Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications
Abstract
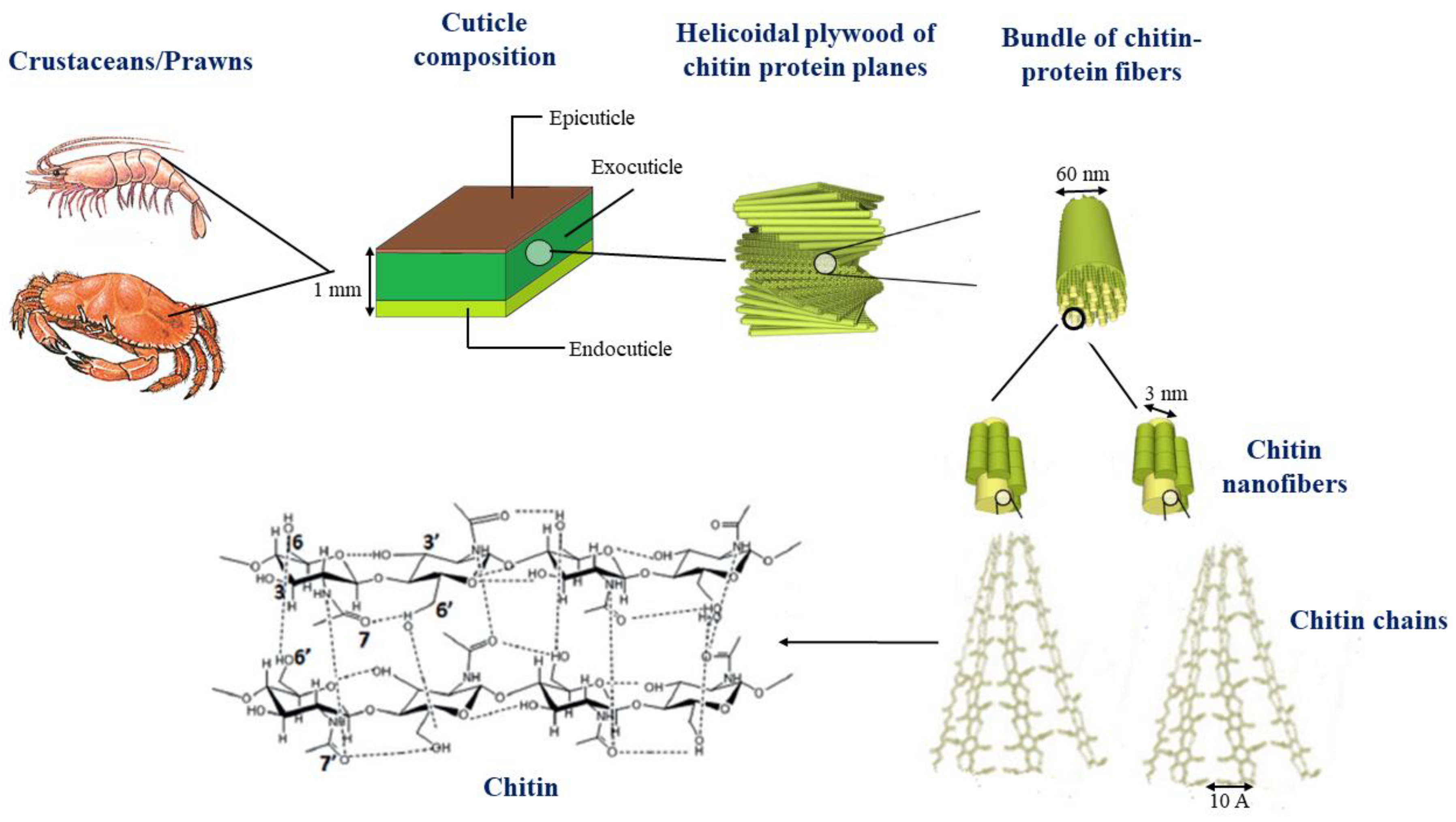
