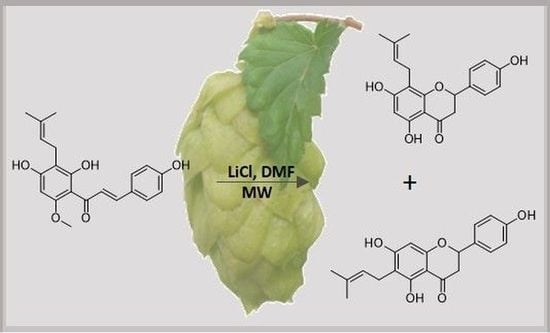
Semi-Synthetic Approach Leading to 8-Prenylnaringenin and 6-Prenylnaringenin: Optimization of the Microwave-Assisted Demethylation of Xanthohumol Using Design of Experiments
Abstract
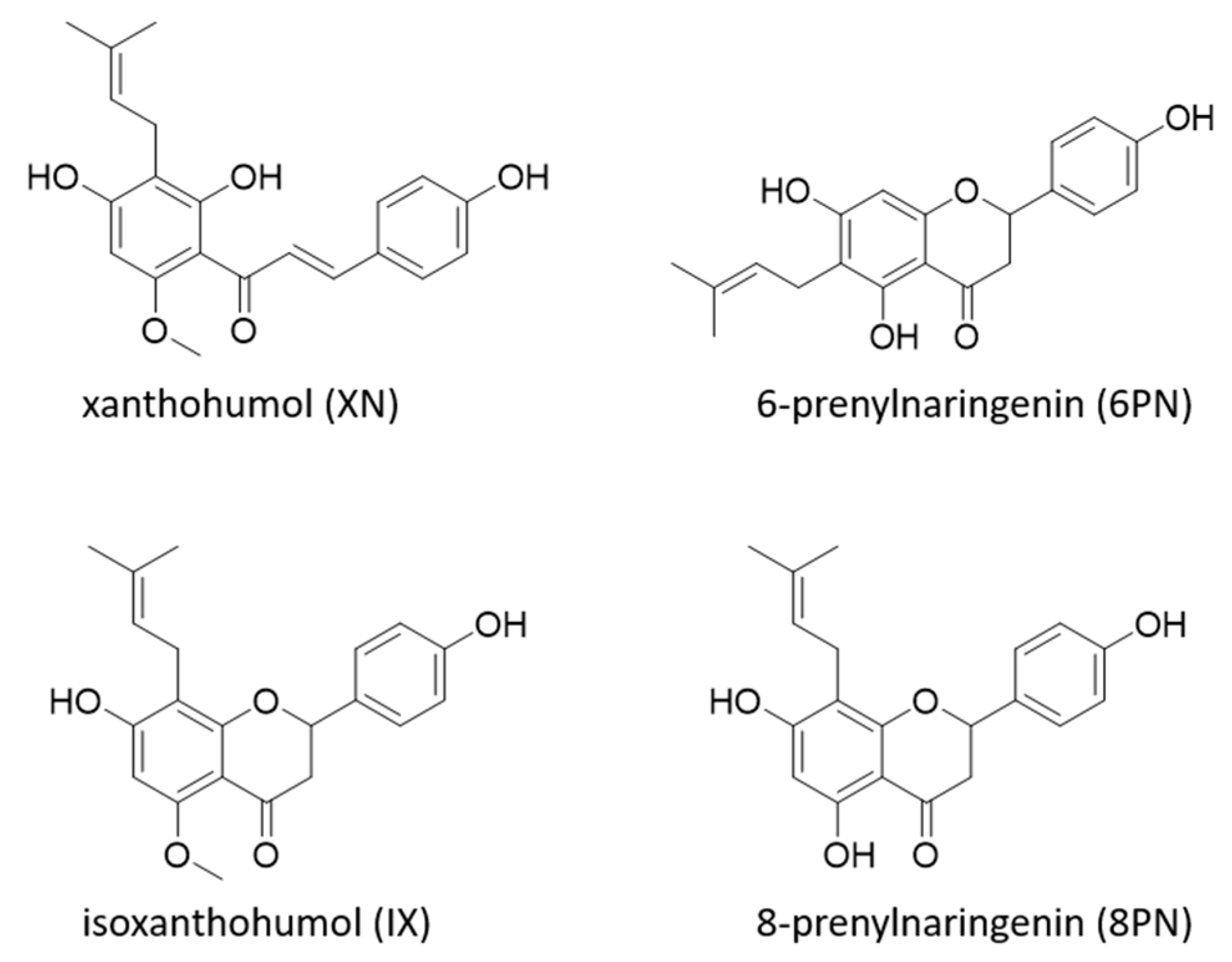
1. Introduction
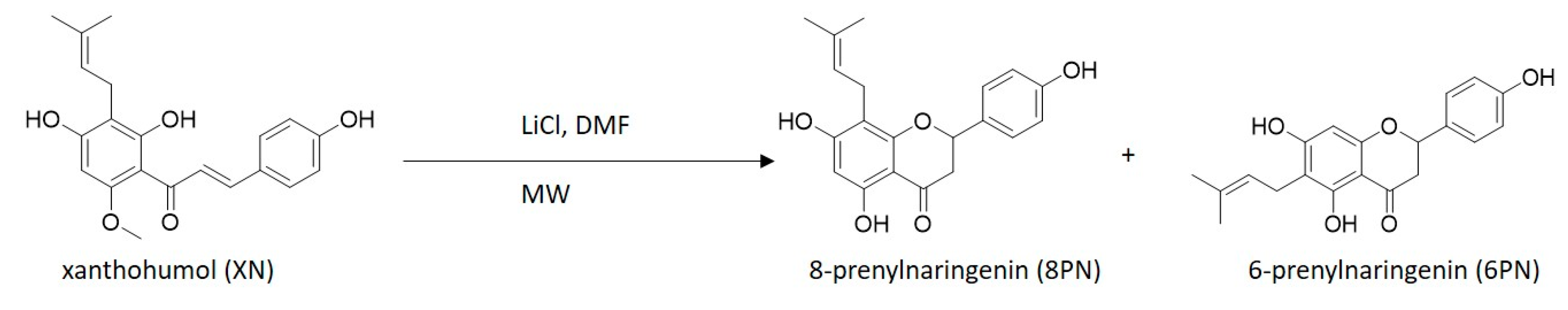
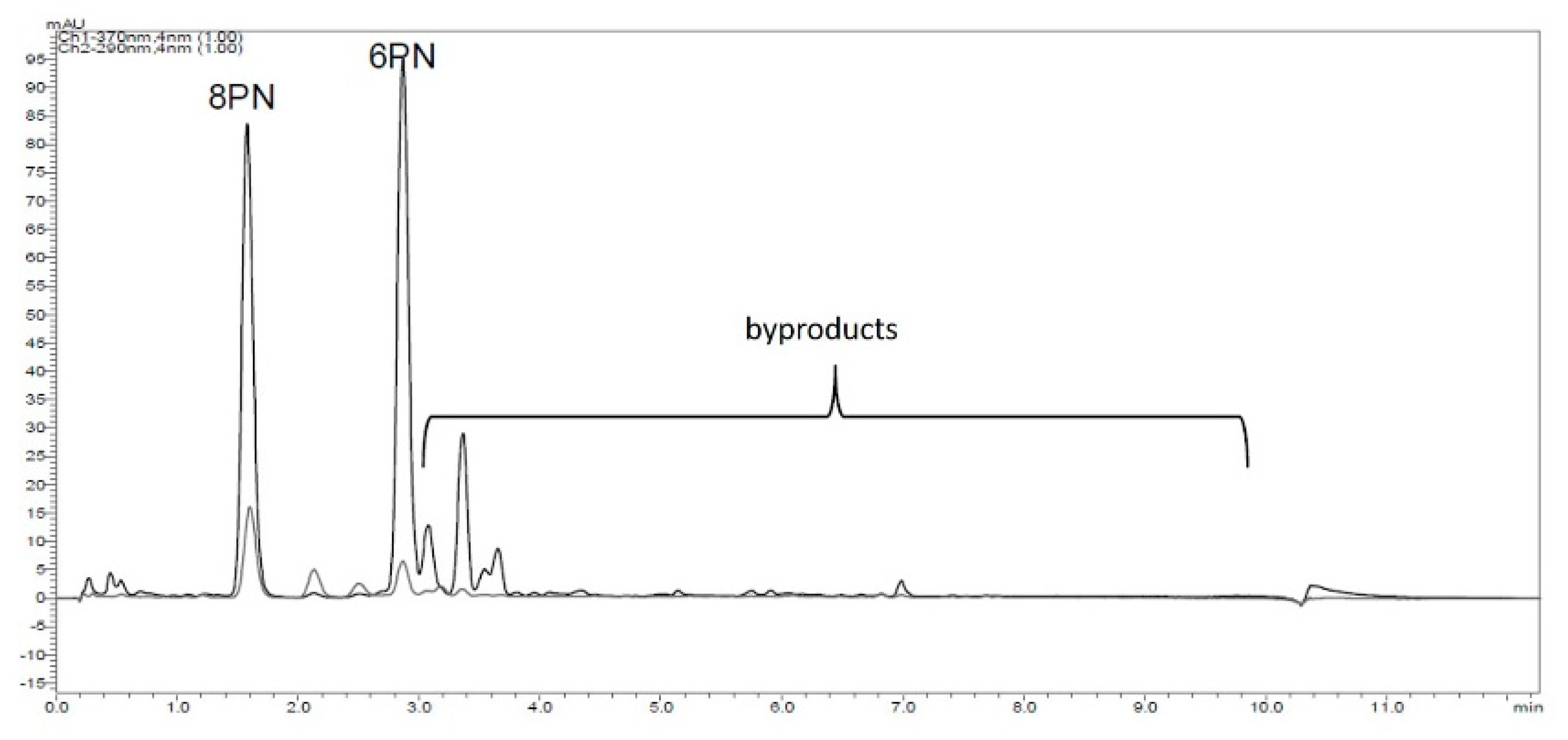
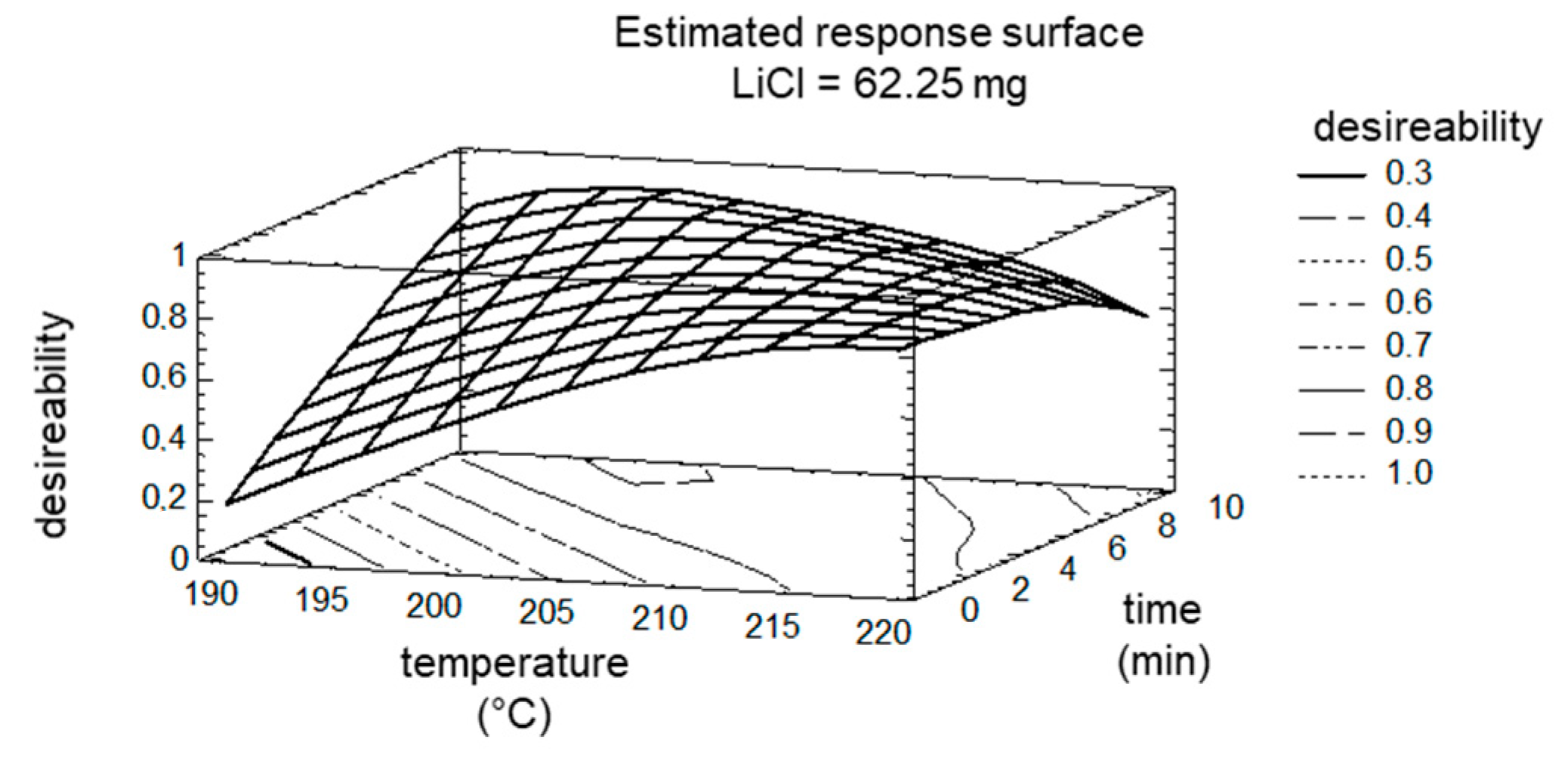
2. Results and Discussion
3. Materials and Methods
3.1. Microwave System
3.2. Microwave Reaction
3.3. Synthesis Reference Compounds
3.4. HPLC-Method
3.5. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Milligan, S.R.; Kalita, J.C.; Heyerick, A.; Rong, H.; De Cooman, L.; De Keukeleire, D. Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J. Clin. Endocrinol. Metab. 1999, 84, 2249–2252. [Google Scholar] [CrossRef]
- Diller, R.A.; Riepl, H.M.; Rose, O.; Frias, C.; Henze, G.; Prokop, A. Ability of prenylflavanones present in hops to induce apoptosis in a human burkitt lymphoma cell line. Planta Med. 2007, 73, 755–761. [Google Scholar] [CrossRef]
- Rad, M.; Humpel, M.; Schaefer, O.; Schoemaker, R.C.; Schleuning, W.D.; Cohen, A.F.; Burggraaf, J. Pharmacokinetics and systemic endocrine effects of the phyto-oestrogen 8-prenylnaringenin after single oral doses to postmenopausal women. Br. J. Clin. Pharmacol. 