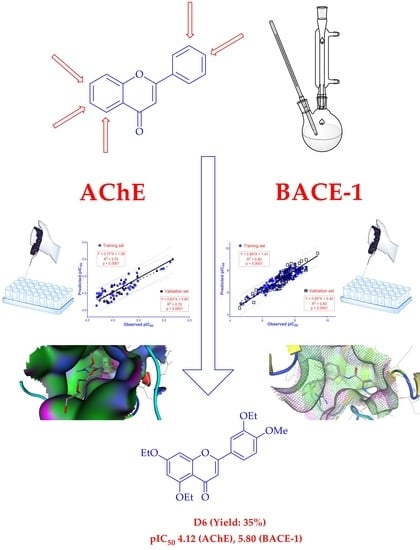
Synthesis, In Silico and In Vitro Evaluation of Some Flavone Derivatives for Acetylcholinesterase and BACE-1 Inhibitory Activity
Abstract
1. Introduction
2. Results
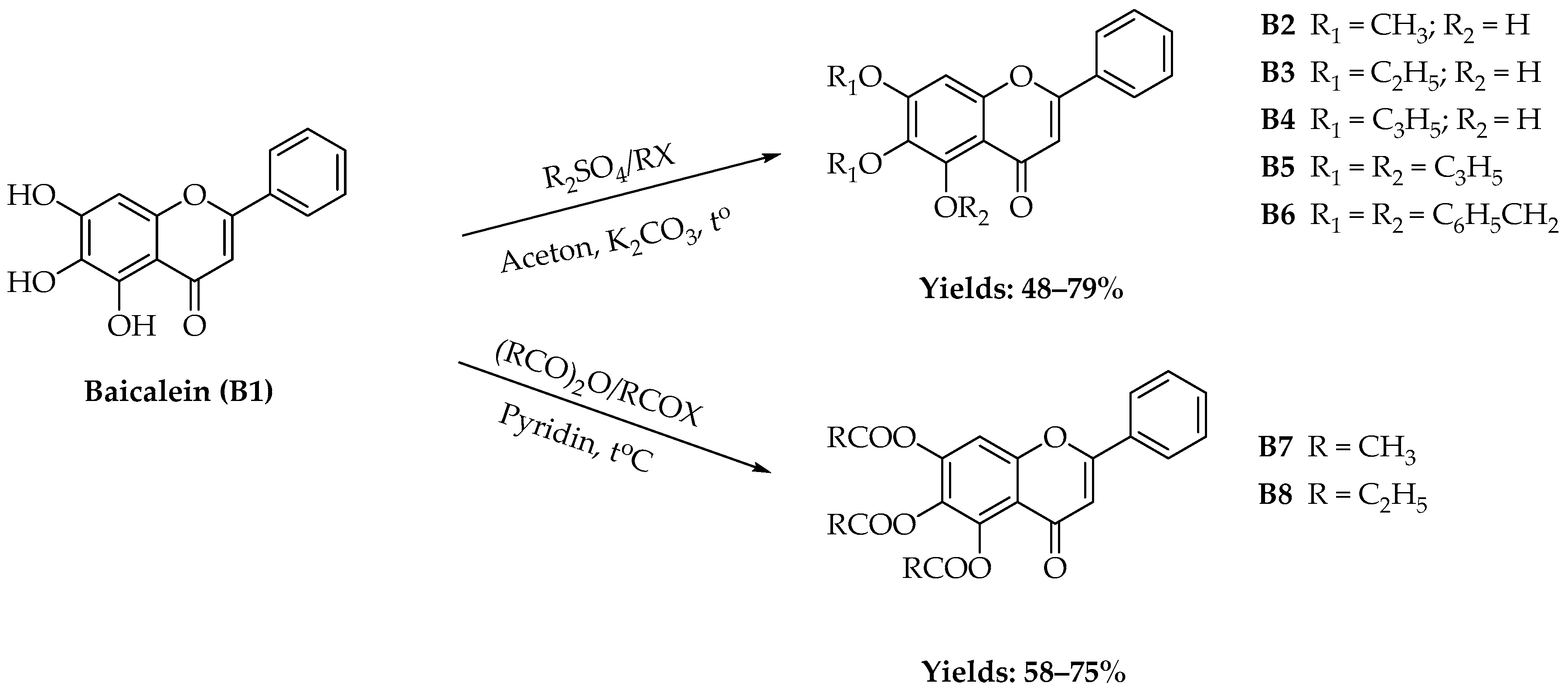
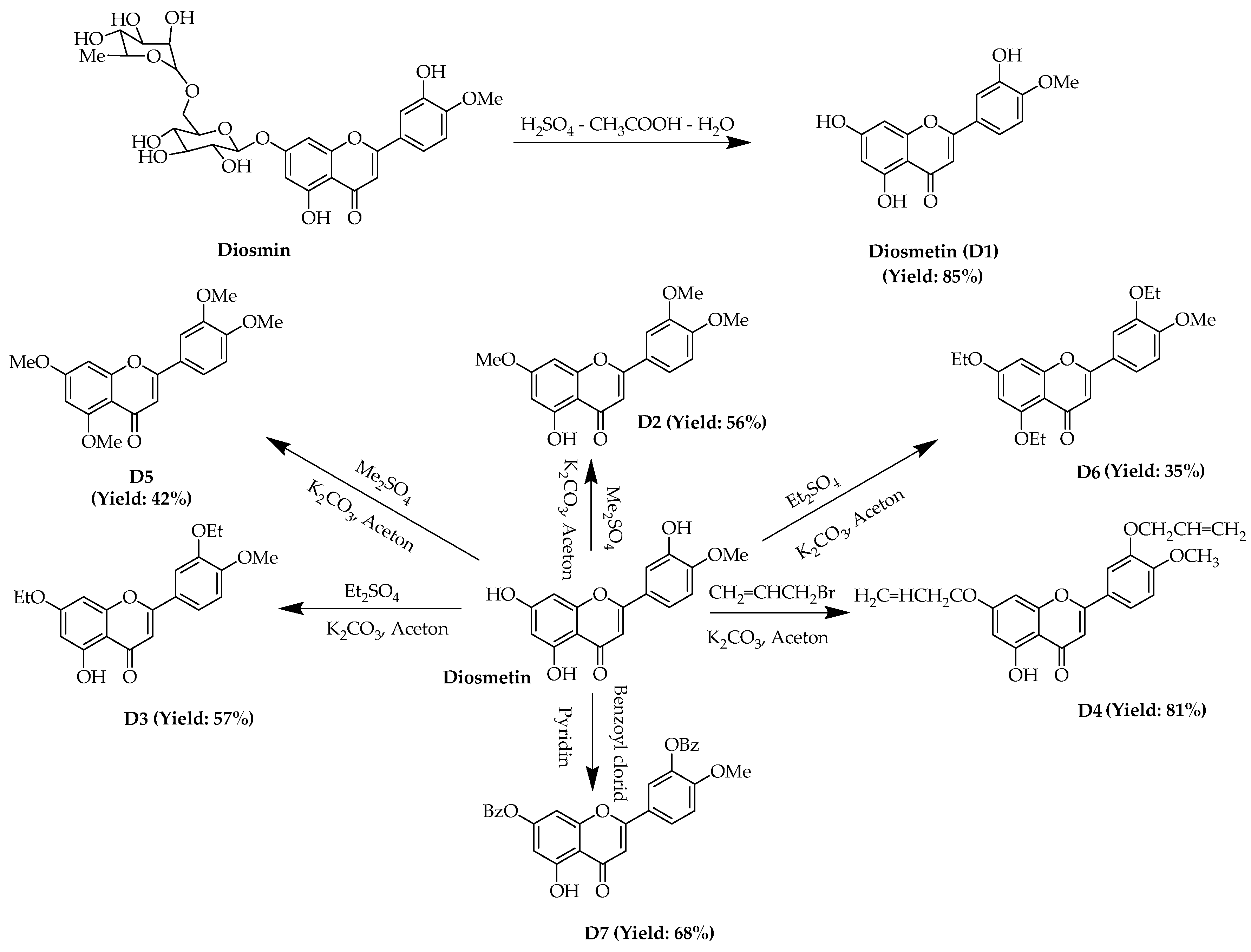
2.1. Chemistry
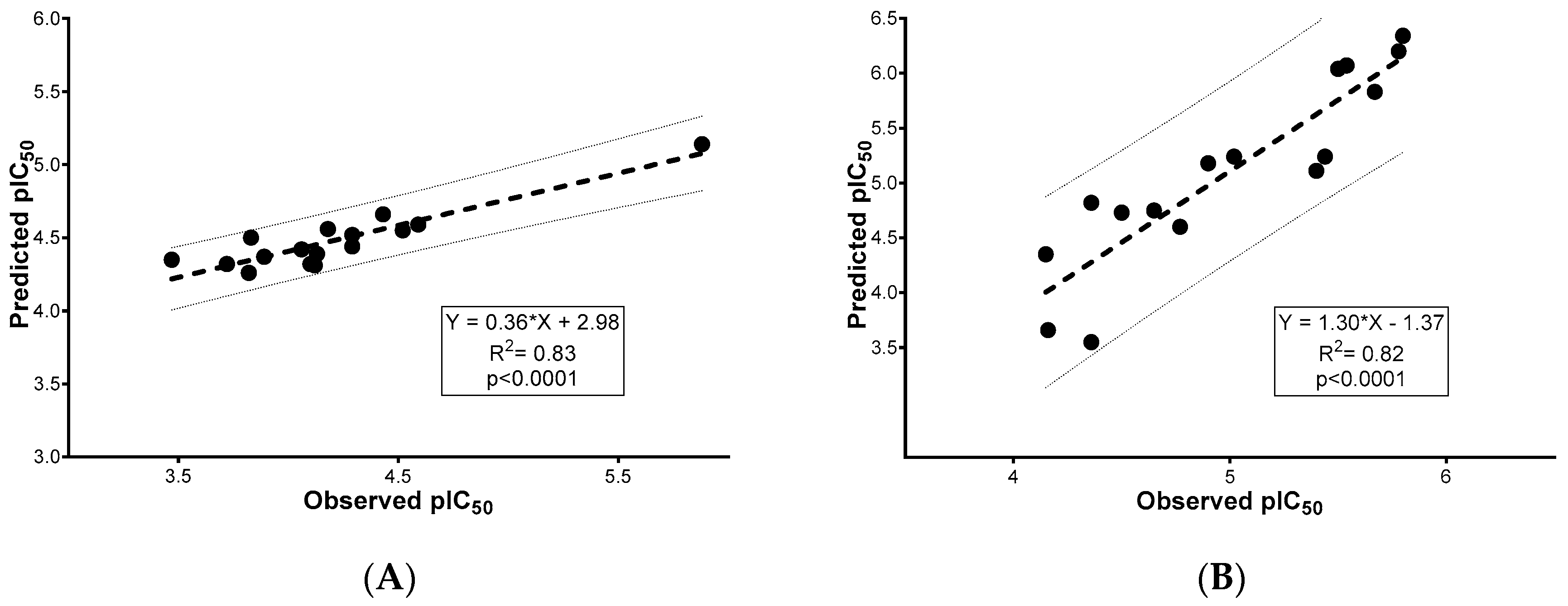
2.2. Enzyme Inhibition and 2D-QSAR Analysis
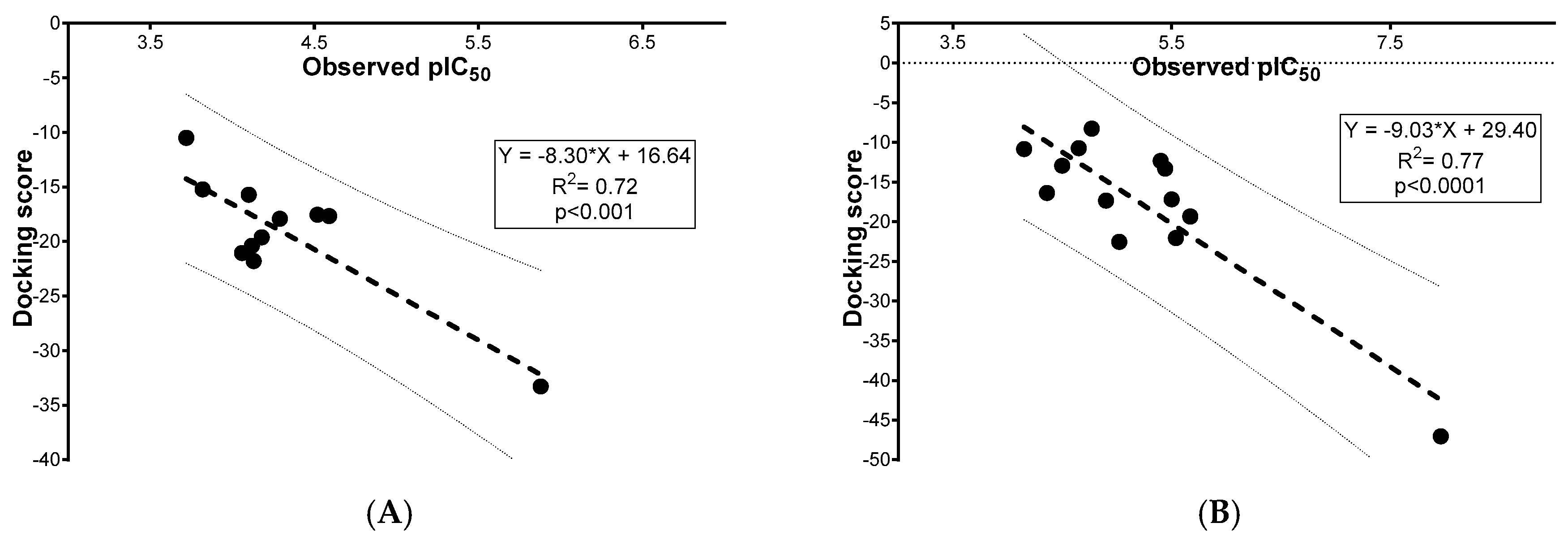
2.3. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Materials and Instruments
4.2. Chemistry
4.2.1. Synthesis of B2–B6
4.2.2. Synthesis of B7 and B8
4.2.3. Synthesis of Diosmetin (D1)
4.2.4. Synthesis of D2–D6
4.2.5. Synthesis of D7
4.3. In Vitro Assays
4.4. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kim, M.; Park, H.E.; Lee, S.-H.; Han, K.; Lee, J.H. Increased risk of Alzheimer’s disease in patients with psoriasis: A nationwide population-based cohort study. Sci. Rep. 2020, 10, 6454. [Google Scholar] [CrossRef] [PubMed]
- Ringman, J.M. Update on Alzheimer’s and the Dementias: Introduction. Neurol. Clin. 2017, 35, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Graham, W.V.; Bonito-Oliva, A.; Sakmar, T.P. Update on Alzheimer’s Disease Therapy and Prevention Strategies. Annu. Rev. Med. 2017, 68, 413–430. [Google Scholar] [CrossRef]
