Overview of Radiolabeled Somatostatin Analogs for Cancer Imaging and Therapy
Abstract
1. Introduction
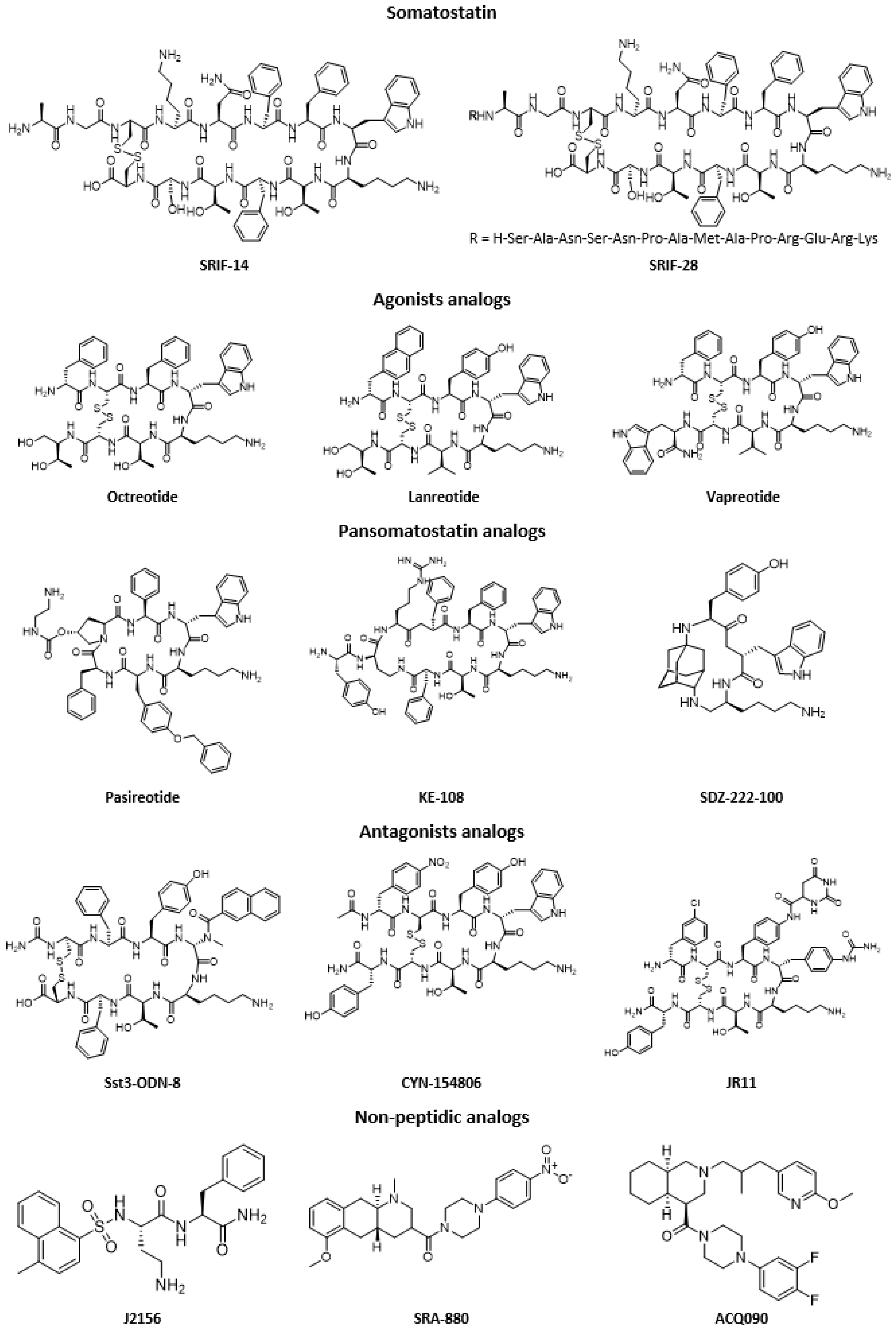
2. Somatostatin Analogs
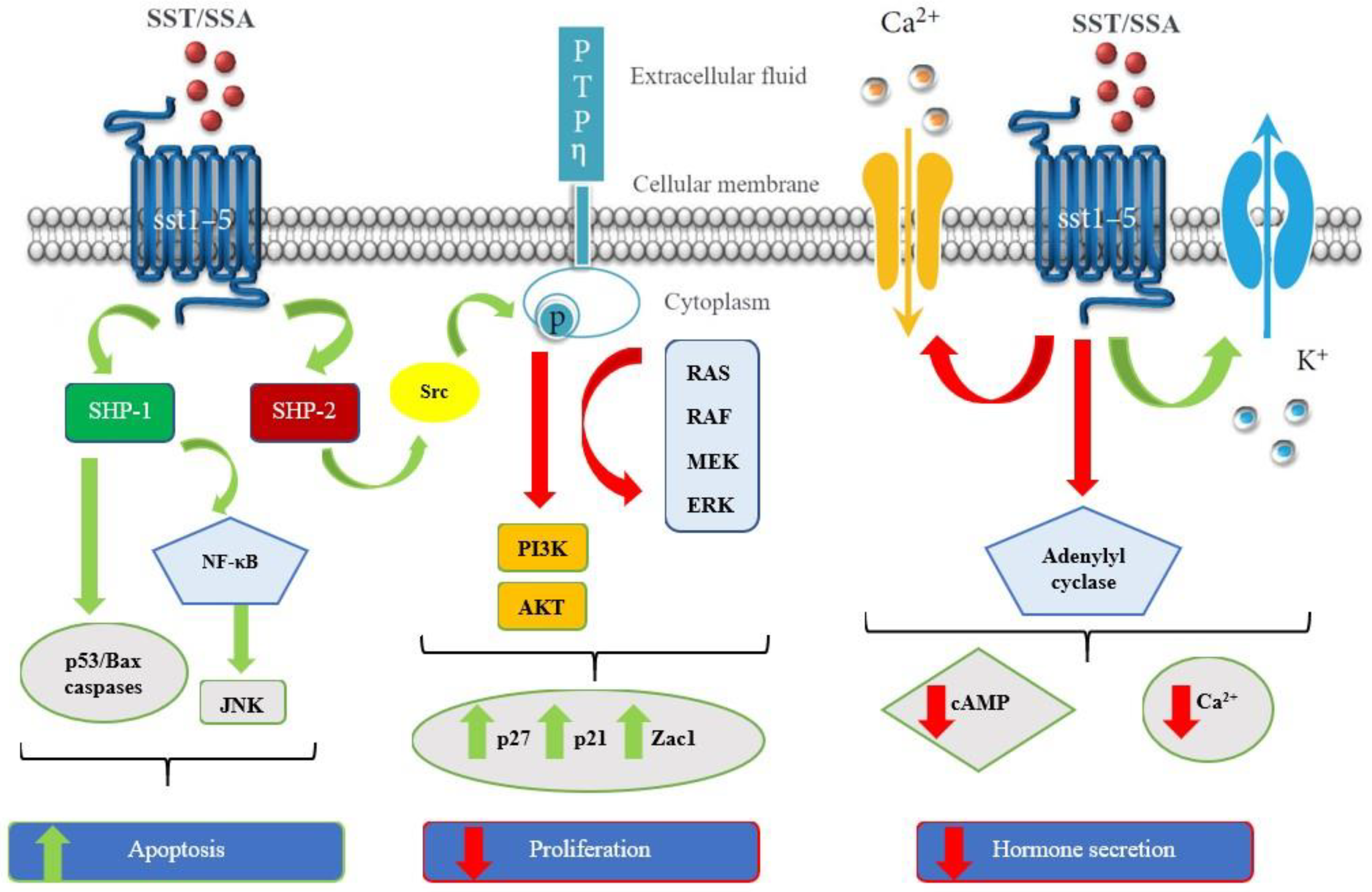
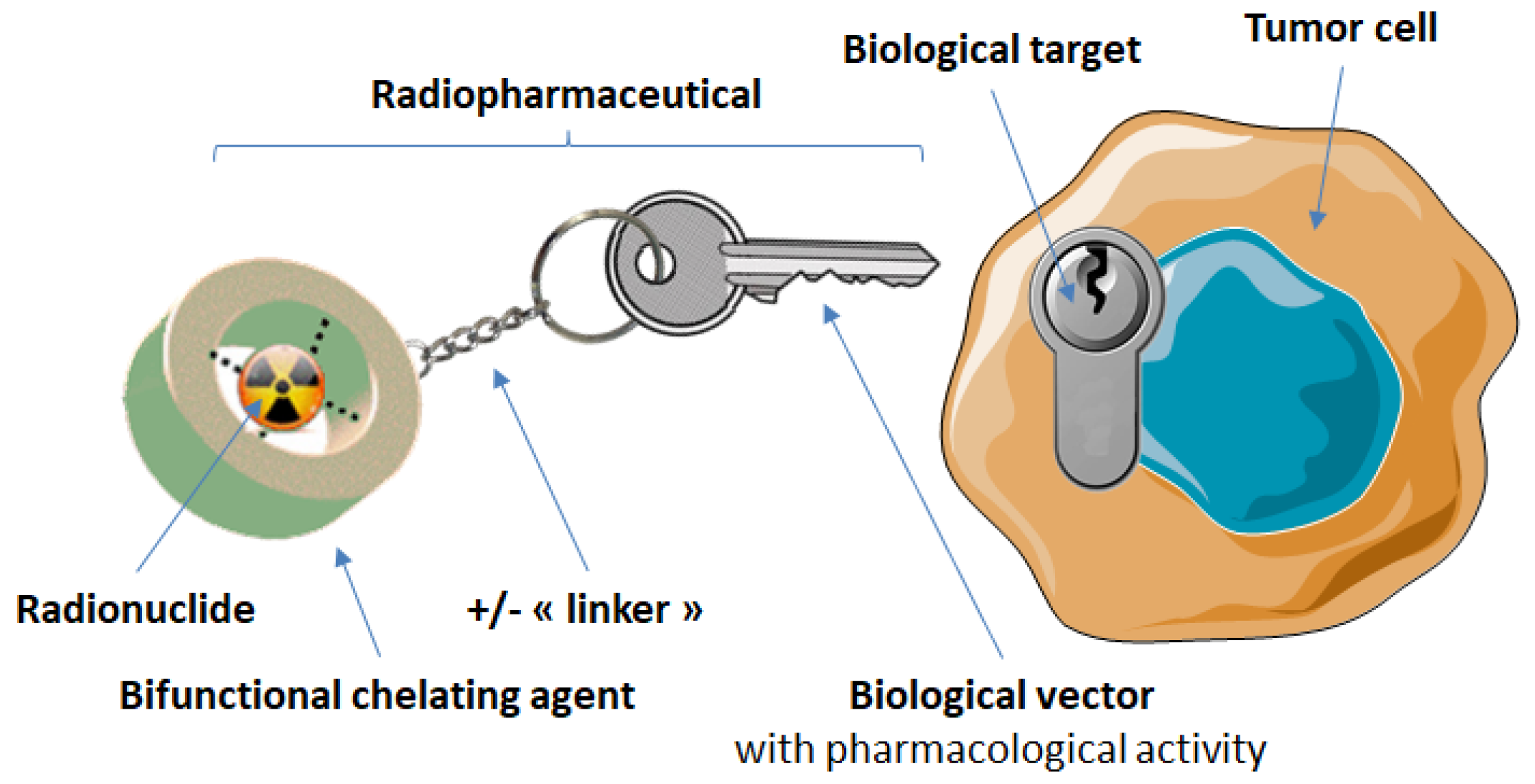
3. Targeting of Somatostatin Receptors with Radiopharmaceuticals
3.1. Radiolabeled Somatostatin Analogs for Imaging
3.1.1. Gallium-68 and Indium-111
3.1.2. Technetium-99m
3.1.3. Copper-64
3.1.4. Other Radiometals
3.1.5. Fluorine-18
3.2. Radiolabeled Somatostatin Analogs for Therapy
3.2.1. Yttrium-90 and Lutetium-177
3.2.2. Rhenium-188 and Other β-Emitting Radionuclides
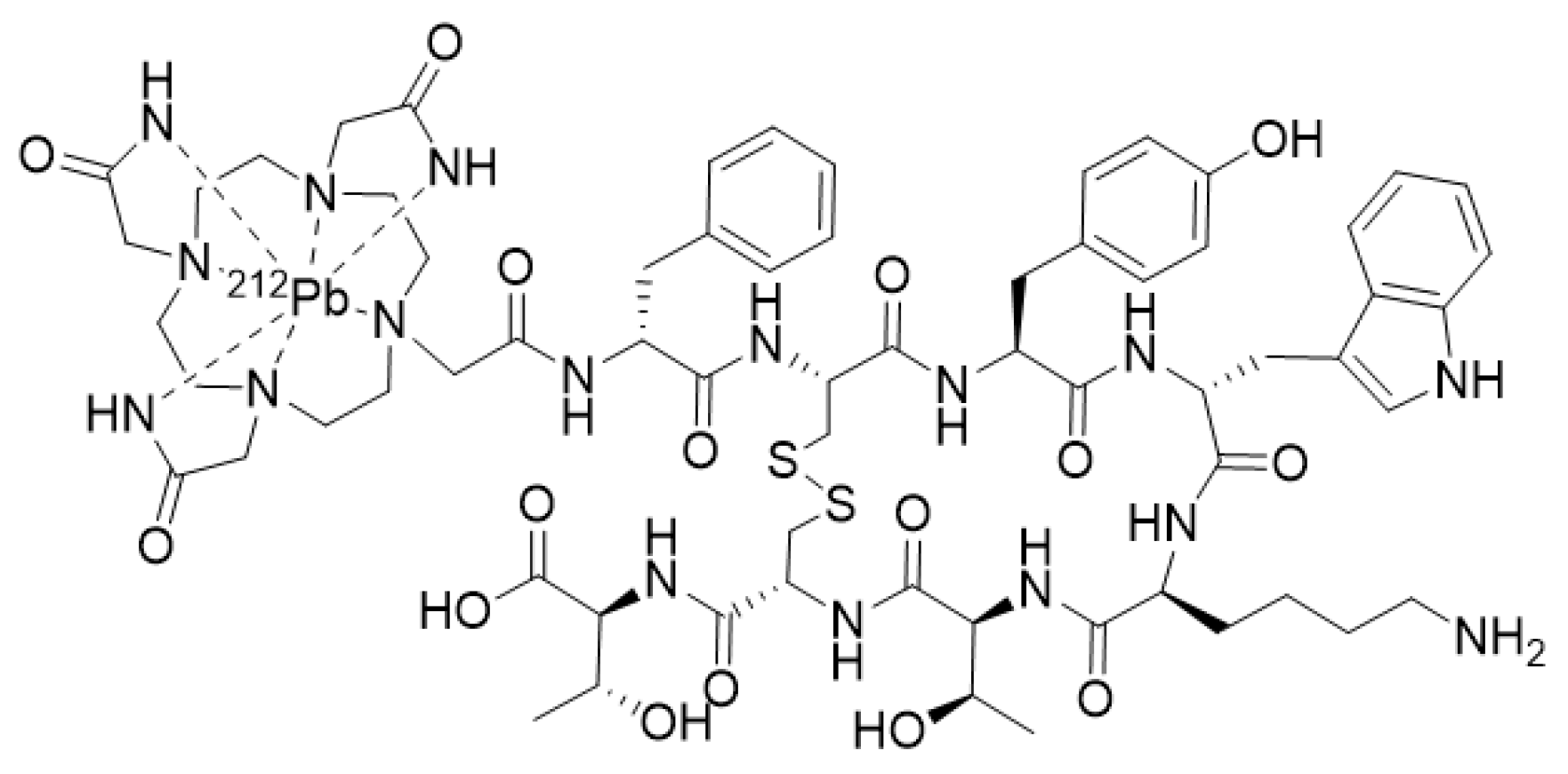
3.2.3. Alpha and Auger Emitters
4. Antagonists vs. Agonists
5. Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brazeau, P.; Vale, W.; Burgus, R.; Ling, N.; Butcher, M.; Rivier, J.; Guillemin, R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 1973, 179, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.C.; Greenwood, M.T.; Panetta, R.; Demchyshyn, L.; Niznik, H.; Srikant, C.B. The somatostatin receptor family. Life Sci. 1995, 57, 1249–1265. [Google Scholar] [CrossRef]
- Günther, T.; Tulipano, G.; Dournaud, P.; Bousquet, C.; Csaba, Z.; Kreienkamp, H.J.; Lupp, A.; Korbonits, M.; Castaño, J.P.; Wester, H.J.; et al. International Union of Basic and Clinical Pharmacology. CV. Somatostatin Receptors: Structure, Function, Ligands, and New Nomenclature. Pharmacol. Rev. 2018, 70, 763–835. [Google Scholar] [CrossRef]
- Patel, Y.C. Somatostatin and Its Receptor Family. Front. Neuroendocrinol. 1999, 20, 157–198. [Google Scholar] [CrossRef] [PubMed]
- Weckbecker, G.; Lewis, I.; Albert, R.; Schmid, H.A.; Hoyer, D.; Bruns, C. Opportunities in somatostatin research: Biological, chemical and therapeutic aspects. Nat. Rev. Drug Discov. 2003, 2, 999–1017. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O.; Lamarca, A.; Valle, J.W.; Hubner, R.A. Somatostatin receptor expression in hepatocellular carcinoma: Prognostic and therapeutic considerations. Endocr. Relat. Cancer 2014, 21, R485–R493. [Google Scholar] [CrossRef][Green Version]
- Pyronnet, S.; Bousquet, C.; Najib, S.; Azar, R.; Laklai, H.; Susini, C. Antitumor effects of somatostatin. Mol. Cell. Endocrinol. 2008, 286, 230–237. [Google Scholar] [CrossRef]
- Barbieri, F.; Bajetto, A.; Pattarozzi, A.; Gatti, M.; Würth, R.; Thellung, S.; Corsaro, A.; Villa, V.; Nizzari, M.; Florio, T. Peptide receptor targeting in cancer: The somatostatin paradigm. Int. J. Pept. 2013, 2013, 926295. [Google Scholar] [CrossRef]
- Rai, U.; Thrimawithana, T.R.; Valery, C.; Young, S.A. Therapeutic uses of somatostatin and its analogues: Current view and potential applications. Pharmacol. Ther. 2015, 152, 98–110. [Google Scholar] [CrossRef]
- Reubi, J.C.; Schaer, J.C.; Laissue, J.A.; Waser, B. Somatostatin receptors and their subtypes in human tumors and in peritumoral vessels. Metabolism 1996, 45, 39–41. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B.; Schaer, J.C.; Laissue, J.A. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur. J. Nucl. Med. 2001, 28, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Guillermet-Guibert, J.; Lahlou, H.; Pyronnet, S.; Bousquet, C.; Susini, C. Somatostatin receptors as tools for diagnosis and therapy: Molecular aspects. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 535551. [Google Scholar] [CrossRef]
- Hasskarl, J.; Kaufmann, M.; Schmid, H.A. Somatostatin receptors in non-neuroendocrine malignancies: The potential role of somatostatin analogs in solid tumors. Future Oncol. 2011, 7, 895–913. [Google Scholar] [CrossRef]
- Gomes-Porras, M.; Cárdenas-Salas, J.; Álvarez-Escolá, C. Somatostatin Analogs in Clinical Practice: A Review. Int. J. Mol. Sci. 2020, 21, 1682. [Google Scholar] [CrossRef]
- Hejna, M.; Schmidinger, M.; Raderer, M. The clinical role of somatostatin analogues as antineoplastic agents: Much ado about nothing? Ann. Oncol. 2002, 13, 653–668. [Google Scholar] [CrossRef]
- Keskin, O.; Yalcin, S. A review of the use of somatostatin analogs in oncology. OncoTargets Ther. 2013, 6, 471–483. [Google Scholar] [CrossRef][Green Version]
- Reubi, J.C. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr. Rev. 2003, 24, 389–427. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, H.; Zhao, N.; Zhu, X.G. Cholangiocarcinoma cells express somatostatin receptor subtype 2 and respond to octreotide treatment. J. Hepatobiliary Pancreat. Surg. 2002, 9, 497–502. [Google Scholar] [CrossRef]
- Bläker, M.; Schmitz, M.; Gocht, A.; Burghardt, S.; Schulz, M.; Bröring, D.C.; Pace, A.; Greten, H.; De Weerth, A. Differential expression of somatostatin receptor subtypes in hepatocellular carcinomas. J. Hepatol. 2004, 41, 112–118. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B. Concomitant expression of several peptide receptors in neuroendocrine tumours: Molecular basis for in vivo multireceptor tumour targeting. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 781–793. [Google Scholar] [CrossRef]
- Reubi, J.C.; Zimmermann, A.; Jonas, S.; Waser, B.; Neuhaus, P.; Läderach, U.; Wiedenmann, B. Regulatory peptide receptors in human hepatocellular carcinomas. Gut 1999, 45, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Reynaert, H.; Rombouts, K.; Vandermonde, A.; Urbain, D.; Kumar, U.; Bioulac-Sage, P.; Pinzani, M.; Rosenbaum, J.; Geerts, A. Expression of somatostatin receptors in normal and cirrhotic human liver and in hepatocellular carcinoma. Gut 2004, 53, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Khac, E.; Ollivier, I.; Aparicio, T.; Moullart, V.; Hugentobler, A.; Lebtahi, R.; Lobry, C.; Susini, C.; Duhamel, C.; Hommel, S.; et al. Somatostatin receptor scintigraphy screening in advanced hepatocarcinoma: A multi-center French study. Cancer Biol. Ther. 2009, 8, 2033–2039. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, C.; van Dekken, H.; Hofland, L.J.; Zondervan, P.E.; de Wilt, J.H.; van Marion, R.; de Man, R.A.; IJzermans, J.N.; van Eijck, C.H. Somatostatin receptors in human hepatocellular carcinomas: Biological, patient and tumor characteristics. Dig. Surg. 2008, 25, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Huo, L.; Wang, L. Octreotide inhibits proliferation and induces apoptosis of hepatocellular carcinoma cells. Acta Pharmacol. Sin. 2004, 25, 1380–1386. [Google Scholar]
- Li, S.; Liu, Y.; Shen, Z. Characterization of somatostatin receptor 2 and 5 expression in operable hepatocellular carcinomas. Hepatogastroenterology 2012, 59, 2054–2058. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, L.; Mu, Y. Somatostatin receptor subtypes 2 and 5 are associated with better survival in operable hepatitis B-related hepatocellular carcinoma following octreotide long-acting release treatment. Oncol. Lett. 2013, 6, 821–828. [Google Scholar] [CrossRef][Green Version]
- Huang, C.Z.; Huang, A.M.; Liu, J.F.; Wang, B.; Lin, K.C.; Ye, Y.B. Somatostatin Octapeptide Inhibits Cell Invasion and Metastasis in Hepatocellular Carcinoma Through PEBP1. Cell Physiol. Biochem. 2018, 47, 2340–2349. [Google Scholar] [CrossRef]
- Hua, Y.P.; Huang, J.F.; Liang, L.J.; Li, S.Q.; Lai, J.M.; Liang, H.Z. The study of inhibition effect of octreotide on the growth of hepatocellular carcinoma xenografts in situ in nude mice. Chin. J. Surg. 2005, 43, 721–725. [Google Scholar]
- Jia, W.D.; Xu, G.L.; Wang, W.; Wang, Z.H.; Li, J.S.; Ma, J.L.; Ren, W.H.; Ge, Y.S.; Yu, J.H.; Liu, W.B. A somatostatin analogue, octreotide, inhibits the occurrence of second primary tumors and lung metastasis after resection of hepatocellular carcinoma in mice. Tohoku J. Exp. Med. 2009, 218, 155160. [Google Scholar] [CrossRef][Green Version]
- Reynaert, H.; Colle, I. Treatment of Advanced Hepatocellular Carcinoma with Somatostatin Analogues: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 4811. [Google Scholar] [CrossRef] [PubMed]