1. Introduction
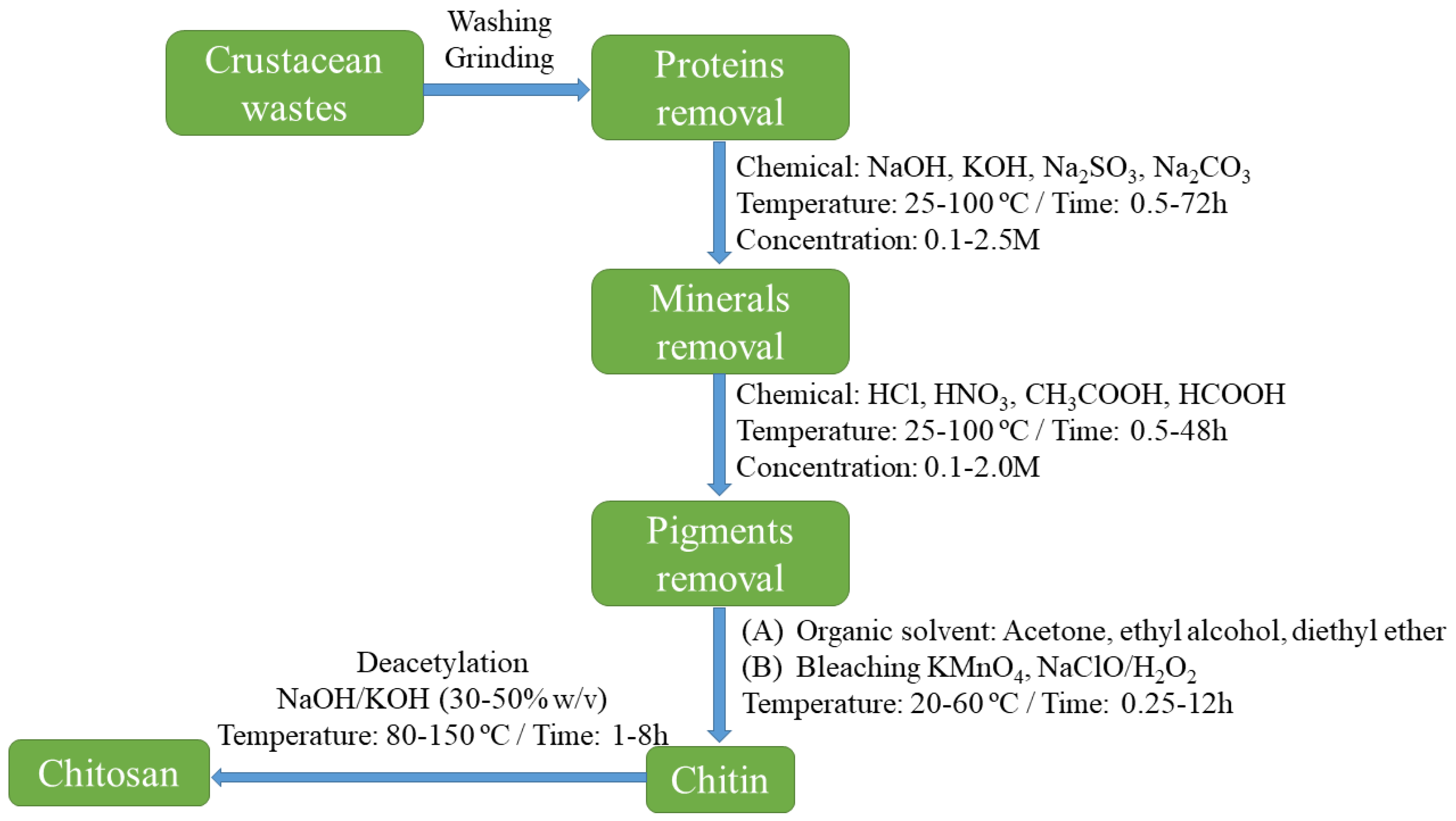
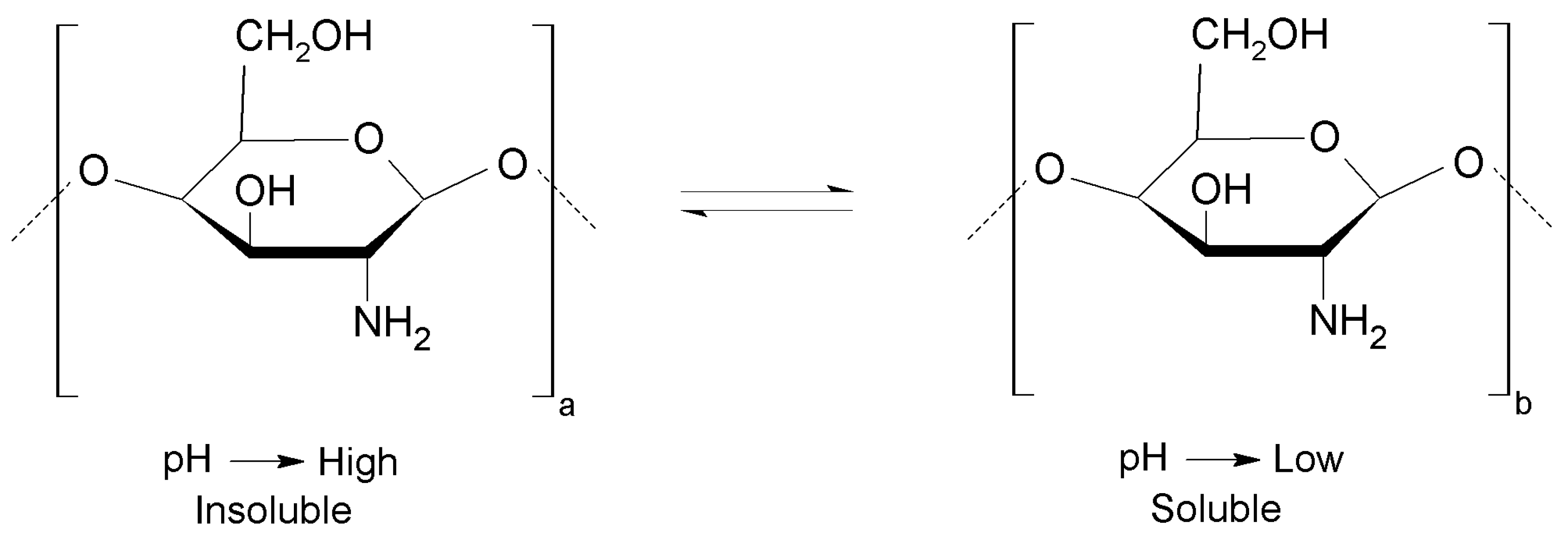
