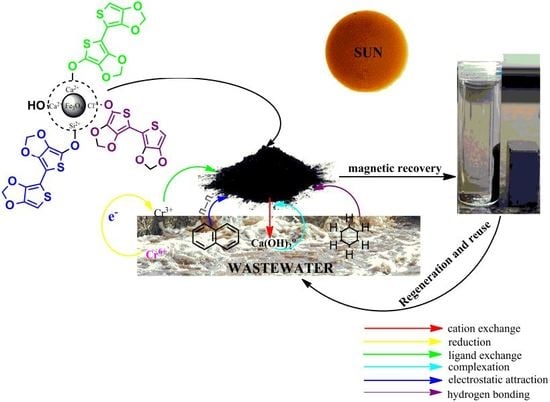
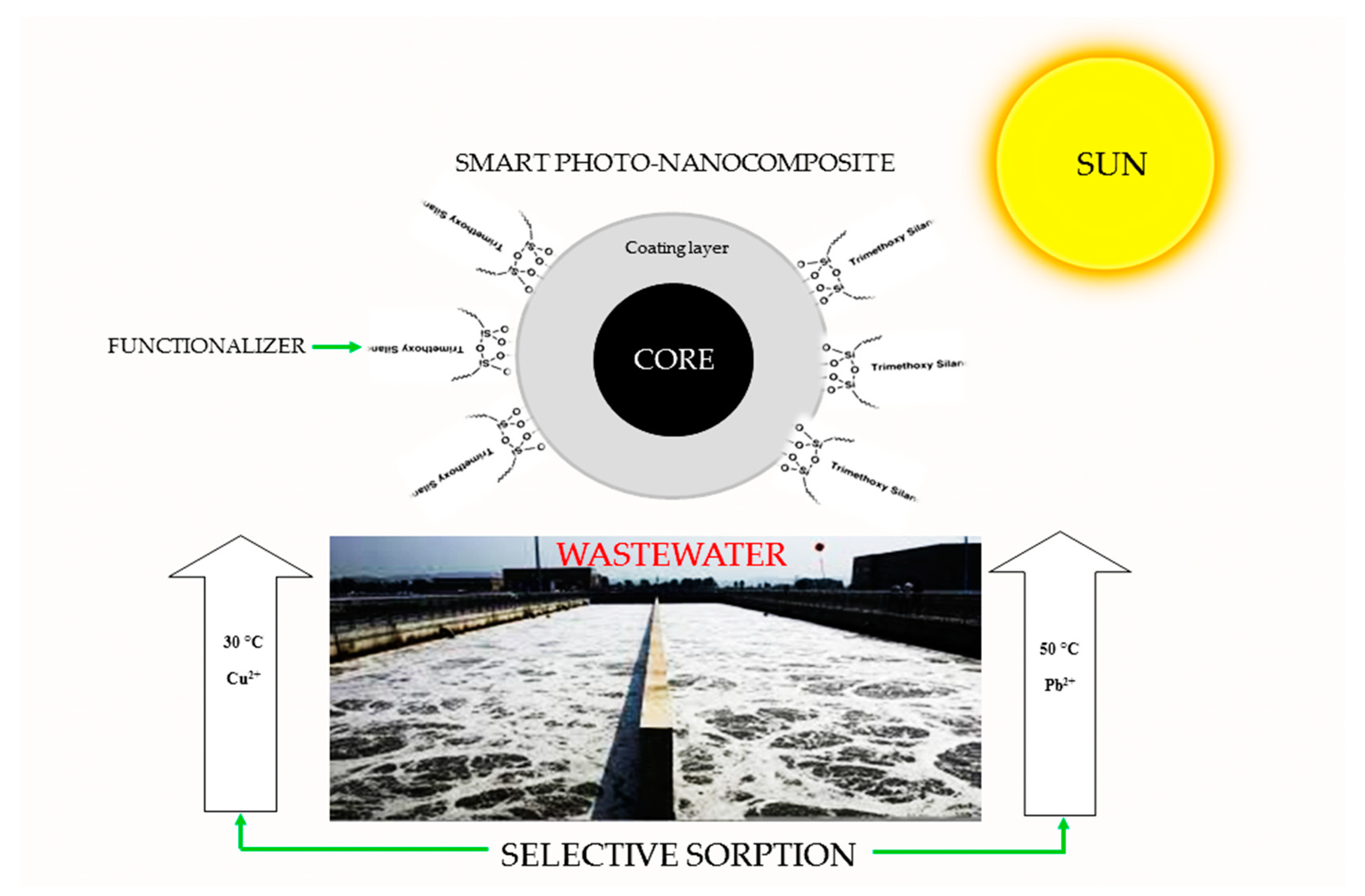
Multifunctional Magnetic Oxide Nanoparticle (MNP) Core-Shell: Review of Synthesis, Structural Studies and Application for Wastewater Treatment
Abstract
1. Introduction
1.1. Magnetic and Magnetic Oxide Nanocomposites
1.2. Smart Magnetic Oxide Nanocomposites for Wastewater Treatment
2. Literature Review
2.1. Preparation Methods for Magnetic Nanoparticles and Composites
2.2. Magnetic Cores for Magnetic Oxide Nanocomposites
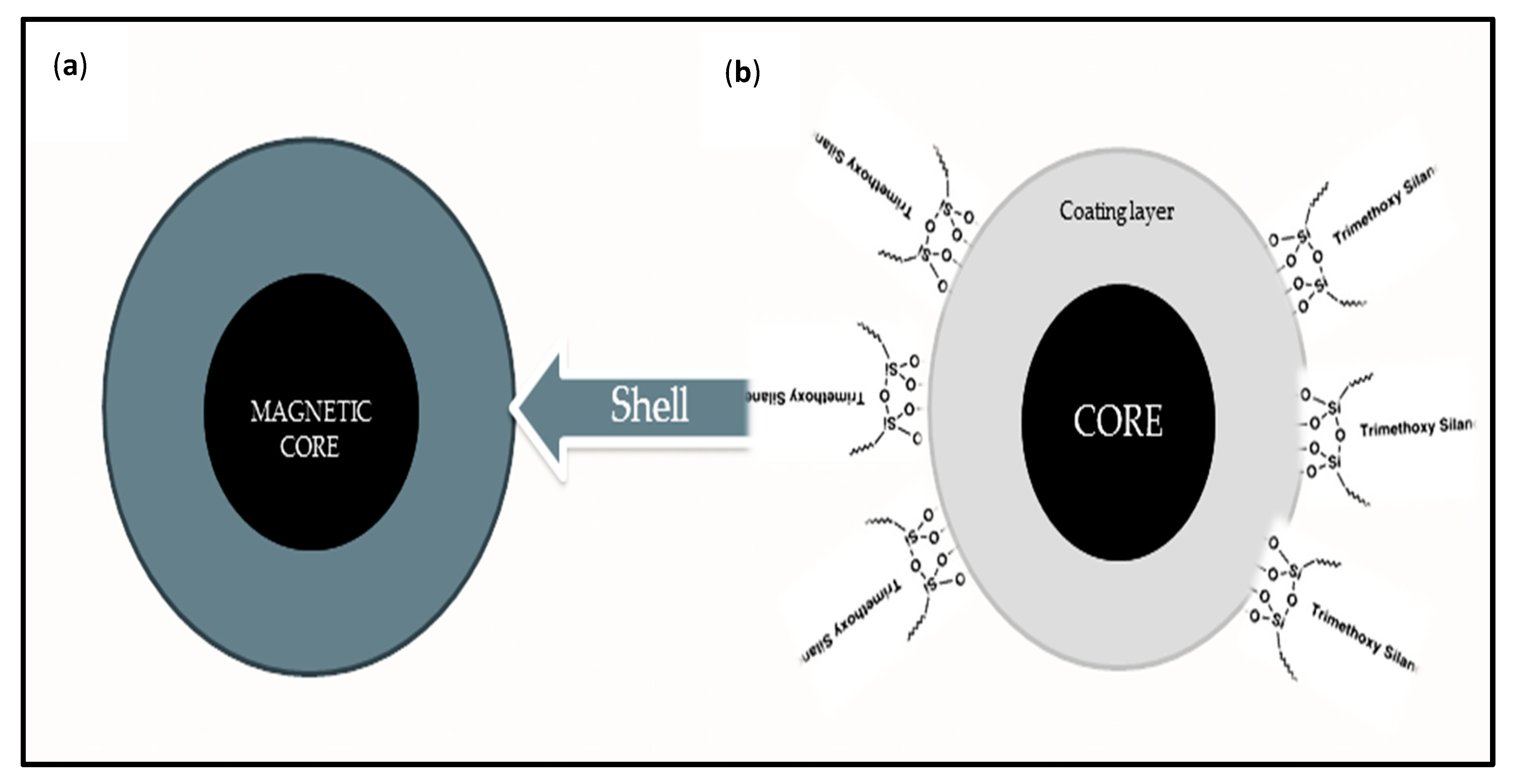
2.3. Protective or Coating Shells for Magnetic Oxide Nanoparticles
2.4. Functionalizers for Magnetic Oxide Nanocomposites
2.5. Magnetic Oxide Nanocomposites for Wastewater Treatment
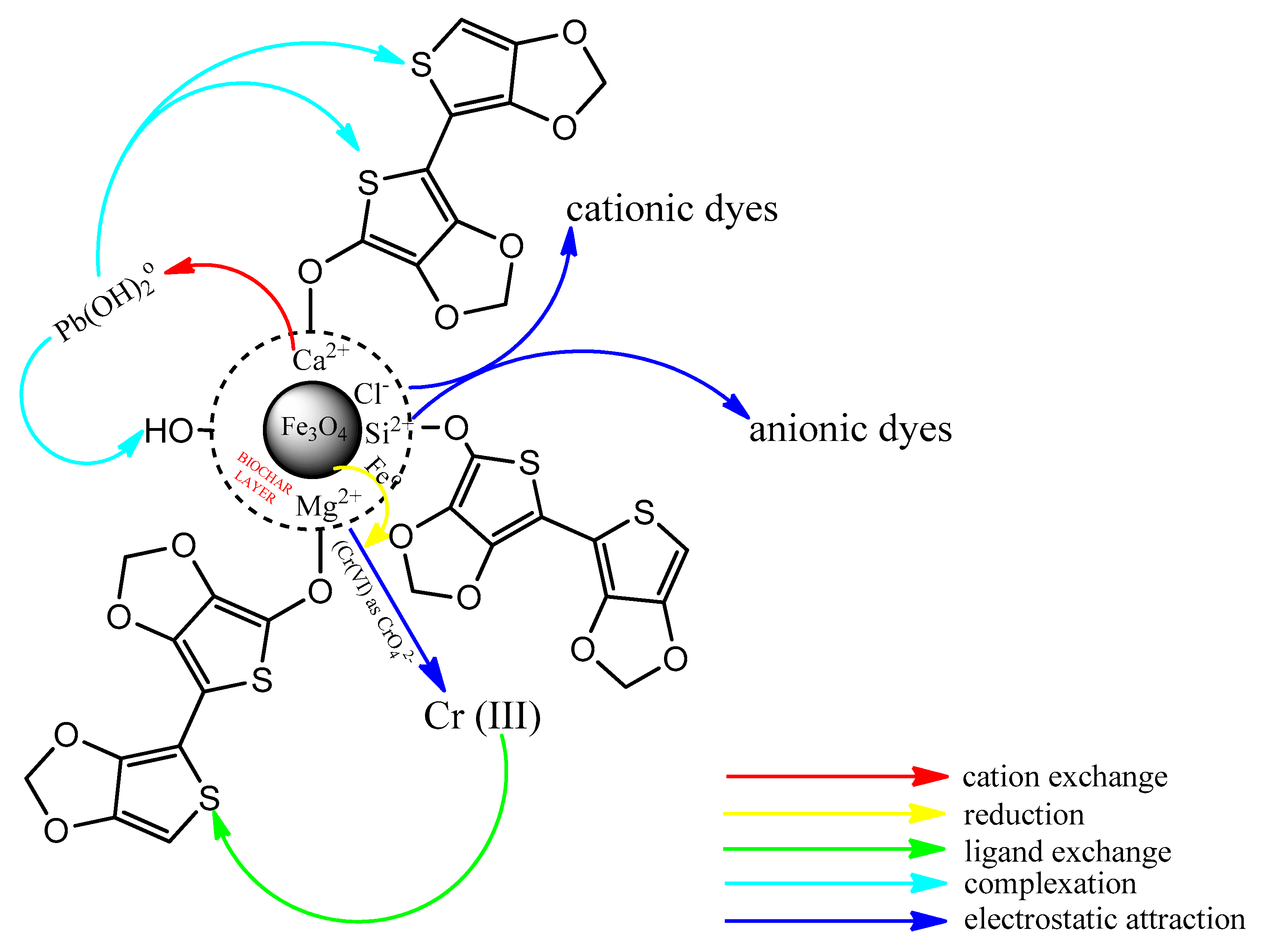
2.6. Mechanism of Pollutant Removal by Metal Oxide Nanocomposites
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Feynman, R.P. There’s plenty of room at the bottom. Eng. Sci. 1959, 23. [Google Scholar]
- Faraji, M.; Yamini, Y.; Rezaee, M. Magnetic nanoparticles: Synthesis, stabilization, functionalization, characterization, and applications. J. Iran. Chem. Soc. 2010, 7, 1–37. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Chen, M.; Gu, H.; Rapole, S.B.; Pallavkar, S.; Ho, T.C.; Hopper, J.; Guo, Z. Magnetic nanocomposites for environmental remediation. Adv. Powder Technol. 2013, 24, 459–467. [Google Scholar] [CrossRef]
- Kanchi, S. Nanotechnology for water treatment. J. Environ. Anal. Chem. 2014, 1, 1–3. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Shawon, Z.B.Z.; Rahman, M.T.; Hao, K.W.; Hidajat, K.; Uddin, M.S. Ionically modified magnetic nanomaterials for arsenic and chromium removal from water. Chem. Eng. J. 2013, 225, 607–615. [Google Scholar] [CrossRef]
- Dubey, S.; Banerjee, S.; Upadhyay, S.N.; Sharma, Y.C. Application of common nano-materials for removal of selected metallic species from water and wastewaters: A critical review. J. Mol. Liq. 2017, 240, 656–677. [Google Scholar] [CrossRef]
- Bitar, A.; Vega-Chacón, J.; Lgourna, Z.; Fessi, H.; Jafelicci Jr, M.; Elaissari, A. Submicron silica shell–magnetic core preparation and characterization. Colloids Surf. A Phys. Eng. Asp. 2018, 537, 318–324. [Google Scholar] [CrossRef]
- Li, K.; Cheng, D.; Yu, H.; Liu, Z. Process optimization and magnetic properties of soft magnetic composite cores based on phosphated and mixed resin coated Fe powders. J. Magn. Magn. Mater. 2020, 501, 166455. [Google Scholar] [CrossRef]
- Fu, Q.-T.; He, T.-T.; Ying, R.-J. Preparation and Properties of Magnetic Hematite Core/Alumina Shell Nanospheres. Destech Trans. Eng. Technol. Res. 2017. [Google Scholar] [CrossRef]
- Tenório-Neto, E.T.; Baraket, A.; Kabbaj, D.; Zine, N.; Errachid, A.; Fessi, H.; Kunita, M.H.; Elaissari, A. Submicron magnetic core conducting polypyrrole polymer shell: Preparation and characterization. Mater. Sci. Eng. C 2016, 61, 688–694. [Google Scholar] [CrossRef]
- Habila, M.A.; ALOthman, Z.A.; El-Toni, A.M.; Labis, J.P.; Khan, A.; Al-Marghany, A.; Elafifi, H.E. One-Step Carbon Coating and Polyacrylamide Functionalization of Fe3O4 Nanoparticles for Enhancing Magnetic Adsorptive-Remediation of Heavy Metals. Molecules 2017, 22, 2074. [Google Scholar] [CrossRef] [PubMed]
- Essandoh, M.; Garcia, R.A. Efficient removal of dyes from aqueous solutions using a novel hemoglobin/iron oxide composite. Chemosphere 2018, 206, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dief, A.M.; Abdel-Fatah, S.M. Development and functionalization of magnetic nanoparticles as powerful and green catalysts for organic synthesis. Beni-Seuf Univ. J. Appl. Sci. 2018, 7, 55–67. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Ala’a, H.; García-Peñas, A.; Mola, G.T.; Si, C.; Stadler, F.J. Bio-inspired and biomaterials-based hybrid photocatalysts for environmental detoxification: A review. Chem. Eng. J. 2019, 122937. [Google Scholar] [CrossRef]
- Aguer, J.-P.; Richard, C. Influence of the excitation wavelength on the photoinductive properties of humic substances. Chemosphere 1999, 38, 2293–2301. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P.; Singh, V.K. Wastewater treatment using low cost activated carbons derived from agricultural byproducts—A case study. J. Hazard. Mater. 2008, 152, 1045–1053. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.E.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Jeong, U.; Teng, X.; Wang, Y.; Yang, H.; Xia, Y. Superparamagnetic colloids: Controlled synthesis and niche applications. Adv. Mater. 2007, 19, 33–60. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ajibade, P.A. Green synthesis and characterization of magnetite (Fe3O4) nanoparticles using Chromolaena odorata root extract for smart nanocomposite. Mater. Lett. 2020, 263, 127145. [Google Scholar] [CrossRef]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Boskovic, L.; Altman, I.S.; Agranovski, I.E.; Braddock, R.D.; Myojo, T.; Choi, M. Influence of particle shape on filtration processes. Aerosol Sci. Technol. 2005, 39, 1184–1190. [Google Scholar] [CrossRef]
- Tran, N.; Webster, T.J. Magnetic nanoparticles: Biomedical applications and challenges. J. Mater. Chem. 2010, 20, 8760–8767. [Google Scholar] [CrossRef]