- Guo, S.; Getsios, D.; Revankar, N.; Xu, P.; Thompson, G.; Bobula, J.; Lacey, L.; Gaudig, M. Evaluating disease-modifying agents: A simulation framework for Alzheimer’s disease. Pharmacoeconomics 2014, 32, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Association, A.S. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 2015, 11, 332–384. [Google Scholar] [CrossRef]
- Lalut, J.; Payan, H.; Davis, A.; Lecoutey, C.; Legay, R.; Santos, J.S.-D.O.; Claeysen, S.; Dallemagne, P.; Rochais, C. Rational design of novel benzisoxazole derivatives with acetylcholinesterase inhibitory and serotoninergic 5-HT4 receptors activities for the treatment of Alzheimer’s disease. Sci. Rep. 2020, 10, 3014. [Google Scholar] [CrossRef]
- Blazer, L.L.; Neubig, R.R. Small molecule protein-protein interaction inhibitors as CNS therapeutic agents: Current progress and future hurdles. Neuropsychopharmacology 2009, 34, 126–141. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef]
- Tumiatti, V.; Minarini, A.; Bolognesi, M.L.; Milelli, A.; Rosini, M.; Melchiorre, C. Tacrine derivatives and Alzheimer’s disease. Curr. Med. Chem. 2010, 17, 1825–1838. [Google Scholar] [CrossRef]
- Gong, C.X.; Liu, F.; Iqbal, K. Multifactorial Hypothesis and Multi-Targets for Alzheimer’s Disease. J. Alzheimers Dis. 2018, 64, S107–S117. [Google Scholar] [CrossRef]