2. Chitosan
2.1. Modification of Chitosan by Functionalization
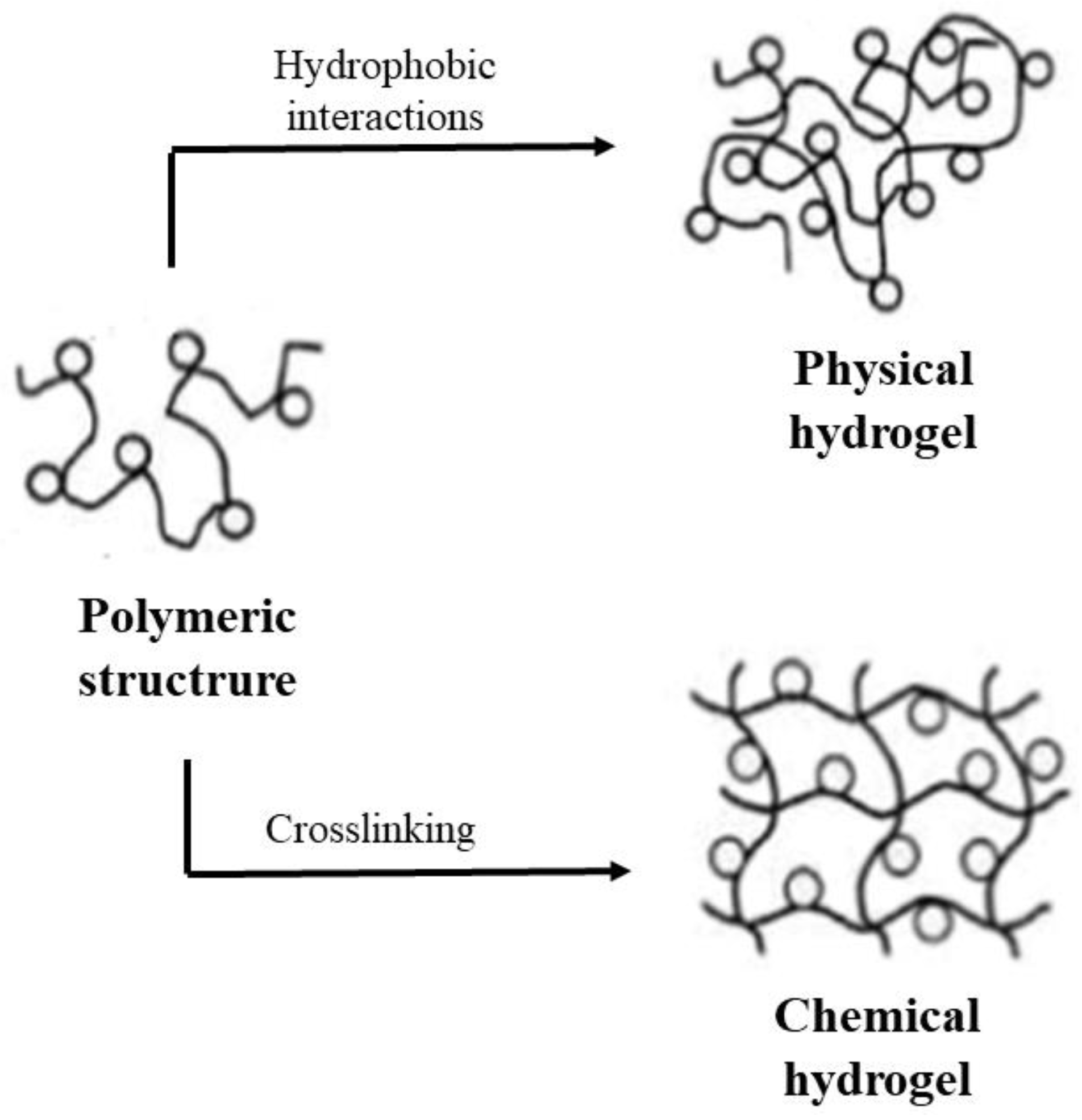
