Tabebuia impetiginosa: A Comprehensive Review on Traditional Uses, Phytochemistry, and Immunopharmacological Properties
Abstract
1. Introduction
2. Methodology
3. Taxonomy and Botanical Traits
3.1. Taxonomy
3.2. Botanical Traits
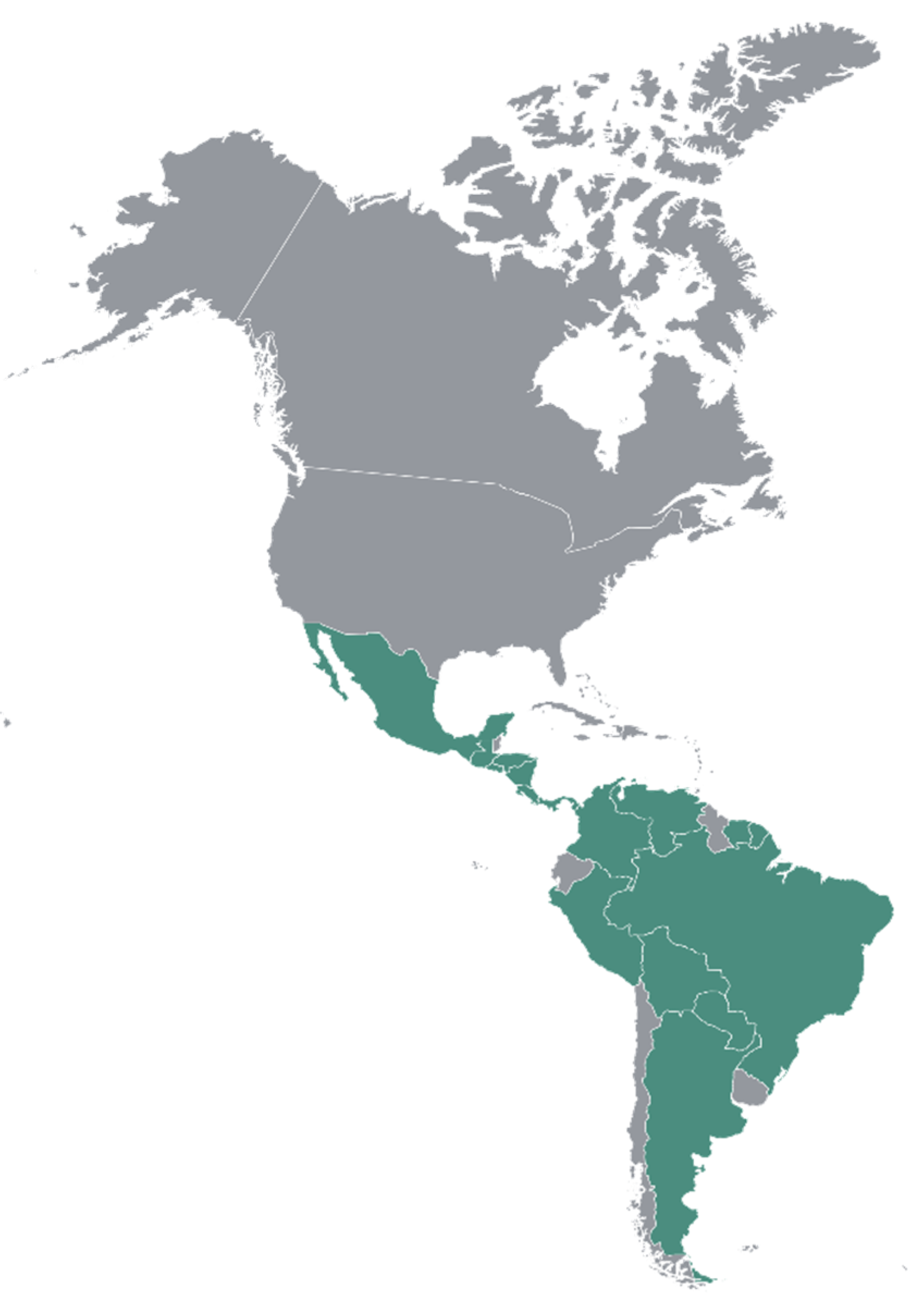
3.3. Distribution
4. Traditional Uses
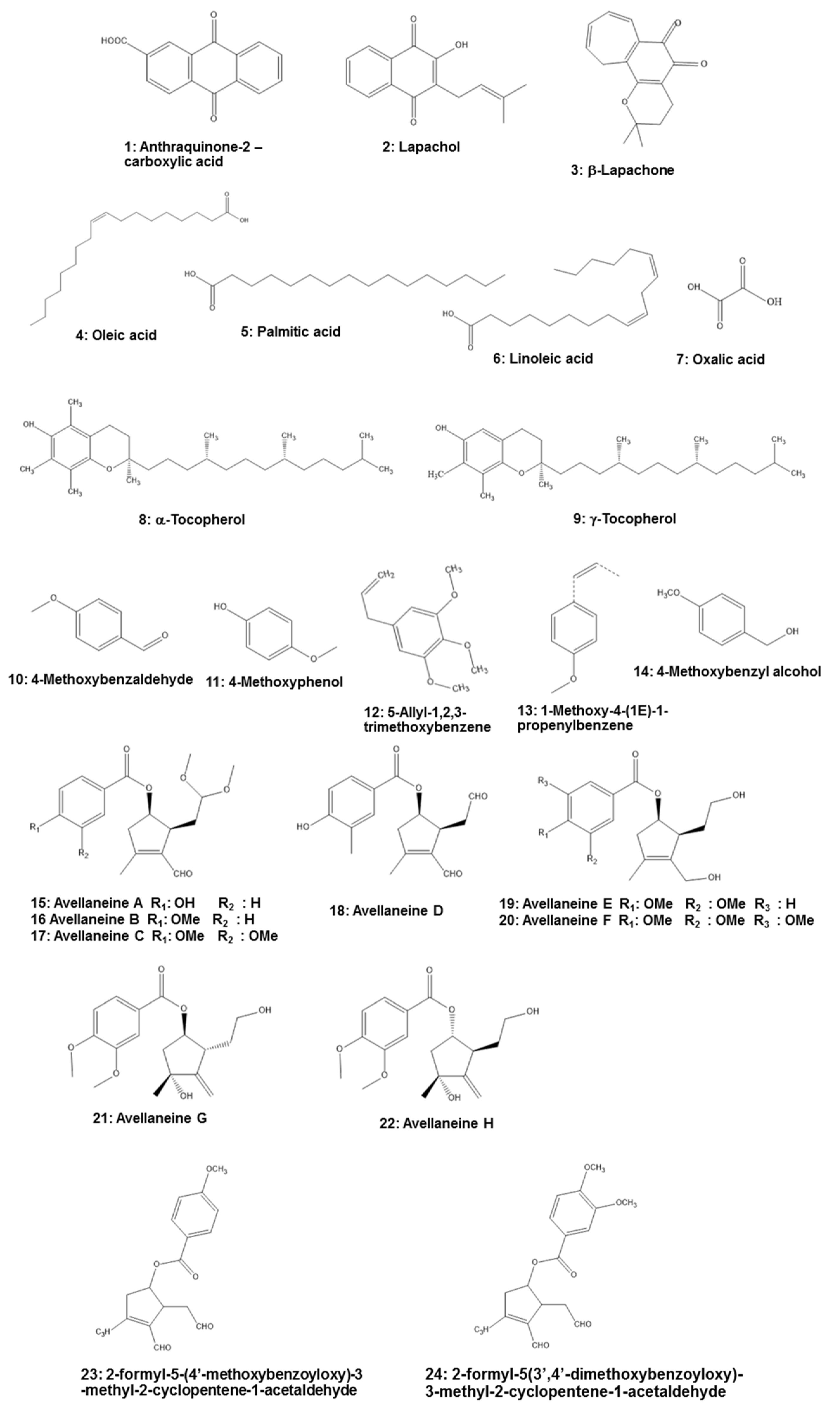
5. Phytochemistry
6. Pharmacological Activities
6.1. Anti-inflammatory Activity
6.1.1. Regulation of Inflammatory Mediators.
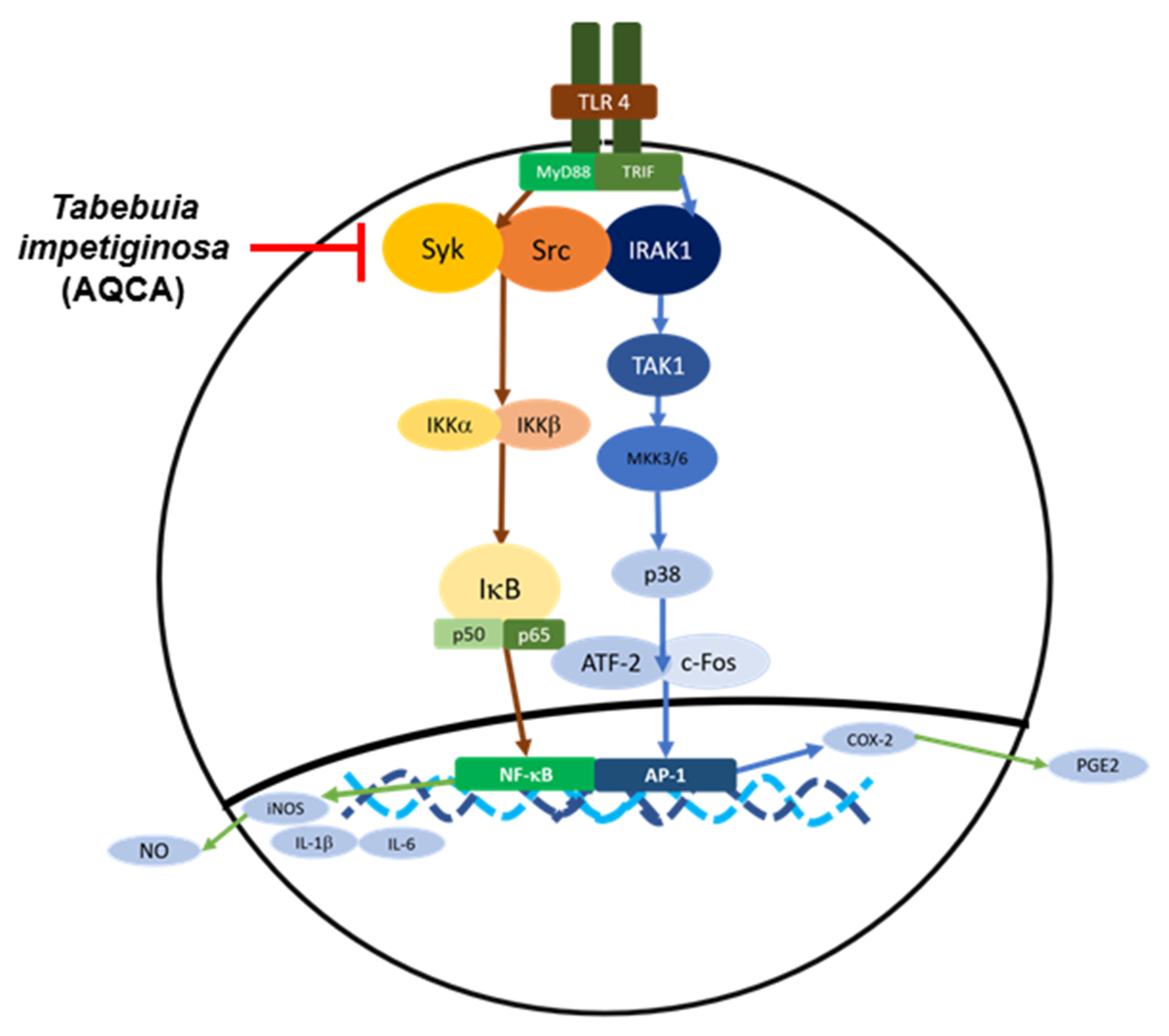
6.1.2. Effects on Inflammatory Signaling
6.2. Anti-Cancer Activity
6.3. Anti-Autoimmune Diseases
7. Clinical Trials
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ngo, L.T.; Okogun, J.I.; Folk, W.R. 21st century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 2013, 30, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, H.; Morris-Natschke, S.L.; Akiyama, T.; Lee, K.-H. Plant-derived natural product research aimed at new drug discovery. J. Nat. Med. 2008, 62, 263–280. [Google Scholar] [CrossRef]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar]
- Pires, T.C.S.P.; Dias, M.I.; Calhelha, R.C.; Carvalho, A.M.; Queiroz, M.-J.R.; Barros, L.; Ferreira, I.C. Bioactive Properties of Tabebuia impetiginosa-Based Phytopreparations and Phytoformulations: A Comparison between Extracts and Dietary Supplements. Molecules 2015, 20, 22863–22871. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, F.G.G.; Vilar, J.C.; Alves, I.A.N.; Cavalcanti, S.C.D.H.; Antoniolli, Â.R. Antinociceptive and antiedematogenic properties and acute toxicity of Tabebuia avellanedae Lor. ex Griseb. inner bark aqueous extract. BMC Pharmacol. 2001, 1, 6. [Google Scholar] [CrossRef]
- Freitas, A.E.; Moretti, M.; Budni, J.; Balen, G.O.; Fernandes, S.C.; Veronezi, P.O.; Heller, M.; Micke, G.A.; Pizzolatti, M.G.; Rodrigues, A.L.S. NMDA Receptors and the l-Arginine-Nitric Oxide-Cyclic Guanosine Monophosphate Pathway Are Implicated in the Antidepressant-Like Action of the Ethanolic Extract fromTabebuia avellanedaein Mice. J. Med. Food 2013, 16, 1030–1038. [Google Scholar] [CrossRef]
- Fernandez, A.; Cock, I.E. Tabebuia impetiginosa (Mart. Ex DC. Mattos) Bark Extracts Inhibit the Growth Gastrointestinal Bacterial Pathogens and Potentiate the Activity of some Conventional Antibiotics. Pharmacogn. Commun. 2020, 10, 75–82. [Google Scholar] [CrossRef]
- Byeon, S.E.; Chung, J.Y.; Lee, Y.G.; Kim, B.H.; Kim, K.H.; Cho, J.Y. In vitro and in vivo anti-inflammatory effects of taheebo, a water extract from the inner bark of Tabebuia avellanedae. J. Ethnopharmacol. 2008, 119, 145–152. [Google Scholar] [CrossRef]
- Sharma, P.K.; Khanna, R.N.; Rohatgi, B.K.; Thomson, R.H. Tecomaquinone-III: A new quinone from Tabebuia pentaphylla. Phytochemistry 1988, 27, 632–633. [Google Scholar] [CrossRef]
- Manners, G.D.; Jurd, L. A new naphthaquinone from Tabebuia guayacan. Phytochemistry 1976, 15, 225–226. [Google Scholar] [CrossRef]
- Blatt, C.T.; Salatino, A.; Salatino, M.L. Flavonoids of Tabebuia caraiba (Bignoniaceae). Biochem. Syst. Ecol. 1996, 24, 89. [Google Scholar] [CrossRef]