- Rees, T.M.; Brimijoin, S. The role of acetylcholinesterase in the pathogenesis of Alzheimer’s disease. Drugs Today (Barc.) 2003, 39, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R. BACE1: The beta-secretase enzyme in Alzheimer’s disease. J. Mol. Neurosci. 2004, 23, 105–114. [Google Scholar] [CrossRef]
- Auffret, G.; Labaied, M.; Frappier, F.; Rasoanaivo, P.; Grellier, P.; Lewin, G. Synthesis and antimalarial evaluation of a series of piperazinyl flavones. Bioorg. Med. Chem. Lett. 2007, 17, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Naik, K.K.; Thangavel, S.; Alam, A.; Kumar, S. Flavone Analogues as Antimicrobial Agents. Recent Pat. Inflamm. Allergy Drug Discov. 2017, 11, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.H.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M.; et al. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar] [CrossRef]
- Thirugnanasambantham, P.; Viswanathan, S.; Mythirayee, C.; Krishnamurty, V.; Ramachandran, S.; Kameswaran, L. Analgesic activity of certain flavone derivatives: A structure-activity study. J. Ethnopharmacol. 1990, 28, 207–214. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A., Jr. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef]
- Mota, K.S.D.L.; Dias, G.E.N.; Pinto, M.E.F.; Luiz-Ferreira, A.; Souza-Brito, A.R.M.; Hiruma-Lima, C.A.; Barbosa-Filho, J.M.; Batista, L.M. Flavonoids with gastroprotective activity. Molecules 2009, 14, 979–1012. [Google Scholar] [CrossRef]