2006, 62, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Sehmisch, S.; Hammer, F.; Christoffel, J.; Seidlova-Wuttke, D.; Tezval, M.; Wuttke, W.; Stuermer, K.M.; Stuermer, E.K. Comparison of the phytohormones genistein, resveratrol and 8-prenylnaringenin as agents for preventing osteoporosis. Planta Med. 2008, 74, 794–801. [Google Scholar] [CrossRef]
- Zierau, O.; Kretzschmar, G.; Moller, F.; Weigt, C.; Vollmer, G. Time dependency of uterine effects of naringenin type phytoestrogens in vivo. Mol. Cell. Endocrinol. 2008, 294, 92–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Venturelli, S.; Niessner, H.; Sinnberg, T.; Berger, A.; Burkard, M.; Urmann, C.; Donaubauer, K.; Bocker, A.; Leischner, C.; Riepl, H.; et al. 6- and 8-Prenylnaringenin, novel natural histone deacetylase inhibitors found in hops, exert antitumor activity on melanoma cells. Cell Physiol. Biochem. 2018, 51, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Castro, L.A.; Burkard, M.; Sus, N.; Scheubeck, G.; Leischner, C.; Lauer, U.M.; Bosy-Westphal, A.; Hund, V.; Busch, C.; Venturelli, S.; et al. The oral bioavailability of 8-prenylnaringenin from hops (Humulus Lupulus L.) in healthy women and men is significantly higher than that of its positional isomer 6-prenylnaringenin in a randomized crossover trial. Mol. Nutr. Food Res. 2018, 62, e1700838. [Google Scholar] [CrossRef] [PubMed]