- Wagner, H.; Kreher, B.; Lotter, H.; Hamburger, M.O.; Cordell, G.A. Structure Determination of New Isomeric Naphtho[2,3-b] furan-4,9-diones fromTabebuia avellanedae by the selective-INEPT technique. Helvetica Chim. Acta 1989, 72, 659–667. [Google Scholar] [CrossRef]
- Woo, H.; Choi, Y. Growth inhibition of A549 human lung carcinoma cells by β-lapachone through induction of apoptosis and inhibition of telomerase activity. Int. J. Oncol. 2005, 26, 1017–1023. [Google Scholar] [CrossRef]
- Tahara, T.; Watanabe, A.; Yutani, M.; Yamano, Y.; Sagara, M.; Nagai, S.; Saito, K.; Yamashita, M.; Ihara, M.; Iida, A. STAT3 inhibitory activity of naphthoquinones isolated from Tabebuia avellanedae. Bioorganic Med. Chem. 2020, 28, 115347. [Google Scholar] [CrossRef] [PubMed]
- Park, J.G.; Yi, Y.-S.; Hong, Y.H.; Yoo, S.; Han, S.Y.; Kim, E.; Jeong, S.-G.; Aravinthan, A.; Baik, K.S.; Choi, S.Y.; et al. Tabetri™ (Tabebuia avellanedae Ethanol Extract) Ameliorates Osteoarthritis Symptoms Induced by Monoiodoacetate through Its Anti-Inflammatory and Chondroprotective Activities. Mediat. Inflamm. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hasegawa, I.; Ohta, T. Anti-inflammatory cyclopentene derivatives from the inner bark of Tabebuia avellanedae. Fitoterapia 2016, 109, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, K.; Fukuda, Y.; Tokikura, C.; Noda, M.; Yamamoto, A.; Yamamoto, M.; Yamashita, M.; Zaima, N.; Iida, A.; Moriyama, T. The anti-obesity effect of Taheebo (Tabebuia avellanedae Lorentz ex Griseb) extract in ovariectomized mice and the identification of a potential anti-obesity compound. Biochem. Biophys. Res. Commun. 2016, 478, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Suo, M.; Ohta, T.; Takano, F.; Jin, S. Bioactive Phenylpropanoid Glycosides from Tabebuia avellanedae. Molecules 2013, 18, 7336–7345. [Google Scholar] [CrossRef] [PubMed]
- The Plant List. 2013. Available online: http://www.theplantlist.org/ (accessed on 20 August 2020).
- Castellanos, J.R.G.; Prieto, J.M.; Heinrich, M. Red Lapacho (Tabebuia impetiginosa)—A global ethnopharmacological commodity? J. Ethnopharmacol. 2009, 121, 1–13. [Google Scholar] [CrossRef]
- University of Florida. 1994. University of Florida, Institute of Food and Agricultural Sciences, Environmental Horticulture Department. Available online: https://hort.ifas.ufl.edu/database/trees/trees_common.shtml/ (accessed on 17 September 2020).
- The New York Botanical Garden. 1995. Available online: http://sweetgum.nybg.org/science/ (accessed on 16 September 2020).
- Missouri Botanical Garden. 2018. Available online: http://www.tropicos.org/ (accessed on 20 August 2020).
- Telang, N.; Mukherjee, B.; Wong, G.Y. Growth inhibition of estrogen receptor positive human breast cancer cells by Taheebo from the inner bark of Tabebuia avellandae tree. Int. J. Mol. Med. 2009, 24, 253–260. [Google Scholar] [CrossRef]
- Kiage-Mokua, B.N.; Roos, N.; Schrezenmeir, J. Lapacho Tea (Tabebuia impetiginosa) Extract Inhibits Pancreatic Lipase and Delays Postprandial Triglyceride Increase in Rats. Phytotherapy Res. 2012, 26, 1878–1883. [Google Scholar] [CrossRef]
