Water-Tree Resistability of UV-XLPE from Hydrophilicity of Auxiliary Crosslinkers
Abstract
1. Introduction
2. Results and Discussion
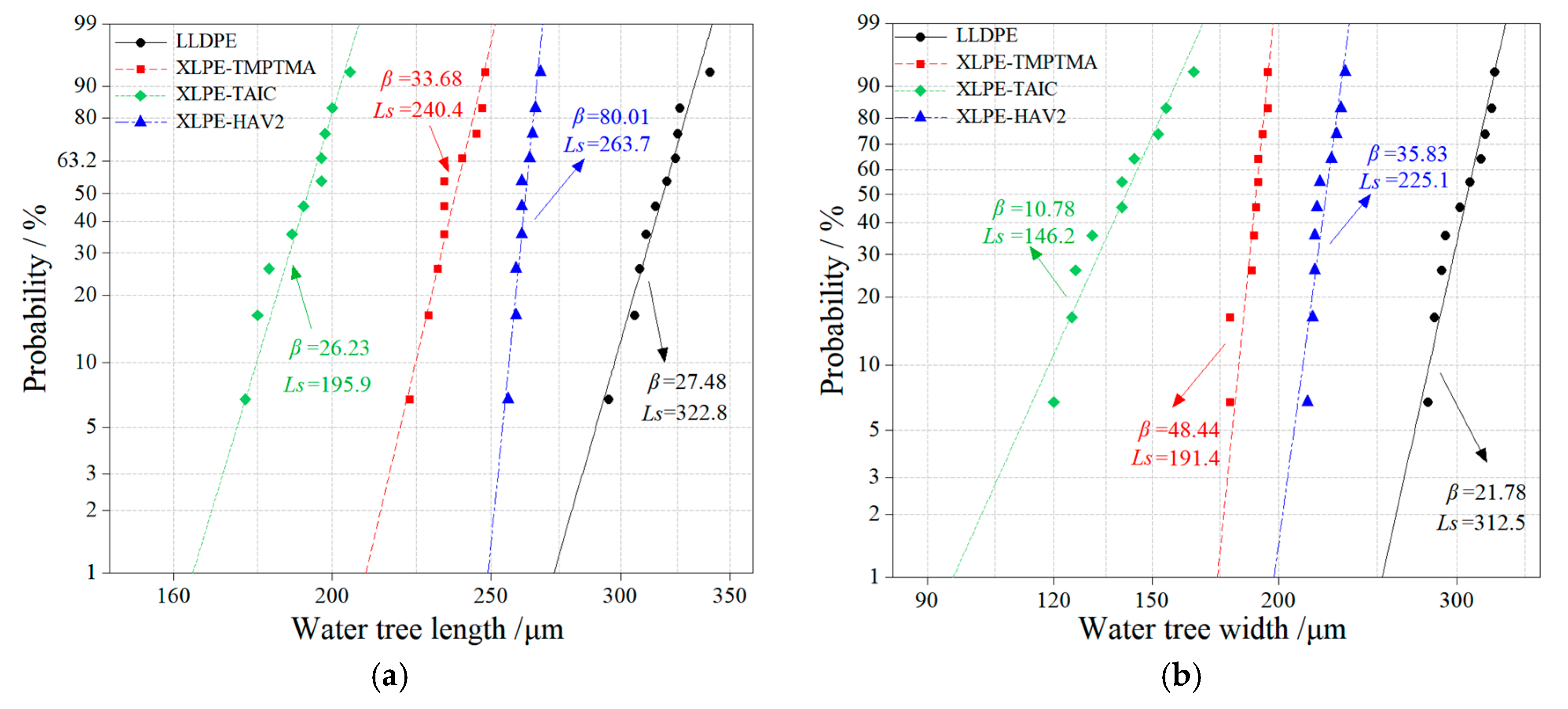
2.1. Crosslinking Degree and Water-Tree Morphology
2.2. Viscoelastic Properties
2.3. Stress–Strain Characteristics
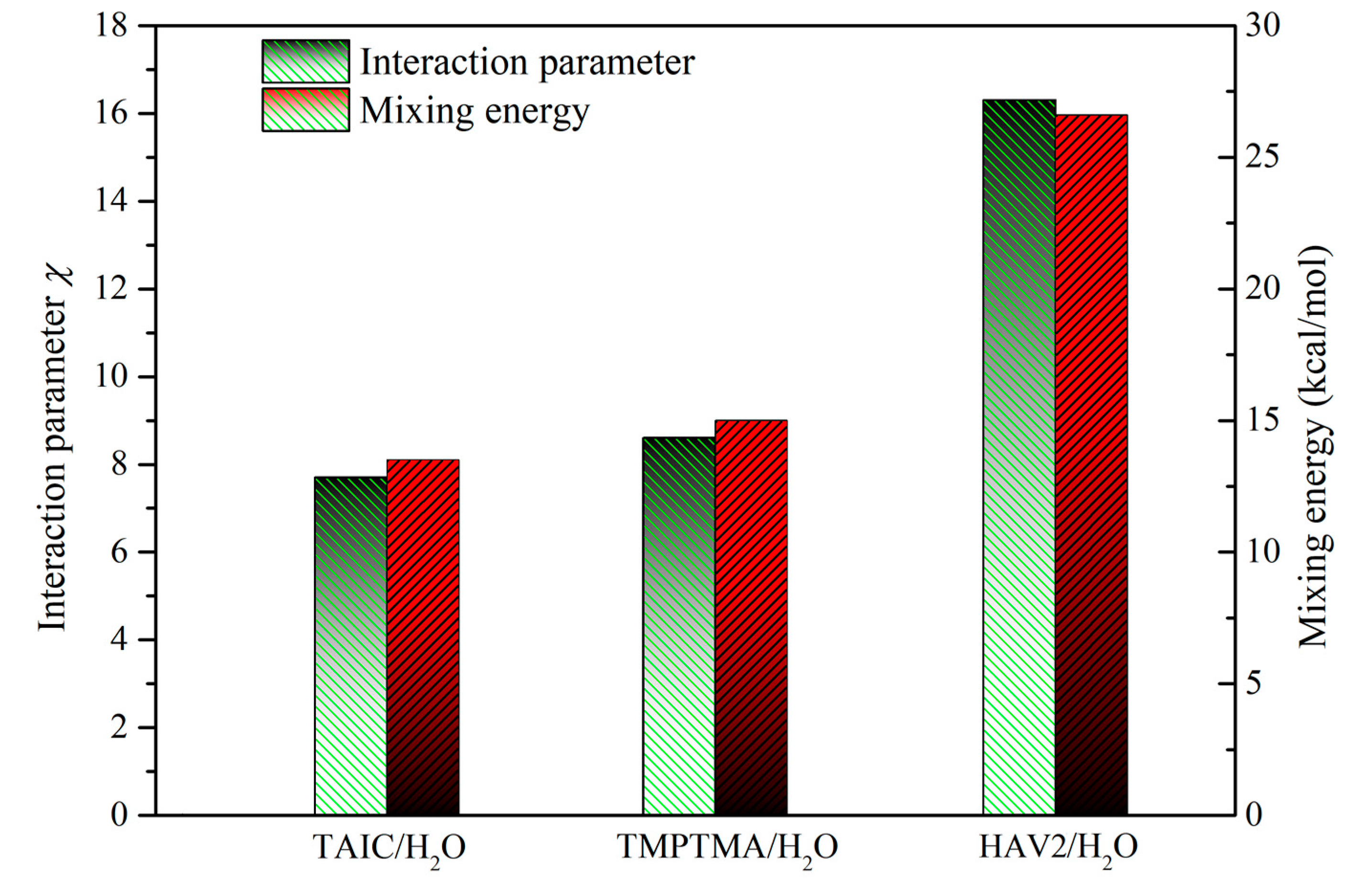
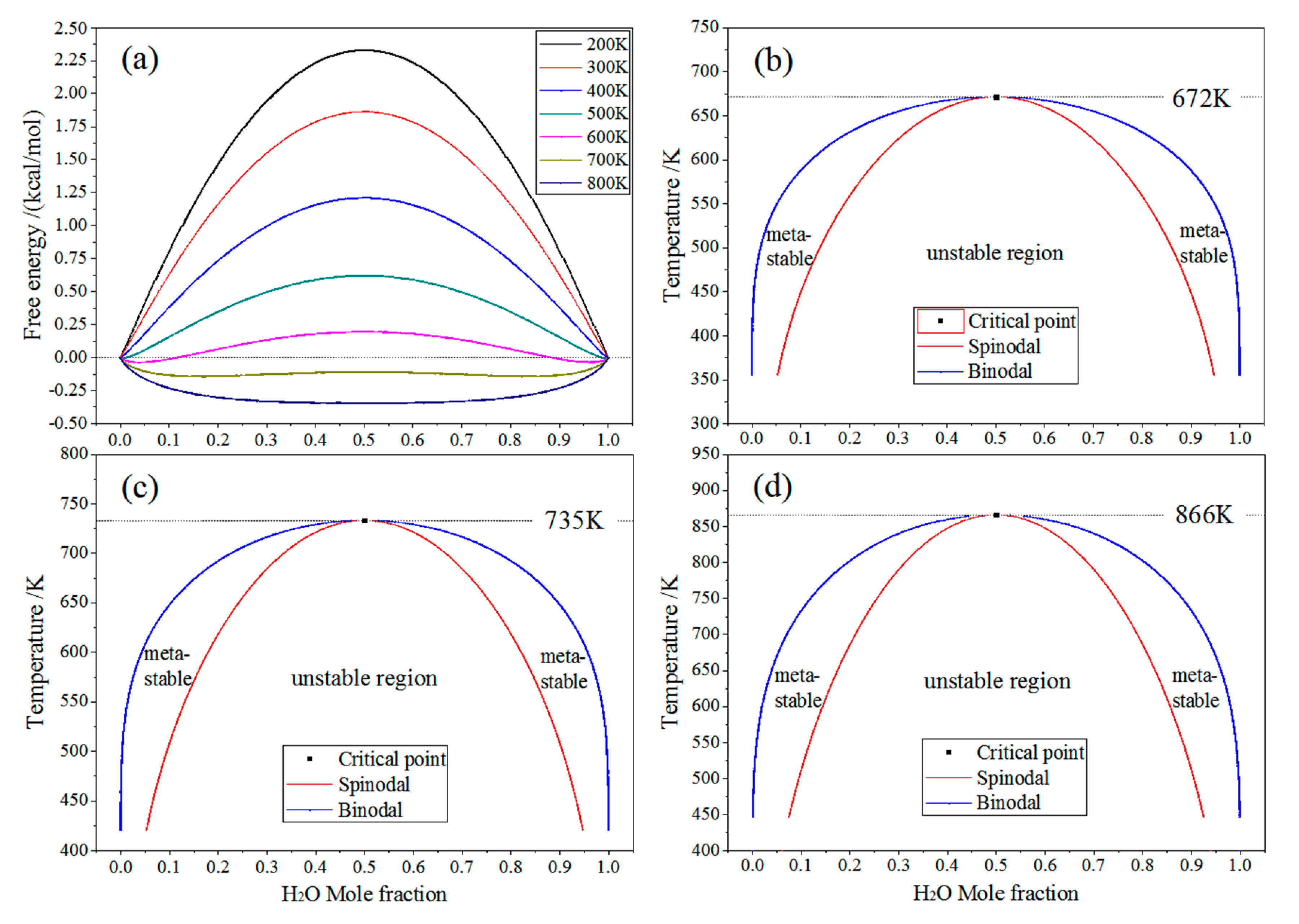
2.4. Miscibility of Crosslinker and Water
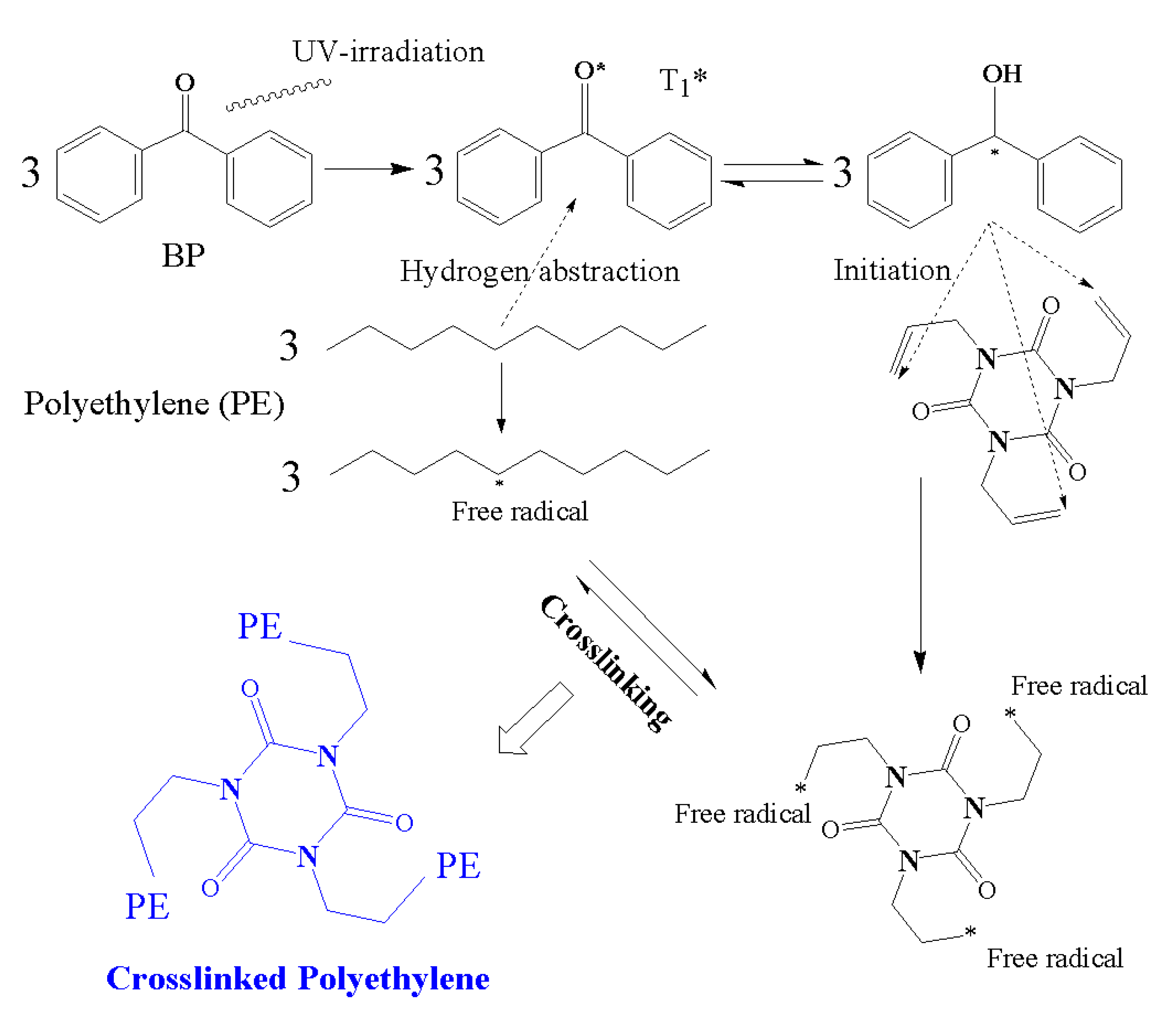
2.5. Initiation and Growth Mechanism of Water-Tree
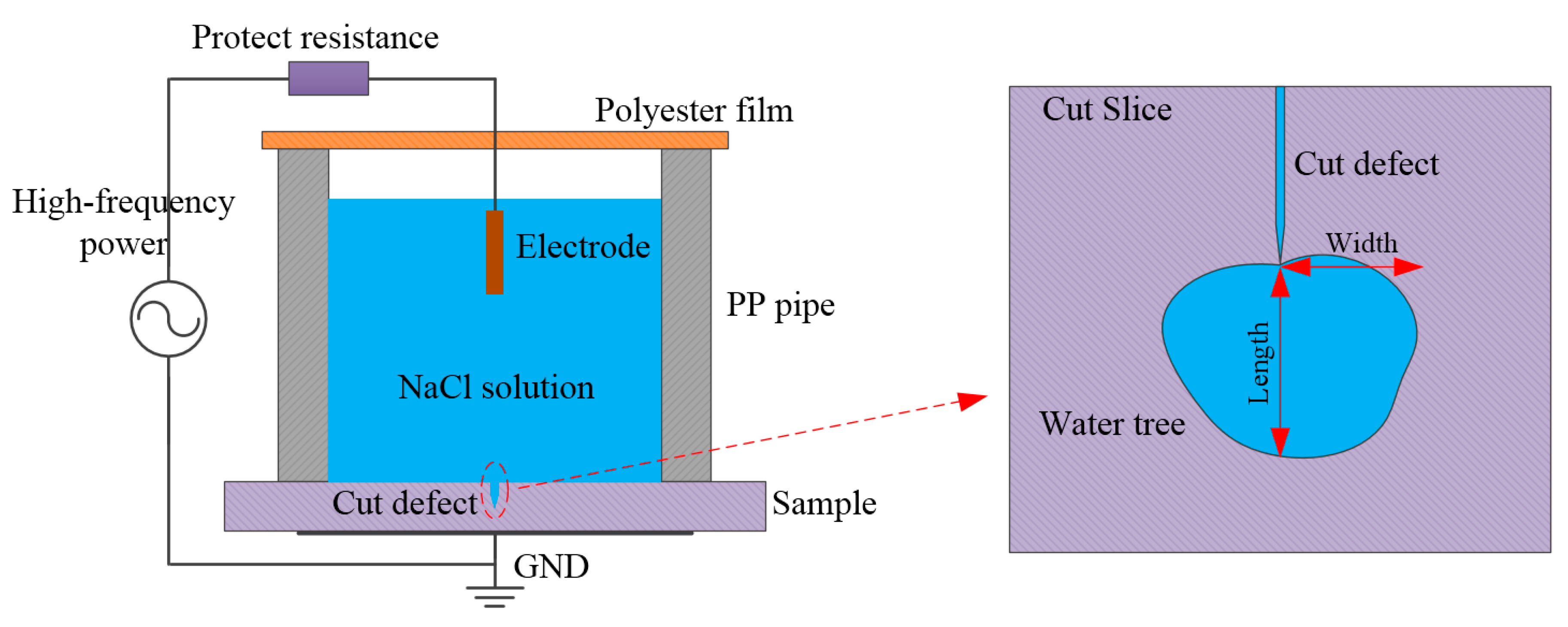
3. Experimental and Theoretical Schemes
3.1. Material Preparation
3.2. Accelerated Water-Tree Aging Experiment
3.3. Characterization and Measurement
3.4. Miscibility Calculation Method
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Zhao, J.; Zhao, W.; Zhong, L.; Hu, L.; Rao, W.; Zheng, M.; Meng, S. Influence of morphological variations on the AC breakdown of XLPE insulation in submarine cable factory joints. High Volt. 2019, 5, 69–75. [Google Scholar] [CrossRef]
- El-Zein, A.; Mohamed, K.; Talaat, M. Water trees in polyethylene insulated power cables: Approach to water trees initiation mechanism. Electr. Power Syst. Res. 2020, 180, 106158. [Google Scholar] [CrossRef]