- Kouroumalis, E.; Skordilis, P.; Thermos, K.; Vasilaki, A.; Moschandrea, J.; Manousos, O.N. Treatment of hepatocellular carcinoma with octreotide: A randomised controlled study. Gut 1998, 42, 442–447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dimitroulopoulos, D.; Xinopoulos, D.; Tsamakidis, K.; Zisimopoulos, A.; Andriotis, E.; Panagiotakos, D.; Fotopoulou, A.; Chrysohoou, C.; Bazinis, A.; Daskalopoulou, D.; et al. Long acting octreotide in the treatment of advanced hepatocellular cancer and overexpression of somatostatin receptors: Randomized placebo-controlled trial. World J. Gastroenterol. 2007, 13, 3164–3170. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.; Allgaier, H.P.; Olschewski, M.; Zähringer, A.; Blum, H.E.; HECTOR Study Group. Long-acting octreotide versus placebo for treatment of advanced HCC: A randomized controlled double-blind study. Hepatology 2007, 45, 9–15. [Google Scholar] [CrossRef]
- Barbare, J.C.; Bouché, O.; Bonnetain, F.; Dahan, L.; Lombard-Bohas, C.; Faroux, R.; Raoul, J.L.; Cattan, S.; Lemoine, A.; Blanc, J.F.; et al. Treatment of advanced hepatocellular carcinoma with long-acting octreotide: A phase III multicenter, randomised, double blind placebo-controlled study. Eur. J. Cancer 2009, 45, 1788–1797. [Google Scholar] [CrossRef]
- Samonakis, D.N.; Notas, G.; Christodoulakis, N.; Kouroumalis, E.A. Mechanisms of action and resistance of somatostatin analogues for the treatment of hepatocellular carcinoma: A message not well taken. Dig. Dis. Sci. 2008, 53, 2359–2365. [Google Scholar] [CrossRef]
- Kaemmerer, D.; Schindler, R.; Mußbach, F.; Dahmen, U.; Altendorf-Hofmann, A.; Dirsch, O.; Sänger, J.; Schulz, S.; Lupp, A. Somatostatin and CXCR4 chemokine receptor expression in hepatocellular and cholangiocellular carcinomas: Tumor capillaries as promising targets. BMC Cancer 2017, 17, 896. [Google Scholar] [CrossRef]
- Rinke, A.; Müller, H.H.; Schade-Brittinger, C.; Klose, K.J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.F.; Bläker, M.; et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID study group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: The CLARINET open-label extension study. Endocr. Relat. Cancer 2016, 23, 191–199. [Google Scholar] [CrossRef]
- Sheppard, M.; Shapiro, B.; Pimstone, B.; Kronheim, S.; Berelowitz, M.; Gregory, M. Metabolic clearance and plasma half-disappearance time of exogenous somatostatin in man. J. Clin. Endocrinol. Metab. 1979, 48, 50–53. [Google Scholar] [CrossRef]
- Lowell, A.; Freda, P.U. From somatostatin to octreotide LAR: Evolution of a somatostatin analogue. Curr. Med. Res. Opin. 2009, 25, 2989–2999. [Google Scholar] [CrossRef]
- Öberg, K.; Lamberts, S.W.J. Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: Past, present and future. Endocr. Relat. Cancer 2016, 23, R551–R566. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.; McBride, A.; Ray, D.; Pulgar, S.; Ramirez, R.A.; Elquza, E.; Favaro, J.P.; Dranitsaris, G. Lanreotide vs. octreotide LAR for patients with advanced gastroenteropancreatic neuroendocrine tumors: An observational time and motion analysis. J. Oncol. Pharm. Pract. 2019, 25, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Feelders, R.A.; Yasothan, U.; Kirkpatrick, P. Pasireotide. Nat. Rev. Drug Discov. 2012, 11, 597–598. [Google Scholar] [CrossRef]
- Crider, A.M. Recent Advances in the Development of Nonpeptide Somatostatin Receptor Ligands. Mini Rev. Med. Chem. 2002, 2, 507–517. [Google Scholar] [CrossRef]
- Correia, J.D.; Paulo, A.; Raposinho, P.D.; Santos, I. Radiometallated peptides for molecular imaging and targeted therapy. Dalton Trans. 2011, 40, 6144–6167. [Google Scholar] [CrossRef]
- Jamous, M.; Haberkorn, U.; Mier, W. Synthesis of Peptide Radiopharmaceuticals for the Therapy and Diagnosis of Tumor Diseases. Molecules 2013, 18, 3379–3409. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Buonaguro, L.; Tornesello, M.L.; Buonaguro, F.M. New Insights in the Design of Bioactive Peptides and Chelating Agents for Imaging and Therapy in Oncology. Molecules 2017, 22, 1282. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Tornesello, M.L.; Buonaguro, F.M. An Overview of Bioactive Peptides for in vivo Imaging and Therapy in Human Diseases. Mini Rev. Med. Chem. 2017, 17, 758–770. [Google Scholar] [CrossRef]
- Cutler, C.S.; Hennkens, H.M.; Sisay, N.; Huclier-Markai, S.; Jurisson, S.S. Radiometals for Combined Imaging and Therapy. Chem. Rev. 2013, 113, 858–883. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Orvig, C. Tumour Targeting with Radiometals for Diagnosis and Therapy. Chem. Commun. 2013, 49, 4720–4739. [Google Scholar] [CrossRef] [PubMed]
- Blower, P.J. A nuclear chocolate box: The periodic table of nuclear medicine. Dalton Trans. 2015, 44, 4819–4844. [Google Scholar] [CrossRef] [PubMed]
- Kostelnik, T.I.; Orvig, C. Radioactive Main Group and Rare Earth Metals for Imaging and Therapy. Chem. Rev. 2019, 119, 902–956. [Google Scholar] [CrossRef] [PubMed]
- Price, E.W.; Orvig, C. Matching Chelators to Radiometals for Radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Packard, A.B. Radioactive Transition Metals for Imaging and Therapy. Chem. Rev. 2019, 119, 870–901. [Google Scholar] [CrossRef]
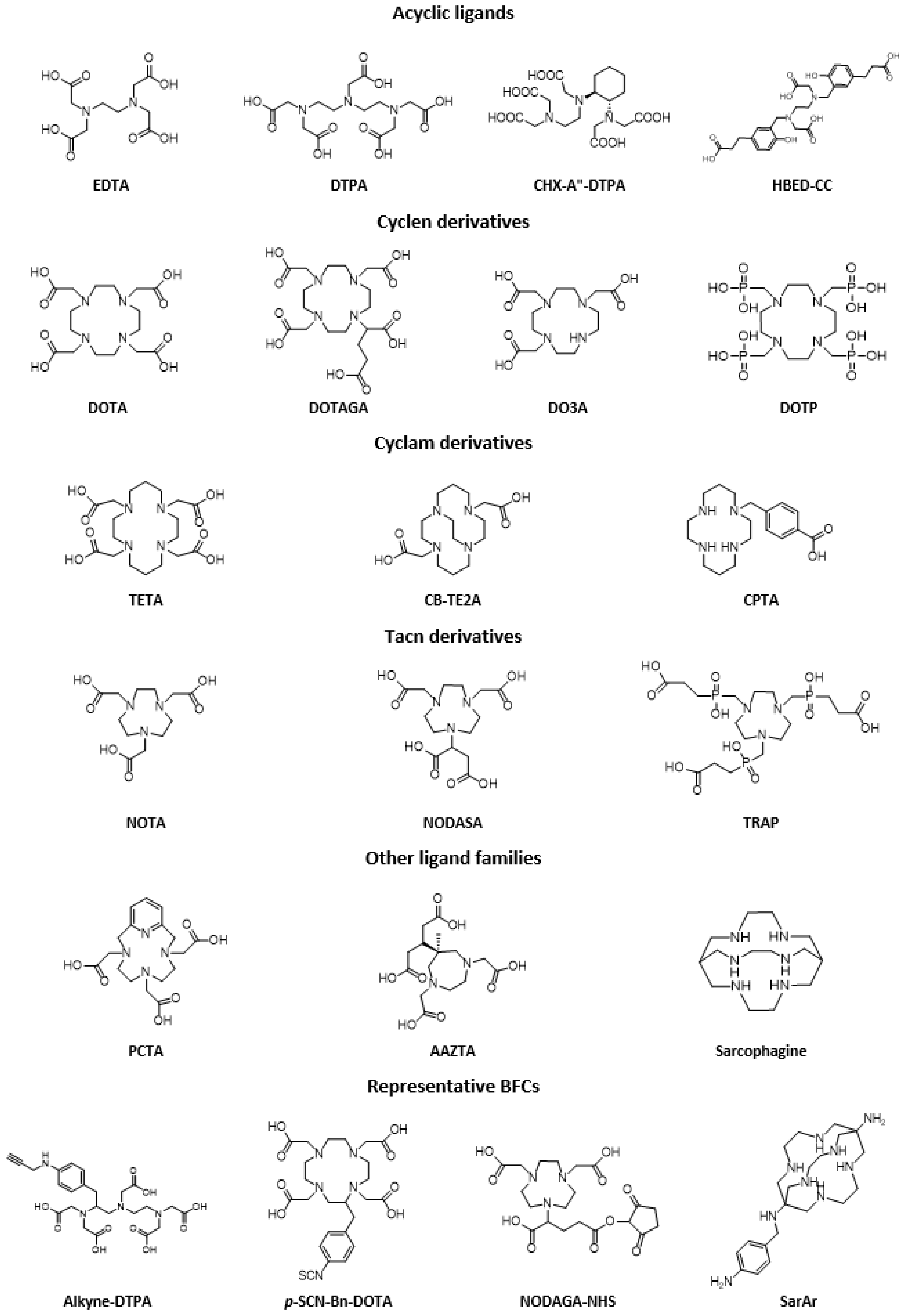
- Hancock, R.D.; Martell, A.E. Ligand design for selective complexation of metal ions in aqueous solution. Chem. Rev. 1989, 89, 1875–1914. [Google Scholar] [CrossRef]
- Stasiuk, G.J.; Long, N.J. The ubiquitous DOTA and its derivatives: The impact of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid on biomedical imaging. Chem. Commun. 2013, 49, 2732–2746. [Google Scholar] [CrossRef]
- Baranyai, Z.; Tircsó, G.; Rösch, F. The Use of the Macrocyclic Chelator DOTA in Radiochemical Separations. Eur. J. Inorg. Chem. 2020, 36–56. [Google Scholar] [CrossRef]
- Sun, X.; Wuest, M.; Weisman, G.R.; Wong, E.H.; Reed, D.P.; Boswell, C.A.; Motekaitis, R.; Martell, A.E.; Welch, M.J.; Anderson, C.J. Radiolabeling and in vivo behavior of copper-64-labeled cross-bridged cyclam ligands. J. Med. Chem. 2002, 45, 469–477. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Dixit, M. Metallic radionuclides in the development of diagnostic and therapeutic radiopharmaceuticals. Dalton Trans. 2011, 40, 6112–6128. [Google Scholar] [CrossRef]
- Mushtaq, S.; Yun, S.J.; Jeon, J. Recent Advances in Bioorthogonal Click Chemistry for Efficient Synthesis of Radiotracers and Radiopharmaceuticals. Molecules 2019, 24, 3567. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Maecke, H.R. Peptide-based probes for cancer imaging. J. Nucl. Med. 2008, 49, 1735–1738. [Google Scholar] [CrossRef] [PubMed]
- Maecke, H.R.; Reubi, J.C. Somatostatin receptors as targets for nuclear medicine imaging and radionuclide treatment. J. Nucl. Med. 2011, 52, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Schär, J.C.; Waser, B.; Wenger, S.; Heppeler, A.; Schmitt, J.S.; Mäcke, H.R. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000, 27, 273–282. [Google Scholar] [CrossRef]
- Fani, M.; Maecke, H.R. Radiopharmaceutical development of radiolabeled peptides. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, S11–S30. [Google Scholar] [CrossRef]
- Lamberts, S.W.; Bakker, W.H.; Reubi, J.C.; Krenning, E.P. Somatostatin-receptor imaging in the localization of endocrine tumors. N. Engl. J. Med. 1990, 323, 1246–1249. [Google Scholar] [CrossRef]
- Bakker, W.H.; Krenning, E.P.; Breeman, W.A.; Kooij, P.P.; Reubi, J.C.; Koper, J.W.; de Jong, M.; Laméris, J.S.; Visser, T.J.; Lamberts, S.W. In vivo use of a radioiodinated somatostatin analogue: Dynamics, metabolism, and binding to somatostatin receptor-positive tumors in man. J. Nucl. Med. 1991, 32, 1184–1189. [Google Scholar]
- Bakker, W.H.; Krenning, E.P.; Breeman, W.A.; Koper, J.W.; Kooij, P.P.; Reubi, J.C.; Klijn, J.G.; Visser, T.J.; Docter, R.; Lamberts, S.W. Receptor scintigraphy with a radioiodinated somatostatin analogue: Radiolabeling, purification, biologic activity, and in vivo application in animals. J. Nucl. Med. 1990, 31, 1501–1509. [Google Scholar]
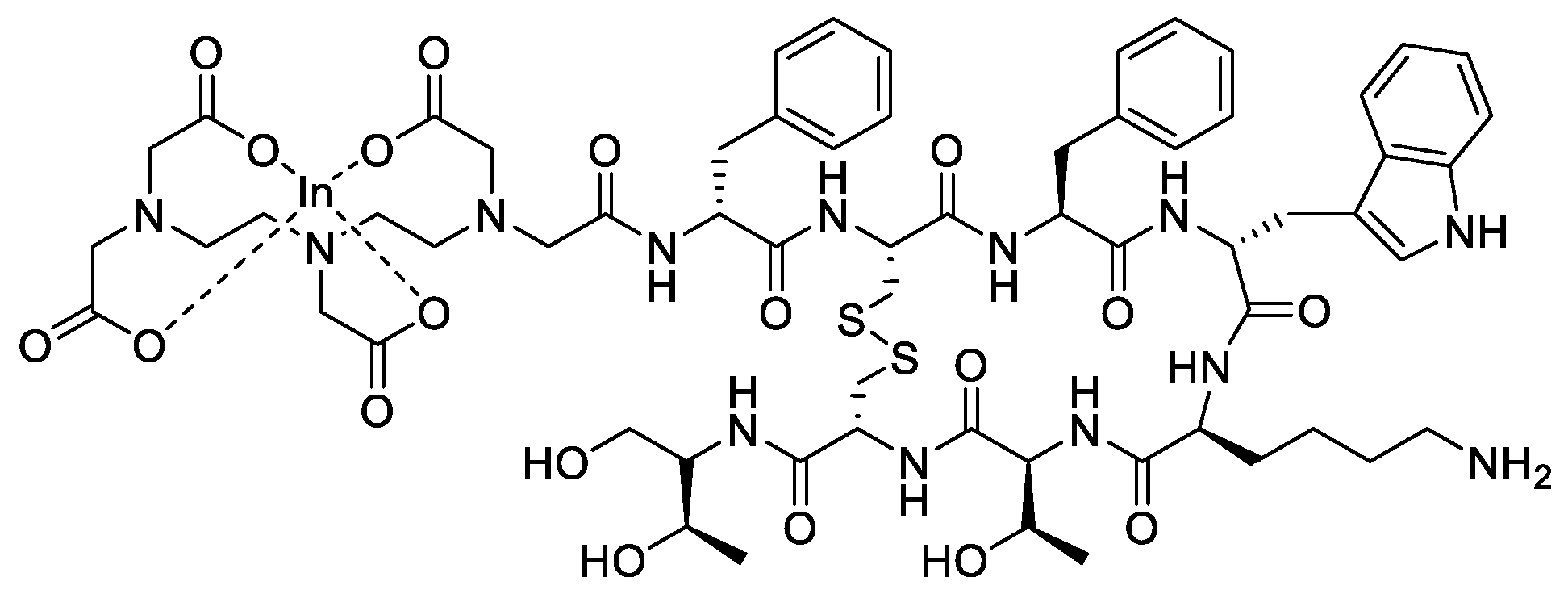
- Bakker, W.H.; Albert, R.; Bruns, C.; Breeman, W.A.; Hofland, L.J.; Marbach, P.; Pless, J.; Pralet, D.; Stolz, B.; Koper, J.W.; et al. [111In-DTPA-D-Phe1]-octreotide, a potential radiopharmaceutical for imaging of somatostatin receptor-positive tumors: Synthesis, radiolabeling and in vitro validation. Life Sci. 1991, 49, 1583–1591. [Google Scholar] [CrossRef]
- Bakker, W.H.; Krenning, E.P.; Reubi, J.C.; Breeman, W.A.; Setyono-Han, B.; de Jong, M.; Kooij, P.P.; Bruns, C.; van Hagen, P.M.; Marbach, P.; et al. In vivo application of [111In-DTPA-D-Phe1]-octreotide for detection of somatostatin receptor-positive tumors in rats. Life Sci. 1991, 49, 1593–1601. [Google Scholar] [CrossRef]