- Freitas, A.E.; Budni, J.; Lobato, K.R.; Binfaré, R.W.; Machado, D.G.; Jacinto, J.; Veronezi, P.O.; Pizzolatti, M.G.; Rodrigues, A.L.S. Antidepressant-like action of the ethanolic extract from Tabebuia avellanedae in mice: Evidence for the involvement of the monoaminergic system. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2010, 34, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, C.M.; Vasconcelos, T.L.C.; Póvoas, F.T.X.; Santos, R.; Maynart, W.; Almeida, T.J.J.O.C.; Research, P. Antimicrobial, antioxidant and cytotoxic activity of extracts of Tabebuia impetiginosa (Mart ex DC.) Standl. J. Chem. Pharm. Res. 2014, 6, 2673–2681. [Google Scholar]
- Lee, M.H.; Choi, H.M.; Hahm, D.-H.; Her, E.; Yang, H.-I.; Yoo, M.-C.; Kim, K.S. Analgesic and anti-inflammatory effects in animal models of an ethanolic extract of Taheebo, the inner bark of Tabebuia avellanedae. Mol. Med. Rep. 2012, 6, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, S.W.; Kwon, D.-J.; Heo, S.-I.; Park, S.-H.; Kim, S.Y.; Hong, S. Oral administration of taheebo (Tabebuia avellanedae Lorentz ex Griseb.) water extract prevents DSS-induced colitis in mice by up-regulating type II T helper immune responses. BMC Complement. Altern. Med. 2017, 17, 448. [Google Scholar] [CrossRef] [PubMed]
- Son, D.J.; Lim, Y.; Park, Y.H.; Chang, S.K.; Yun, Y.P.; Hong, J.T.; Takeoka, G.R.; Lee, K.G.; Lee, S.E.; Kim, M.R.; et al. Inhibitory effects of Tabebuia impetiginosa inner bark extract on platelet aggregation and vascular smooth muscle cell proliferation through suppressions of arachidonic acid liberation and ERK1/2 MAPK activation. J. Ethnopharmacol. 2006, 108, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Mistrangelo, M.; Cornaglia, S.; Pizzio, M.; Rimonda, R.; Gavello, G.; Conte, I.D.; Mussa, A. Immunostimulation to reduce recurrence after surgery for anal condyloma acuminata: A prospective randomized controlled trial. Color. Dis. 2009, 12, 799–803. [Google Scholar] [CrossRef]
- Camiel, L.D.; Whelan, J. Tropical American Plants in the Treatment of Infectious Diseases. J. Diet. Suppl. 2008, 5, 349–372. [Google Scholar] [CrossRef]
- Malange, K.F.; Dos Santos, G.G.; Parada, C.A.; Kato, N.N.; Toffoli-Kadri, M.C.; Carollo, C.A.; Silva, D.B.; Portugal, L.C.; Alves, F.M.; Rita, P.H.S.; et al. Tabebuia aurea decreases hyperalgesia and neuronal injury induced by snake venom. J. Ethnopharmacol. 2019, 233, 131–140. [Google Scholar] [CrossRef]
- De Melo, J.G.; Santos, A.G.; De Amorim, E.L.C.; Nascimento, S.C.D.; Albuquerque, U.P. Medicinal Plants Used as Antitumor Agents in Brazil: An Ethnobotanical Approach. Evidence-Based Complement. Altern. Med. 2011, 2011, 1–14. [Google Scholar] [CrossRef]
- Warashina, T.; Nagatani, Y.; Noro, T. Constituents from the bark of Tabebuia impetiginosa. Phytochemistry 2004, 65, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Jeong, K.M.; Lee, J.; Zhao, J.; Choi, S.-Y.; Baek, K.-S. Development and Validation of an Analytical Method Readily Applicable for Quality Control of Tabebuia impetiginosa (Taheebo) Ethanolic Extract. J. AOAC Int. 2018, 101, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-S.; Lee, K.-G.; Shibamoto, T.; Lee, S.-E.; Takeoka, G.R. Antioxidant Activity and Characterization of Volatile Constituents of Taheebo (Tabebuia impetiginosaMartius ex DC). J. Agric. Food Chem. 2003, 51, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Koyama, J.; Morita, I.; Tagahara, K.; Hirai, K.-I. Cyclopentene dialdehydes from Tabebuia impetiginosa. Phytochemitry 2000, 53, 869–872. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, J.; Kim, K.H.; Cho, J.Y. Regulation of macrophage and monocyte immune responses by water extract from the inner bark of Tabebuia avellanedae. JMPR 2010, 4, 431–438. [Google Scholar]