- Kurihara, T.; Okamoto, T.; Hozumi, N.; Miyajima, K.; Uchida, K. Evaluation of relationship between residual charge signal and AC breakdown strength of water-tree degraded 22 to 77 kV classes XLPE cables removed from service using pulsed voltages. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 656–665. [Google Scholar] [CrossRef]
- Mugala, G.; Eriksson, R.; Pettersson, P. High frequency characteristics of water-tree degraded XLPE insulation in power cables. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 1271–1277. [Google Scholar] [CrossRef]
- Hvidsten, S.; Ildstad, E.; Holmgren, B.; Werelius, P. Correlation between AC breakdown strength and low frequency dielectric loss of water tree aged XLPE cables. IEEE Trans. Power Deliv. 1998, 13, 40–45. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, X.; Li, Y.; Wu, J. The influence of temperature on water treeing in polyethylene. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 544–551. [Google Scholar] [CrossRef]
- Al-Arainy, A.; Ahaideb, A.; Qureshi, M.; Malik, N. Statistical evaluation of water tree lengths in XLPE cables at different temperatures. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 995–1006. [Google Scholar] [CrossRef]
- Pelissou, S.; Lessard, G. Underground medium-voltage cable rejuvenation-part I: Laboratory tests on cables. IEEE Trans. Power Deliv. 2011, 26, 2324–2332. [Google Scholar] [CrossRef]