- Zahran, E.M.; Abdelmohsen, U.R.; Hussein, A.S.; Salem, M.A.; Khalil, H.E.; Yehia Desoukey, S.; Fouad, M.A.; Kamel, M.S. Antiulcer potential and molecular docking of flavonoids from Ocimum forskolei Benth., family Lamiaceae. Nat. Prod. Res. 2019, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.D.; Tran, T.D.; Le, M.T.; Thai, K.M. Machine learning-, rule- and pharmacophore-based classification on the inhibition of P-glycoprotein and NorA. SAR QSAR Environ. Res. 2016, 27, 747–780. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.-D.; Tran, T.-D.; Le, M.-T.; Thai, K.-M. Computational predictive models for P-glycoprotein inhibition of in-house chalcone derivatives and drug-bank compounds. Mol. Divers. 2016, 20, 945–961. [Google Scholar] [CrossRef] [PubMed]
- Thai, K.M.; Huynh, N.T.; Ngo, T.D.; Mai, T.T.; Nguyen, T.H.; Tran, T.D. Three- and four-class classification models for P-glycoprotein inhibitors using counter-propagation neural networks. SAR QSAR Environ. Res. 2015, 26, 139–163. [Google Scholar] [CrossRef]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure-activity relationship study. J. Enzym. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Alshali, K.Z.; Haidari, R.A.A.; El-Kholy, A.A.; Zayed, M.F. Lipoxygenase inhibitors flavonoids from Cyperus rotundus aerial parts. Rev. Bras. Farm. 2018, 28, 320–324. [Google Scholar] [CrossRef]