- Böhler, T.; Nolting, J.; Gurragchaa, P.; Lupescu, A.; Neumayer, H.-H.; Budde, K.; Kamar, N.; Klupp, J. Tabebuia avellanedae extracts inhibit IL-2-independent T-lymphocyte activation and proliferation. Transpl. Immunol. 2008, 18, 319–323. [Google Scholar] [CrossRef]
- Suo, M.; Isao, H.; Kato, H.; Takano, F.; Ohta, T. Anti-inflammatory constituents from Tabebuia avellanedae. Fitoterapia 2012, 83, 1484–1488. [Google Scholar] [CrossRef]
- Woo, H.J.; Park, K.-Y.; Rhu, C.-H.; Lee, W.H.; Choi, B.T.; Kim, G.Y.; Park, Y.-M.; Choi, Y.H. β-Lapachone, a Quinone Isolated from Tabebuia avellanedae, Induces Apoptosis in HepG2 Hepatoma Cell Line Through Induction of Bax and Activation of Caspase. J. Med. Food 2006, 9, 161–168. [Google Scholar] [CrossRef]
- Eun, L.S.; Soo, P.B.; Jong, H.Y. Antioxidative activity of taheebo (Tabebuia impetiginosa Martius ex DC.) extracts on the H2O2-induced NIH3T3 cells. J. Med. Plants Res. 2012, 6, 5258–5265. [Google Scholar] [CrossRef]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2012, 35, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Oh, D.Y.; Bandyopadhyay, G.; Lagakos, W.S.; Talukdar, S.; Osborn, O.; Johnson, A.; Chung, H.; Mayoral, R.; Maris, M.; et al. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat. Med. 2015, 21, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yada, K.; Lee, H.; Fukuda, Y.; Iida, A.; Suzuki, K. Taheebo Polyphenols Attenuate Free Fatty Acid-Induced Inflammation in Murine and Human Macrophage Cell Lines as Inhibitor of Cyclooxygenase-2. Front. Nutr. 2017, 4, 4. [Google Scholar] [CrossRef]
- Ren, K.; Torres, R. Role of interleukin-1β during pain and inflammation. Brain Res. Rev. 2009, 60, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Dunster, J. The macrophage and its role in inflammation and tissue repair: Mathematical and systems biology approaches. Wiley Interdiscip. Rev. Syst. Biol. Med. 2015, 8, 87–99. [Google Scholar] [CrossRef]
- Ohno, S.; Ohno, Y.; Suzuki, Y.; Miura, S.; Yoshioka, H.; Mori, Y.; Suzuki, K. Ingestion of Tabebuia avellanedae (Tahee-bo) Inhibits Production of Reactive Oxygen Species from Human Pe-ripheral Blood Neutrophils. Int. J. Food Sci. Nutr. Diet. 2015, 6, 1–4. [Google Scholar]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free. Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Agrawal, A.; Agrawal, S.; Gupta, S. Role of Dendritic Cells in Inflammation and Loss of Tolerance in the Elderly. Front. Immunol. 2017, 8, 896. [Google Scholar] [CrossRef]