- Purbia, R.; Paria, S. Yolk/shell nanoparticles: Classifications, synthesis, properties, and applications. Nanoscale 2015, 7, 19789–19873. [Google Scholar] [CrossRef] [PubMed]
- Taka, A.L.; Pillay, K.; Mbianda, X.Y. Nanosponge cyclodextrin polyurethanes and their modification with nanomaterials for the removal of pollutants from waste water: A review. Carbohydr. Polym. 2017, 159, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Orha, C.; Pode, R.; Manea, F.; Lazau, C.; Bandas, C. Titanium dioxide-modified activated carbon for advanced drinking water treatment. Process Saf. Environ. Prot. 2017, 108, 26–33. [Google Scholar] [CrossRef]
- Lin, S.-H.; Teng, M.-Y.; Juang, R.-S. Adsorption of surfactants from water onto raw and HCl-activated clays in fixed beds. Desalination 2009, 249, 116–122. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Negm, N.A.; Hefni, H.H.; Wahab, M.M.A. Metal adsorption by agricultural biosorbents: Adsorption isotherm, kinetic and biosorbents chemical structures. Int. J. Biol. Macromol. 2015, 81, 400–409. [Google Scholar] [CrossRef]
- Samrat, M.V.N.; Rao, K.K.; SenGupta, A.K.; Riotte, J.; Mudakavi, J. Defluoridation of reject water from a reverse osmosis unit and synthetic water using adsorption. J. Water Process Eng. 2018, 23, 327–337. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Hussain, Y.A.; Al-Zoubi, H.; Batiha, M.; Hammouri, E. Hybrid precipitation-nanofiltration treatment of effluent pond water from phosphoric acid industry. Desalination 2017, 406, 88–97. [Google Scholar] [CrossRef]
- Mendow, G.; Grosso, C.I.; Sánchez, A.; Querini, C.A. Hybrid process for the purification of water contaminated with nitrites: Ion exchange plus catalytic reduction. Chem. Eng. Res. Des. 2017, 125, 348–360. [Google Scholar] [CrossRef]
- Gonzalez, A.; Grágeda, M.; Ushak, S. Assessment of pilot-scale water purification module with electrodialysis technology and solar energy. Appl. Energy 2017, 206, 1643–1652. [Google Scholar] [CrossRef]
- Chang, H.; Liu, B.; Liang, H.; Yu, H.; Shao, S.; Li, G. Effect of filtration mode and backwash water on hydraulically irreversible fouling of ultrafiltration membrane. Chemosphere 2017, 179, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; He, Q.; Li, J. Smart core/shell nanocomposites: Intelligent polymers modified gold nanoparticles. Adv. Colloid Interface Sci. 2009, 149, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Saber, O.; Kotb, H.M. Designing Dual-Function Nanostructures for Water Purification in Sunlight. Appl. Sci. 2020, 10, 1786. [Google Scholar] [CrossRef]
- Lai, C.; Huang, F.; Zeng, G.; Huang, D.; Qin, L.; Cheng, M.; Zhang, C.; Li, B.; Yi, H.; Liu, S. Fabrication of novel magnetic MnFe2O4/bio-char composite and heterogeneous photo-Fenton degradation of tetracycline in near neutral pH. Chemosphere 2019, 224, 910–921. [Google Scholar] [CrossRef]
- El Ghissassi, F.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L. A review of human carcinogens—Part D: Radiation. Lancet Oncol. 2009, 10, 751–752. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Shen, S.; Wu, D. Progress of recyclable magnetic particles for biomedical applications. J. Mater. Chem. B 2018, 6, 366–380. [Google Scholar] [CrossRef]
- Bohara, R.A.; Thorat, N.D.; Pawar, S.H. Role of functionalization: Strategies to explore potential nano-bio applications of magnetic nanoparticles. RSC Adv. 2016, 6, 43989–44012. [Google Scholar] [CrossRef]
- De Matteis, L.; Custardoy, L.; Fernández-Pacheco, R.; Magén, C.; de la Fuente, J.M.; Marquina, C.; Ibarra, M.R. Ultrathin MgO coating of superparamagnetic magnetite nanoparticles by combined coprecipitation and sol–gel synthesis. Chem. Mater. 2012, 24, 451–456. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuřitka, I.; Vilcakova, J.; Havlica, J.; Kalina, L.; Urbánek, P.; Machovsky, M.; Skoda, D.; Masař, M.; Holek, M. Sonochemical synthesis of Gd3+ doped CoFe2O4 spinel ferrite nanoparticles and its physical properties. Ultrason. Sonochem. 2018, 40, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zheng, C.; Zhang, F.; Yang, Y.; Wu, G.; Yu, A.; Guan, N. Size-controlled synthesis of magnetite (Fe3O4) nanoparticles coated with glucose and gluconic acid from a single Fe (III) precursor by a sucrose bifunctional hydrothermal method. J. Phys. Chem. C 2009, 113, 16002–16008. [Google Scholar] [CrossRef]
- Veintemillas-Verdaguer, S.; Morales, M.; Serna, C. Effect of the oxidation conditions on the maghemites produced by laser pyrolysis. Appl. Organomet. Chem. 2001, 15, 365–372. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Namvar, F.; Nadi, B.; Rahman, M.Z.A.; Amin, J. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [PubMed]
- Bibi, I.; Kamal, S.; Ahmed, A.; Iqbal, M.; Nouren, S.; Jilani, K.; Nazar, N.; Amir, M.; Abbas, A.; Ata, S. Nickel nanoparticle synthesis using Camellia Sinensis as reducing and capping agent: Growth mechanism and photo-catalytic activity evaluation. Int. J. Biol. Macromol. 2017, 103, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Kumar, S.R.; Paulpandi, M.; ManivelRaja, M.; Mangalaraj, D.; Viswanathan, C.; Kannan, S.; Ponpandian, N. An in vitro analysis of H1N1 viral inhibition using polymer coated superparamagnetic Fe3O4 nanoparticles. RSC Adv. 2014, 4, 13409–13418. [Google Scholar] [CrossRef]