- Krenning, E.P.; Bakker, W.H.; Kooij, P.P.; Breeman, W.A.; Oei, H.Y.; de Jong, M.; Reubi, J.C.; Visser, T.J.; Bruns, C.; Kwekkeboom, D.J.; et al. Somatostatin receptor scintigraphy with indium-111-DTPA-D-Phe-1-octreotide in man: Metabolism, dosimetry and comparison with iodine-123-Tyr-3-octreotide. J. Nucl. Med. 1992, 33, 652–658. [Google Scholar] [PubMed]
- Otte, A.; Jermann, E.; Behe, M.; Goetze, M.; Bucher, H.C.; Roser, H.W.; Heppeler, A.; Mueller-Brand, J.; Maecke, H.R. DOTATOC: A powerful new tool for receptor-mediated radionuclide therapy. Eur. J. Nucl. Med. 1997, 24, 792–795. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Forrer, F.; Uusijärvi, H.; Waldherr, C.; Cremonesi, M.; Bernhardt, P.; Mueller-Brand, J.; Maecke, H.R. A comparison of 111In-DOTATOC and 111In-DOTATATE: Biodistribution and dosimetry in the same patients with metastatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Schmitt, J.S.; Ginj, M.; Mäcke, H.R.; Bernard, B.F.; Krenning, E.; De Jong, M.; Wenger, S.; Reubi, J.C. DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labeling with various radiometals. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1338–1347. [Google Scholar] [CrossRef]
- Virgolini, I.; Ambrosini, V.; Bomanji, J.B.; Baum, R.P.; Fanti, S.; Gabriel, M.; Papathanasiou, N.D.; Pepe, G.; Oyen, W.; De Cristoforo, C.; et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2004–2010. [Google Scholar] [CrossRef]
- Hofmann, M.; Maecke, H.; Börner, R.; Weckesser, E.; Schöffski, P.; Oei, L.; Schumacher, J.; Henze, M.; Heppeler, A.; Meyer, J.; et al. Biokinetics and imaging with the somatostatin receptor PET radioligand 68Ga-DOTATOC: Preliminary data. Eur. J. Nucl. Med. 2001, 28, 1751–1757. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lau, W.F.; Hicks, R.J. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: Clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics 2015, 35, 500–516. [Google Scholar] [CrossRef]
- Wild, D.; Mäcke, H.R.; Waser, B.; Reubi, J.C.; Ginj, M.; Rasch, H.; Müller-Brand, J.; Hofmann, M. 68Ga-DOTANOC: A first compound for PET imaging with high affinity for somatostatin receptor subtypes 2 and 5. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 724. [Google Scholar] [CrossRef]
- Antunes, P.; Ginj, M.; Walter, M.A.; Chen, J.; Reubi, J.C.; Maecke, H.R. Influence of different spacers on the biological profile of a DOTA-Somatostatin analogue. Bioconjugate Chem. 2007, 18, 84–92. [Google Scholar] [CrossRef]
- Eisenwiener, K.P.; Prata, M.I.; Buschmann, I.; Zhang, H.W.; Santos, A.C.; Wenger, S.; Reubi, J.C.; Mäcke, H.R. NODAGATOC, a new chelator-coupled somatostatin analogue labeled with [67/68Ga] and [111In] for SPECT, PET, and targeted therapeutic applications of somatostatin receptor (hsst2) expressing tumors. Bioconjugate Chem. 2002, 13, 530–541. [Google Scholar] [CrossRef]
- Laznickova, A.; Laznicek, M.; Trejtnar, F.; Maecke, H.R.; Eisenwiener, K.P.; Reubi, J.C. Biodistribution of two octreotate analogs radiolabeled with indium and yttrium in rats. Anticancer Res. 2010, 30, 2177–2184. [Google Scholar] [PubMed]
- Ginj, M.; Chen, J.; Walter, M.A.; Eltschinger, V.; Reubi, J.C.; Maecke, H.R. Preclinical evaluation of new and highly potent analogues of octreotide for predictive imaging and targeted radiotherapy. Clin. Cancer Res. 2005, 11, 1136–1145. [Google Scholar] [PubMed]
- Boubaker, A.; Prior, J.O.; Willi, J.P.; Champendal, M.; Kosinski, M.; Bischof-Delaloye, A.; Maecke, H.R.; Ginj, M.; Baechler, S.; Buchegger, F. Biokinetics and dosimetry of 111In-DOTA-NOC-ATE compared with 111In-DTPA-octreotide. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1868–1875. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, M.T.; Cullinane, C.; Waldeck, K.; Roselt, P.; Hicks, R.J.; Blower, P.J. Rapid kit-based 68Ga-labeling and PET imaging with THP-Tyr3-octreotate: A preliminary comparison with DOTA-Tyr3octreotate. EJNMMI Res. 2015, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Mueller, A.; Tamma, M.L.; Nicolas, G.; Rink, H.R.; Cescato, R.; Reubi, J.C.; Maecke, H.R. Radiolabeled bicyclic somatostatin-based analogs: A novel class of potential radiotracers for SPECT/PET of neuroendocrine tumors. J. Nucl. Med. 2010, 51, 1771–1779. [Google Scholar] [CrossRef][Green Version]
- Ginj, M.; Zhang, H.; Eisenwiener, K.P.; Wild, D.; Schulz, S.; Rink, H.; Cescato, R.; Reubi, J.C.; Maecke, H.R. New pansomatostatin ligands and their chelated versions: Affinity profile, agonist activity, internalization, and tumor targeting. Clin. Cancer Res. 2008, 14, 2019–2027. [Google Scholar] [CrossRef][Green Version]
- Liu, F.; Liu, T.; Xu, X.; Guo, X.; Li, N.; Xiong, C.; Li, C.; Zhu, H.; Yang, Z. Design, Synthesis, and Biological Evaluation of 68Ga-DOTA-PA1 for Lung Cancer: A Novel PET Tracer for Multiple Somatostatin Receptor Imaging. Mol. Pharm. 2018, 15, 619–628. [Google Scholar] [CrossRef]
- Tatsi, A.; Maina, T.; Cescato, R.; Waser, B.; Krenning, E.P.; de Jong, M.; Cordopatis, P.; Reubi, J.C.; Nock, B.A. [111In-DOTA]Somatostatin-14 analogs as potential pansomatostatin-like radiotracers—First results of a preclinical study. EJNMMI Res. 2012, 2, 25. [Google Scholar] [CrossRef]
- Maina, T.; Cescato, R.; Waser, B.; Tatsi, A.; Kaloudi, A.; Krenning, E.P.; de Jong, M.; Nock, B.A.; Reubi, J.C. [111In-DOTA]LTT-SS28, a first pansomatostatin radioligand for in vivo targeting of somatostatin receptor-positive tumors. J. Med. Chem. 2014, 57, 6564–6571. [Google Scholar] [CrossRef]
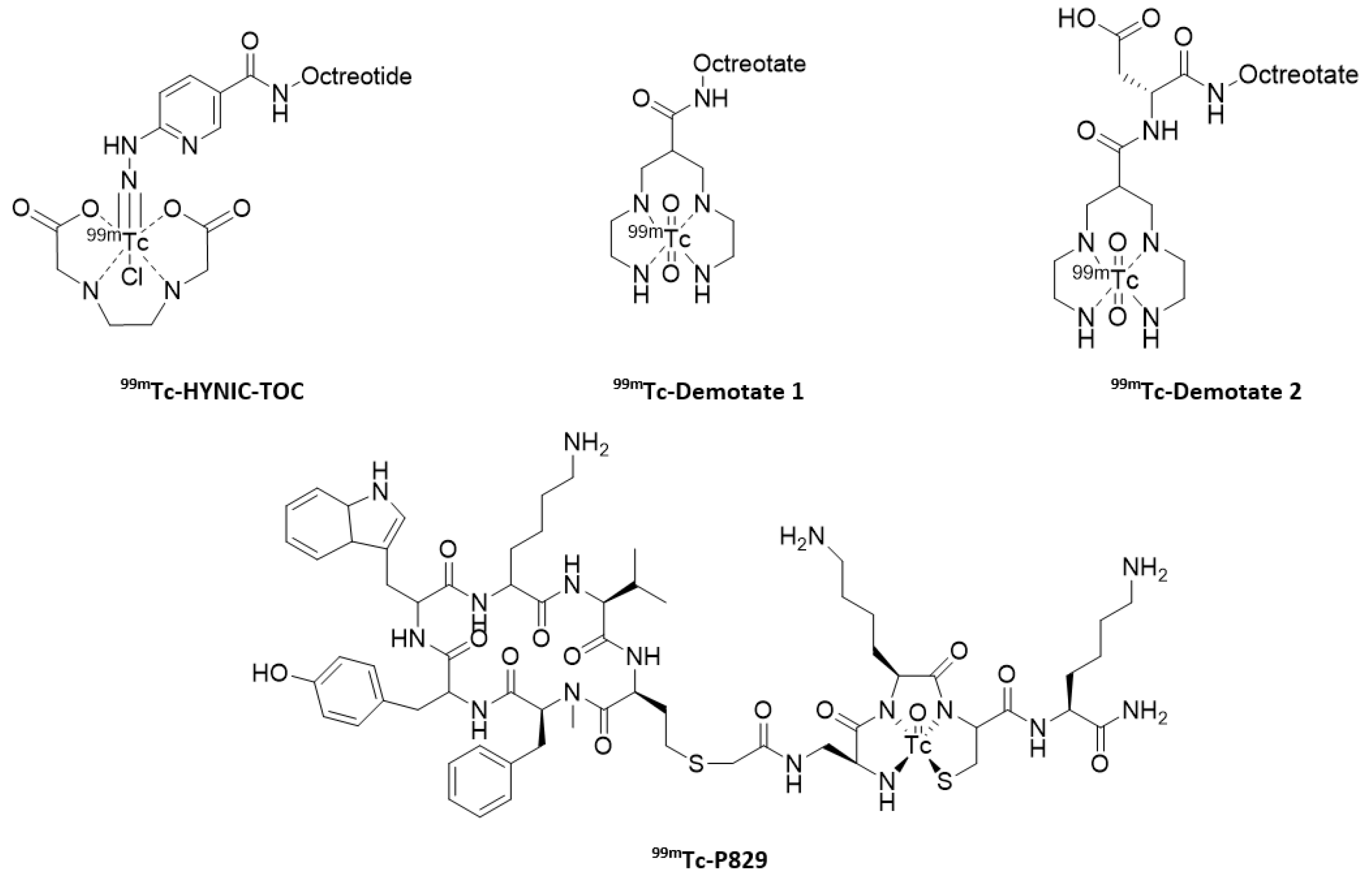
- Pearson, D.A.; Lister-James, J.; McBride, W.J.; Wilson, D.M.; Martel, L.J.; Civitello, E.R.; Taylor, J.E.; Moyer, B.R.; Dean, R.T. Somatostatin receptor-binding peptides labeled with technetium-99m: Chemistry and initial biological studies. J. Med. Chem. 1996, 39, 1361–1371. [Google Scholar] [CrossRef]
- Decristoforo, C.; Mather, S.J. Preparation, 99mTc-labeling and in vitro characterization of HYNIC and N3S modified RC-160 and [Tyr3]octreotide. Bioconjugate Chem. 1999, 10, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Maina, T.; Stolz, B.; Albert, R.; Bruns, C.; Koch, P.; Mäcke, H. Synthesis, radiochemistry and biological evaluation of a new somatostatin analogue (SDZ 219-387) labeled with technetium-99m. Eur. J. Nucl. Med. 1994, 21, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.L.; Kolan, H.; Li, J.; Wiaderkiewicz, R.; Pallela, V.R.; Duggaraju, R.; Schally, A.V. Radiolabeled somatostatin analogues in prostate cancer. Nucl. Med. Biol. 1997, 24, 105–113. [Google Scholar] [CrossRef]
- Abiraj, K.; Ursillo, S.; Tamma, M.L.; Rylova, S.N.; Waser, B.; Constable, E.C.; Fani, M.; Nicolas, G.P.; Reubi, J.C.; Maecke, H.R. The tetraamine chelator outperforms HYNIC in a new technetium-99m-labeled somatostatin receptor 2 antagonist. EJNMMI Res. 2018, 8, 75. [Google Scholar] [CrossRef]
- Spradau, T.W.; Edwards, W.B.; Anderson, C.J.; Welch, M.J.; Katzenellenbogen, J.A. Synthesis and biological evaluation of Tc-99m-cyclopentadienyltricarbonyltechnetium-labeled octreotide. Nucl. Med. Biol. 1999, 26, 1–7. [Google Scholar] [CrossRef]
- Makris, G.; Kuchuk, M.; Gallazzi, F.; Jurisson, S.S.; Smith, C.J.; Hennkens, H.M. Somatostatin receptor targeting with hydrophilic [99mTc/186Re]Tc/Re-tricarbonyl NODAGA and NOTA complexes. Nucl. Med. Biol. 2019, 71, 39–46. [Google Scholar] [CrossRef]
- Abrams, M.J.; Juweid, M.; tenKate, C.I.; Schwartz, D.A.; Hauser, M.M.; Gaul, F.E.; Fuccello, A.J.; Rubin, R.H.; Strauss, H.W.; Fischman, A.J. Technetium-99m-human polyclonal IgG radiolabeled via the hydrazino nicotinamide derivative for imaging focal sites of infection in rats. J. Nucl. Med. 1990, 31, 2022–2028. [Google Scholar]
- Mikolajczak, R.; Maecke, H.R. Radiopharmaceuticals for somatostatin receptor imaging. Nucl. Med. Rev. Cent. East Eur. 2016, 19, 126–132. [Google Scholar] [CrossRef]
- Decristoforo, C.; Melendez-Alafort, L.; Sosabowski, J.K.; Mather, S.J. 99mTc-HYNIC-[Tyr3]octreotide for imaging somatostatin receptor positive tumors: Preclinical evaluation and comparison with 111In-octreotide. J. Nucl. Med. 2000, 41, 1114–1119. [Google Scholar]
- Gabriel, M.; Decristoforo, C.; Donnemiller, E.; Ulmer, H.; Watfah Rychlinski, C.; Mather, S.J.; Moncayo, R. An intrapatient comparison of 99mTc-EDDA/HYNIC-TOC with 111In-DTPA-Octreotide for diagnosis of somatostatin receptor expressing tumors. J. Nucl. Med. 2003, 44, 708–716. [Google Scholar]