- Morva, A.; Lemoine, S.; Achour, A.; Pers, J.-O.; Youinou, P.; Jamin, C. Maturation and function of human dendritic cells are regulated by B lymphocytes. Blood 2012, 119, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Hunto, S.T.; Shin, K.K.; Kim, H.G.; Park, S.H.; Oh, J.; Sung, G.-H.; Hossain, M.A.; Rho, H.S.; Lee, J.; Kim, J.-H.; et al. Phosphatidylinositide 3-Kinase Contributes to the Anti-Inflammatory Effect of Abutilon crispum L. Medik Methanol Extract. Evidence-Based Complement. Altern. Med. 2018, 2018, 1935902. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Osadebe, P.O.; Okoye, F.B.C. Anti-inflammatory effects of crude methanolic extract and fractions of Alchornea cordifolia leaves. J. Ethnopharmacol. 2003, 89, 19–24. [Google Scholar] [CrossRef]
- Park, J.G.; Son, Y.-J.; Kim, M.-Y.; Cho, J.Y. Syk and IRAK1 Contribute to Immunopharmacological Activities of Anthraquinone-2-carboxlic Acid. Molecules 2016, 21, 809. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.K. MAP Kinase Pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef]
- Park, J.G.; Kim, S.C.; Kim, Y.H.; Yang, W.S.; Kim, Y.; Hong, S.; Kim, K.-H.; Yoo, B.C.; Kim, S.H.; Kim, J.-H.; et al. Anti-Inflammatory and Antinociceptive Activities of Anthraquinone-2-Carboxylic Acid. Mediat. Inflamm. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- da Silva Junior, E.N.; de Souza, M.C.; Pinto, A.V.; Pinto Mdo, C.; Goulart, M.O.; Barros, F.W.; Pessoa, C.; Costa-Lotufo, L.V.; Montenegro, R.C.; de Moraes, M.O.; et al. Synthesis and potent antitumor activity of new arylamino derivatives of nor-beta-lapachone and nor-alpha-lapachone. Bioorg. Med. Chem. 2007, 15, 7035–7041. [Google Scholar] [CrossRef]
- Queiroz, M.L.; Valadares, M.C.; Torello, C.O.; Ramos, A.L.; Oliveira, A.B.; Rocha, F.D.; Arruda, V.A.; Accorci, W.R. Comparative studies of the effects of Tabebuia avellanedae bark extract and β-lapachone on the hematopoietic response of tumour-bearing mice. J. Ethnopharmacol. 2008, 117, 228–235. [Google Scholar] [CrossRef]
- Müller, K.; Sellmer, A.; Wiegrebe, W. Potential Antipsoriatic Agents: Lapacho Compounds as Potent Inhibitors of HaCaT Cell Growth. J. Nat. Prod. 1999, 62, 1134–1136. [Google Scholar] [CrossRef]
| Synonym Name | Remarks |
|---|---|
| Handroanthus impetiginosus (Mart. ex Dc.) Mattos | Accepted name |
| Tabebuia ipe var. integra (Sprague) Sandwith | One confidence level |
| Tecoma avellanedae var. alba Lillo | One confidence level |
| Tecoma ipe var. integra Sprague | One confidence level |
| Tecoma ipe var. integrifolia Hassl. | One confidence level |
| Tecoma ipe f. leucotricha Hassl. | One confidence level |
| Gelseminum avellanedae (Lorentz ex Griseb.) Kuntze | Three confidence levels |
| Handroanthus avellanedae (Lorentz ex Griseb.) Mattos | Three confidence levels |
| Tabebuia avellanedae Lorentz ex Griseb. | Three confidence levels |
| Tabebuia dugandii Standl. | Three confidence levels |
| Tabebuia impetiginosa (Mart. ex DC.) Standl. | Three confidence levels |
| Tabebuia nicaraguensis S.F. Blake | Three confidence levels |
| Tabebuia palmeri Rose | Three confidence levels |
| Tabebuia schunkevigoi D.R. Simpson | Three confidence levels |
| Tecoma adenophylla Bureau and K. Schum | Three confidence levels |
| Tecoma avellanedae (Lorentz ex Griseb.) Speg. | Three confidence levels |
| Tecoma impetiginosa Mart. ex DC. | Three confidence levels |
| Tecoma integra (Sprague) Hassl | Three confidence levels |