- Liu, X.L.; Fan, H.M.; Yi, J.B.; Yang, Y.; Choo, E.S.G.; Xue, J.M.; Di Fan, D.; Ding, J. Optimization of surface coating on Fe3O4 nanoparticles for high performance magnetic hyperthermia agents. J. Mater. Chem. 2012, 22, 8235–8244. [Google Scholar] [CrossRef]
- Hernández-Hernández, A.A.; Álvarez-Romero, G.A.; Castañeda-Ovando, A.; Mendoza-Tolentino, Y.; Contreras-López, E.; Galán-Vidal, C.A.; Páez-Hernández, M.E. Optimization of microwave-solvothermal synthesis of Fe3O4 nanoparticles. Coating, modification, and characterization. Mater. Chem. Phys. 2018, 205, 113–119. [Google Scholar] [CrossRef]
- Lakshmanan, R.; Okoli, C.; Boutonnet, M.; Järås, S.; Rajarao, G.K. Microemulsion prepared magnetic nanoparticles for phosphate removal: Time efficient studies. J. Environ. Chem. Eng. 2014, 2, 185–189. [Google Scholar] [CrossRef]
- Vereda, F.; de Vicente, J.; Hidalgo-Alvarez, R. Oxidation of ferrous hydroxides with nitrate: A versatile method for the preparation of magnetic colloidal particles. J. Colloid Interface Sci. 2013, 392, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Schärtl, W. Current directions in core–shell nanoparticle design. Nanoscale 2010, 2, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Wahajuddin, S.A. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445. [Google Scholar] [CrossRef]
- Kumar, P.; Khanduri, H.; Pathak, S.; Singh, A.; Basheed, G.A.; Pant, R.P. Temperature selectivity for single phase hydrothermal synthesis of PEG-400 coated magnetite nanoparticle. Dalton Trans. 2020, 49, 8672–8683. [Google Scholar] [CrossRef]
- Dudric, R.; Souca, G.; Szatmári, Á.; Szilárd, T.; Nitica, S.; Iacovita, C.; Moldovan, A.I.; Stiufiuc, R.; Tetean, R.; Burzo, E. Magnetite Nanoparticles for Medical Applications. In Proceedings of the AIP Conference, Tamil Nadu, India, 21–22 February 2020. [Google Scholar]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room temperature co-precipitation synthesis of magnetite nanoparticles in a large pH window with different bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef] [PubMed]
- Takai, Z.I.; Mustafa, M.K.; Asman, S.; Sekak, K.A. Preparation and characterization of magnetite (Fe3O4) nanoparticles by sol-gel method. J. Hum. Dev. Commun. 2019, 12, 37–46. [Google Scholar]
- Abbas, M.; Takahashi, M.; Kim, C. Facile sonochemical synthesis of high-moment magnetite (Fe3O4) nanocube. J. Nanopart. Res. 2013, 15, 1354. [Google Scholar] [CrossRef]
- Vega-Chacón, J.; Picasso, G.; Avilés-Félix, L.; Jafelicci Jr, M. Influence of synthesis experimental parameters on the formation of magnetite nanoparticles prepared by polyol method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 015014. [Google Scholar] [CrossRef]
- Fajaroh, F.; Setyawan, H.; Widiyastuti, W.; Winardi, S. Synthesis of magnetite nanoparticles by surfactant-free electrochemical method in an aqueous system. Adv. Powder Technol. 2012, 23, 328–333. [Google Scholar] [CrossRef]
- Asab, G.; Zereffa, E.A.; Abdo Seghne, T. Synthesis of Silica-Coated Fe3O4 Nanoparticles by Microemulsion Method: Characterization and Evaluation of Antimicrobial Activity. Int. J. Biomater. 2020, 4783612. [Google Scholar] [CrossRef]
- Chanteau, B.; Fresnais, J.; Berret, J.-F. Electrosteric enhanced stability of functional sub-10 nm cerium and iron oxide particles in cell culture medium. Langmuir 2009, 25, 9064–9070. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, S.; Shao, Y.; Liu, J.; Xu, Z.; Zhu, D. Amino-functionalized Fe3O4@SiO2 core-shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J. Colloid Interface Sci. 2010, 349, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, Y.; Zhang, Z.; Shen, Y.; Zhao, M.; Pan, G. Study on the adsorption of Ca2+, Cd2+ and Pb2+ by magnetic Fe3O4 yeast treated with EDTA dianhydride. Chem. Eng. J. 2011, 168, 737–745. [Google Scholar] [CrossRef]
- Mao, G.-Y.; Yang, W.-J.; Bu, F.-X.; Jiang, D.-M.; Zhao, Z.-J.; Zhang, Q.-H.; Fang, Q.-C.; Jiang, J.-S. One-step hydrothermal synthesis of Fe3O4@ C nanoparticles with great performance in biomedicine. J. Mater. Chem. B 2014, 2, 4481–4488. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Ye, H.; Li, M.-M.; Zhao, B.-X. Efficient removal of cationic dyes from aqueous solution by polymer-modified magnetic nanoparticles. Chem. Eng. J. 2012, 198–199, 11–17. [Google Scholar] [CrossRef]
- Cheng, J.; Tan, G.; Li, W.; Li, J.; Wang, Z.; Jin, Y. Preparation, characterization and in vitro photodynamic therapy of a pyropheophorbide-a-conjugated Fe3O4 multifunctional magnetofluorescence photosensitizer. RSC Adv. 2016, 6, 37610–37620. [Google Scholar] [CrossRef]
- Cheng, J.; Tan, G.; Li, W.; Zhang, H.; Wu, X.; Wang, Z.; Jin, Y. Facile synthesis of chitosan assisted multifunctional magnetic Fe3O4@SiO2@CS@pyropheophorbide-a fluorescent nanoparticles for photodynamic therapy. New J. Chem. 2016, 40, 8522–8534. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, D.; Li, H.; Liu, G. Magnetically recoverable nanoparticles as efficient catalysts for organic transformations in aqueous medium. Green Chem. 2014, 16, 3401–3427. [Google Scholar] [CrossRef]
