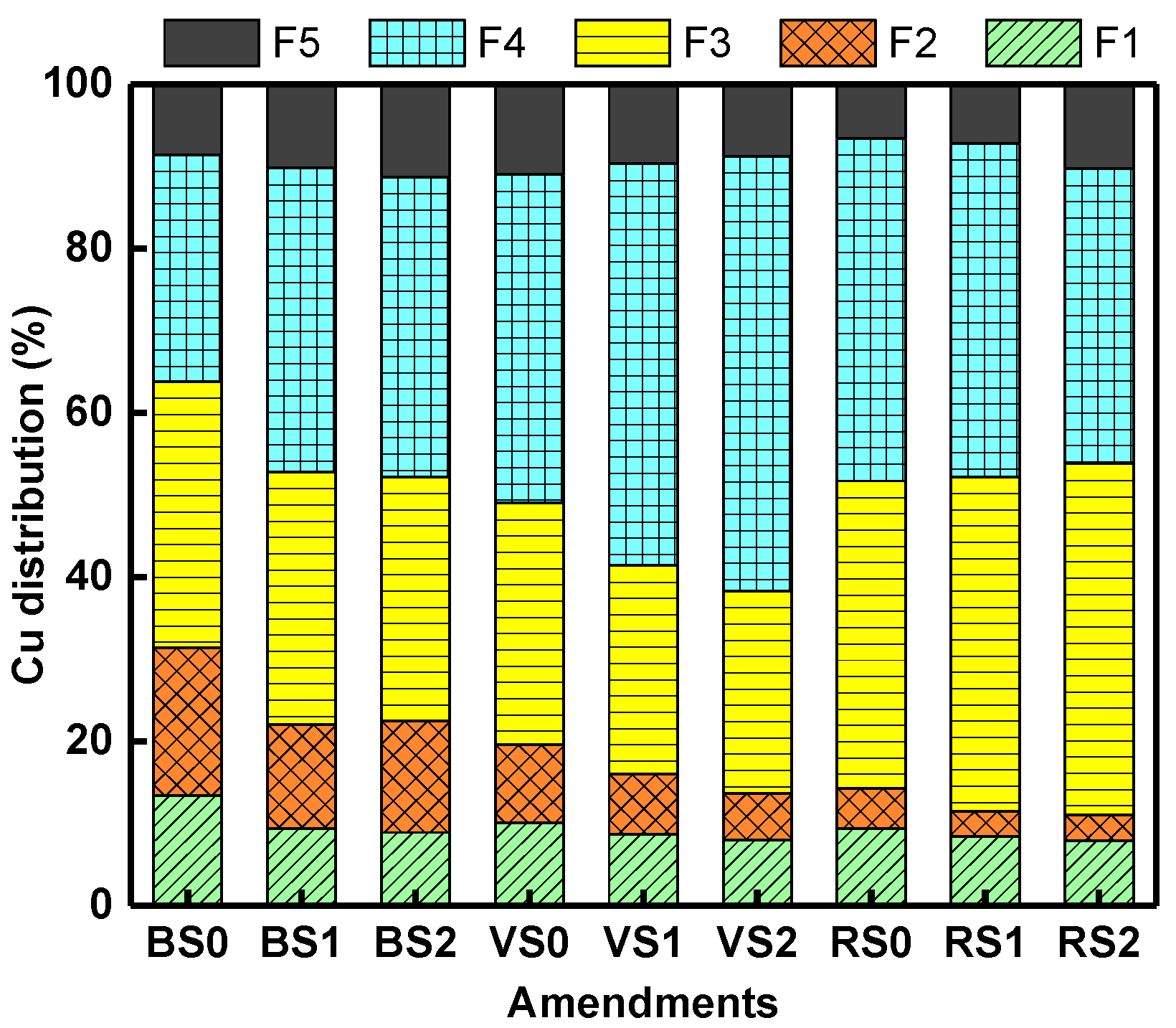
3.6. Tessier Cu Fractions in the Soil
3.7. Limitation and Environmental Implication
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xu, X.; Huang, R.; Liu, J.; Shu, Y. Fractionation and release of Cd, Cu, Pb, Mn, and Zn from historically contaminated river sediment in Southern China: Effect of time and Ph. Environ. Toxicol. Chem. 2019, 38, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Shi, W.; Guo, J.; Fang, B.; Wang, S.; Giesy, J.P.; Holm, P.E. China’s soil pollution control: Choices and challenges. Environ. Sci. Technol. 2016, 50, 13181–13183. [Google Scholar] [CrossRef]
- Niu, L.; Yang, F.; Xu, C.; Yang, H.; Liu, W. Status of metal accumulation in farmland soils across China: From distribution to risk assessment. Environ. Pollut. 2013, 176, 55–62. [Google Scholar] [CrossRef]
- Lu, A.; Wang, J.; Qin, X.; Wang, K.; Han, P.; Zhang, S. Multivariate and geostatistical analyses of the spatial distribution and origin of heavy metals in the agricultural soils in Shunyi, Beijing, China. Sci. Total Environ. 2012, 425, 66–74. [Google Scholar] [CrossRef]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512–513, 143–153. [Google Scholar] [CrossRef]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wu, J.; Wang, J. Source apportionment and health risk assessment of trace metals in surface soils of Beijing metropolitan, China. Chemosphere 2016, 144, 1002–1011. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, Y.; Zhu, Y.G.; Tang, Z.; McGrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
- He, B.Y.; Yu, D.P.; Chen, Y.; Shi, J.L.; Xia, Y.; Li, Q.S.; Wang, L.L.; Ling, L.; Zeng, E.Y. Use of low-calcium cultivars to reduce cadmium uptake and accumulation in edible amaranth (Amaranthus mangostanus L.). Chemosphere 2017, 171, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.D.; Ma, L.N.; Gao, B.; Bandara, W.H. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 2009, 43, 3285–3291. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Zhu, L. Transformation, morphology, and dissolution of silicon and carbon in rice straw-derived biochars under different pyrolytic temperatures. Environ. Sci. Technol. 2014, 48, 3411–3419. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, X.; Xu, Y.; Chen, B. Environmental effects of silicon within biochar (Sichar) and carbon-silicon coupling mechanisms: A critical review. Environ. Sci. Technol. 2019, 53, 13570–13582. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Jin, F.; Wang, F.; McMillan, O.; Al-Tabbaa, A. Sorption of lead by Salisbury biochar produced from British broadleaf hardwood. Bioresour. Technol. 2015, 193, 553–556. [Google Scholar] [CrossRef]
- Shen, Z.; McMillan, O.; Jin, F.; Al-Tabbaa, A. Salisbury biochar did not affect the mobility or speciation of lead in kaolin in a short-term laboratory study. J. Hazard. Mater. 2016, 316, 214–220. [Google Scholar] [CrossRef]