- Cwikla, J.B.; Mikolajczak, R.; Pawlak, D.; Buscombe, J.R.; Nasierowska-Guttmejer, A.; Bator, A.; Maecke, H.R.; Walecki, J. Initial direct comparison of 99mTc-TOC and 99mTc-TATE in identifying sites of disease in patients with proven GEP NETs. J. Nucl. Med. 2008, 49, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Decristoforo, C.; Maina, T.; Nock, B.; Gabriel, M.; Cordopatis, P.; Moncayo, R. 99mTc-Demotate 1: First data in tumour patients-results of a pilot/phase I study. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Maina, T.; Nock, B.A.; Cordopatis, P.; Bernard, B.F.; Breeman, W.A.; van Gameren, A.; van den Berg, R.; Reubi, J.C.; Krenning, E.P.; de Jong, M. [99mTc]Demotate 2 in the detection of sst2-positive tumours: A preclinical comparison with [111In]DOTA-tate. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Virgolini, I.; Leimer, M.; Handmaker, H.; Lastoria, S.; Bischof, C.; Muto, P.; Pangerl, T.; Gludovacz, D.; Peck-Radosavljevic, M.; Lister-James, J.; et al. Somatostatin receptor subtype specificity and in vivo binding of a novel tumor tracer, 99mTc-P829. Cancer Res. 1998, 58, 1850–1859. [Google Scholar]
- Lebtahi, R.; Le Cloirec, J.; Houzard, C.; Daou, D.; Sobhani, I.; Sassolas, G.; Mignon, M.; Bourguet, P.; Le Guludec, D. Detection of neuroendocrine tumors: 99mTc-P829 scintigraphy compared with 111In-pentetreotide scintigraphy. J. Nucl. Med. 2002, 43, 889–895. [Google Scholar]
- Blum, J.E.; Handmaker, H.; Rinne, N.A. The utility of a somatostatin-type receptor binding peptide radiopharmaceutical (P829) in the evaluation of solitary pulmonary nodules. Chest 1999, 115, 224–232. [Google Scholar] [CrossRef]
- Bååth, M.; Kolbeck, K.G.; Danielsson, R. Somatostatin receptor scintigraphy with 99mTc-Depreotide (NeoSpect) in discriminating between malignant and benign lesions in the diagnosis of lung cancer: A pilot study. Acta Radiol. 2004, 45, 833–839. [Google Scholar] [CrossRef]
- Axelsson, R.; Herlin, G.; Bååth, M.; Aspelin, P.; Kolbeck, K.G. Role of scintigraphy with technetium-99m depreotide in the diagnosis and management of patients with suspected lung cancer. Acta Radiol. 2008, 49, 295–302. [Google Scholar] [CrossRef]
- Menda, Y.; Kahn, D. Somatostatin receptor imaging of non-small cell lung cancer with 99mTc depreotide. Semin. Nucl. Med. 2002, 32, 92–96. [Google Scholar] [CrossRef]
- Van Den Bossche, B.; D’haeninck, E.; Bacher, K.; Thierens, H.; Van Belle, S.; Dierckx, R.A.; Van de Wiele, C. Biodistribution and dosimetry of 99mTc-depreotide (P829) in patients suffering from breast carcinoma. Cancer Biother. Radiopharm. 2004, 19, 776–783. [Google Scholar] [CrossRef]
- Briganti, V.; Cuccurullo, V.; Di Stasio, G.D.; Mansi, L. Gamma Emitters in Pancreatic Endocrine Tumors Imaging in the PET Era: Is there a Clinical Space for 99mTc-peptides? Curr. Radiopharm. 2019, 12, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Boschi, A.; Uccelli, L.; Martini, P. A Picture of Modern Tc-99m Radiopharmaceuticals: Production, Chemistry, and Applications in Molecular Imaging. Appl. Sci. 2019, 9, 2526. [Google Scholar] [CrossRef]
- Boschi, A.; Martini, P.; Janevik-Ivanovska, E.; Duatti, A. The emerging role of copper-64 radiopharmaceuticals as cancer theranostics. Drug Discov. Today 2018, 23, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.J.; Dehdashti, F.; Cutler, P.D.; Schwarz, S.W.; Laforest, R.; Bass, L.A.; Lewis, J.S.; McCarthy, D.W. 64Cu-TETA-octreotide as a PET imaging agent for patients with neuroendocrine tumors. J. Nucl. Med. 2001, 42, 213–221. [Google Scholar]
- Sprague, J.E.; Peng, Y.; Sun, X.; Weisman, G.R.; Wong, E.H.; Achilefu, S.; Anderson, C.J. Preparation and biological evaluation of copper-64-labeled Tyr3-octreotate using a cross-bridged macrocyclic chelator. Clin. Cancer Res. 2004, 10, 8674–8682. [Google Scholar] [CrossRef]
- Edwards, W.B.; Fields, C.G.; Anderson, C.J.; Pajeau, T.S.; Welch, M.J.; Fields, G.B. Generally Applicable, Convenient Solid-Phase Synthesis and Receptor Affinities of Octreotide Analogs. J. Med. Chem. 1994, 37, 3749–3757. [Google Scholar] [CrossRef]
- Paterson, B.M.; Roselt, P.; Denoyer, D.; Cullinane, C.; Binns, D.; Noonan, W.; Jeffery, C.M.; Price, R.I.; White, J.M.; Hicks, R.J.; et al. PET imaging of tumours with a 64Cu labeled macrobicyclic cage amine ligand tethered to Tyr3-octreotate. Dalton Trans. 2014, 43, 1386–1396. [Google Scholar] [CrossRef]
- Marciniak, A.; Brasuń, J. Somatostatin analogues labeled with copper radioisotopes: Currrent status. J. Radioanal. Nucl. Chem. 2017, 313, 279–289. [Google Scholar] [CrossRef]
- Pfeifer, A.; Knigge, U.; Binderup, T.; Mortensen, J.; Oturai, P.; Loft, A.; Berthelsen, A.K.; Langer, S.W.; Rasmussen, P.; Elema, D.; et al. 64Cu-DOTATATE PET for Neuroendocrine Tumors: A Prospective Head-to-Head Comparison with 111In-DTPA-Octreotide in 112 Patients. J. Nucl. Med. 2015, 56, 847–854. [Google Scholar] [CrossRef]
- Johnbeck, C.B.; Knigge, U.; Loft, A.; Berthelsen, A.K.; Mortensen, J.; Oturai, P.; Langer, S.W.; Elema, D.R.; Kjaer, A. Head-to-head comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: A prospective study of 59 patients with neuroendocrine tumors. J. Nucl. Med. 2017, 58, 451–458. [Google Scholar] [CrossRef]
- Carlsen, E.A.; Johnbeck, C.B.; Binderup, T.; Loft, M.; Pfeifer, A.; Mortensen, J.; Oturai, P.; Loft, A.; Berthelsen, A.K.; Langer, S.W.; et al. 64Cu-DOTATATE PET/CT and prediction of overall and progression-free survival in patients with neuroendocrine neoplasms. J. Nucl. Med. 2020. [Google Scholar] [CrossRef]
- Andersen, T.L.; Baun, C.; Olsen, B.B.; Dam, J.H.; Thisgaard, H. Improving Contrast and Detectability: Imaging with [55Co]Co-DOTATATE in Comparison with [64Cu]Cu-DOTATATE and [68Ga]Ga-DOTATATE. J. Nucl. Med. 2020, 61, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Revheim, M.; Raynor, W.; Zehetner, W.; Knoll, P.; Zandieh, S.; Alavi, A. 64Cu-DOTATOC PET-CT in Patients with Neuroendocrine Tumors. Oncol. Ther. 2019, 8, 125–131. [Google Scholar] [CrossRef]
- Hicks, R.J.; Jackson, P.; Kong, G.; Ware, R.E.; Hofman, M.S.; Pattison, D.A.; Akhurst, T.A.; Drummond, E.; Roselt, P.; Callahan, J.; et al. 64Cu-SARTATE PET Imaging of Patients with Neuroendocrine Tumors Demonstrates High Tumor Uptake and Retention, Potentially Allowing Prospective Dosimetry for Peptide Receptor Radionuclide Therapy. J. Nucl. Med. 2019, 60, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Delpassand, E.S.; Ranganathan, D.; Wagh, N.; Shafie, A.; Gaber, A.; Abbasi, A.; Kjaer, A.; Tworowska, I.; Núñez, R. 64Cu-DOTATATE PET/CT for Imaging Patients with Known or Suspected Somatostatin Receptor-Positive Neuroendocrine Tumors: Results of the First US Prospective, Reader-Blinded Clinical Trial. J. Nucl. Med. 2020, 61, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Heppeler, A.; André, J.P.; Buschmann, I.; Wang, X.; Reubi, J.C.; Hennig, M.; Kaden, T.A.; Maecke, H.R. Metal-ion-dependent biological properties of a chelator-derived somatostatin analogue for tumour targeting. Chem. Eur. J. 2008, 14, 3026–3034. [Google Scholar] [CrossRef] [PubMed]
- Thisgaard, H.; Olsen, B.B.; Dam, J.H.; Bollen, P.; Mollenhauer, J.; Høilund-Carlsen, P.F. Evaluation of cobalt-labeled octreotide analogs for molecular imaging and Auger electron-based radionuclide therapy. J. Nucl. Med. 2014, 55, 1311–1316. [Google Scholar] [CrossRef]
- Thisgaard, H.; Olesen, M.; Dam, J.H. Radiosynthesis of Co-55- and Co-58m-labeled DOTATOC for positron emission tomography imaging and targeted radionuclide therapy. J. Label. Compd. Radiopharm. 2011, 54, 758–762. [Google Scholar] [CrossRef]
- Müller, C.; Domnanich, K.A.; Umbricht, C.A.; van der Meulen, N.P. Scandium and terbium radionuclides for radiotheranostics: Current state of development towards clinical application. Br. J. Radiol. 2018, 91, 20180074. [Google Scholar] [CrossRef]
- Pruszyński, M.; Majkowska-Pilip, A.; Loktionova, N.S.; Eppard, E.; Roesch, F. Radiolabeling of DOTATOC with the long-lived positron emitter 44Sc. Appl. Radiat. Isot. 2012, 70, 974–979. [Google Scholar] [CrossRef]