- Ge, F.; Li, M.-M.; Ye, H.; Zhao, B.-X. Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J. Hazard. Mater. 2012, 211–212, 366–372. [Google Scholar] [CrossRef]
- Rajput, S.; Singh, L.P.; Pittman Jr, C.U.; Mohan, D. Lead (Pb2+) and copper (Cu2+) remediation from water using superparamagnetic maghemite (γ-Fe2O3) nanoparticles synthesized by Flame Spray Pyrolysis (FSP). J. Colloid Interface Sci. 2017, 492, 176–190. [Google Scholar] [CrossRef]
- Wu, X.; Ding, Z.; Song, N.; Li, L.; Wang, W. Effect of the rare-earth substitution on the structural, magnetic and adsorption properties in cobalt ferrite nanoparticles. Ceram. Int. 2016, 42, 4246–4255. [Google Scholar] [CrossRef]
- Kim, E.-J.; Lee, C.-S.; Chang, Y.-Y.; Chang, Y.-S. Hierarchically structured manganese oxide-coated magnetic nanocomposites for the efficient removal of heavy metal ions from aqueous systems. ACS Appl. Mater. Interfaces 2013, 5, 9628–9634. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-F.; Zhao, Z.-s.; Jiang, G.-b. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Jang, J. Thiol containing polymer encapsulated magnetic nanoparticles as reusable and efficiently separable adsorbent for heavy metal ions. ChemComm 2007, 41, 4230–4232. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, D.; Sun, H.; Chen, J.; Wu, S.; Na, P. Synthesis, characterization, and adsorptive properties of magnetic cellulose nanocomposites for arsenic removal. Water Air Soil Pollut. 2014, 225, 1945. [Google Scholar] [CrossRef]
- Wanjeri, V.; Sheppard, C.; Prinsloo, A.; Ngila, J.; Ndungu, P. Isotherm and kinetic investigations on the adsorption of organophosphorus pesticides on graphene oxide based silica coated magnetic nanoparticles functionalized with 2-phenylethylamine. J. Environ. Chem. Eng. 2018, 6, 1333–1346. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhang, Y.; Zeng, T.; Jia, C.; Chang, L. Loading Cu-doped magnesium oxide onto surface of magnetic nanoparticles to prepare magnetic disinfectant with enhanced antibacterial activity. Colloids Surf. B 2018, 161, 433–441. [Google Scholar] [CrossRef]
- Ali, A.; Hira Zafar, M.Z.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49. [Google Scholar] [CrossRef]
- Abdulla-Al-Mamun, M.; Kusumoto, Y.; Zannat, T.; Horie, Y.; Manaka, H. Au-ultrathin functionalized core-shell (Fe3O4@Au) monodispersed nanocubes for a combination of magnetic/plasmonic photothermal cancer cell killing. RSC Adv. 2013, 3, 7816–7827. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397. [Google Scholar] [CrossRef]
- Nidhin, M.; Indumathy, R.; Sreeram, K.; Nair, B.U. Synthesis of iron oxide nanoparticles of narrow size distribution on polysaccharide templates. Bull. Mater. Sci. 2008, 31, 93–96. [Google Scholar] [CrossRef]
- Chen, S.; Feng, J.; Guo, X.; Hong, J.; Ding, W. One-step wet chemistry for preparation of magnetite nanorods. Mater. Lett. 2005, 59, 985–988. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Lo, I.M. Removal and recovery of Cr (VI) from wastewater by maghemite nanoparticles. Water Res. 2005, 39, 4528–4536. [Google Scholar] [CrossRef] [PubMed]
- Aivazoglou, E.; Metaxa, E.; Hristoforou, E. Microwave-assisted synthesis of iron oxide nanoparticles in biocompatible organic environment. AIP Adv. 2018, 8, 048201. [Google Scholar] [CrossRef]
- Parveen, S.; Wani, A.H.; Shah, M.A.; Devi, H.S.; Bhat, M.Y.; Koka, J.A. Preparation, characterization and antifungal activity of iron oxide nanoparticles. Microb. Pathog. 2018, 115, 287–292. [Google Scholar] [CrossRef]
- Mandel, K.; Hutter, F.; Gellermann, C.; Sextl, G. Modified superparamagnetic nanocomposite microparticles for highly selective HgII or CuII separation and recovery from aqueous solutions. ACS Appl. Mater. Interfaces 2012, 4, 5633–5642. [Google Scholar] [CrossRef]
- Brown, P.; Hope-Weeks, L.J. The synthesis and characterization of zinc ferrite aerogels prepared by epoxide addition. J. Solgel Sci. Technol. 2009, 51, 238–243. [Google Scholar] [CrossRef]
- Osborne, E.A.; Atkins, T.M.; Gilbert, D.A.; Kauzlarich, S.M.; Liu, K.; Louie, A.Y. Rapid microwave-assisted synthesis of dextran-coated iron oxide nanoparticles for magnetic resonance imaging. Nanotechnology 2012, 23, 215602. [Google Scholar] [CrossRef]
- Tsuji, M.; Hashimoto, M.; Nishizawa, Y.; Kubokawa, M.; Tsuji, T. Microwave-assisted synthesis of metallic nanostructures in solution. Chem. Eur. J. 2005, 11, 440–452. [Google Scholar] [CrossRef]
- Cao, W.; Chen, Z.; Gao, T.; Zhou, D.; Leng, X.; Niu, F.; Zhu, Y.; Qin, L.; Wang, J.; Huang, Y. Rapid synthesis of single-phase bismuth ferrite by microwave-assisted hydrothermal method. Mater. Chem. Phys. 2016, 175, 1–5. [Google Scholar] [CrossRef]
- Grindi, B.; Beji, Z.; Viau, G.; BenAli, A. Microwave-assisted synthesis and magnetic properties of M-SrFe12O19 nanoparticles. J. Magn. Magn. Mater. 2018, 449, 119–126. [Google Scholar] [CrossRef]
- Kombaiah, K.; Vijaya, J.J.; Kennedy, L.J.; Bououdina, M.; Ramalingam, R.J.; Al-Lohedan, H.A. Okra extract-assisted green synthesis of CoFe2O4 nanoparticles and their optical, magnetic, and antimicrobial properties. Mater. Chem. Phys. 2018, 204, 410–419. [Google Scholar] [CrossRef]