- Shen, Z.; Som, A.M.; Wang, F.; Jin, F.; McMillan, O.; Al-Tabbaa, A. Long-term impact of biochar on the immobilisation of nickel (II) and zinc (II) and the revegetation of a contaminated site. Sci. Total Environ. 2016, 542, 771–776. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Y.; McMillan, O.; Jin, F.; Al-Tabbaa, A. Characteristics and mechanisms of nickel adsorption on biochars produced from wheat straw pellets and rice husk. Environ. Sci. Pollut. Res. Int. 2017, 24, 12809–12819. [Google Scholar] [CrossRef]
- Shen, Z.; Hou, D.; Zhao, B.; Xu, W.; Ok, Y.S.; Bolan, N.S.; Alessi, D.S. Stability of heavy metals in soil washing residue with and without biochar addition under accelerated ageing. Sci. Total Environ. 2018, 619–620, 185–193. [Google Scholar] [CrossRef]
- Shen, Z.; Tian, D.; Zhang, X.; Tang, L.; Su, M.; Zhang, L.; Li, Z.; Hu, S.; Hou, D. Mechanisms of biochar assisted immobilization of Pb(2+) by bioapatite in aqueous solution. Chemosphere 2018, 190, 260–266. [Google Scholar] [CrossRef]
- Rees, F.; Simonnot, M.O.; Morel, J.L. Short-term effects of biochar on soil heavy metal mobility are controlled by intra-particle diffusion and soil pH increase. Eur. J. Soil Sci. 2014, 65, 149–161. [Google Scholar] [CrossRef]
- Uchimiya, M.; Bannon, D.I.; Wartelle, L.H. Retention of heavy metals by carboxyl functional groups of biochars in small arms range soil. J. Agric. Food Chem. 2012, 60, 1798–1809. [Google Scholar] [CrossRef]
- Bian, R.; Joseph, S.; Cui, L.; Pan, G.; Li, L.; Liu, X.; Zhang, A.; Rutlidge, H.; Wong, S.; Chia, C.; et al. A three-year experiment confirms continuous immobilization of cadmium and lead in contaminated paddy field with biochar amendment. J. Hazard. Mater. 2014, 272, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, X.; Geng, Z.; Zhou, H.; Guo, X.; Zhang, Y.; Zhao, H.; Wang, G. The influence of biochar type on long-term stabilization for Cd and Cu in contaminated paddy soils. J. Hazard. Mater. 2016, 304, 40–48. [Google Scholar] [CrossRef]
- Lucchini, P.; Quilliam, R.S.; Deluca, T.H.; Vamerali, T.; Jones, D.L. Does biochar application alter heavy metal dynamics in agricultural soil? Agric. Ecosyst. Environ. 2014, 184, 149–157. [Google Scholar] [CrossRef]
- Karami, N.; Clemente, R.; Moreno-Jimenez, E.; Lepp, N.W.; Beesley, L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef]
- Ren, X.; Wang, F.; Zhang, P.; Guo, J.; Sun, H. Aging effect of minerals on biochar properties and sorption capacities for atrazine and phenanthrene. Chemosphere 2018, 206, 51–58. [Google Scholar] [CrossRef]
- Dong, X.; Li, G.; Lin, Q.; Zhao, X. Quantity and quality changes of biochar aged for 5 years in soil under field conditions. Catena 2017, 159, 136–143. [Google Scholar] [CrossRef]
- Lawrinenko, M.; Laird, D.A.; Johnson, R.L.; Jing, D. Accelerated aging of biochars: Impact on anion exchange capacity. Carbon 2016, 103, 217–227. [Google Scholar] [CrossRef]