- Proença, C.; Freitas, M.; Ribeiro, D.; Tomé, S.M.; Oliveira, E.F.T.; Viegas, M.F.; Araújo, A.N.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; et al. Evaluation of a flavonoids library for inhibition of pancreatic α-amylase towards a structure-activity relationship. J. Enzym. Inhib. Med. Chem. 2019, 34, 577–588. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, G.; Liao, Y.; Pan, J.; Gong, D. Dietary Flavonoids as Xanthine Oxidase Inhibitors: Structure–Affinity and Structure–Activity Relationships. J. Agric. Food Chem. 2015, 63, 7784–7794. [Google Scholar] [CrossRef]
- Chimenti, F.; Fioravanti, R.; Bolasco, A.; Chimenti, P.; Secci, D.; Rossi, F.; Yáñez, M.; Orallo, F.; Ortuso, F.; Alcaro, S.; et al. A new series of flavones, thioflavones, and flavanones as selective monoamine oxidase-B inhibitors. Bioorg. Med. Chem. 2010, 18, 1273–1279. [Google Scholar] [CrossRef]
- Khan, H.; Marya; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and flavones as BACE-1 inhibitors: Structure-activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta 2008, 1780, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ji, Y.; Youn, K.; Lim, G.; Lee, J.; Kim, D.H.; Jun, M. Baicalein as a Potential Inhibitor against BACE1 and AChE: Mechanistic Comprehension through In Vitro and Computational Approaches. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- SciFinder. Available online: https://sso.cas.org/as/YpCJE/resume/as/authorization.ping (accessed on 8 July 2020).