- Zhou, K.; Tao, X.; Wang, X.; Zhao, W.; Tao, W. Insight into the new role of titanium isopropoxide catalyst on rejuvenation for water tree aged cables. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 611–618. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, X.; Yang, J.; Jiang, P. Synergetic effects of silane-grafting and EVA on water tree resistance of LDPE. Chin. J. Polym. Sci. 2010, 28, 1–11. [Google Scholar] [CrossRef]
- Huang, X.; Liu, F.; Jiang, P. Effect of nanoparticle surface treatment on morphology, electrical and water treeing behavior of LLDPE composites. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 1697–1704. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, X.; Jiang, P. A comparative study of effects of SEBS and EPDM on the water tree resistance of cross-linked polyethylene. Polym. Degrad. Stab. 2010, 95, 1943–1949. [Google Scholar] [CrossRef]
- Ciuprina, F.; Teissedre, G.; Filippini, J.C.; Smedberg, A.; Campus, A.; Hampton, N. Chemical crosslinking of polyethylene and its effect on water tree initiation and propagation. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 709–715. [Google Scholar] [CrossRef]
- Crine, J.P. Electrical, chemical and mechanical processes in water treeing. IEEE Trans. Dielectr. Electr. Insul. 1998, 5, 681–694. [Google Scholar] [CrossRef]