- Sorrenti, G.; Masiello, C.A.; Dugan, B.; Toselli, M. Biochar physico-chemical properties as affected by environmental exposure. Sci. Total Environ. 2016, 563–564, 237–246. [Google Scholar] [CrossRef]
- Hale, S.; Hanley, K.; Lehmann, J.; Zimmerman, A.; Cornelissen, G. Effects of chemical, biological, and physical aging as well as soil addition on the sorption of pyrene to activated carbon and biochar. Environ. Sci. Technol. 2011, 45, 10445–10453. [Google Scholar] [CrossRef]
- Liu, G.; Chen, L.; Jiang, Z.; Zheng, H.; Dai, Y.; Luo, X.; Wang, Z. Aging impacts of low molecular weight organic acids, (LMWOAs) on furfural production residue-derived biochars: Porosity, functional properties, and inorganic minerals. Sci. Total Environ. 2017, 607–608, 1428–1436. [Google Scholar] [CrossRef]
- Zhang, X.; Sarmah, A.K.; Bolan, N.S.; He, L.; Lin, X.; Che, L.; Tang, C.; Wang, H. Effect of aging process on adsorption of diethyl phthalate in soils amended with bamboo biochar. Chemosphere 2016, 142, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, X.; Chen, B. Biochar impacts on soil silicon dissolution kinetics and their interaction mechanisms. Sci. Rep. 2018, 8, 8040. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, X.; Zhang, K.; Chen, B. Effects of biochar amendment on the soil silicon cycle in a soil-rice ecosystem. Environ. Pollut. 2019, 248, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Farmer, V.C.; Delbos, E.; Miller, J.D. The role of phytolith formation and dissolution in controlling concentrations of silica in soil solutions and streams. Geoderma 2005, 127, 71–79. [Google Scholar] [CrossRef]
- Bruun Hansen, H.C.; Raben-Lange, B.; Raulund-Rasmussen, K.; Borggaard, O.K. Monosilicate adsorption by ferrihydrite and goethite at pH 3–6. Soil Sci. 1994, 158, 40–46. [Google Scholar] [CrossRef]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Bian, R.; Chen, D.; Qu, J.; Wanjiru Kibue, G.; Pan, G.; Zhang, X.; Zheng, J.; Zheng, J. Effect of biochar amendment on soil-silicon availability and rice uptake. J. Plant Nutr. Soil Sci. 2014, 177, 91–96. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, S.; Chen, D.; Dai, G.; Wei, D.; Shu, Y. Activation of persulfate with biochar for degradation of bisphenol A in soil. Chem. Eng. J. 2020, 381, 122637. [Google Scholar] [CrossRef]
- Lin, N.; Zhang, H.; Jia, Z.Z.; Huang, R.L.; Shu, Y.H. Adsorption of Pb(II) by biochars derived from three types of biomass. J. Agro-Environ. Sci. 2016, 35, 992–998. [Google Scholar]
- Lu, R. Analytical Methods of Soil Agrochemistry; China Agricultural Science and Technology Publishing House: Beijing, China, 1999; pp. 18–99. [Google Scholar]