- Balkrishna, A.; Pokhrel, S.; Tomer, M.; Verma, S.; Kumar, A.; Nain, P.; Gupta, A.; Varshney, A. Anti-Acetylcholinesterase Activities of Mono-Herbal Extracts and Exhibited Synergistic Effects of the Phytoconstituents: A Biochemical and Computational Study. Molecules 2019, 24, 4175. [Google Scholar] [CrossRef] [PubMed]
- Paudel, P.; Seong, S.H.; Zhou, Y.; Ha, M.T.; Min, B.S.; Jung, H.A.; Choi, J.S. Arylbenzofurans from the Root Bark of Morus alba as Triple Inhibitors of Cholinesterase, β-Site Amyloid Precursor Protein Cleaving Enzyme 1, and Glycogen Synthase Kinase-3β: Relevance to Alzheimer’s Disease. ACS Omega 2019, 4, 6283–6294. [Google Scholar] [CrossRef]
- Jannat, S.; Balupuri, A.; Hong, S.S.; Choi, C.; Choi, Y.-H.; Ku, J.-M.; Kim, W.; Leem, J.; Kim, J.; Shrestha, A.; et al. Inhibition of β-site amyloid precursor protein cleaving enzyme 1 and cholinesterases by pterosins via a specific structure−activity relationship with a strong BBB permeability. Exp. Mol. Med. 2019, 51. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.-S.; Le, M.-T.; Tran, T.-D.; Tran, T.-H.; Thai, K.-M. Design of Curcumin and Flavonoid Derivatives with Acetylcholinesterase and Beta-Secretase Inhibitory Activities Using in Silico Approaches. Molecules 2020, 25, 3644. [Google Scholar] [CrossRef]
- Neumann, U.; Ufer, M.; Jacobson, L.H.; Rouzade-Dominguez, M.-L.; Huledal, G.; Kolly, C.; Lüönd, R.M.; Machauer, R.; Veenstra, S.J.; Hurth, K.; et al. The BACE-1 inhibitor CNP520 for prevention trials in Alzheimer’s disease. EMBO Mol. Med. 2018, 10, e9316. [Google Scholar] [CrossRef]
- Ojha, P.K.; Mitra, I.; Das, R.N.; Roy, K. Further exploring rm2 metrics for validation of QSPR models. Chemometr. Intell. Lab. Syst. 2011, 107, 194–205. [Google Scholar] [CrossRef]
- Enyedy, I.J.; Egan, W.J. Can we use docking and scoring for hit-to-lead optimization? J. Comput. Aided Mol. Des. 2008, 22, 161–168. [Google Scholar] [CrossRef]
- Sussman, J.L.; Silman, I. Acetylcholinesterase: Structure and use as a model for specific cation—protein interactions. Curr. Opin. Struct. Biol. 1992, 2, 721–729. [Google Scholar] [CrossRef]
- Guedes, I.A.; Pereira, F.S.S.; Dardenne, L.E. Empirical Scoring Functions for Structure-Based Virtual Screening: Applications, Critical Aspects, and Challenges. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Burlingham, B.T.; Widlanski, T.S. An Intuitive Look at the Relationship of Ki and IC50: A More General Use for the Dixon Plot. J. Chem. Educ. 2003, 80, 214. [Google Scholar] [CrossRef]
- Copeland, R.A. Reversible Modes of Inhibitor Interactions with Enzymes. In Evaluation of Enzyme Inhibitors in Drug Discovery; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 57–121. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Tran, T.-D.; Nguyen, T.-C.-V.; Nguyen, N.-S.; Nguyen, D.-M.; Nguyen, T.-T.-H.; Le, M.-T.; Thai, K.-M. Synthesis of Novel Chalcones as Acetylcholinesterase Inhibitors. Appl. Sci. 2016, 6, 198. [Google Scholar] [CrossRef]
- Nuthakki, V.K.; Sharma, A.; Kumar, A.; Bharate, S.B. Identification of embelin, a 3-undecyl-1,4-benzoquinone from Embelia ribes as a multitargeted anti-Alzheimer agent. Drug Dev. Res. 2019, 80, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chemical characterization, antioxidant properties, cholinesterase inhibitory and anti-amyloidogenic activities of sulfated polysaccharides from some seaweeds. Bioact. Carbohydr. Diet. Fibre 2019, 18, 100182. [Google Scholar] [CrossRef]
- Sybyl X 2.0. Available online: https://sybyl-x.software.informer.com/2.0/ (accessed on 20 May 2019).