- Wang, Z.; Marcolongo, P.; Lemberg, J.A.; Panganiban, B.; Evans, J.W.; Ritchie, R.O.; Wright, P.K. Mechanical fatigue as a mechanism of water tree propagation in TR-XLPE. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 321–330. [Google Scholar] [CrossRef]
- Li, K.; Zhou, K.; Zhu, G. Toward understanding the relationship between the microstructure and propagation behavior of water trees. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 1116–1124. [Google Scholar] [CrossRef]
- Ye, L.; Wu, Q.; Qu, B. Photocrosslinking and related properties of intumescent flame-retardant LLDPE/EVA/IFR blends. Polym. Adv. Technol. 2012, 23, 858–865. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, Y.; Li, M.; Zhao, H.; Wang, X.; Han, B. Theoretical study on the reaction mechanism in the UV radiation cross-linking process of polyethylene. RSC Adv. 2016, 6, 110831–110839. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Zhang, H.; Shang, Y.; Wang, X.; Han, B.; Li, Z. Theoretical study on the reaction of triallyl isocyanurate in the UV radiation cross-linking of polyethylene. RSC Adv. 2017, 7, 37095–37104. [Google Scholar] [CrossRef]
- Qu, B.; Shi, W.; Liang, R.; Jin, S.; Xu, Y.; Wang, Z.; Ranby, B. Phtocrosslinking of polyethylene for production of thin wall insulated wire. Polym. Eng. Sci. 1995, 35, 1005–1010. [Google Scholar] [CrossRef]