- Oberbauer, E.; Urmann, C.; Steffenhagen, C.; Bieler, L.; Brunner, D.; Furtner, T.; Humpel, C.; Baumer, B.; Bandtlow, C.; Couillard-Despres, S.; et al. Chroman-like cyclic prenylflavonoids promote neuronal differentiation and neurite outgrowth and are neuroprotective. J. Nutr. Biochem. 2013, 24, 1953–1962. [Google Scholar] [CrossRef]
- Itoigawa, M.; Ito, C.; Wu, T.S.; Enjo, F.; Tokuda, H.; Nishino, H.; Furukawa, H. Cancer chemopreventive activity of acridone alkaloids on Epstein-Barr virus activation and two-stage mouse skin carcinogenesis. Cancer Lett. 2003, 193, 133–138. [Google Scholar] [CrossRef]
- McCormick, S.; Robson, K.; Bohm, B. Flavonoids of Wyethia angustifolia and W. helenioides. Phytochemistry 1986, 25, 1723–1726. [Google Scholar] [CrossRef]
- Kitaoka, M.; Kadokawa, H.; Sugano, M.; Ichikawa, K.; Taki, M.; Takaishi, S.; Iijima, Y.; Tsutsumi, S.; Boriboon, M.; Akiyama, T. Prenylflavonoids: A new class of non-steroidal phytoestrogen (Part 1) Isolation of 8-isopentenylnaringenin and an initial study on its structure-activity relationship. Planta Med. 1998, 64, 511–515. [Google Scholar] [CrossRef]
- Jain, A.C.; Gupta, R.C.; Sarpal, P.D. Synthesis of (+/−) Lupinifolin, Di-O-Methyl Xanthohumol and Isoxanthohumol and Related Compounds. Tetrahedron 1978, 34, 3563–3567. [Google Scholar] [CrossRef]