- Protein Data Bank. Available online: https://www.rcsb.org/ (accessed on 20 May 2019).
- Hevener, K.E.; Zhao, W.; Ball, D.M.; Babaoglu, K.; Qi, J.; White, S.W.; Lee, R.E. Validation of molecular docking programs for virtual screening against dihydropteroate synthase. J. Chem. Inf. Model. 2009, 49, 444–460. [Google Scholar] [CrossRef]
- LeadIT 2.0.2. Available online: https://www.biosolveit.de/LeadIT/ (accessed on 20 May 2019).
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef]
- Pagadala, N.S.; Syed, K.; Tuszynski, J. Software for molecular docking: A review. Biophys. Rev. 2017, 9, 91–102. [Google Scholar] [CrossRef]
- MOE. 2008.10 Edition. Chemical Computing Group Inc., 1010 Sherbrooke St. W, Suite 910, Montreal, Quebec, Canada H3A 2R7. Available online: https://www.chemcomp.com/ (accessed on 20 May 2019).
Sample Availability: Samples of the compounds are not available from the authors. |

| Compound | AChE | BACE-1 | ||||||
|---|---|---|---|---|---|---|---|---|
| IC50 (µM) | Obs. pIC50 | Pre. pIC50 * | Docking Score (kJ·mol−1) ** | IC50 (µM) | Obs. pIC50 | Pre. pIC50 * | Docking Score (kJ·mol−1) ** | |
| B1 (Baicalein) | 37.15 | 4.43 | 4.66 | −27.06 | 69.18 | 4.16 | 3.66 | −17.64 |
| B2 | 25.51 | 4.59 | 4.59 | −17.67 | 43.65 | 4.36 | 4.82 | −16.37 |
| B3 | 30.01 | 4.52 | 4.55 | −17.55 | 3.98 | 5.40 | 5.11 | −12.35 |
| B4 | 51.81 | 4.29 | 4.44 | −10.36 | 70.79 | 4.15 | 4.35 | −10.84 |
| B5 | 191.47 | 3.72 | 4.32 | −10.49 | 16.98 | 4.77 | 4.60 | −8.29 |
| B6 | 80.32 | 4.10 | 4.32 | −15.71 | 12.59 | 4.90 | 5.18 | −17.35 |
| B7 | 51.29 | 4.29 | 4.52 | −17.91 | 31.62 | 4.50 | 4.73 | −12.95 |
| B8 | 66.24 | 4.18 | 4.56 | −19.61 | 22.39 | 4.65 | 4.75 | −10.75 |
| D1 (Diosmetin) | 147.91 | 3.83 | 4.50 | −22.86 | 43.65 | 4.36 | 3.55 | −19.32 |
| D2 | 87.10 | 4.06 | 4.42 | −21.05 | 2.14 | 5.67 | 5.83 | −19.33 |
| D3 | 128.82 | 3.89 | 4.37 | −20.90 | 3.16 | 5.50 | 6.04 | −17.18 |
| D4 | 151.36 | 3.82 | 4.26 | −15.22 | 3.60 | 5.44 | 5.24 | –13.31 |
| D5 | 73.82 | 4.13 | 4.39 | −21.80 | 1.66 | 5.78 | 6.20 | −13.46 |
| D6 | 75.91 | 4.12 | 4.31 | −20.39 | 1.58 | 5.80 | 6.34 | −12.02 |
| D7 | 340.09 | 3.47 | 4.35 | −25.35 | 2.86 | 5.54 | 6.07 | −22.07 |
| Galanthamine | 1.31 | 5.88 | 5.14 | −33.28 | - | - | - | - |
| Umibecestat | - | - | - | - | 0.01 | 7.96 [38] | 7.67 | −47.04 |