- Jarlborg, T.; Peter, M. Electronic structure, magnetism and Curie temperatures in Fe, Co and Ni. J. Magn. Magn. Mater. 1984, 42, 89–99. [Google Scholar] [CrossRef]
- Haw, C.; Chiu, W.; Rahman, S.A.; Khiew, P.; Radiman, S.; Shukor, R.A.; Hamid, M.A.A.; Ghazali, N. The design of new magnetic-photocatalyst nanocomposites (CoFe2O4–TiO2) as smart nanomaterials for recyclable-photocatalysis applications. New J. Chem. 2016, 40, 1124–1136. [Google Scholar] [CrossRef]
- Mahdavian, A.R.; Mirrahimi, M.A.-S. Efficient separation of heavy metal cations by anchoring polyacrylic acid on superparamagnetic magnetite nanoparticles through surface modification. Chem. Eng. J. 2010, 159, 264–271. [Google Scholar] [CrossRef]
- Mattila, P.; Heinonen, H.; Loimula, K.; Forsman, J.; Johansson, L.-S.; Tapper, U.; Mahlberg, R.; Hentze, H.-P.; Auvinen, A.; Jokiniemi, J. Scalable synthesis and functionalization of cobalt nanoparticles for versatile magnetic separation and metal adsorption. J. Nanopart. Res. 2014, 16, 2606. [Google Scholar] [CrossRef]
- Rao, K.S.; Rao, P.A.; Varma, M.C.; Rao, K. Development of least sized magnetic nanoparticles with high saturation magnetization. J. Alloy. Compd. 2018, 750, 838–847. [Google Scholar] [CrossRef]
- Santos, C.; Pereira, L.; Carmezim, M. Reduced graphene oxide nanoplatform loaded with nickel-cobalt oxide nanoparticles: Controllable synthesis and physical chemical properties. Mater. Des. 2018, 142, 66–73. [Google Scholar] [CrossRef]
- Zou, J.; Peng, Y.-G.; Tang, Y.-Y. A facile bi-phase synthesis of Fe3O4@ SiO2 core–shell nanoparticles with tunable film thicknesses. RSC Adv. 2014, 4, 9693–9700. [Google Scholar] [CrossRef]
- Kong, L.; Lu, X.; Bian, X.; Zhang, W.; Wang, C. Constructing carbon-coated Fe3O4 microspheres as antiacid and magnetic support for palladium nanoparticles for catalytic applications. ACS Appl. Mater. Interfaces 2010, 3, 35–42. [Google Scholar] [CrossRef]
- Yang, D.; Hu, J.; Fu, S. Controlled synthesis of magnetite-silica nanocomposites via a Seeded Sol-gel approach. J. Phys. Chem. C 2009, 113, 7646–7651. [Google Scholar] [CrossRef]
- Im, S.H.; Herricks, T.; Lee, Y.T.; Xia, Y. Synthesis and characterization of monodisperse silica colloids loaded with superparamagnetic iron oxide nanoparticles. Chem. Phys. Lett. 2005, 401, 19–23. [Google Scholar] [CrossRef]
- Guivar, J.A.R.; Bustamante, A.; Gonzalez, J.; Sanches, E.A.; Morales, M.; Raez, J.M.; López-Muñoz, M.-J.; Arencibia, A. Adsorption of arsenite and arsenate on binary and ternary magnetic nanocomposites with high iron oxide content. Appl. Surf. Sci. 2018, 454, 87–100. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Kuwano, N.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Lee, K.X. Green synthesis of magnetite (Fe3O4) nanoparticles using seaweed (Kappaphycus alvarezii) extract. Nanoscale Res. Lett. 2016, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Kotal, A.; Mandal, T.K.; Giri, S.; Nakamura, H.; Kohara, T. Size-controlled synthesis of magnetite nanoparticles in the presence of polyelectrolytes. Chem. Mater. 2004, 16, 3489–3496. [Google Scholar] [CrossRef]
- Dozier, D.; Palchoudhury, S.; Bao, Y. Synthesis of iron oxide nanoparticles with biological coatings. J. Sci. Health Univ Ala. 2010, 7, 16–18. [Google Scholar]
- Cai, Z.; Dwivedi, A.D.; Lee, W.-N.; Zhao, X.; Liu, W.; Sillanpää, M.; Zhao, D.; Huang, C.-H.; Fu, J. Application of nanotechnologies for removing pharmaceutically active compounds from water: Development and future trends. Environ. Sci. Nano 2018, 5, 27–47. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar]
- Wibowo, J.A.; Djaja, N.F.; Saleh, R. Cu-and Ni-Doping effect on structure and magnetic properties of Fe-doped ZnO nanoparticles. Adv. Mater. Phys. Chem. 2013, 3, 48. [Google Scholar] [CrossRef]
- Shakir, M.; Faraz, M.; Sherwani, M.A.; Al-Resayes, S.I. Photocatalytic degradation of the Paracetamol drug using Lanthanum doped ZnO nanoparticles and their in-vitro cytotoxicity assay. J. Lumin. 2016, 176, 159–167. [Google Scholar] [CrossRef]
- Razieh, S. A Simple Method for Preparation of Nanosized ZnO. Res. J. Chem. Sci. 2016, 5, 45–49. [Google Scholar]
- Ghorbani, H.R.; Mehr, F.P.; Pazoki, H.; Rahmani, B.M. Synthesis of ZnO Nanoparticles by Precipitation Method. Orient. J. Chem. 2015, 31, 1219–1221. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energ. Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Sopian, K. Visible light photocatalytic activity of Fe3+-doped ZnO nanoparticle prepared via sol-gel technique. Chemosphere 2013, 91, 1604–1611. [Google Scholar] [CrossRef]
- Shoueir, K.; El-Sheshtawy, H.; Misbah, M.; El-Hosainy, H.; El-Mehasseb, I.; El-Kemary, M. Fenton-like nanocatalyst for photodegradation of methylene blue under visible light activated by hybrid green DNSA@ Chitosan@ MnFe2O4. Carbohydr. Polym. 2018, 197, 17–28. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated titanates and TiO2 nanostructured materials: Synthesis, properties, and applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Cai, Z.; Zhao, X.; Wang, T.; Liu, W.; Zhao, D. Reusable platinum-deposited anatase/hexa-titanate nanotubes: Roles of reduced and oxidized platinum on enhanced solar-light-driven photocatalytic activity. ACS Sustain. Chem. Eng. 2016, 5, 547–555. [Google Scholar] [CrossRef]