- Philbin, C.S.; Schwartz, S.J. Resolution of diastereomeric flavonoid (1S)-(−)-camphanic acid esters via reversed-phase HPLC. Phytochemistry 2007, 68, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Gester, S.; Metz, P.; Zierau, O.; Vollmer, G. An efficient synthesis of the potent phytoestrogens 8-prenylnaringenin and 6-(1,1-dimethylallyl)naringenin by europium(III)-catalyzed Claisen rearrangement. Tetrahedron 2001, 57, 1015–1018. [Google Scholar] [CrossRef]
- Tischer, S.; Metz, P. Selective C-6 prenylation of flavonoids via europium(III)-catalyzed Clasien rearrangement and cross-metathesis. Adv. Synth. Catal. 2007, 349, 147–151. [Google Scholar] [CrossRef]
- Possemiers, S.; Heyerick, A.; Robbens, V.; De Keukeleire, D.; Verstraete, W. Activation of proestrogens from hops (Humulus lupulus L.) by intestinal microbiota; conversion of isoxanthohumol into 8-prenylnaringenin. J. Agric. Food Chem. 2005, 53, 6281–6288. [Google Scholar] [CrossRef] [PubMed]
- Possemiers, S.; Rabot, S.; Espin, J.C.; Bruneau, A.; Philippe, C.; Gonzalez-Sarrias, A.; Heyerick, A.; Tomas-Barberan, F.A.; De Keukeleire, D.; Verstraete, W. Eubacterium limosum activates isoxanthohumol from hops (Humulus lupulus L.) into the potent phytoestrogen 8-prenylnaringenin in vitro and in rat intestine. J. Nutr. 2008, 138, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.L.; Wang, W.; Chen, F.; Dong, Y.C.; Liu, X.J.; Ni, H.; Chen, Q.H. Production of 8-prenylnaringenin from isoxanthohumol through biotransformation by fungi cells. J. Agric. Food Chem. 2011, 59, 7419–7426. [Google Scholar] [CrossRef]
- Wilhelm, H.; Wessjohann, L.A. An efficient synthesis of the phytoestrogen 8-prenylnaringenin from xanthohumol by a novel demethylation process. Tetrahedron 2006, 62, 6961–6966. [Google Scholar] [CrossRef]
- Aniol, M.; Szymanska, K.; Zolnierczyk, A. An efficient synthesis of the phytoestrogen 8-prenylnaringenin from isoxanthohumol with magnesium iodide etherate. Tetrahedron 2008, 64, 95449547. [Google Scholar] [CrossRef]
- Fang, Z.; Zhou, G.-C.; Zheng, S.-L.; He, G.-L.; Li, J.-L.; He, L.; Bei, D. Lithium chloride-catalyzed selective demethylation of aryl methyl ethers under microwave irradiation. J. Mol. Catal. A Chem. 2007, 274, 16–23. [Google Scholar] [CrossRef]
- Brückner, R. Reaktionsmechanismen; Elsevier GmbH: München, Germany, 2004; Volume 3. [Google Scholar]
- Kappe, C.O.; Dallinger, D. Controlled microwave heating in modern organic synthesis: Highlights from the 2004–2008 literature. Mol. Divers. 2009, 13, 71–193. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O.; Pieber, B.; Dallinger, D. Microwave effects in organic synthesis: Myth or reality? Angew. Chem. Int. Ed. 2013, 52, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Hänsel, R.; Schulz, J. Desmethylxanthohumol: Isolierung aus hopfen und cyclisierung zu den flavonen. Arch. Der Pharm. 1988, 321, 37–40. [Google Scholar] [CrossRef]
- Hänsel, R.; Keller, K.; Rimpler, H.; Schneider, G. Drogen E-O; Springer: Berlin/Heidelberg, Germany, 1993; Volume 5. [Google Scholar]
- Chadwick, L.R.; Nikolic, D.; Burdette, J.E.; Overk, C.R.; Bolton, J.L.; van Breemen, R.B.; Frohlich, R.; Fong, H.H.S.; Farnsworth, N.R.; Pauli, G.F. Estrogens and congeners from spent hops (Humulus lupulus). J. Nat. Prod. 2004, 67, 2024–2032. [Google Scholar] [CrossRef]
- Bernard, A.; Ghiani, R.; Piras, P.; Rivoldini, A. Dealkylation of activated alkyl aryl ethres using Lithium chloride in dimethylformamide. Synthesis 1989, 4, 287–289. [Google Scholar] [CrossRef]
- Chen, P.K.; Rosana, M.R.; Dudley, G.B.; Stiegman, A.E. Parameters affecting the microwave-specific acceleration of a chemical reaction. J. Org. Chem. 2014, 79, 7425–7436. [Google Scholar] [CrossRef]
- Milligan, S.; Kalita, J.; Pocock, V.; Heyerick, A.; De Cooman, L.; Rong, H.; De Keukeleire, D. Oestrogenic activity of the hop phyto-oestrogen, 8-prenylnaringenin. Reproduction 2002, 123, 235–242. [Google Scholar] [CrossRef]
