In Vitro Antimicrobial Activity of Isopimarane-Type Diterpenoids
Abstract
1. Introduction
2. Results and Discussion
2.1. Cytotoxicity Evaluation
2.2. Structure-Activity Modeling
3. Materials and Methods
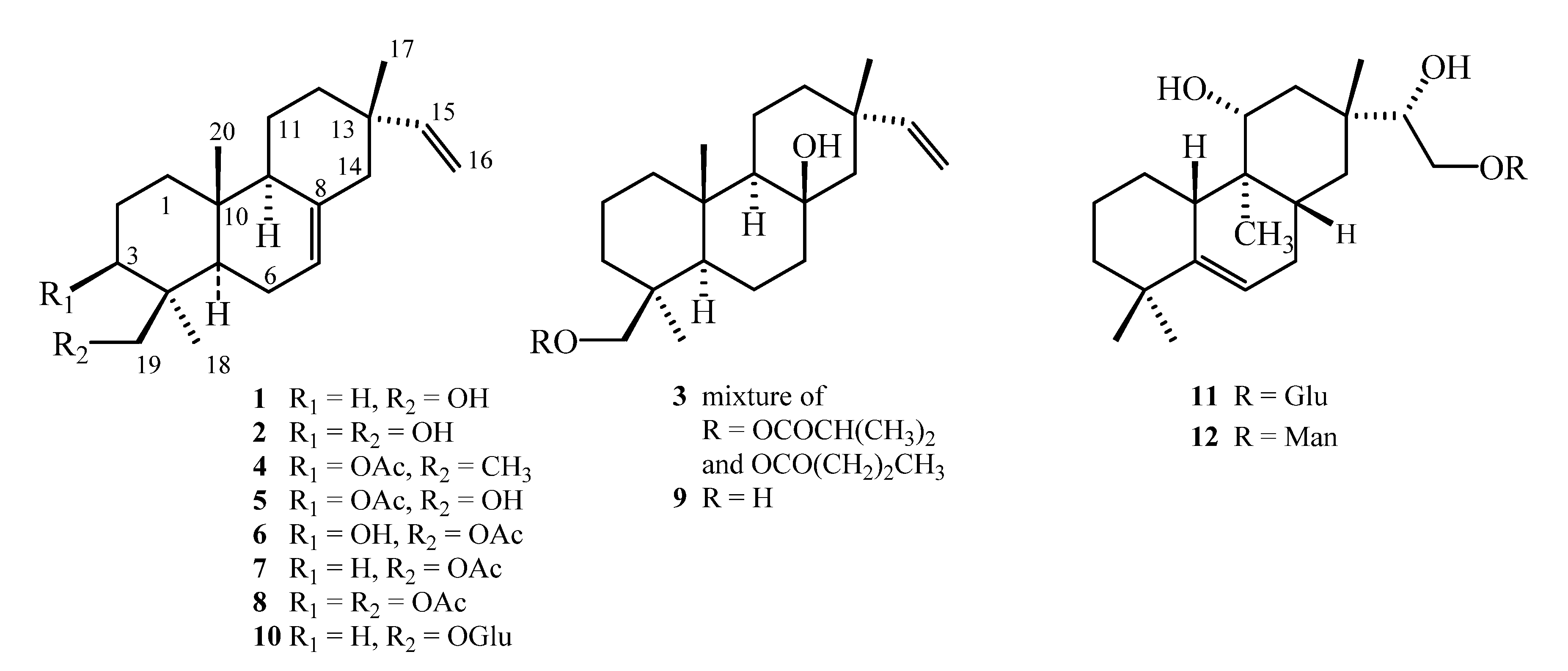
3.1. General Experimental Procedures
3.2. Compounds Tested
Preparation of Isopimarane Derivatives 7 and 8
3.3. General Procedure for Debenzoylation
3.4. Antimicrobial Assays
3.4.1. Microbial Strains
3.4.2. Microdilution Method
3.5. Cytotoxicity Assays
3.5.1. Cell Culture
3.5.2. Crystal Violet (CV) Staining Assay
3.6. Structure-Activity Modelling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization, Antimicrobial resistance. Available online: http://www.who.int/mediacentre/factsheets/fs194/en/ (accessed on 15 March 2020).