- Murgolo, S.; Petronella, F.; Ciannarella, R.; Comparelli, R.; Agostiano, A.; Curri, M.; Mascolo, G. UV and solar-based photocatalytic degradation of organic pollutants by nano-sized TiO2 grown on carbon nanotubes. Catal. Today 2015, 240, 114–124. [Google Scholar] [CrossRef]
- Huang, G.; Zhu, X.; Li, H.; Wang, L.; Chi, X.; Chen, J.; Wang, X.; Chen, Z.; Gao, J. Facile integration of multiple magnetite nanoparticles for theranostics combining efficient MRI and thermal therapy. Nanoscale 2015, 7, 2667–2675. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211, 317–331. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, J.; Kailasa, S.K.; Kwon, E.E.; Tsang, Y.F.; Ok, Y.S.; Kim, K.-H. A critical review of ferrate (VI)-based remediation of soil and groundwater. Environ. Res. 2018, 160, 420–448. [Google Scholar] [CrossRef] [PubMed]
- Tosco, T.; Papini, M.P.; Viggi, C.C.; Sethi, R. Nanoscale zerovalent iron particles for groundwater remediation: A review. J. Clean. Prod. 2014, 77, 10–21. [Google Scholar] [CrossRef]
- Sposito, G. The Surface Chemistry of Soils; Oxford University Press: Oxford, UK, 1984. [Google Scholar]
- Othman, N.H.; Alias, N.H.; Shahruddin, M.Z.; Bakar, N.F.A.; Him, N.R.N.; Lau, W.J. Adsorption Kinetics of Methylene Blue Dyes onto Magnetic Graphene Oxide. J. Environ. Chem. Eng. 2018, 6, 2803–2811. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Das, R.; Ho, W.H.; Srinivasu, V.; Maity, A. A novel method for removal of Cr (VI) using polypyrrole magnetic nanocomposite in the presence of unsteady magnetic fields. Sep. Purif. Technol. 2018, 194, 377–387. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G. Sewage sludge-derived TiO2/Fe/Fe3C-biochar composite as an efficient heterogeneous catalyst for degradation of methylene blue. Chemosphere 2019, 215, 101–114. [Google Scholar] [CrossRef]
- Wu, H.; Feng, Q.; Lu, P.; Chen, M.; Yang, H. Degradation mechanisms of cefotaxime using biochar supported Co/Fe bimetallic nanoparticles. Environ. Sci. Water Res. Technol. 2018, 4, 964–975. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.; Yousaf, B.; Fu, B.; Ahmed, R.; Abbas, Q.; Munir, M.A.M.; Ruijia, L. One-step synthesis of N-doped metal/biochar composite using NH3-ambiance pyrolysis for efficient degradation and mineralization of Methylene Blue. J. Environ. Sci. (China) 2019, 78, 29–41. [Google Scholar] [CrossRef]
- Sun, Y.; Iris, K.; Tsang, D.C.; Cao, X.; Lin, D.; Wang, L.; Graham, N.J.; Alessi, D.S.; Komárek, M.; Ok, Y.S. Multifunctional iron-biochar composites for the removal of potentially toxic elements, inherent cations, and hetero-chloride from hydraulic fracturing wastewater. Environ. Int. 2019, 124, 521–532. [Google Scholar] [CrossRef]
- Hatamie, A.; Parham, H.; Zargar, B.; Heidari, Z. Evaluating magnetic nano-ferrofluid as a novel coagulant for surface water treatment. J. Mol. Liq. 2016, 219, 694–702. [Google Scholar] [CrossRef]
- Richard, F.C.; Bourg, A.C. Aqueous geochemistry of chromium: A review. Water Res. 1991, 25, 807–816. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.; Liu, S.; Yin, Y.; Zeng, G.; Tan, X.; Hu, X.; Hu, X.; Jiang, L.; Ding, Y. Investigation of the adsorption-reduction mechanisms of hexavalent chromium by ramie biochars of different pyrolytic temperatures. Bioresour. Technol. 2016, 218, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Ucun, H.; Bayhana, Y.K.; Kaya, Y.; Cakici, A.; Algur, O.F. Biosorption of lead (II) from aqueous solution by cone biomass of Pinus sylvestris. Desalination 2003, 154, 233–238. [Google Scholar] [CrossRef]
- Xiang, B.; Ling, D.; Lou, H.; Gu, H. 3D hierarchical flower-like nickel ferrite/manganese dioxide toward lead (II) removal from aqueous water. J. Hazard. Mater. 2017, 325, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman Oyekanmi, A.; Abd Latiff, A.A.; Daud, Z.; Saphira Radin Mohamed, R.M.; Ismail, N.; Ab Aziz, A.; Rafatullah, M.; Hossain, K.; Ahmad, A.; Kamoldeen Abiodun, A. Adsorption of cadmium and lead from palm oil mill effluent using bone-composite: Optimisation and isotherm studies. Int. J. Environ. Anal. Chem. 2019, 99, 707–725. [Google Scholar] [CrossRef]
- Yu, F.; Ma, J.; Wang, J.; Zhang, M.; Zheng, J. Magnetic iron oxide nanoparticles functionalized multi-walled carbon nanotubes for toluene, ethylbenzene and xylene removal from aqueous solution. Chemosphere 2016, 146, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Saǧ, Y.; Kutsal, T. Determination of the biosorption heats of heavy metal ions on Zoogloea ramigera and Rhizopus arrhizus. Biochem. Eng. J. 2000, 6, 145–151. [Google Scholar] [CrossRef]
- Khosa, M.; Ullah, A. Mechanistic insight into protein supported biosorption complemented by kinetic and thermodynamics perspectives. Adv. Colloid Interface Sci. 2018, 261, 28–40. [Google Scholar] [CrossRef]
- Lakshmi, U.R.; Srivastava, V.C.; Mall, I.D.; Lataye, D.H. Rice husk ash as an effective adsorbent: Evaluation of adsorptive characteristics for Indigo Carmine dye. J. Environ. Manag. 2009, 90, 710–720. [Google Scholar] [CrossRef]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Pore-and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind. Eng. Chem. Fundam. 1966, 5, 212–223. [Google Scholar] [CrossRef]