- Müller, C.; Vermeulen, C.; Johnston, K.; Köster, U.; Schmid, R.; Türler, A.; van der Meulen, N.P. Preclinical in vivo application of 152Tb-DOTANOC: A radiolanthanide for PET imaging. EJNMMI Res. 2016, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; van der Meulen, N.P.; Muller, C.; Klette, I.; Kulkarni, H.R.; Türler, A.; Schibli, R.; Baum, R.P. First-in-human PET/CT imaging of metastatic neuroendocrine neoplasms with cyclotron-produced 44Sc-DOTATOC: A proof-of-concept study. Cancer Biother. Radiopharm. 2017, 32, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Singh, A.; Benešová, M.; Vermeulen, C.; Gnesin, S.; Köster, U.; Johnston, K.; Müller, D.; Senftleben, S.; Kulkarni, H.R.; et al. Clinical evaluation of the radiolanthanide terbium-152: First-in-human PET/CT with 152Tb-DOTATOC. Dalton Trans. 2017, 46, 14638–14646. [Google Scholar] [CrossRef] [PubMed]
- Koumarianou, E.; Pawlak, D.; Korsak, A.; Mikolajczak, R. Comparison of receptor affinity of natSc-DOTA-TATE versus natGa-DOTA-TATE. Nucl. Med. Rev. Cent. East Eur. 2011, 14, 85–89. [Google Scholar] [CrossRef]
- Domnanich, K.A.; Müller, C.; Farkas, R.; Schmid, R.M.; Ponsard, B.; Schibli, R.; Türler, A.; van der Meulen, N.P. 44Sc for labeling of DOTA- and NODAGA-functionalized peptides: Preclinical in vitro and in vivo investigations. EJNMMI Radiopharm. Chem. 2017, 1, 8. [Google Scholar] [CrossRef]
- Sinnes, J.P.; Nagel, J.; Rösch, F. AAZTA5/AAZTA5-TOC: Synthesis and radiochemical evaluation with 68Ga, 44Sc and 177Lu. EJNMMI Radiopharm. Chem. 2019, 4, 18. [Google Scholar] [CrossRef]
- Müller, C.; Fischer, E.; Behe, M.; Köster, U.; Dorrer, H.; Reber, J.; Haller, S.; Cohrs, S.; Blanc, A.; Grünberg, J.; et al. Future prospects for SPECT imaging using the radiolanthanide terbium-155—Production and preclinical evaluation in tumor-bearing mice. Nucl. Med. Biol. 2014, 41, e58–e65. [Google Scholar] [CrossRef]
- Walrand, S.; Jamar, F.; Mathieu, I.; De Camps, J.; Lonneux, M.; Sibomana, M.; Labar, D.; Michel, C.; Pauwels, S. Quantitation in PET using isotopes emitting prompt single gammas: Application to yttrium-86. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 354–361. [Google Scholar] [CrossRef]
- Helisch, A.; Förster, G.J.; Reber, H.; Buchholz, H.G.; Arnold, R.; Göke, B.; Weber, M.M.; Wiedenmann, B.; Pauwels, S.; Haus, U.; et al. Pre-therapeutic dosimetry and biodistribution of 86Y-DOTA-Phe1-Tyr3-octreotide versus 111In-pentetreotide in patients with advanced neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1386–1392. [Google Scholar] [CrossRef]
- Clifford, T.; Boswell, C.A.; Biddlecombe, G.B.; Lewis, J.S.; Brechbiel, M.W. Validation of a novel CHX-A “derivative suitable for peptide conjugation: Small animal PET/CT imaging using yttrium-86-CHX-A”-octreotide. J. Med. Chem. 2006, 49, 4297–4304. [Google Scholar] [CrossRef]
- Jamar, F.; Barone, R.; Mathieu, I.; Walrand, S.; Labar, D.; Carlier, P.; de Camps, J.; Schran, H.; Chen, T.; Smith, M.C.; et al. 86Y-DOTA0-D-Phe1-Tyr3-octreotide (SMT487)—A phase 1 clinical study: Pharmacokinetics, biodistribution and renal protective effect of different regimens of amino acid co-infusion. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczak, R.; van der Meulen, N.P.; Lapi, S.E. Radiometals for imaging and theranostics, current production, and future perspectives. J. Label. Compd. Radiopharm. 2019, 62, 615–634. [Google Scholar] [CrossRef] [PubMed]
- do Carmo, S.J.C.; Scott, P.J.H.; Alves, F. Production of radiometals in liquid targets. EJNMMI Radiopharm. Chem. 2020, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Talip, Z.; Favaretto, C.; Geistlich, S.; Meulen, N.P.V. A Step-by-Step Guide for the Novel Radiometal Production for Medical Applications: Case Studies with 68Ga, 44Sc, 177Lu and 161Tb. Molecules 2020, 25, 966. [Google Scholar] [CrossRef]
- Waldmann, C.M.; Stuparu, A.D.; van Dam, R.M.; Slavik, R. The search for an alternative to [68Ga]Ga-DOTA-TATE in neuroendocrine tumor theranostics: Current state of 18F-labeled somatostatin analog development. Theranostics 2019, 9, 1336–1347. [Google Scholar] [CrossRef]
- Guhlke, S.; Wester, H.J.; Bruns, C.; Stöcklin, G. (2-[18F]Fluoropropionyl-(D)phe1)-octreotide, a potential radiopharmaceutical for quantitative somatostatin receptor imaging with PET: Synthesis, Radiolabeling, in vitro validation and biodistribution in mice. Nucl. Med. Biol. 1994, 21, 819–825. [Google Scholar] [CrossRef]
- Hostetler, E.D.; Edwards, W.B.; Anderson, C.J.; Welch, M.J. Synthesis of 4-[18F]fluorobenzoyl octreotide and biodistribution in tumour-bearing Lewis rats. J. Label. Compd Radiopharm. 1999, 42, S720–S722. [Google Scholar]
- Wester, H.J.; Schottelius, M.; Poethko, T.; Bruus-Jensen, K.; Schwaiger, M. Radiolabeled Carbohydrated Somatostatin Analogs: A Review of the Current Status. Cancer Biother. Radiopharm. 2004, 19, 231–244. [Google Scholar] [CrossRef]
- Maschauer, S.; Heilmann, M.; Wängler, C.; Schirrmacher, R.; Prante, O. Radiosynthesis and preclinical evaluation of 18F-fluoroglycosylated octreotate for somatostatin receptor imaging. Bioconjugate Chem. 2016, 27, 2707–2714. [Google Scholar] [CrossRef]
- Liu, Z.; Pourghiasian, M.; Bénard, F.; Pan, J.; Lin, K.S.; Perrin, D.M. Preclinical evaluation of a high-affinity 18F-trifluoroborate octreotate derivative for somatostatin receptor imaging. J. Nucl. Med. 2014, 55, 1499–1505. [Google Scholar] [CrossRef]
- Niedermoser, S.; Chin, J.; Wängler, C.; Kostikov, A.; Bernard-Gauthier, V.; Vogler, N.; Soucy, J.P.; McEwan, A.J.; Schirrmacher, R.; Wängler, B. In vivo evaluation of 18F-SiFAlin-Modified TATE: A potential challenge for 68Ga-DOTATATE, the clinical gold standard for somatostatin receptor imaging with PET. J. Nucl. Med. 2015, 56, 1100–1105. [Google Scholar] [CrossRef]
- Allott, L.; Dubash, S.; Aboagye, E.O. [18F]FET-βAG-TOCA: The Design, Evaluation and Clinical Translation of a Fluorinated Octreotide. Cancers 2020, 12, 865. [Google Scholar] [CrossRef] [PubMed]
- Laverman, P.; McBride, W.J.; Sharkey, R.M.; Eek, A.; Joosten, L.; Oyen, W.J.G.; Goldenberg, D.M.; Boerman, O.C. A novel facile method of labeling octreotide with 18F-fluorine. J. Nucl. Med. 2010, 51, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Meisetschlaeger, G.; Poethko, T.; Stahl, A.; Wolf, I.; Scheidhauer, K.; Schottelius, M.; Herz, M.; Wester, H.J.; Schwaiger, M. Gluc-Lys([18F]FP)-TOCA PET in patients with SSTR-positive tumors: Biodistribution and diagnostic evaluation compared with [111In]DTPA-octreotide. J. Nucl. Med. 2006, 47, 566–573. [Google Scholar]
- Ilhan, H.; Lindner, S.; Todica, A.; Cyran, C.C.; Tiling, R.; Auernhammer, C.J.; Spitzweg, C.; Boeck, S.; Unterrainer, M.; Gildehaus, F.J.; et al. Biodistribution and first clinical results of 18F-SiFAlin-TATE PET: A novel 18F-labeled somatostatin analog for imaging of neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.; Cleeren, F.; Tshibangu, T.; Koole, M.; Serdons, K.; Dekervel, J.; Van Cutsem, E.; Verslype, C.; Van Laere, K.; Bormans, G.; et al. [18F]AlF-NOTA-octreotide PET imaging: Biodistribution, dosimetry and first comparison with [68Ga]Ga-DOTATATE in neuroendocrine tumour patients. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef]
- Kemerink, G.J.; Visser, M.G.; Franssen, R.; Beijer, E.; Zamburlini, M.; Halders, S.G.; Brans, B.; Mottaghy, F.M.; Teule, G.J. Effect of the positron range of 18F, 68Ga and 124I on PET/CT in lung-equivalent materials. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 940–948. [Google Scholar] [CrossRef]
- Uccelli, L.; Martini, P.; Cittanti, C.; Carnevale, A.; Missiroli, L.; Giganti, M.; Bartolomei, M.; Boschi, A. Therapeutic Radiometals: Worldwide Scientific Literature Trend Analysis (2008–2018). Molecules 2019, 24, 640. [Google Scholar] [CrossRef]
- Cremonesi, M.; Ferrari, M.E.; Bodei, L.; Chiesa, C.; Sarnelli, A.; Garibaldi, C.; Pacilio, M.; Strigari, L.; Summers, P.E.; Orecchia, R.; et al. Correlation of dose with toxicity and tumour response to 90Y- and 177Lu-PRRT provides the basis for optimization through individualized treatment planning. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2426–2441. [Google Scholar] [CrossRef]
- Otte, A.; Mueller-Brand, J.; Dellas, S.; Nitzsche, E.U.; Herrmann, R.; Maecke, H.R. Yttrium-90 labeled somatostatin-analogue for cancer treatment. Lancet 1998, 351, 417–418. [Google Scholar] [CrossRef]