- Reddy, P.N.; Srirama, K.; Dirisala, V.R. An Update on Clinical Burden, Diagnostic Tools, and Therapeutic Options of Staphylococcus aureus. Infect. Dis. Res. Treat. 2017, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, Antimicrobial Resistance—Global Report on Surveillance. Available online: https://www.who.int/antimicrobial-resistance/publications/surveillancereport/en/ (accessed on 17 March 2020).
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Moloney, M.G. Natural Products as a Source for Novel Antibiotics. Trends Pharmacol. Sci. 2016, 37, 689–701. [Google Scholar] [CrossRef]
- Wu, Y.B.; Ni, Z.Y.; Shi, Q.W.; Dong, M.; Kiyota, H.; Gu, Y.C.; Cong, B. Constituents fromSalviaSpecies and Their Biological Activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, W.; Piao, H.; Xu, W.; Shi, H.; Zhao, C. The Genus Gnaphalium L. (Compositae): Phytochemical and Pharmacological Characteristics. Molecules 2013, 18, 8298–8318. [Google Scholar] [CrossRef]
- Rayanil, K.O.; Limpanawisut, S.; Tuntiwachwuttikul, P. Ent-pimarane and ent-trachylobane diterpenoids from Mitrephora alba and their cytotoxicity against three human cancer cell lines. Phytochemistry 2013, 89, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Qian, R.; Xiao, J.; Xu, N.; Fu, H.; Chen, Y. Kirenol, a compound from Herba Siegesbeckiae, induces apoptosis in human chronic myeloid leukemia K562 cells. Die Pharm. 2014, 69, 148–153. [Google Scholar]
- Cai, X.F.; Shen, G.; Dat, N.T.; Kang, O.H.; A Kim, J.; Lee, Y.M.; Lee, J.J.; Kim, Y. Inhibitory Effect of TNF-α and IL-8 Secretion by Pimarane-Type Diterpenoids from Acanthopanax koreanum. Chem. Pharm. Bull. 2003, 51, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Heras, B.D.L.; Hortelano, S. Molecular basis of the anti-inflammatory effects of terpenoids. Inflamm. Allergy -Drug Targets 2009, 8, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Zhou, Y.M.; Ye, Y.J.; Shang, X.M.; Cai, Y.L.; Xiong, C.; Wu, Y.X.; Xu, H.X. Topical anti-inflammatory and analgesic activity of kirenol isolated from Siegesbeckia orientalis. J. Ethnopharmacol. 2011, 137, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Cho, Y.S.; Oh, S.H.; Lee, S.; Min, B.S.; Moon, K.H.; Choi, J.S. Kinetics and molecular docking studies of pimarane-type diterpenes as protein tyrosine phosphatase (PTP1B) inhibitors from Aralia continentalis roots. Arch. Pharmacal Res. 2013, 36, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.C.; Simão, M.R.; Ambrósio, S.R.; Furtado, N.A.J.C.; Veneziani, R.C.S.; Heleno, V.C.G.; Da Costa, F.B.; Gomes, B.P.F.D.A.; Souza, M.G.M.; Dos Reis, E.B.; et al. Antimicrobial Activity of Diterpenes from Viguiera arenaria against Endodontic Bacteria. Molecules 2011, 16, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Porto, T.S.; Rangel, R.; Furtado, N.A.J.C.; De Carvalho, T.C.; Martins, C.H.G.; Veneziani, R.C.S.; Da Costa, F.B.; Vinholis, A.H.C.; Cunha, W.R.; Heleno, V.C.G.; et al. Pimarane-type Diterpenes: Antimicrobial Activity against