- Yu, H.Y.; Ding, X.; Li, F.; Wang, X.; Zhang, S.; Yi, J.; Liu, C.; Xu, X.; Wang, Q. The availabilities of arsenic and cadmium in rice paddy fields from a mining area: The role of soil extractable and plant silicon. Environ. Pollut. 2016, 215, 258–265. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Ok, Y.S.; Niazi, N.K.; Xu, S.; Yuan, G.; Chen, X.; Zhang, X.; et al. Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd, Cu, Pb and Zn) in contaminated soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particular trace elements. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, J.; Wu, J.; Dai, G.; Wei, D.; Shu, Y. Assessing biochar application to immobilize Cd and Pb in a contaminated soil: A field experiment under a cucumber-sweet potato-rape rotation. Environ. Geochem. Health 2020, 1–12. [Google Scholar] [CrossRef]
- Xiao, R.; Wang, P.; Mi, S.; Ali, A.; Liu, X.; Li, Y.; Guan, W.; Li, R.; Zhang, Z. Effects of crop straw and its derived biochar on the mobility and bioavailability in Cd and Zn in two smelter-contaminated alkaline soils. Ecotoxicol. Environ. Saf. 2019, 181, 155–163. [Google Scholar] [CrossRef]
- Zhao, Z.; Nie, T.; Zhou, W. Enhanced biochar stabilities and adsorption properties for tetracycline by synthesizing silica-composited biochar. Environ. Pollut. 2019, 254, 113015. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, M.; Usman, A.R.A.; Al-Faraj, A.S.; Abduljabbar, A.; Yong, S.O.; Al-Wabel, M.I. Date palm waste-derived biochar composites with silica and zeolite: Synthesis, characterization and implication for carbon stability and recalcitrant potential. Environ. Geochem. Health 2019, 41, 1687–1704. [Google Scholar] [CrossRef]
- Zhuo, S.H.; Meng, W.; Xu, T.; Deng, Y.Y.; Lin, Z.B.; Wang, X.G. Transport and transformation of Cd between biochar and soil under combined dry-wet and freeze-thaw aging. Environ. Pollut. 2020, 263, 114449. [Google Scholar]
- Xu, Z.; Xu, X.; Tsang, D.C.W.; Cao, X. Contrasting impacts of pre- and post-application aging of biochar on the immobilization of Cd in contaminated soils. Environ. Pollut. 2018, 242, 1362–1370. [Google Scholar] [CrossRef]
- Wagner, A.; Kaupenjohann, M. Suitability of biochars (pyro- and hydrochars) for metal immobilization on former sewage-field soils. Eur. J. Soil Sci. 2014, 65, 139–148. [Google Scholar] [CrossRef]
- Li, G.; Khan, S.; Ibrahim, M.; Sun, T.R.; Tang, J.F.; Cotner, J.B.; Xu, Y.Y. Biochars induced modification of dissolved organic matter (DOM) in soil and its impact on mobility and bioaccumulation of arsenic and cadmium. J. Hazard. Mater. 2018, 348, 100–108. [Google Scholar] [CrossRef]
- Sui, F.; Wang, J.; Zuo, J.; Joseph, S.; Munroe, P.; Drosos, M.; Li, L.; Pan, G. Effect of amendment of biochar supplemented with Si on Cd mobility and rice uptake over three rice growing seasons in an acidic Cd-tainted paddy from central South China. Sci. Total Environ. 2019, 709, 136101. [Google Scholar] [CrossRef]
- He, E.; Yang, Y.; Xu, Z.; Qiu, H.; Yang, F.; Peijnenburg, W.; Zhang, W.; Qiu, R.; Wang, S.Z. Two years of aging influences the distribution and lability of metal(loid)s in a contaminated soil amended with different biochars. Sci. Total Environ. 2019, 673, 245–253. [Google Scholar] [CrossRef]