| Tecoma impetiginosa Mart | Invalid name |
| Species | Distribution |
|---|---|
| Tabebuia avellanedae Lorentz ex Griseb. | Argentina |
| Handroanthus avellanedae (Lorentz ex Griseb.) Mattos | Bolivia |
| Bignonia heptaphylla Vell. | Brazil |
| Handroanthus impetiginosus (Mart. ex DC.) Mattos | Bolivia, Brazil, Mexico |
| Gelseminum avellanedae (Lorentz ex Griseb.) Kuntze | Bolivia |
| Tabebuia avellanedae var. paulensis Toledo | Brazil |
| Tabebuia dugandii Standl. | Colombia |
| Tabebuia eximia (Miq.) Sandwith | Bolivia, Panama |
| Tabebuia heptaphylla (Vell.) Toledo | Argentina, Bolivia, Brazil, Paraguay |
| Tabebuia hypodictyon (A. DC.) Standl. | Bolivia, Panama |
| * Tabebuia ipe (Mart.) Standl. | Panama |
| Tabebuia ipe var. integra (Sprague) Sandwith | Bolivia, Paraguay |
| Tabebuia nicaraguensis S.F. Blake | Nicaragua |
| Tabebuia palmeri Rose | Costa Rica, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama |
| Tabebuia schunkevigoi D.R. Simpson | Peru |
| Tecoma adenophylla Bureau ex K. Schum. | Brazil |
| Tecoma avellanedae (Lorentz ex Griseb.) Speg. | Honduras |
| Tecoma avellanedae var. alba Lillo | Argentina |
| Tecoma eximia Miq. | Brazil |
| Tecoma hassleri Sprague | Paraguay |
| ! Tecoma heptaphylla (Vell.) Mart. | Panama |
| Tecoma hypodictyon A. DC. | Brazil |
| ** Tecoma impetiginosa Mart. | Panama |
| !Tecoma impetiginosa Mart. ex DC. | Brazil |
| Tecoma impetiginosa var. lepidota Bureau | Brazil |
| Tecoma integra (Sprague) Chodat | Bolivia, Panama |
| Tecoma ipe fo. leucotricha Hassl. | Paraguay |
| ** Tecoma ipe Mart. | Bolivia, Panama |
| Tecoma ipe var. integra Sprague | Paraguay |
| Tecoma ipe var. integrifolia Hassl. | Bolivia |
| Tecoma ochracea Cham. | Brazil |
| Pharmacological Activity | Extract/Isolated Compounds | Model | Concentration/Dose | Results | Ref. |
|---|---|---|---|---|---|
| Immunomodulatory | Water extract | RAW264.7 (murine macrophage cell), U937 (human promonocytic cell) | 50, 100, 200, and 400 μg/mL | Maintained cluster formation of RAW264.7 cells even after lipopolysaccharide (LPS) treatment. Downregulated the phagocytic uptake of FITC-labeled dextran. Upregulated cell-cell interactions by decreasing migration of cells and enhancing CD-29-mediated cell-cell adhesion and the surface levels of adhesion molecules and costimulatory molecules linked to macrophage stimulation, as seen in upregulation of reaction oxygen species (ROS) release. Suppressed an alteration in the membrane level of macrophages (phagocytic uptake and morphological changes). | [39] |
| Ethanol extract | IL-2-independent T-lymphocyte | 0.25, 0.5, 0.75, 0.9, and 1.0, mg/mL | Inhibited activation and proliferation of IL-2-independent T-lymphocyte | [40] | |
| Anti-inflammatory | Water extract | LPS-stimulated macrophages, arachidonic acid, or croton oil-induced mouse ear edema models | 0–400 μg/mL, 100–400 mg/kg | Inhibited the production of NO and PGE2 and suppressed the mRNA levels of COX-2 and iNOS. Curative effect in an in vivo PGE2-based inflammatory symptoms model induced by arachidonic acid. | [8] |