- Otte, A.; Herrmann, R.; Heppeler, A.; Behe, M.; Jermann, E.; Powell, P.; Maecke, H.R.; Muller, J. Yttrium-90 DOTATOC: First clinical results. Eur. J. Nucl. Med. 1999, 26, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Vinjamuri, S.; Gilbert, T.M.; Banks, M.; McKane, G.; Maltby, P.; Poston, G.; Weissman, H.; Palmer, D.H.; Vora, J.; Pritchard, D.M.; et al. Peptide Receptor Radionuclide Therapy With 90Y-DOTATATE/90Y-DOTATOC in Patients with Progressive Metastatic Neuroendocrine Tumours: Assessment of Response, Survival and Toxicity. Br. J. Cancer 2013, 108, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Virgolini, I.; Britton, K.; Buscombe, J.; Moncayo, R.; Paganelli, G.; Riva, P. In- and Y-DOTA-lanreotide: Results and implications of the MAURITIUS trial. Semin. Nucl. Med. 2002, 32, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Cremonesi, M.; Grana, C.M.; Chinol, M.; Baio, S.M.; Severi, S.; Paganelli, G. Yttrium-labeled peptides for therapy of NET. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, S93–S102. [Google Scholar] [CrossRef]
- Gabriel, M.; Nilica, B.; Kaiser, B.; Virgolini, I.J. Twelve-Year Follow-up After Peptide Receptor Radionuclide Therapy. J. Nucl. Med. 2019, 60, 524–529. [Google Scholar] [CrossRef]
- Baum, R.P.; Kluge, A.W.; Kulkarni, H.; Schorr-Neufing, U.; Niepsch, K.; Bitterlich, N.; van Echteld, C.J. [177Lu-DOTA]0-D-Phe1-Tyr3-Octreotide (177Lu-DOTATOC) For Peptide Receptor Radiotherapy in Patients with Advanced Neuroendocrine Tumours: A Phase-II Study. Theranostics 2016, 6, 501–510. [Google Scholar] [CrossRef]
- Esser, J.P.; Krenning, E.P.; Teunissen, J.J.; Kooij, P.P.; van Gameren, A.L.; Bakker, W.H.; Kwekkeboom, D.J. Comparison of [177Lu-DOTA0,Tyr3]octreotate and [177Lu-DOTA0,Tyr3]octreotide: Which peptide is preferable for PRRT? Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1346–1351. [Google Scholar] [CrossRef]
- de Jong, M.; Breeman, W.A.; Valkema, R.; Bernard, B.F.; Krenning, E.P. Combination radionuclide therapy using 177Lu- and 90Y-labeled somatostatin analogs. J. Nucl. Med. 2005, 46, 13S–17S. [Google Scholar]
- Kunikowska, J.; Królicki, L.; Hubalewska-Dydejczyk, A.; Mikołajczak, R.; Sowa-Staszczak, A.; Pawlak, D. Clinical results of radionuclide therapy of neuroendocrine tumours with 90Y-DOTATATE and tandem 90Y/177Lu-DOTATATE: Which is a better therapy option? Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1788–1797. [Google Scholar] [CrossRef]
- Kunikowska, J.; Zemczak, A.; Kołodziej, M.; Gut, P.; Łoń, I.; Pawlak, D.; Mikołajczak, R.; Kamiński, G.; Ruchała, M.; Kos-Kudła, B.; et al. Tandem peptide receptor radionuclide therapy using 90Y/177Lu-DOTATATE for neuroendocrine tumors efficacy and side-effects—Polish multicenter experience. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 922–933. [Google Scholar] [CrossRef]
- Brabander, T.; Nonnekens, J.; Hofland, J. The next generation of peptide receptor radionuclide therapy. Endocr. Relat. Cancer 2019, 26, C7–C11. [Google Scholar] [CrossRef] [PubMed]
- Adant, S.; Shah, G.M.; Beauregard, J.M. Combination treatments to enhance peptide receptor radionuclide therapy of neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Kopka, K. Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef]
- Werner, R.A.; Bluemel, C.; Allen-Auerbach, M.S.; Higuchi, T.; Herrmann, K. 68Gallium- and 90Yttrium-/177Lutetium: “theranostic twins” for diagnosis and treatment of NETs. Ann. Nucl. Med. 2015, 29, 1–7. [Google Scholar] [CrossRef]
- Waseem, N.; Aparici, C.M.; Kunz, P.L. Evaluating the Role of Theranostics in Grade 3 Neuroendocrine Neoplasms. J. Nucl. Med. 2019, 60, 882–891. [Google Scholar] [CrossRef]
- Sorbye, H.; Kong, G.; Grozinsky-Glasberg, S. PRRT in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Endocr. Relat. Cancer 2020, 27, R67–R77. [Google Scholar] [CrossRef]
- Mak, I.Y.F.; Hayes, A.R.; Khoo, B.; Grossman, A. Peptide Receptor Radionuclide Therapy as a Novel Treatment for Metastatic and Invasive Phaeochromocytoma and Paraganglioma. Neuroendocrinology 2019, 109, 287–298. [Google Scholar] [CrossRef]
- Vyakaranam, A.R.; Crona, J.; Norlén, O.; Granberg, D.; Garske-Román, U.; Sandström, M.; Fröss-Baron, K.; Thiis-Evensen, E.; Hellman, P.; Sundin, A. Favorable Outcome in Patients with Pheochromocytoma and Paraganglioma Treated with 177Lu-DOTATATE. Cancers 2019, 11, 909. [Google Scholar] [CrossRef]
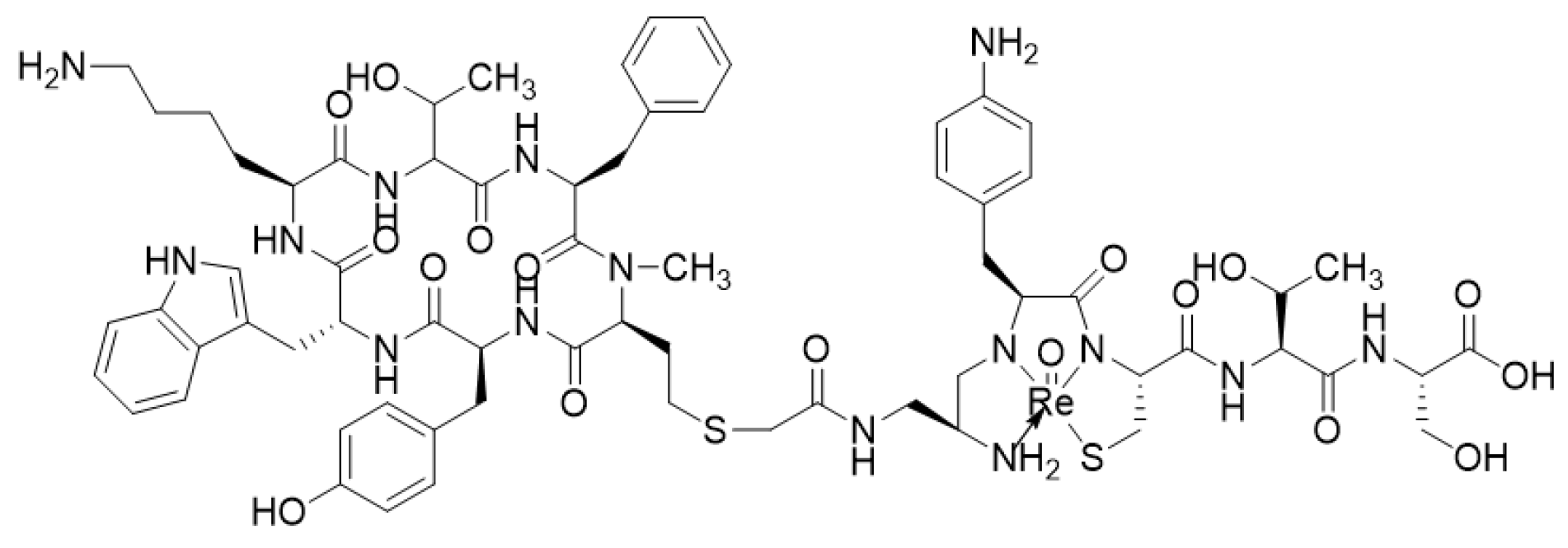
- Lepareur, N.; Lacœuille, F.; Bouvry, C.; Hindré, F.; Garcion, E.; Chérel, M.; Noiret, N.; Garin, E.; Knapp, F.F.R., Jr. Rhenium-188 Labeled Radiopharmaceuticals: Current Clinical Applications in Oncology and Promising Perspectives. Front. Med. 2019, 6, 132. [Google Scholar] [CrossRef]
- Zamora, P.O.; Gulhke, S.; Bender, H.; Diekmann, D.; Rhodes, B.A.; Biersack, H.J.; Knapp, F.F., Jr. Experimental radiotherapy of receptor-positive human prostate adenocarcinoma with 188Re-RC-160, a directly-radiolabeled somatostatin analogue. Int. J. Cancer 1996, 65, 214–220. [Google Scholar] [CrossRef]
- Zamora, P.O.; Bender, H.; Gulhke, S.; Marek, M.J.; Knapp, F.F., Jr.; Rhodes, B.A.; Biersack, H.J. Pre-clinical experience with Re-188-RC-160, a radiolabeled somatostatin analog for use in peptide-targeted radiotherapy. Anticancer Res. 1997, 17, 1803–1808. [Google Scholar] [PubMed]
- Arteaga de Murphy, C.; Pedraza-López, M.; Ferro-Flores, G.; Murphy-Stack, E.; Chávez-Mercado, L.; Ascencio, J.A.; García-Salinas, L.; Hernández-Gutiérrez, S. Uptake of 188Re-beta-naphthyl-peptide in cervical carcinoma tumours in athymic mice. Nucl. Med. Biol. 2001, 28, 319–326. [Google Scholar] [CrossRef]
- Molina-Trinidad, E.M.; de Murphy, C.A.; Ferro-Flores, G.; Murphy-Stack, E.; Jung-Cook, H. Radiopharmacokinetic and dosimetric parameters of 188Re-lanreotide in athymic mice with induced human cancer tumors. Int. J. Pharm. 2006, 310, 125–130. [Google Scholar] [CrossRef]
- Molina-Trinidad, E.M.; de Murphy, C.A.; Jung-Cook, H.; Stack, E.M.; Pedraza-Lopez, M.; Morales-Marquez, J.L.; Serrano, G.V. Therapeutic 188Re-lanreotide: Determination of radiopharmacokinetic parameters in rats. J. Pharm. Pharmacol. 2010, 62, 456–461. [Google Scholar] [CrossRef]
- Cyr, J.E.; Pearson, D.A.; Wilson, D.M.; Nelson, C.A.; Guaraldi, M.; Azure, M.T.; Lister-James, J.; Dinkelborg, L.M.; Dean, R.T. Somatostatin receptor-binding peptides suitable for tumour radiotherapy with Re-188 or Re-186. Chemistry and initial biological studies. J. Med. Chem. 2007, 50, 1354–1364. [Google Scholar] [CrossRef]
- Edelman, M.J.; Clamon, G.; Kahn, D.; Magram, M.; Lister-James, J.; Line, B.R. Targeted radiopharmaceutical therapy for advanced lung cancer: Phase I trial of rhenium Re188 P2045, a somatostatin analog. J. Thorac. Oncol. 2009, 4, 1550–1554. [Google Scholar] [CrossRef]
- Nelson, C.A.; Azure, M.T.; Adams, C.T.; Zinn, K.R. The somatostatin analog 188Re-P2045 inhibits the growth of AR42J pancreatic tumor xenografts. J. Nucl. Med. 2014, 55, 2020–2025. [Google Scholar] [CrossRef][Green Version]
- Champion, C.; Quinto, M.A.; Morgat, C.; Zanotti-Fregonara, P.; Hindié, E. Comparison between Three Promising ß-emitting Radionuclides, 67Cu, 47Sc and 161Tb, with Emphasis on Doses Delivered to Minimal Residual Disease. Theranostics 2016, 6, 1611–1618. [Google Scholar] [CrossRef]
- De Jong, M.; Breeman, W.A.; Bernard, B.F.; Rolleman, E.J.; Hofland, L.J.; Visser, T.J.; Setyono-Han, B.; Bakker, W.H.; van der Pluijm, M.E.; Krenning, E.P. Evaluation in vitro and in rats of 161Tb-DTPA-octreotide, a somatostatin analogue with potential for intraoperative scanning and radiotherapy. Eur. J. Nucl. Med. 1995, 22, 608–616. [Google Scholar] [CrossRef]
- Loveless, C.S.; Radford, L.L.; Ferran, S.J.; Queern, S.L.; Shepherd, M.R.; Lapi, S.E. Photonuclear production, chemistry, and in vitro evaluation of the theranostic radionuclide 47Sc. EJNMMI Res. 2019, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Tafreshi, N.K.; Doligalski, M.L.; Tichacek, C.J.; Pandya, D.N.; Budzevich, M.M.; El-Haddad, G.; Khushalani, N.I.; Moros, E.G.; McLaughlin, M.L.; Wadas, T.J.; et al. Development of Targeted Alpha Particle Therapy for Solid Tumors. Molecules 2019, 24, 4314. [Google Scholar] [CrossRef] [PubMed]