- Beesley, L.; Dickinson, N. Carbon and trace element fluxes in the pore water of an urban soil following green waste compost, woody and biochar amendments, inoculated with the earthworm Lumbricus terrestris. Soil. Biol. Biochem. 2011, 43, 188–196. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds, such as Sodium silicate, biochars and soils, are available from the authors. |


| Treatment | BS0 | BS1 | BS2 | VS0 | VS1 | VS2 | RS0 | RS1 | RS2 |
|---|---|---|---|---|---|---|---|---|---|
| SOC (%) | 3.32 | 5.07 | 4.38 | 1.53 | 3.27 | 2.37 | 0.90 | 2.41 | 1.85 |
| Time | BS | VS | RS | ||||
|---|---|---|---|---|---|---|---|
| BC300 | BC600 | BC300 | BC600 | BC300 | BC600 | ||
| IE(Cu,%) | 1 d | 4 ± 0.25 f | 12 ± 0.85 h | 18 ± 0.17 d | 25 ± 0.53 d | 12 ± 0.29 d | 19 ± 0.24 e |
| 3 d | 10 ± 0.53 d | 17 ± 0.66 f,g | 6 ± 0.54 i | 14 ± 0.57 g | 12 ± 0.37 d | 18 ± 0.37 e,f | |
| 5 d | 11 ± 0.33 d | 16 ± 0.33 f,g | 13 ± 0.16 e | 19 ± 0.33 f | 17 ± 0.41 b | 20 ± 0.76 d | |
| 7 d | 3 ± 0.21 g | 18 ± 0.57 d,e | 7 ± 0.21 h | 17 ± 0.16 g | 7 ± 0.34 e | 14 ± 0.54 i | |
| 14 d | 3 ± 0.08 g | 17 ± 0.49 e,f | 11 ± 0.22 f | 22 ± 0.24 e | 14 ± 0.49 c | 15 ± 0.37 h,i | |
| 21 d | 5 ± 0.29 e | 12 ± 0.24 h | 8 ± 0.47 g | 15 ± 0.49 i | 8 ± 0.29 e | 16 ± 0.24 g,h | |
| 28 d | 12 ± 0.22 c | 19 ± 0.57 c,d | 5 ± 0.21 j | 32 ± 0.21 c | 6 ± 0.17 f | 17 ± 0.24 f,g | |
| 60 d | 12 ± 0.25 c | 15 ± 0.93 g | 26 ± 0.53 c | 48 ± 0.33 b | 11 ± 0.29 d | 28 ± 0.33 c | |
| 180 d | 16 ± 0.22 b | 22 ± 0.24 b | 29 ± 0.45 b | 53 ± 0.08 a | 12 ± 0.17 d | 30 ± 0.54 b | |
| 1 a | 19 ± 0.12 a | 26 ± 0.41 a | 32 ± 0.39 a | 53 ± 0.49 a | 24 ± 0.37 a | 41 ± 0.76 a | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Wu, J.; Duan, L.; Cheng, S.; Huang, J.; Chen, T. Investigating the Aging Effects of Biochar on Soil C and Si Dissolution and the Interactive Impact on Copper Immobilization. Molecules 2020, 25, 4319. https://doi.org/10.3390/molecules25184319
Jiang S, Wu J, Duan L, Cheng S, Huang J, Chen T. Investigating the Aging Effects of Biochar on Soil C and Si Dissolution and the Interactive Impact on Copper Immobilization. Molecules. 2020; 25(18):4319. https://doi.org/10.3390/molecules25184319
Chicago/Turabian StyleJiang, Shaojun, Jiachen Wu, Lianxin Duan, Sheng Cheng, Jian Huang, and Tao Chen. 2020. "Investigating the Aging Effects of Biochar on Soil C and Si Dissolution and the Interactive Impact on Copper Immobilization" Molecules 25, no. 18: 4319. https://doi.org/10.3390/molecules25184319
APA StyleJiang, S., Wu, J., Duan, L., Cheng, S., Huang, J., & Chen, T. (2020). Investigating the Aging Effects of Biochar on Soil C and Si Dissolution and the Interactive Impact on Copper Immobilization. Molecules, 25(18), 4319. https://doi.org/10.3390/molecules25184319