- Qiu, P.; Chen, J.; Sun, W.; Zhao, H. Improved dc dielectric performance of photon-initiated crosslinking polyethylene with TMPTMA auxiliary agent. Materials 2019, 12, 3540. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, W.; Zhao, H. Significantly improved electrical properties of photo-initiated auxiliary crosslinking EPDM used for cable termination. Polymers 2019, 11, 2083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, C.; Nie, L.; Jing, Z.; Han, B. Research on the water blade electrode method for assessing water tree resistance of cross-linked polyethylene. Polym. Test. 2016, 50, 235–240. [Google Scholar] [CrossRef]
- Sun, K.; Chen, J.; Zhao, H.; Sun, W.; Chen, Y.; Luo, Z. Dynamic thermomechanical analysis on water tree resistance of crosslinked polyethylene. Materials 2019, 12, 746. [Google Scholar] [CrossRef]
- Stadler, F.J. Dynamic-mechanical behavior of polyethylenes and ethene/alpha-olefin- copolymers: Part II. alpha- and beta-relaxation. Korean J. Chem. Eng. 2011, 28, 954–963. [Google Scholar] [CrossRef]
- Yash, P.K.; Edith, A.T.; Thomas, J.T.; Virgil, V.V.; Richard, F.A. Dynamic mechanical relaxations in polyethylene. Macromolecules 1985, 18, 1302–1309. [Google Scholar]