- Nayak, T.; Norenberg, J.; Anderson, T.; Atcher, R. A comparison of high- versus low-linear energy transfer somatostatin receptor targeted radionuclide therapy in vitro. Cancer Biother. Radiopharm. 2005, 20, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Nayak, T.K.; Norenberg, J.P.; Anderson, T.L.; Prossnitz, E.R.; Stabin, M.G.; Atcher, R.W. Somatostatin-receptor-targeted α-emitting 213Bi is therapeutically more effective than β--emitting 177Lu in human pancreatic adenocarcinoma cells. Nucl. Med. Biol. 2007, 34, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.S.; de Blois, E.; Morgenstern, A.; Bruchertseifer, F.; de Jong, M.; Breeman, W.; Konijnenberg, M. In Vitro comparison of 213Bi- and 177Lu-radiation for peptide receptor radionuclide therapy. PLoS ONE 2017, 12, e0181473. [Google Scholar] [CrossRef]
- Norenberg, J.P.; Krenning, B.J.; Konings, I.R.H.M.; Kusewitt, D.F.; Nayak, T.K.; Anderson, T.L.; de Jong, M.; Garmestani, K.; Brechbiel, M.W.; Kvols, L.K. 213Bi-[DOTA0, Tyr3]Octreotide Peptide Receptor Radionuclide Therapy of Pancreatic Tumors in a Preclinical Animal Model. Clin. Cancer Res. 2006, 12, 897–903. [Google Scholar] [CrossRef]
- Miederer, M.; Henriksen, G.; Alke, A.; Mossbrugger, I.; Quintanilla-Martinez, L.; Senekowitsch-Schmidtke, R.; Essler, M. Preclinical evaluation of the alpha-particle generator nuclide 225Ac for somatostatin receptor radiotherapy of neuroendocrine tumors. Clin. Cancer Res. 2008, 14, 3555–3561. [Google Scholar] [CrossRef]
- Chan, H.S.; Konijnenberg, M.W.; de Blois, E.; Koelewijn, S.; Baum, R.P.; Morgenstern, A.; Bruchertseifer, F.; Breeman, W.A.; de Jong, M. Influence of tumour size on the efficacy of targeted alpha therapy with 213Bi-[DOTA0,Tyr3]-octreotate. EJNMMI Res. 2016, 6, 6. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef]
- Zhang, J.; Singh, A.; Kulkarni, H.R.; Schuchardt, C.; Müller, D.; Wester, H.J.; Maina, T.; Rösch, F.; van der Meulen, N.P.; Müller, C.; et al. From Bench to Bedside-The Bad Berka Experience with First-in-Human Studies. Semin. Nucl. Med. 2019, 49, 422–437. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Bal, C.; Sahoo, R.K.; Tripathi, M. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: First clinical experience on the efficacy and safety. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Stallons, T.A.R.; Saidi, A.; Tworowska, I.; Delpassand, E.S.; Torgue, J.J. Preclinical Investigation of 212Pb-DOTAMTATE for Peptide Receptor Radionuclide Therapy in a Neuroendocrine Tumor Model. Mol. Cancer Ther. 2019, 18, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Tworowska, I.; Delpassand, E.S.; Bolek, L.; Shanoon, F.; Sgouros, G.; Frey, E.; He, B.; Muzammil, A.; Ghaly, M.; Stallons, T.; et al. Targeted Alpha-emitter Therapy of Neuroendocrine Tumors using 212Pb-octreotate (AlphaMedix™). J. Med. Imaging Radiat. Sci. 2019, 50, S34. [Google Scholar] [CrossRef]
- Guérard, F.; Gestin, J.F.; Brechbiel, M.W. Production of [211At]-astatinated radiopharmaceuticals and applications in targeted α-particle therapy. Cancer Biother. Radiopharm. 2013, 28, 1–20. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Boskovitz, A.; Shankar, S.; Zalutsky, M.R. Radioiodine and 211At-labeled guanidinomethyl halobenzoyl octreotate conjugates: Potential peptide radiotherapeutics for somatostatin receptor-positive cancers. Peptides 2004, 25, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, G.; Affleck, D.J.; Schottelius, M.; Wester, H.; Friedman, H.S.; Zalutsky, M.R. Synthesis and evaluation of glycosylated octreotate analogues labeled with radioiodine and 211At via a tin precursor. Bioconjugate Chem. 2006, 17, 195–203. [Google Scholar] [CrossRef]
- Zhao, B.; Qin, S.; Chai, L.; Lu, G.; Yang, Y.; Cai, H.; Yuan, X.; Fan, S.; Huang, Q.; Yu, F. Evaluation of astatine-211-labeled octreotide as a potential radiotherapeutic agent for NSCLC treatment. Bioorg. Med. Chem. 2018, 26, 1086–1091. [Google Scholar] [CrossRef]
- Krenning, E.P.; Valkema, R.; Kooij, P.P.; Breeman, W.A.; Bakker, W.H.; de Herder, W.W.; van Eijck, C.H.; Kwekkeboom, D.J.; de Jong, M.; Pauwels, S. Scintigraphy and radionuclide therapy with [indium-111-labeled-diethyltriamine penta-acetic acid-D-Phe1]-octreotide. Ital. J. Gastroenterol. Hepatol. 1999, 31, S219–S223. [Google Scholar]
- Valkema, R.; De Jong, M.; Bakker, W.H.; Breeman, W.A.; Kooij, P.P.; Lugtenburg, P.J.; De Jong, F.H.; Christiansen, A.; Kam, B.L.; De Herder, W.W.; et al. Phase I study of peptide receptor radionuclide therapy with [In-DTPA]octreotide: The Rotterdam experience. Semin. Nucl. Med. 2002, 32, 110–122. [Google Scholar] [CrossRef]
- Anthony, L.B.; Woltering, E.A.; Espenan, G.D.; Cronin, M.D.; Maloney, T.J.; McCarthy, K.E. Indium-111-pentetreotide prolongs survival in gastroenteropancreatic malignancies. Semin. Nucl. Med. 2002, 32, 123–132. [Google Scholar] [CrossRef]
- Buscombe, J.R.; Caplin, M.E.; Hilson, A.J. Long-term efficacy of high-activity 111In-pentetreotide therapy in patients with disseminated neuroendocrine tumors. J. Nucl. Med. 2003, 44, 1–6. [Google Scholar] [PubMed]
- Lewington, V.J. Targeted radionuclide therapy for neuroendocrine tumours. Endocr. Relat. Cancer 2003, 10, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Capello, A.; Krenning, E.; Bernard, B.; Reubi, J.C.; Breeman, W.; de Jong, M. 111In-labeled somatostatin analogues in a rat tumour model: Somatostatin receptor status and effects of peptide receptor radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 1288–1295. [Google Scholar] [CrossRef]
- Ginj, M.; Zhang, H.; Waser, B.; Cescato, R.; Wild, D.; Wang, X.; Erchegyi, J.; Rivier, J.; Mäcke, H.R.; Reubi, J.C. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc. Natl. Acad. Sci. USA 2006, 103, 16436–16441. [Google Scholar] [CrossRef] [PubMed]
- Waser, B.; Tamma, M.L.; Cescato, R.; Maecke, H.R.; Reubi, J.C. Highly efficient in vivo agonist induced internalization of sst2 receptors in somatostatin target tissues. J. Nucl. Med. 2009, 50, 936941. [Google Scholar] [CrossRef] [PubMed]
- Cescato, R.; Schulz, S.; Waser, B.; Eltschinger, V.; Rivier, J.E.; Wester, H.J.; Culler, M.; Ginj, M.; Liu, Q.; Schonbrunn, A.; et al. Internalization of sst2, sst3, and sst5 receptors: Effects of somatostatin agonists and antagonists. J. Nucl. Med. 2006, 47, 502–511. [Google Scholar] [PubMed]
- Fani, M.; Peitl, P.K.; Velikyan, I. Current status of radiopharmaceuticals for the theranostics of neuroendocrine neoplasms. Pharmaceuticals 2017, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Waser, B.; Mäcke, H.; Rivier, J. Highly Increased 125I-JR11 Antagonist Binding In Vitro Reveals Novel Indications for sst2 Targeting in Human Cancers. J. Nucl. Med. 2017, 58, 300–306. [Google Scholar] [CrossRef]
- Dude, I.; Zhang, Z.; Rousseau, J.; Hundal-Jabal, N.; Colpo, N.; Merkens, H.; Lin, K.S.; Bénard, F. Evaluation of agonist and antagonist radioligands for somatostatin receptor imaging of breast cancer using positron emission tomography. EJNMMI Radiopharm. Chem. 2017, 2, 4. [Google Scholar] [CrossRef]
- Rylova, S.N.; Stoykow, C.; Del Pozzo, L.; Abiraj, K.; Tamma, M.L.; Kiefer, Y.; Fani, M.; Maecke, H.R. The somatostatin receptor 2 antagonist 64Cu-NODAGA-JR11 outperforms 64Cu-DOTA-TATE in a mouse xenograft model. PLoS ONE 2018, 13, e0195802. [Google Scholar] [CrossRef]
- Krebs, S.; Pandit-Taskar, N.; Reidy, D.; Beattie, B.J.; Lyashchenko, S.K.; Lewis, J.S.; Bodei, L.; Weber, W.A.; O’Donoghue, J.A. Biodistribution and radiation dose estimates for 68Ga-DOTA-JR11 in patients with metastatic neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Fani, M.; Behe, M.; Brink, I.; Rivier, J.E.; Reubi, J.C.; Maecke, H.R.; Weber, W.A. First clinical evidence that imaging with somatostatin receptor antagonists is feasible. J. Nucl. Med. 2011, 52, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Del Pozzo, L.; Abiraj, K.; Mansi, R.; Tamma, M.L.; Cescato, R.; Waser, B.; Weber, W.A.; Reubi, J.C.; Maecke, H.R. PET of somatostatin receptor-positive tumors using 64Cu- and 68Ga-somatostatin antagonists: The chelate makes the difference. J. Nucl. Med. 2011, 52, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Braun, F.; Waser, B.; Beetschen, K.; Cescato, R.; Erchegyi, J.; Rivier, J.E.; Weber, W.A.; Maecke, H.R.; Reubi, J.C. Unexpected sensitivity of sst2 antagonists to N-terminal radiometal modifications. J. Nucl. Med. 2012, 53, 1481–1489. [Google Scholar] [CrossRef]
- Reubi, J.C.; Erchegyi, J.; Cescato, R.; Waser, B.; Rivier, J.E. Switch from antagonist to agonist after addition of a DOTA chelator to a somatostatin analog. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 15511558. [Google Scholar] [CrossRef]