- Shikuku, V.O.; Kowenje, C.O.; Kengara, F.O. Errors in parameters estimation using linearized adsorption isotherms: Sulfadimethoxine adsorption onto kaolinite clay. Chem. Sci. Int. J. 2018, 23, 1–6. [Google Scholar] [CrossRef]



| Nos | Method | Advantages | Disadvantages | * Cost | Ref |
|---|---|---|---|---|---|
| 1. | Hydrothermal | Single step synthesis; high Ms value; highly crystalline particles | Cubic particles; large particle sizes; long reaction time; polydispered size distribution; high reaction temperature | $44.01 | [54] |
| 2. | Thermal Decomposition | Medium particle sizes; high Ms; short reaction time | Cubic–spherical shapes; polydispered size distribution | $73.84 | [55] |
| 3. | Co-precipitation | Spherical and small particles; high Ms; low reaction temperature; one-step synthesis; monodispered size distribution | Agglomerated particles | $39.50 | [56] |
| 4. | Sol-gel | Spherical nanoparticles; small particle sizes | Polydispered size distribution; long reaction time; multi-step reaction process | $99.12 | [57] |
| 5. | Sonochemical | High Ms; short reaction time; one-step reaction; | Nanocubes; large particle sizes; polydispered size distribution | $84.39 | [58] |
| 6. | Polyol | Spherical and small particles; moderate Ms; monodispered size distribution | Multi-precursors and reaction steps. | $141.07 | [59] |
| 7. | Electrochemical | High Ms; small particle size; monodispered size distribution; room temperature synthesis | Prone to impurity; quasi-spherical particles; hydrophobic nanoparticles | $32.55 | [60] |
| 8. | Microemulsion | Small particle size; monophased product; monodispered size distribution | Agglomerated and cubic particles; multi-precursors | $242.72 | [61] |
| Material | Method of Synthesis | * Functionalizer | Comment | Ref. |
|---|---|---|---|---|
| Fe3O4 | Co-precipitation | PPhSi | Saturation magnetization (Ms) value of 68 emug−1 is close to Ms value of magnetite. Less bulky phosphonium group must have enhanced Ms value. TEM images showed particles were an average of 12 nm and spherical. Morphology is desirable for wastewater treatment. | [5] |
| Fe3O4@C | Hydrothermal | polyacrylamide | Cubic particle size in the range of 50–70 nm are limitations for the application of the composite for wastewater treatment. Furthermore, polyacrylamide decreased Ms value from 67 emug−1 to 31 emug−1. | [11] |
| Fe3O4@SiO2 | Co-precipitation | APTMS | Low Ms value of 34 emug−1 is attributed to the bulky silanol group; however, an average particle size of 18 nm is desirable for a superparamagnetic composite. | [63] |
| Fe3O4@yeast | Co-precipitation | EDTAD | Particle size, saturation magnetization, and shape were not reported. The presence of EDTAD as a functionalizer is desirable in the complexation and adsorption of Pb2+, Ca2+, Cd2+. | [64] |
| Fe3O4@APS | Co-precipitation | AA-co-CA | The cubic shape of the particles can limit its application in wastewater treatment; however, Ms value of 52 emug−1 and size of 18 nm are good merits. | [66] |
| Fe3O4@APS | Co-precipitation | APS | Average particle size of 15 nm and Ms value of 67 emug−1 are desirable for wastewater treatment. Cubic-structured particles can pose limitations during clearance. | [70] |
| γ-Fe2O3 | Flame spray pyrolysis | - | The comparatively low saturation magnetization of 44.5 emug−1 and platelet shape of the particles are quite a limitation. However, average particle size of 10 nm is desirable. | [71] |
| CoFe2O4 | Hydrothermal | Pr3+, Sm3+, Tb3+, Ho3+ | High reaction temperature gives little control over the properties of the particles, resulting in quasi-spherical agglomerated particles. Nevertheless, particle size of 9.2 nm and Ms value of 60 emug−1 are desirable. | [72] |
| Fe3O4 | Hydrothermal | MnO2 | Reported average size of 60 nm and slightly agglomerated quasi-shaped particles are limitations of nanocomposite application in wastewater treatment. Ms value of 40 emug−1 should be enough for magnetic recovery. | [73] |
| Fe3O4 | Co-precipitation | Humic acid | High Ms value of 79.6 emug−1 and particle size of 10 nm showed humic acid sparingly affected the magnetic properties of the nanoparticles. TEM images of composites were spherical and showed no agglomeration. | [74] |
| Fe3O4 | Chemical precipitation | PEDOT | Monodispersed and spherical particles of 11 nm are desirable for wastewater treatment. However, saturation magnetization value of 24 emug−1 is relatively small when compared to bare magnetite nanoparticles. | [75] |
| Fe-oxide | Biological | Cellulose | Reported mixture of rod- and cone-shaped particles with grain size range of 40–110 nm can affect sustained magnetic response and clearance from wastewater. | [76] |
| Fe3O4@SiO2 | Co-precipitation | GO-PEA | Spherical particles with an average size of 22 nm are desirable for wastewater treatment. A major limitation is the sharp decrease in Ms value from 77 to 33 emug−1 upon coating and functionalization. | [77] |