- Milligan, S.R.; Kalita, J.C.; Pocock, V.; Van De Kauter, V.; Stevens, J.F.; Deinzer, M.L.; Rong, H.; De Keukeleire, D. The endocrine activities of 8-prenylnaringenin and related hop (Humulus lupulus L.) flavonoids. J. Clin. Endocrinol. Metab. 2000, 85, 4912–4915. [Google Scholar] [CrossRef]
- Effenberger, K.E.; Johnsen, S.A.; Monroe, D.G.; Spelsberg, T.C.; Westendorf, J.J. Regulation of osteoblastic phenotype and gene expression by hop-derived phytoestrogens. J. Steroid Biochem. Mol. Biol. 2005, 96, 387–399. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds xanthohumol, 8-prenylnaringein, 6prenylnaringein, isoxanthohumol and further prenylated flavonoids are available from the authors. |
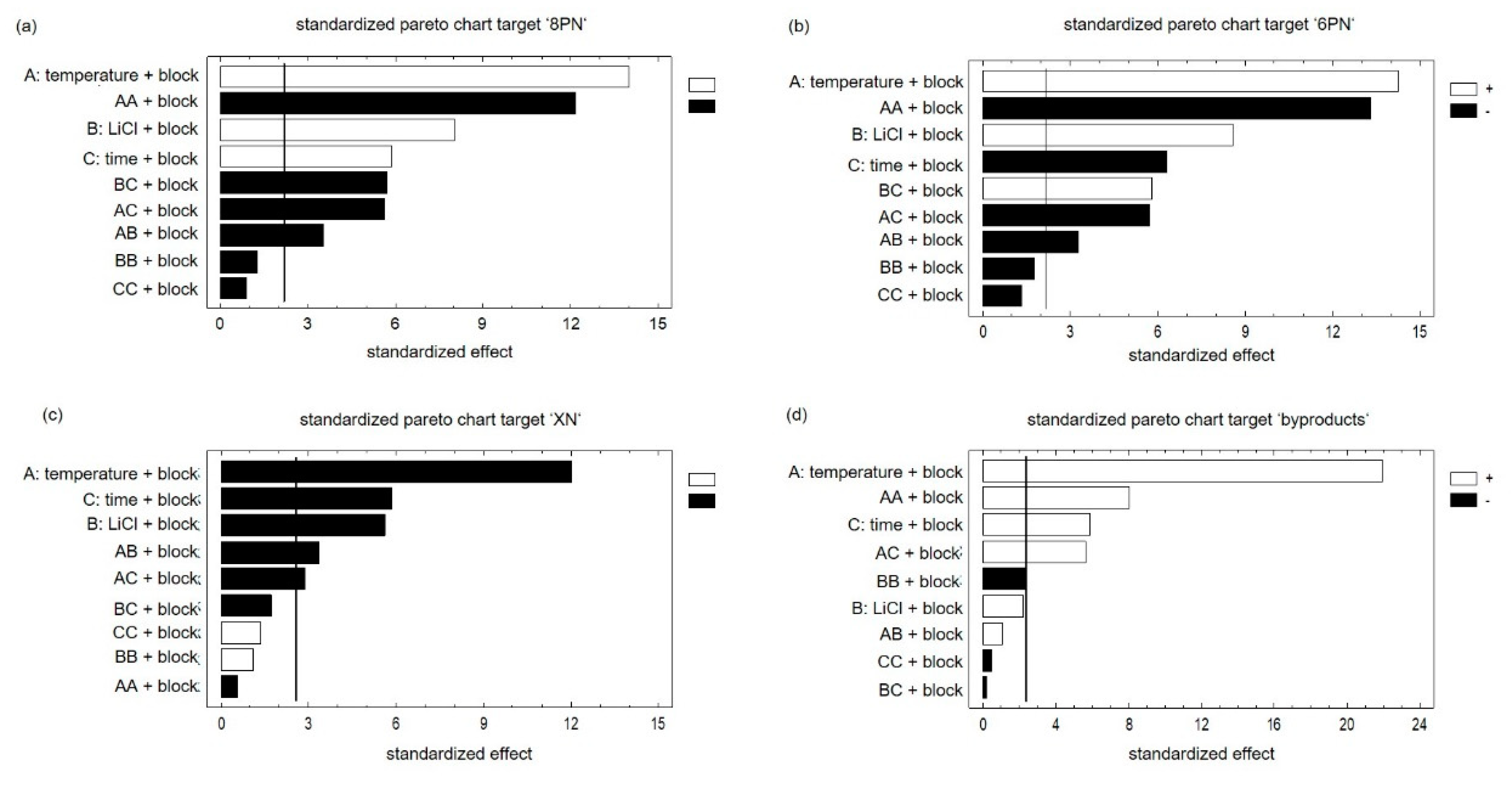
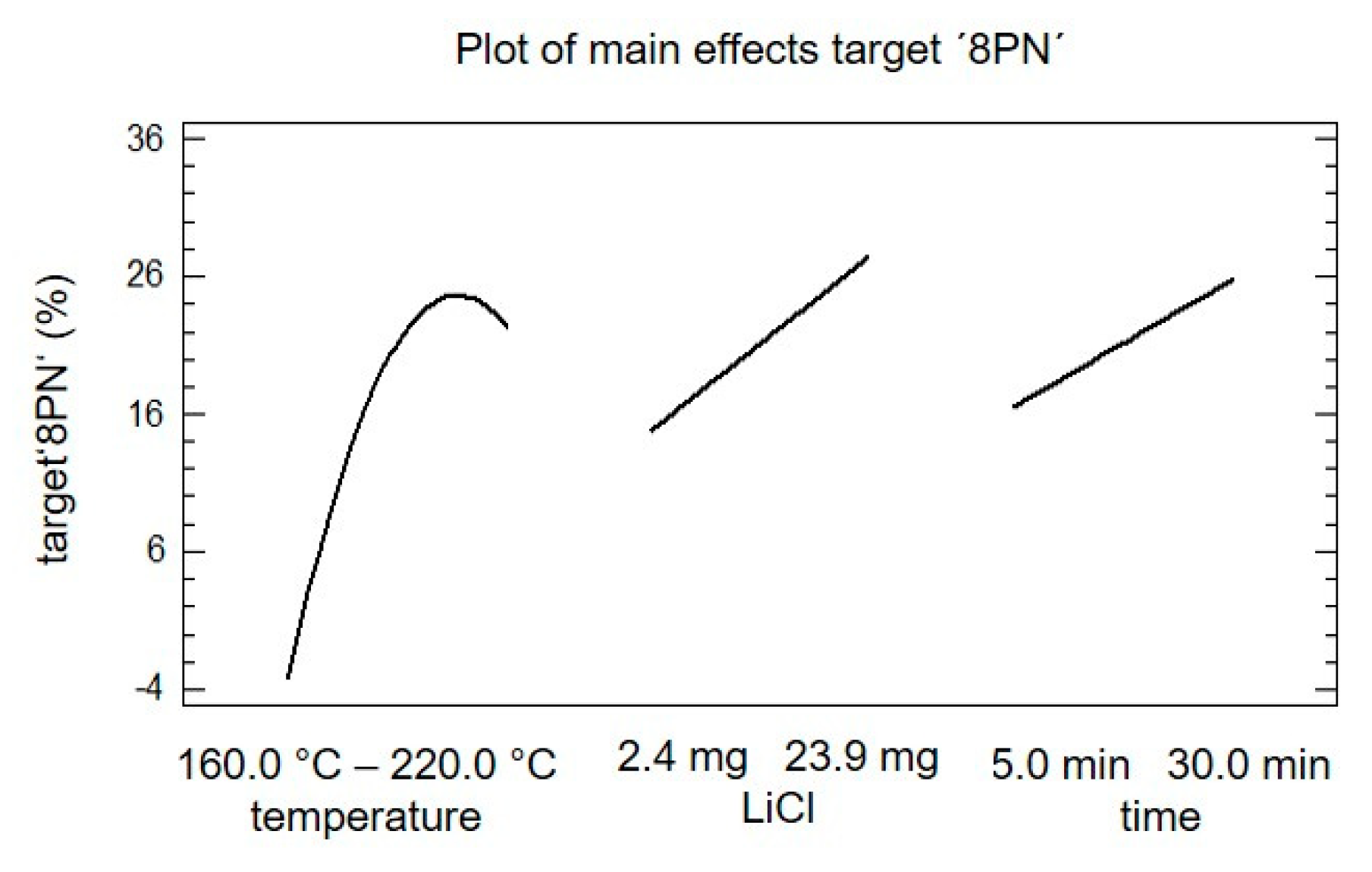
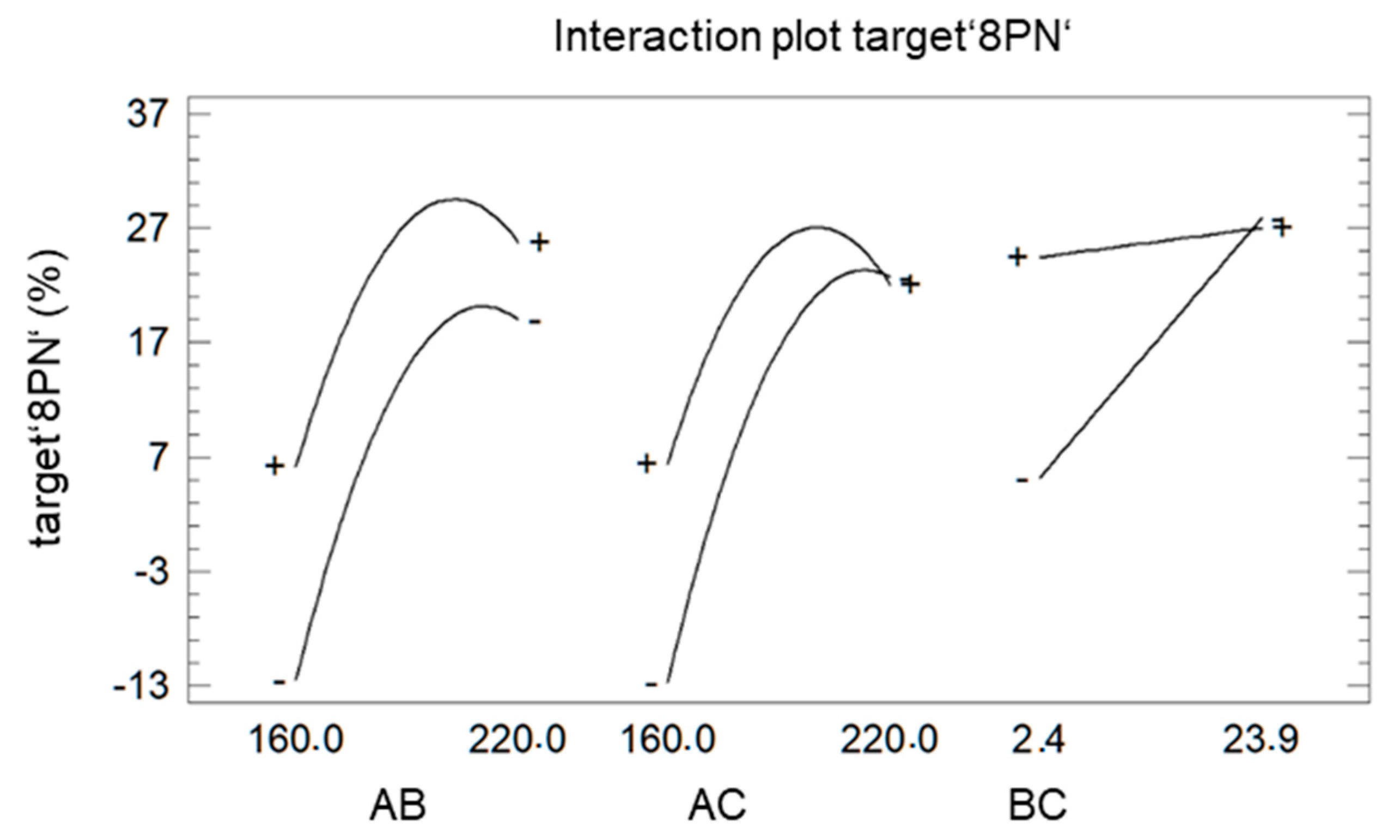
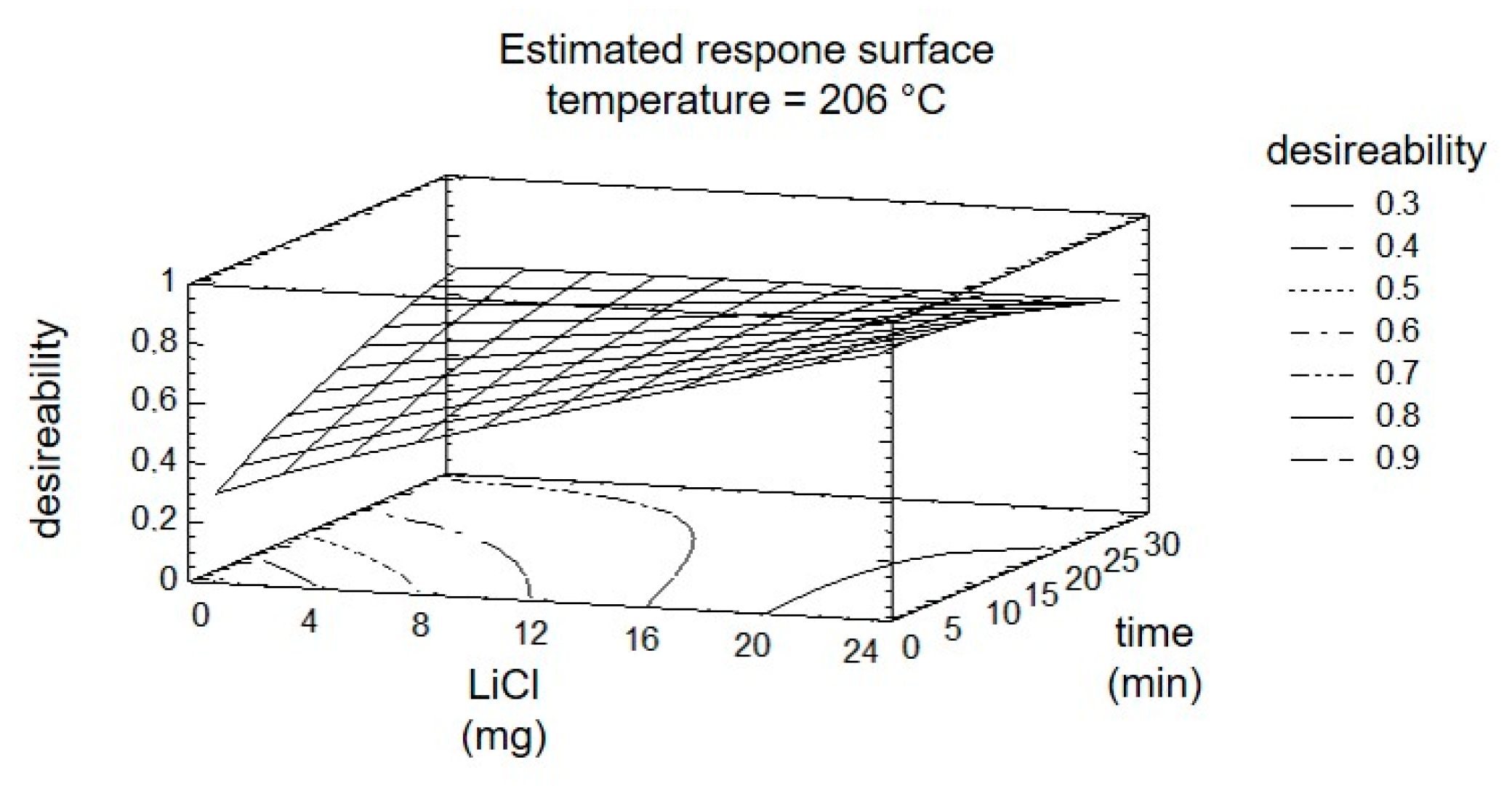
| Step. | A Temperature °C | B LiCl mg | C Time min |
|---|---|---|---|
| low | 160 | 2.4 | 5 |
| high | 220 | 23.9 | 30 |
| Target | R2 (%) | R2corrected |
|---|---|---|
| 8PN | 0.951 | 0.929 |
| 6PN | 0.947 | 0.923 |
| XN | 0.950 | 0.924 |
| By products | 0.986 | 0.977 |
| Step | A Temperature °C | B LiCl mg | C Time min |
|---|---|---|---|
| low | 190.0 | 37.1 | 1.0 |
| high | 220.0 | 55.87 | 9.0 |
| Temperature °C | LiCl mg | Time min | Yield 8PN % | Yield 6PN % | |
|---|---|---|---|---|---|