Oral Pathogens. Molecules 2009, 14, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Porto, T.S.; Simão, M.R.; Carlos, L.Z.; Martins, C.H.G.; Furtado, N.A.J.C.; Said, S.; Arakawa, N.S.; Dos Santos, R.A.; Veneziani, R.C.S.; Ambrosio, S.R. Pimarane-type Diterpenes Obtained by Biotransformation: Antimicrobial Properties Against Clinically Isolated Gram-positive Multidrug-resistant Bacteria. Phytother. Res. 2012, 27, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Porto, T.S.; Furtado, N.A.J.C.; Heleno, V.C.G.; Martins, C.H.G.; Da Costa, F.B.; Severiano, M.E.; Silva, A.N.; Veneziani, R.C.S.; Ambrosio, S.R. Antimicrobial ent-pimarane diterpenes from Viguiera arenaria against Gram-positive bacteria. Fitoterapia 2009, 80, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Woldemichael, G.M.; Wächter, G.; Singh, M.P.; Maiese, W.M.; Timmermann, B.N. Antibacterial Diterpenes fromCalceolariapinifolia. J. Nat. Prod. 2003, 66, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Rijo, P.; Simões, M.F.; Duarte, A.; Rodríguez, B. Isopimarane diterpenoids from Aeollanthus rydingianus and their antimicrobial activity. Phytochemistry 2009, 70, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Uriel, C.; Rijo, P.; Fernandes, A.S.; Gómez, A.M.; Fraser-Reid, B.; Lopez, J.C. Methyl 1,2-Orthoesters in Acid-Washed Molecular Sieves Mediated Glycosylations. Chem. Sel. 2016, 1, 6011–6015. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Available online: https://clsi.org/standards/products/microbiology/documents/m07/ (accessed on 20 March 2020).
- Guerreiro, P.S.; Fernandes, A.S.; Costa, J.G.; Castro, M.; Miranda, J.P.; Oliveira, N.G. Differential effects of methoxyamine on doxorubicin cytotoxicity and genotoxicity in MDA-MB-231 human breast cancer cells. Mutat. Res. Toxicol. Environ. Mutagen. 2013, 757, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.S.; Gaspar, J.; Cabral, M.F.; Rueff, J.; Castro, M.; Batinic-Haberle, I.; Costa, J.; Oliveira, N.G. Protective role of ortho-substituted Mn(III) N-alkylpyridylporphyrins against the oxidative injury induced by tert-butylhydroperoxide. Free Radic. Res. 2010, 44, 430–440. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| S. aureus | E. faecalis | E. faecium | E. flavescens | E. hirae | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATCC 25923 | ATCC 43866 | ATCC 700699 | CIP 106760 | FFHB 29593 | ATCC 6538 | ATCC 51299 | CIP 106996 | FFHB 427483 | FFHB 435628 | ATCC 49996 | CIP 5855 | |
| 1 | 13.55 | 6.76 | 27.07 | 13.55 | 13.55 | 13.55 | 54.14 | 27.07 | 216.62 | >433.25 | 216.62 | 27.07 |
| 2 | 410.55 | 51.30 | >821.10 | 410.55 | 410.55 | 51.30 | >410.55 | >410.55 | nt | >410.55 | >410.55 | >410.55 |
| 3 | >676.71 | >676.71 | >676.71 | >676.71 | >676.71 | nt | >338.36 | nt | >338.36 | >338.36 | >338.36 | >338.36 |
| 4 | >725.61 | 181.40 | >725.61 | >725.61 | >725.61 | nt | 181.40 | nt | 181.40 | >362.80 | 181.40 | >362.80 |
| 5 | >721.38 | >721.38 | >721.38 | >721.38 | >721.38 | nt | >360.69 | nt | 180.34 | >360.69 | >360.69 | >360.69 |