| Ethanol extract | TPA- or arachidonic acid-induced ear edema, hot plate, acetic acid-induced vascular permeability in rats | 100, 200, or 400 mg/kg | Inhibited inflammation of paw edema, ear inflammation, and dye leakage in the vasculature using various animal models including acetic acid-induced vascular permeability, 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced ear edema, arachidonic acid-induced mouse ear edema, and carrageenan-induced paw. | [28] | |
| Five novel compounds | Human myeloma THP-1 cells | 25 μM | Showed inhibitory activity on production of the inflammatory cytokines, such as TNF-α and IL-1β. | [41] | |
| Cyclopentene derivatives | RAW264.7 cells | 12.5, 25, 50 μg/mL | Suppressed the production of NO and PGE2. | [16] | |
| Anti-cancer | Naphthoquinones | MDA-BB-231, MCF7, and A549 cells | 0–30 μM | Inhibited growth of cancer cell lines and STAT3 phosphorylation activity. | [14] |
| Water extract | Estrogen receptor (ER)+ human mammary carcinoma MCF-7 cell line | 0.05, 0.125, 0.25, 0.5, 0.75, 1.5 mg/mL | Exhibited dose-dependent growth inhibition of MCF-7 cells. | [24] | |
| β-lapachone | A549 human lung carcinoma cells | Inhibited growth of A549 cells and telomerase activity; induced apoptosis by reducing the expression of Bcl-2, increasing the expression of Bax, and activating caspase-3 and caspase-9. | [13] | ||
| β-lapachone | HepG2 hepatoma cell line | Inhibited the activity of HepG2 by inducing apoptosis; downregulation of Bcl-2 and Bcl-XL, upregulation of Bax expression; induced apoptosis by activating caspase-3 and caspase-9 and degrading poly (ADP-ribose) polymerase protein. | [42] | ||
| Methanol extract | Human tumor cell lines MCF-7, NCI-H460, HeLa, and HepG2; porcine liver primary cells (PLP2). | GI50 values: 110.76 ± 5.33 µg/mL (MCF-7), 76.67 ± 7.09 µg/mL (NCI-H460), 93.18 ± 1.46 µg/mL (HeLa), 83.61 ± 6.61 µg/mL (HepG2), and >400 µg/mL (PLP2). | Showed cytotoxic effects on MCF-7, NCI-H460, HeLa, and HepG2 cells. | [4] | |
| Antinociceptive | Ethanol extract | Acetic acid-induced writhing response in rats | 100, 200, or 400 mg/kg | Increased the pain threshold in a mouse model when assessed through the hot plate test and inhibited the number of writhes compared to controls in the acetic acid-induced writhing responses mouse model. | [28] |
| Osteoarthritis | Ethanol extract | RAW264.7 cells and chondrosarcoma cell line (SW1353); monoiodoacetate (MIA)-induced osteoarthritis in rats | 75, 150, and 300 μg/mL | Showed a chondroprotective effect by preventing cartilage degradation through targeting of NF-κB and AP-1 signaling pathways in macrophage and chondrocyte cells. Downregulated MMP2, MMP9, and MMP13 in a PMA-induced, dose-dependent manner; no effect on the gene expression of COL2A1 and CHSY1. | [15] |
| Colitis | Water extract | RAW264.7 cells Dextran sulfate sodium (DSS)-induced colitis in mice | 100, 300, 900, and 2700 μg/mL 2 mg/day, a total of 5 days | Activated DC to produce immunosuppressive IL10; upregulated anti-inflammatory Th2 and Foxp3+ Treg cells in mesenteric lymph node (MLN) and downregulated pro-inflammatory Th1 and Th17 cells. By upregulating type II T-assisted immune response, weight loss and inflammation of colon tissue were downregulated in DSS-induced colitis mice. | [29] |