- Fakirow, S.; Krasteva, B. On the Glass Transition Temperature of Polyethylene as Revealed by Microhardness Measurements. J. Macromol. Sci. Part B 2000, 39, 297–301. [Google Scholar] [CrossRef]
- Colson, J.P. Alpha relaxation in polyethylene. J. Appl. Phys. 1971, 42, 5902–5903. [Google Scholar] [CrossRef]
- Celina, M.; George, G.A. Characterisation and degradation studies of peroxide and silane crosslinked polyethylene. Polym. Degrad. Stab. 1995, 48, 297–312. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Song, Y.; Zheng, Q. Influence of crosslinking on physical properties of low density polyethylene. Chin. J. Polym. Sci. 2012, 30, 837–844. [Google Scholar] [CrossRef]
- Blanco, M. Molecular silverware. I. General solutions to excluded volume constrained problems. J. Comput. Chem. 1991, 12, 237–247. [Google Scholar] [CrossRef]
- Fan, C.F.; Olafson, B.D.; Blanco, M.; Hsu, S.L. Application of molecular simulation to derive phase diagrams of binary mixtures. Macromolecules 1992, 25, 3667–3676. [Google Scholar] [CrossRef]
Sample Availability: Samples of the specific XLPE materials are available from the authors. |




| Samples | Thermal Elongation/% | Gel Content/% |
| XLPE-TMPTMA | 40 | 88 |
| XLPE-TAIC | 45 | 85 |
| XLPE-HAV2 | 30 | 91 |
| Samples | Loss Modulus/MPa | Loss Factor tanθ | Characteristic Length/μm | Characteristic Width/μm |