| Quercetin | - | - | - | - | 9.55 | 5.02 | 5.24 | −22.55 |
| R2 = 0.83, RMSE = 0.44 | R2 = 0.82, RMSE = 0.40 | |||||||
| AChE * | ||||||||||||
| pIC50 = −0.928 + (2.348 × BCUT_SLOGP_3) − (0.150 × reactive) − (0.002 × SlogP_VSA2) − (0.004 × PEOE_VSA+1) − (0.004 × SMR_VSA2) − (0.005 × PEOE_VSA–3) | ||||||||||||
| Internal Validation | External Validation | |||||||||||
| N | RMSE | R2 | RMSELOO | Q2LOO | N | RMSE | R2 | R2(PRED) | CCC | |||
| 50 | 0.18 | 0.70 | 0.22 | 0.57 | 22 | 0.16 | 0.78 | 0.78 | 0.65 | 0.69 | 0.11 | 0.88 |
| BACE-1 * | ||||||||||||
| pIC50 = 1.268 + (6.370 × BCUT_PEOE_1) + (3.305 × a_ICM) + (0.870 × petitjean) + (0.157 × a_Nn) + (0.085 × rings) + (0.022 × PEOE_VSA–6) + (0.006 × PEOE_VSA–0) + (0.009 × SlogP_VSA3) + (0.009 × SlogP_VSA5) – (0.478 × chiral_u) − (0.260 × logS) | ||||||||||||
| Internal Validation | External Validation | |||||||||||
| N | RMSE | R2 | RMSELOO | Q2LOO | N | RMSE | R2 | R2(PRED) | CCC | |||
| 150 | 0.37 | 0.80 | 0.40 | 0.77 | 65 | 0.41 | 0.83 | 0.81 | 0.79 | 0.76 | 0.05 | 0.91 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.-S.; Tran, T.-D.; Tran, T.-H.; Mai, T.-T.; Nguyen, N.-L.; Thai, K.-M.; Le, M.-T. Synthesis, In Silico and In Vitro Evaluation of Some Flavone Derivatives for Acetylcholinesterase and BACE-1 Inhibitory Activity. Molecules 2020, 25, 4064. https://doi.org/10.3390/molecules25184064
Tran T-S, Tran T-D, Tran T-H, Mai T-T, Nguyen N-L, Thai K-M, Le M-T. Synthesis, In Silico and In Vitro Evaluation of Some Flavone Derivatives for Acetylcholinesterase and BACE-1 Inhibitory Activity. Molecules. 2020; 25(18):4064. https://doi.org/10.3390/molecules25184064
Chicago/Turabian StyleTran, Thai-Son, Thanh-Dao Tran, The-Huan Tran, Thanh-Tan Mai, Ngoc-Le Nguyen, Khac-Minh Thai, and Minh-Tri Le. 2020. "Synthesis, In Silico and In Vitro Evaluation of Some Flavone Derivatives for Acetylcholinesterase and BACE-1 Inhibitory Activity" Molecules 25, no. 18: 4064. https://doi.org/10.3390/molecules25184064
APA StyleTran, T.-S., Tran, T.-D., Tran, T.-H., Mai, T.-T., Nguyen, N.-L., Thai, K.-M., & Le, M.-T. (2020). Synthesis, In Silico and In Vitro Evaluation of Some Flavone Derivatives for Acetylcholinesterase and BACE-1 Inhibitory Activity. Molecules, 25(18), 4064. https://doi.org/10.3390/molecules25184064