| Antioxidant | Methanol extract | EC50 values: 0.68 ± 0.03 (DPPH scavenging activity), 0.27 ± 0.01 (Reducing power), 0.23 ± 0.04 (β-carotene bleaching inhibition), 0.14 ± 0.01 (thiobarbituric acid Thiobarbituric acid reactive substances (TBARS) inhibition). | Showed the highest antioxidant activity, which may be related to its total phenol content. | [4] | |
| Methanol, butanol, and water extracts | H2O2-induced NIH3T3 cells | 0–2 mg/mL | Regenerated superoxide dismutase (SOD), catalase, and glucose 6-phosphate dehydrogenase activities; enhanced the concentration of glutathione in the cell; protected proteins from oxidative attack of H2O2, reduced formation of malondialdehyde in the cell, and protected NIH3T3 cells from H2O2-induced oxidative stress. | [43] | |
| Volatile constituents | 5, 10, 50, 100, and 500 μg/mL | Displayed dose-dependent activity in antioxidant assays | [37] | ||
| Phenylpropanoid glycosides | Compound 5 had the highest antioxidant activity, with an IC50 of 0.12 µM | Had inhibitory effects on cytochrome CYP3A4 enzyme | [18] | ||
| Anti-obesity | n-butanol extract | Ovariectomized (OVX) mice. 3T3-L1 cells | A total of 16 weeks | Preventing the accumulation of adipocyte in mice, weight loss and fat mass ↓ in ovariectomized mice. | [17] |
| Ethanol extract | Triton WR-1339-treated Wistar rats | A total of 24,700 kJ/kg energy | Decreased postprandial triglycerides in rats given a fatty meal. | [25] | |
| Anti-allergic | Five novel compounds | RBL-2H3 cells | 100 μM | Inhibited release of β-hexosaminidase of the allergy marker. | [41] |
| Antidepressant | Ethanol extract | Forced swimming test (FST) and tail suspension test (TST) in mice. | 100 mg/kg, p.o. (in the FST) and 10–300 mg/kg, p.o. (in the TST) | Produced antidepressant effects in the tail suspension test and forced swimming test. | [26] |
| Antiplatelet | Methanol extract | Rabbit platelets and cultured rat aortic vascular smooth muscle cells (VSMCs) | 10, 50, 100, and 200 μg/mL | Reduced platelet aggregation by inhibiting arachidonic acid release and ERK1/2 MAPK activation. | [30] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Hunto, S.T.; Yang, Y.; Lee, J.; Cho, J.Y. Tabebuia impetiginosa: A Comprehensive Review on Traditional Uses, Phytochemistry, and Immunopharmacological Properties. Molecules 2020, 25, 4294. https://doi.org/10.3390/molecules25184294
Zhang J, Hunto ST, Yang Y, Lee J, Cho JY. Tabebuia impetiginosa: A Comprehensive Review on Traditional Uses, Phytochemistry, and Immunopharmacological Properties. Molecules. 2020; 25(18):4294. https://doi.org/10.3390/molecules25184294
Chicago/Turabian StyleZhang, Jianmei, Stephanie Triseptya Hunto, Yoonyong Yang, Jongsung Lee, and Jae Youl Cho. 2020. "Tabebuia impetiginosa: A Comprehensive Review on Traditional Uses, Phytochemistry, and Immunopharmacological Properties" Molecules 25, no. 18: 4294. https://doi.org/10.3390/molecules25184294
APA StyleZhang, J., Hunto, S. T., Yang, Y., Lee, J., & Cho, J. Y. (2020). Tabebuia impetiginosa: A Comprehensive Review on Traditional Uses, Phytochemistry, and Immunopharmacological Properties. Molecules, 25(18), 4294. https://doi.org/10.3390/molecules25184294