| LLDPE | 77 | 0.076 | 322.8 | 312.5 |
| XLPE-TMPTMA | 82 | 0.084 | 240.4 | 191.4 |
| XLPE-TAIC | 86 | 0.080 | 195.9 | 146.2 |
| XLPE-HAV2 | 89 | 0.089 | 263.7 | 225.1 |
| Abbreviation | TAIC | TMPTMA | HAV2 |
| Molecular structure | 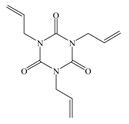 |  | 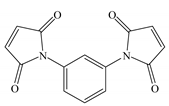 |
| Chemical formula | C12H15N3O3 | C18H26O6 | C14H8N2O4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-Q.; Wang, X.; Sun, W.-F.; Zhao, H. Water-Tree Resistability of UV-XLPE from Hydrophilicity of Auxiliary Crosslinkers. Molecules 2020, 25, 4147. https://doi.org/10.3390/molecules25184147
Chen J-Q, Wang X, Sun W-F, Zhao H. Water-Tree Resistability of UV-XLPE from Hydrophilicity of Auxiliary Crosslinkers. Molecules. 2020; 25(18):4147. https://doi.org/10.3390/molecules25184147
Chicago/Turabian StyleChen, Jun-Qi, Xuan Wang, Wei-Feng Sun, and Hong Zhao. 2020. "Water-Tree Resistability of UV-XLPE from Hydrophilicity of Auxiliary Crosslinkers" Molecules 25, no. 18: 4147. https://doi.org/10.3390/molecules25184147
APA StyleChen, J.-Q., Wang, X., Sun, W.-F., & Zhao, H. (2020). Water-Tree Resistability of UV-XLPE from Hydrophilicity of Auxiliary Crosslinkers. Molecules, 25(18), 4147. https://doi.org/10.3390/molecules25184147