| 6 | 45.07 | 22.54 | 45.07 | 22.54 | 22.54 | nt | 45.07 | nt | 22.54 | 45.07 | 45.07 | 45.07 |
| 7 | 378.15 | 11.83 | >756.29 | 378.15 | 378.15 | 189.07 | >378.15 | >378.15 | >378.15 | >378.15 | nt | >378.15 |
| 8 | 321.67 | 321.67 | >643.34 | 321.67 | 321.67 | 160.83 | >321.67 | >321.67 | >321.67 | >321.67 | nt | >321.67 |
| 9 | >815.69 | >815.69 | >815.69 | >815.69 | >815.69 | nt | >407.84 | nt | >407.84 | >407.84 | >407.84 | >407.84 |
| 10 | 138.68 | 277.36 | >554.72 | 277.36 | 277.36 | 138.68 | nt | >277.36 | nt | nt | nt | nt |
| 11 | 257.89 | 257.89 | 257.89 | 257.89 | 257.89 | 128.95 | >257.89 | >257.89 | >257.89 | 128.95 | nt | >257.89 |
| 12 | 257.89 | 257.89 | >515.78 | 257.89 | 257.89 | 128.95 | >257.89 | >257.89 | >257.89 | >257.89 | nt | >257.89 |
| Vancomycin | 1.35 | 2.70 | 5.39 | 2.70 | 2.70 | - | nt | 21.56 | 1.35 | 0.68 | 2.70 | 0.68 |
| Tetracycline | <1.10 | 281.26 | 140.63 | 70.31 | <1.10 | - | - | <1.10 | 70.31 | <1.10 | <1.10 | <1.10 |
| Ampicillin | <1.40 | >715.51 | <1.40 | >715.51 | 357.76 | - | - | <1.40 | <1.40 | >357.76 | <1.40 | <1.40 |
| Methicillin | 2.58 | 5.13 | 41.06 | >657.17 | >657.17 | - | - | - | - | - | - | - |
| DMSO | 3199.80 | 3199.80 | 3199.80 | 3199.80 | 3199.80 | - | - | 1599.90 | 1599.90 | 1599.90 | 1599.90 | 1599.90 |
| Compound | MW | nBM | RBN | MAXDP | ASP | Gu | nCp | LogMIC |
|---|---|---|---|---|---|---|---|---|
| 1 | 288.52 | 2 | 2.0 | 3.987 | 0.502 | 0.172 | 4 | 1.131 |
| 6 | 346.56 | 3 | 4.0 | 4.786 | 0.489 | 0.185 | 4 | 1.654 |
| 7 | 330.56 | 3 | 4.0 | 4.358 | 0.491 | 0.164 | 4 | 2.578 |
| 8 | 388.60 | 4 | 6.0 | 4.759 | 0.461 | 0.212 | 5 | 2.507 |
| 10 | 450.68 | 2 | 5.0 | 4.385 | 0.499 | 0.181 | 4 | 2.142 |
| 11/12 | 484.70 | 1 | 5.0 | 5.420 | 0.708 | 0.179 | 5 | 2.411 |
| r | 0.560 | 0.208 | 0.813 | 0.506 | 0.201 | 0.236 | 0.528 | |
| p-value | 0.248 | 0.692 | 0.049 | 0.306 | 0.703 | 0.653 | 0.281 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isca, V.M.S.; Andrade, J.; Fernandes, A.S.; Paixão, P.; Uriel, C.; Gómez, A.M.; Duarte, N.; Rijo, P. In Vitro Antimicrobial Activity of Isopimarane-Type Diterpenoids. Molecules 2020, 25, 4250. https://doi.org/10.3390/molecules25184250
Isca VMS, Andrade J, Fernandes AS, Paixão P, Uriel C, Gómez AM, Duarte N, Rijo P. In Vitro Antimicrobial Activity of Isopimarane-Type Diterpenoids. Molecules. 2020; 25(18):4250. https://doi.org/10.3390/molecules25184250
Chicago/Turabian StyleIsca, Vera M. S., Joana Andrade, Ana Sofia Fernandes, Paulo Paixão, Clara Uriel, Ana María Gómez, Noélia Duarte, and Patrícia Rijo. 2020. "In Vitro Antimicrobial Activity of Isopimarane-Type Diterpenoids" Molecules 25, no. 18: 4250. https://doi.org/10.3390/molecules25184250
APA StyleIsca, V. M. S., Andrade, J., Fernandes, A. S., Paixão, P., Uriel, C., Gómez, A. M., Duarte, N., & Rijo, P. (2020). In Vitro Antimicrobial Activity of Isopimarane-Type Diterpenoids. Molecules, 25(18), 4250. https://doi.org/10.3390/molecules25184250