| Fe3O4@Cu | Hydrothermal | MgO-Cu | Particles were agglomerated though spherical; however, average particle size of 50 nm can be a limitation. Diamagnetic MgO coating reduced Ms value to 29 emug−1. | [78] |
| Adsorbent | * Mass (g/L) | Surface Area (m2/g) | Adsorbates | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|---|---|---|
| Fe3O4 | 12 | 105.7 | As5+ | 50.5 | [5] |
| Cr6+ | 35.2 | ||||
| Fe3O4@C/PAA | 0.3 | 8.2 | Cu2+ | 194 | [11] |
| Ni2+ | 144.3 | ||||
| Co2+ | 128 | ||||
| Cd2+ | 161 | ||||
| Fe3O4@haemoglobin | 2 | 12.43 | Eriochrome black T | 178.6 | [12] |
| Indigo carmine | 104.2 | ||||
| Naphthol blue black | 114.9 | ||||
| Tartrazine | 80 | ||||
| Erythrosine | 178.6 | ||||
| Bromophenol blue | 101 | ||||
| Magnetic Iron oxide | 0.4 | 142 | PO42− | 40.0 | [50] |
| Fe3O4@SiO2/NH2 | 0.4 | 216.2 | Cu2+ | 43.8 | [63] |
| Pb2+ | 111.9 | ||||
| Cd2+ | 37 | ||||
| Fe3O4@yeast/EDTAD | 1 | - | Pb2+ | 88.16 | [64] |
| Ca2+ | 27.19 | ||||
| Cd2+ | 40.70 | ||||
| Fe3O4@APS/AA-co-AA | 1 | - | Methylene blue | 124 | [66] |
| Crystal violet | 180.5 | ||||
| Alkali blue | 17.8 | ||||
| Fe3O4@APS/AA-co-CA | 1 | - | Cd2+ | 29.6 | [70] |
| Zn2+ | 43.4 | ||||
| Pb2+ | 166.1 | ||||
| Cu2+ | 126.9 | ||||
| γ-Fe2O3 | 0.2 | 79.4 | Pb2+ | 68.9 | [71] |
| 0.1 | Cu2+ | 34.0 | |||
| CoFe1.9RE0.1O4 | 0.5 | 82 | Congo red | 152.0 | [72] |
| Fe3O4@MnO2 | 1 | 118 | Cd2+ | 53.2 | [73] |
| Fe3O4@humic | 0.1 | 64 | Cu2+ | 46.3 | [74] |
| Cd2+ | 50.4 | ||||
| Pb2+ | 92.4 | ||||
| Hg2+ | 97.7 | ||||
| Fe3O4@PEDOT | - | - | Ag+ | 3016 | [75] |
| Hg2+ | 3200 | ||||
| Pb2+ | 3105.9 | ||||
| Fe-oxide@cellulose | 1 | - | As3+ | 92.95 | [76] |
| Fe3O4@SiO2/GO-PEA | 1 | 133 | Chlorpyrifos | 87 | [77] |
| Malathion | 74 | ||||
| Parathion | 86 | ||||
| Fe2O3@TiO2/GO | 0.6 | 82 | As3+ | 110.4 | [104] |
| As5+ | 90.2 | ||||
| Fe3O4-polypyrrole | 4 | 28.77 | Cr6+ | 208.7 | [126] |
| Fe3O4@MWCNT-KOH | 0.4 | 662.1 | Toluene | 63.34 | [137] |
| Ethylbenzene | 249.44 | ||||
| Xylene (meta) | 227.05 | ||||
| Xylene (otho) | 138.04 | ||||
| Xylene (para) | 105.59 | ||||
| Adsorbent | * Mass (g/L) | Surface area (m2/g) | Adsorbates | Removal efficiency (%) | Ref. |
| Fe3O4@Cu/MgO-Cu | 0.32 | 90.1 | Escherichia coli Staphylococcus aureus | 99% | [78] |
| CoFe2O4@TiO2 | 4 | 44.38 | Methylene blue | 98.89% | [95] |
| DNSA@CS/MnFe2O4 | 0.06 | 219 | Methylene blue | 98.9% | [116] |
| TiO2/Fe/FeC-biochar | - | 267.30 | Methylene blue | 94.4% | [127] |
| Co/Fe/MB | 0.8 | 262 | Cefotaxime | 82.48% | [128] |
| N-TiO2-Fe3O4-C | 1 | - | Methylene blue | 99% | [129] |
| Fe-biochar | 2 | 138.4 | Na+,Ca2+,K+,Mg2+, | [130] | |
| Sr2+,Ba2+ | <30% | ||||
| Cr (VI) | 58.4% | ||||
| As (V) | 65.9% | ||||
| 1,1,2-trichlorethane | 91% |
| Magnetic composite | a pH | c Time | Kinetics | Isotherm | b Ms (emug−1) | Ref. |
|---|---|---|---|---|---|---|
| Fe3O4-PPhSi | 6.0 | 0.83 h | PSO | Langmuir | 68.2 | [5] |
| Fe3O4@C@PAA | - | 1.5 h | PSO | Langmuir and Freundlich | 31 | [11] |
| Magnetic iron oxide | - | 0.5 h | - | Langmuir | - | [50] |
| Fe3O4@SiO2/NH2 | 6.0 | 24 h | - | Langmuir | 34 | [63] |
| Fe3O4@yeast/EDTAD | - | 2 h | PSO | Langmuir and Freundlich | - | [64] |
| Fe3O4@APS/AA-co-CA | 3.5 | 0.75 h | PSO | Langmuir | 52 | [66] |
| Fe3O4@APS@AA-co-CA | 3.5 | 0.75 h | PSO | Langmuir and Freundlich | 52 | [70] |
| γ-Fe2O3 | 6.3 | 3 h | PSO | Langmuir and Freundlich | 44.5 | [71] |
| CoFe1.9RE0.1O4 | - | 1.5 h | PSO | Langmuir model | 60 | [72] |
| Fe3O4@MnO2 | 3.7 | 0.5 h | PSO | Langmuir | 40 | [73] |
| Fe3O4@humic | 6.0 | 0.25 h | - | - | 79.6 | [74] |
| Fe3O4@PEDOT | - | 24 h | - | - | 24 | [75] |
| Fe-oxide@cellulose | 7.8 | 3 h | PSO | Langmuir | 57.2 | [76] |
| Fe3O4@SiO2/GO-PEA | 0.25 h | PSO | Sips | 33 | [77] | |
| CoFe2O4@TiO2 | - | 6 h | PFO | - | 0.211 | [95] |
| Fe2O3@TiO2/GO | 8.6 | 20 h | PSO | Langmuir | 60 | [104] |
| DNSA@CS@MnFe2O4 | - | 0.5 h | - | Langmuir-Hinshelwood | 29.95 | [116] |
| Fe3O4-polypyrrole | - | 24 h | - | Langmuir | 14 | [126] |
| TiO2/Fe/FeC-biochar | 5 h | - | - | 47.60 | [127] | |
| Co/Fe/MB | - | 1.6 h | PSO | - | - | [128] |
| N-TiO2-Fe3O4-C | 5 h | - | - | 19.26 | [129] | |
| Fe-biochar | 8 h | PSO | - | - | [130] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nnadozie, E.C.; Ajibade, P.A. Multifunctional Magnetic Oxide Nanoparticle (MNP) Core-Shell: Review of Synthesis, Structural Studies and Application for Wastewater Treatment. Molecules 2020, 25, 4110. https://doi.org/10.3390/molecules25184110
Nnadozie EC, Ajibade PA. Multifunctional Magnetic Oxide Nanoparticle (MNP) Core-Shell: Review of Synthesis, Structural Studies and Application for Wastewater Treatment. Molecules. 2020; 25(18):4110. https://doi.org/10.3390/molecules25184110
Chicago/Turabian StyleNnadozie, Ebenezer C., and Peter A. Ajibade. 2020. "Multifunctional Magnetic Oxide Nanoparticle (MNP) Core-Shell: Review of Synthesis, Structural Studies and Application for Wastewater Treatment" Molecules 25, no. 18: 4110. https://doi.org/10.3390/molecules25184110
APA StyleNnadozie, E. C., & Ajibade, P. A. (2020). Multifunctional Magnetic Oxide Nanoparticle (MNP) Core-Shell: Review of Synthesis, Structural Studies and Application for Wastewater Treatment. Molecules, 25(18), 4110. https://doi.org/10.3390/molecules25184110