- Radford, L.; Gallazzi, F.; Watkinson, L.; Carmack, T.; Berendzen, A.; Lewis, M.R.; Jurisson, S.S.; Papagiannopoulou, D.; Hennkens, H.M. Synthesis and evaluation of a 99mTc tricarbonyl-labeled somatostatin receptor-targeting antagonist peptide for imaging of neuroendocrine tumors. Nucl. Med. Biol. 2017, 47, 4–9. [Google Scholar] [CrossRef]
- Wild, D.; Fani, M.; Fischer, R.; Del Pozzo, L.; Kaul, F.; Krebs, S.; Fischer, R.; Rivier, J.E.; Reubi, J.C.; Maecke, H.R.; et al. Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: A pilot study. J. Nucl. Med. 2014, 55, 1248–1252. [Google Scholar] [CrossRef]
- Fani, M.; Nicolas, G.P.; Wild, D. Somatostatin Receptor Antagonists for Imaging and Therapy. J. Nucl. Med. 2017, 58, 61S–66S. [Google Scholar] [CrossRef]
- Mansi, R.; Fani, M. Design and development of the theranostic pair 177Lu-OPS201/68Ga-OPS202 for targeting somatostatin receptor expressing tumors. J. Label. Compd. Radiopharm. 2019, 62, 635–645. [Google Scholar] [CrossRef]
- Nicolas, G.P.; Beykan, S.; Bouterfa, H.; Kaufmann, J.; Bauman, A.; Lassmann, M.; Reubi, J.C.; Rivier, J.E.F.; Maecke, H.R.; Fani, M.; et al. Safety, Biodistribution, and Radiation Dosimetry of 68Ga-OPS202 in Patients with Gastroenteropancreatic Neuroendocrine Tumors: A Prospective Phase I Imaging Study. J. Nucl. Med. 2018, 59, 909–914. [Google Scholar] [CrossRef]
- Nicolas, G.P.; Schreiter, N.; Kaul, F.; Uiters, J.; Bouterfa, H.; Kaufmann, J.; Erlanger, T.E.; Cathomas, R.; Christ, E.; Fani, M.; et al. Sensitivity Comparison of 68Ga-OPS202 and 68Ga-DOTATOC PET/CT in Patients with Gastroenteropancreatic Neuroendocrine Tumors: A Prospective Phase II Imaging Study. J. Nucl. Med. 2018, 59, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Reidy-Lagunes, D.; Pandit-Taskar, N.; O’Donoghue, J.A.; Krebs, S.; Staton, K.D.; Lyashchenko, S.K.; Lewis, J.S.; Raj, N.; Gönen, M.; Lohrmann, C.; et al. Phase I Trial of Well-Differentiated Neuroendocrine Tumors (NETs) with Radiolabeled Somatostatin Antagonist 177Lu-Satoreotide Tetraxetan. Clin. Cancer Res. 2019, 25, 6939–6947. [Google Scholar] [CrossRef] [PubMed]
- Rangger, C.; Haubner, R. Radiolabeled Peptides for Positron Emission Tomography and Endoradiotherapy in Oncology. Pharmaceuticals 2020, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Villard, L.; Romer, A.; Marincek, N.; Brunner, P.; Koller, M.T.; Schindler, C.; Ng, Q.K.T.; Mäcke, H.R.; Müller-Brand, J.; Rochlitz, C.; et al. Cohort study of somatostatin-based radiopeptide therapy with [90Y-DOTA]-TOC versus [90Y-DOTA]-TOC plus [177Lu-DOTA]-TOC in neuroendocrine cancers. J. Clin. Oncol. 2012, 30, 1100–1106. [Google Scholar] [CrossRef]
- Gill, M.R.; Falzone, N.; Du, Y.; Vallis, K.A. Targeted radionuclide therapy in combined-modality regimens. Lancet Oncol. 2017, 18, e414–e423. [Google Scholar] [CrossRef]
- Hartrampf, P.E.; Hänscheid, H.; Kertels, O.; Schirbel, A.; Kreissl, M.C.; Flentje, M.; Sweeney, R.A.; Buck, A.K.; Polat, B.; Lapa, C. Long-term results of multimodal peptide receptor radionuclide therapy and fractionated external beam radiotherapy for treatment of advanced symptomatic meningioma. Clin. Transl. Radiat. Oncol. 2020, 22, 29–32. [Google Scholar] [CrossRef]
- Reubi, J.C.; Maecke, H.R. Approaches to multireceptor targeting: Hybrid radioligands, radioligand cocktails, and sequential radioligand applications. J. Nucl. Med. 2017, 58, 10S–16S. [Google Scholar] [CrossRef]
- Ghosh, S.C.; Rodriguez, M.; Carmon, K.S.; Voss, J.; Wilganowski, N.L.; Schonbrunn, A.; Azhdarinia, A. A Modular Dual-Labeling Scaffold That Retains Agonistic Properties for Somatostatin Receptor Targeting. J. Nucl. Med. 2017, 58, 1858–1864. [Google Scholar] [CrossRef]
- Langbein, T.; Weber, W.A.; Eiber, M. Future of Theranostics: An Outlook on Precision Oncology in Nuclear Medicine. J. Nucl. Med. 2019, 60, 13S–19S. [Google Scholar] [CrossRef]
- Jones, W.; Griffiths, K.; Barata, P.C.; Paller, C.J. PSMA Theranostics: Review of the Current Status of PSMA-Targeted Imaging and Radioligand Therapy. Cancers 2020, 12, 1367. [Google Scholar] [CrossRef]
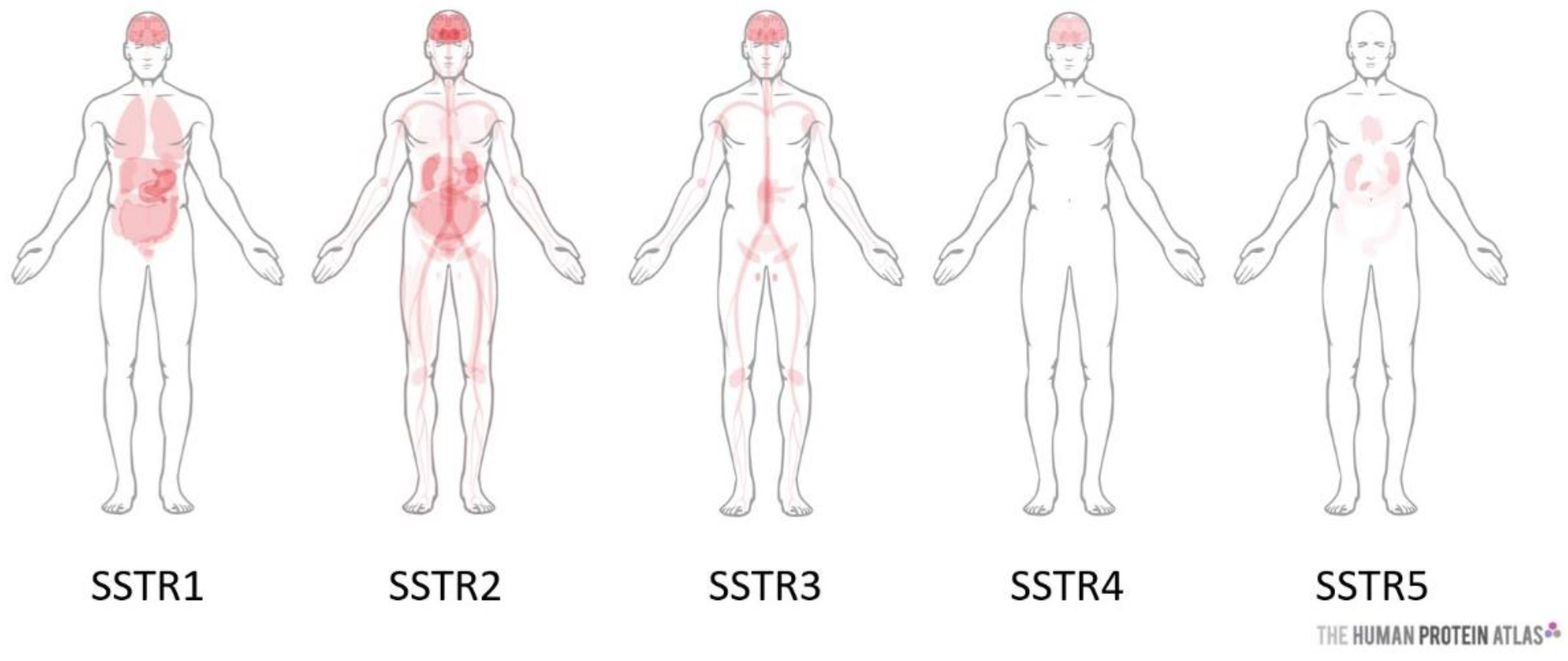




| Tumor Type | SSTR Expression | Ref |
|---|---|---|
| Astrocytoma | + | [17] |
| Breast carcinoma | + (SSTR2) | [11] |
| Cholangiocarcinoma | + (SSTR2) | [18] |
| Colorectal carcinoma | - | [17] |
| Endometrial carcinoma | - | [17] |
| Ependymoma | + (SSTR1, SSTR5) | [11] |
| Esophageal carcinoma | - | [17] |
| Ewing sarcoma | - | [17] |
| Exocrine pancreatic tumor | - | [17] |
| Gastric carcinoma | + (SSTR1 > SSTR2, SSTR5) | [11] |
| Gastrinoma | + (SSTR2) | [17] |
| Glioblastoma | - | [17] |
| Growth hormone-producing pituitary adenoma | + (SSTR2, SSTR5) | [17] |
| Gut carcinoid | + (SSTR2 > SSTR1, SSTR5) | [17] |
| Hepatocellular carcinoma | + (SSTR2, SSTR5) | [19] |
| Insulinoma | + (SSTR1, SSTR2, SSTR3) | [20] |
| Leiomyoma | + | [17] |
| Lymphoma | + (SSTR2) | [11] |
| Medullary thyroid carcinoma | + (SSTR2) | [11] |
| Medulloblastoma | + (SSTR2) | [17] |
| Meningioma | + (SSTR2) | [17] |
| Neuroblastoma | + (SSTR2) | [17] |
| Non-functioning pituitary adenoma | + (SSTR3 > SSTR2) | [17] |
| Non-small cell lung cancer | - | [17] |
| Ovarian carcinoma | + | [17] |
| Paraganglioma | + (SSTR2) | [17] |
| Pheochromocytoma | + (SSTR1, SSTR2) | [17] |
| Prostate carcinoma | + (SSTR1) | [17] |
| Renal cell carcinoma | + (SSTR2) | [11] |
| Small cell lung cancer | + (SSTR2) | [17] |
| Urinary bladder carcinoma | - | [17] |
| Radionuclide | Half-Life (h) | Type of Emission | Energy of Emitted Radiation (keV) | Source | Application |
|---|---|---|---|---|---|
| 99mTc | 6.01 | γ | 140 | Generator | SPECT imaging |
| 111In | 67.4 | γ | 172, 245 | Cyclotron | SPECT imaging |
| 18F | 1.83 | β+ | 634 | Cyclotron | PET imaging |
| 64Cu | 12.7 | β+/γ/β- | 653 | Cyclotron | PET imaging |
| 68Ga | 1.1 | β+ | 1190 | Generator/Cyclotron | PET imaging |
| 90Y | 64.1 | β− | 2284 | Generator | Therapy |
| 177Lu | 160.8 | β−/γ | 497 | Cyclotron | Therapy |
| 188Re | 17 | β−/γ | 2118 | Generator | Therapy |
| 211At | 7.2 | α | 5870 | Cyclotron | Therapy |
| 225Ac | 238 | α | 5830 | Generator | Therapy |
| Peptide | Peptidic Sequence |
|---|---|
| OC Octreotide | d-Phe1-cyclo(Cys2-Phe3-d-Trp4-Lys5-Thr6-Cys7)Thr(ol)8 |
| LAN Lanreotide | β-d-Nal1-cyclo(Cys2-Tyr3-d-Trp4-Lys5-Val6-Cys7)Thr8-NH2 |
| VAP Vapreotide | d-Phe1-cyclo(Cys2-Phe3-d-Trp4-Lys5-Val6-Cys7)Trp8-NH2 |
| TOC [Tyr3]-Octreotide | d-Phe1-cyclo(Cys2-Tyr3-d-Trp4-Lys5-Thr6-Cys7)Thr(ol)8 |
| TATE [Tyr3]-Octreotate | d-Phe1-cyclo(Cys2-Tyr3-d-Trp4-Lys5-Thr6-Cys7)Thr8 |
| NOC [1-Nal3]-Octreotide | d-Phe1-cyclo(Cys2-1-Nal3-d-Trp4-Lys5-Thr6-Cys7)Thr(ol)8 |
| NOC-ATE [1-Nal3, Thr8]-Octreotide | d-Phe1-cyclo(Cys2-1-Nal3-d-Trp4-Lys5-Thr6-Cys7)Thr8 |
| BOC [BzThi3]-Octreotide | d-Phe1-cyclo(Cys2-BzThi3-d-Trp4-Lys5-Thr6-Cys7)Thr(ol)8 |
| BOC-ATE [BzThi3, Thr8]-Octreotide | d-Phe1-cyclo(Cys2-BzThi3-d-Trp4-Lys5-Thr6-Cys7)Thr8 |
| Antagonist Peptide | Peptidic Sequence |
|---|---|
| Sst2-ANT (BASS) | p-NO2-Phe1-cyclo(d-Cys2-Tyr3-d-Trp4-Lys5-Thr6-Cys7)d-Tyr8-NH2 |
| LM3 | p-Cl-Phe1-cyclo(d-Cys2-Tyr3-d-Aph4(Cbm)-Lys5-Thr6-Cys7)d-Tyr8-NH2 |
| JR10 | p-NO2-Phe1-cyclo(d-Cys2-Tyr3-d-Aph4(Cbm)-Lys5-Thr6-Cys7)d-Tyr8-NH2 |
| JR11 (Satoreotide) | p-Cl-Phe1-cyclo(d-Cys2-Aph3(Hor)-d-Aph4(Cbm)-Lys5-Thr6-Cys7)d-Tyr8-NH2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eychenne, R.; Bouvry, C.; Bourgeois, M.; Loyer, P.; Benoist, E.; Lepareur, N. Overview of Radiolabeled Somatostatin Analogs for Cancer Imaging and Therapy. Molecules 2020, 25, 4012. https://doi.org/10.3390/molecules25174012
Eychenne R, Bouvry C, Bourgeois M, Loyer P, Benoist E, Lepareur N. Overview of Radiolabeled Somatostatin Analogs for Cancer Imaging and Therapy. Molecules. 2020; 25(17):4012. https://doi.org/10.3390/molecules25174012
Chicago/Turabian StyleEychenne, Romain, Christelle Bouvry, Mickael Bourgeois, Pascal Loyer, Eric Benoist, and Nicolas Lepareur. 2020. "Overview of Radiolabeled Somatostatin Analogs for Cancer Imaging and Therapy" Molecules 25, no. 17: 4012. https://doi.org/10.3390/molecules25174012
APA StyleEychenne, R., Bouvry, C., Bourgeois, M., Loyer, P., Benoist, E., & Lepareur, N. (2020). Overview of Radiolabeled Somatostatin Analogs for Cancer Imaging and Therapy. Molecules, 25(17), 4012. https://doi.org/10.3390/molecules25174012