| Calculated optima | 198 | 65.35 | 9.34 | 38.0 | 38.2 |
| Experimental results | 198 | 65.75 | 9.34 | 37.6 | 37.6 |
| Average ± standard deviation | 37.7 ± 0.85 % | 38.4 ± 0.88 % |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urmann, C.; Riepl, H. Semi-Synthetic Approach Leading to 8-Prenylnaringenin and 6-Prenylnaringenin: Optimization of the Microwave-Assisted Demethylation of Xanthohumol Using Design of Experiments. Molecules 2020, 25, 4007. https://doi.org/10.3390/molecules25174007
Urmann C, Riepl H. Semi-Synthetic Approach Leading to 8-Prenylnaringenin and 6-Prenylnaringenin: Optimization of the Microwave-Assisted Demethylation of Xanthohumol Using Design of Experiments. Molecules. 2020; 25(17):4007. https://doi.org/10.3390/molecules25174007
Chicago/Turabian StyleUrmann, Corinna, and Herbert Riepl. 2020. "Semi-Synthetic Approach Leading to 8-Prenylnaringenin and 6-Prenylnaringenin: Optimization of the Microwave-Assisted Demethylation of Xanthohumol Using Design of Experiments" Molecules 25, no. 17: 4007. https://doi.org/10.3390/molecules25174007
APA StyleUrmann, C., & Riepl, H. (2020). Semi-Synthetic Approach Leading to 8-Prenylnaringenin and 6-Prenylnaringenin: Optimization of the Microwave-Assisted Demethylation of Xanthohumol Using Design of Experiments. Molecules, 25(17), 4007. https://doi.org/10.3390/molecules25174007