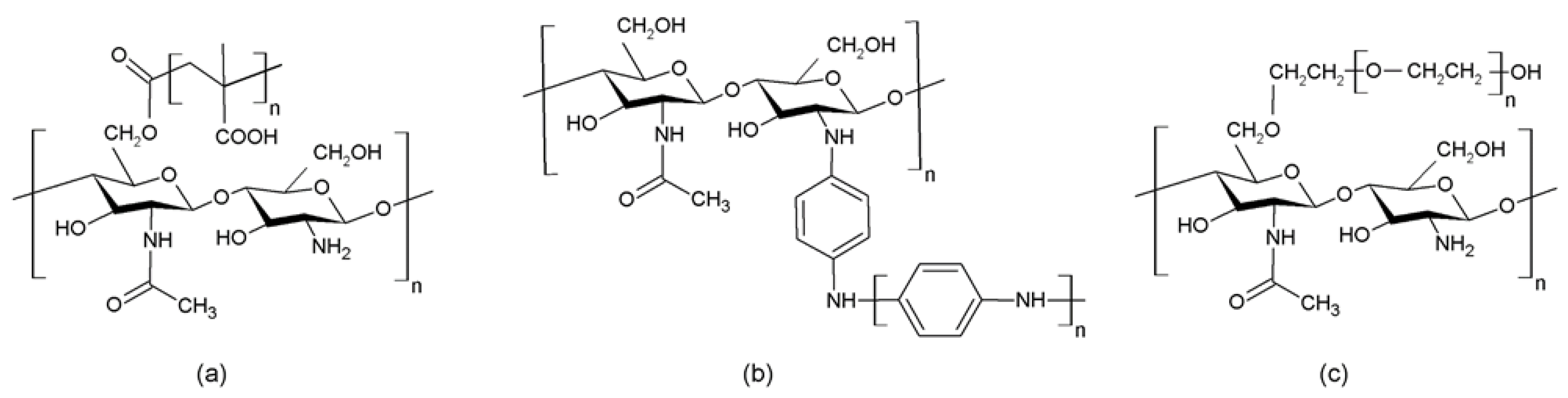
2.1.1. Cross-Linking/Hydrophobic Interactions
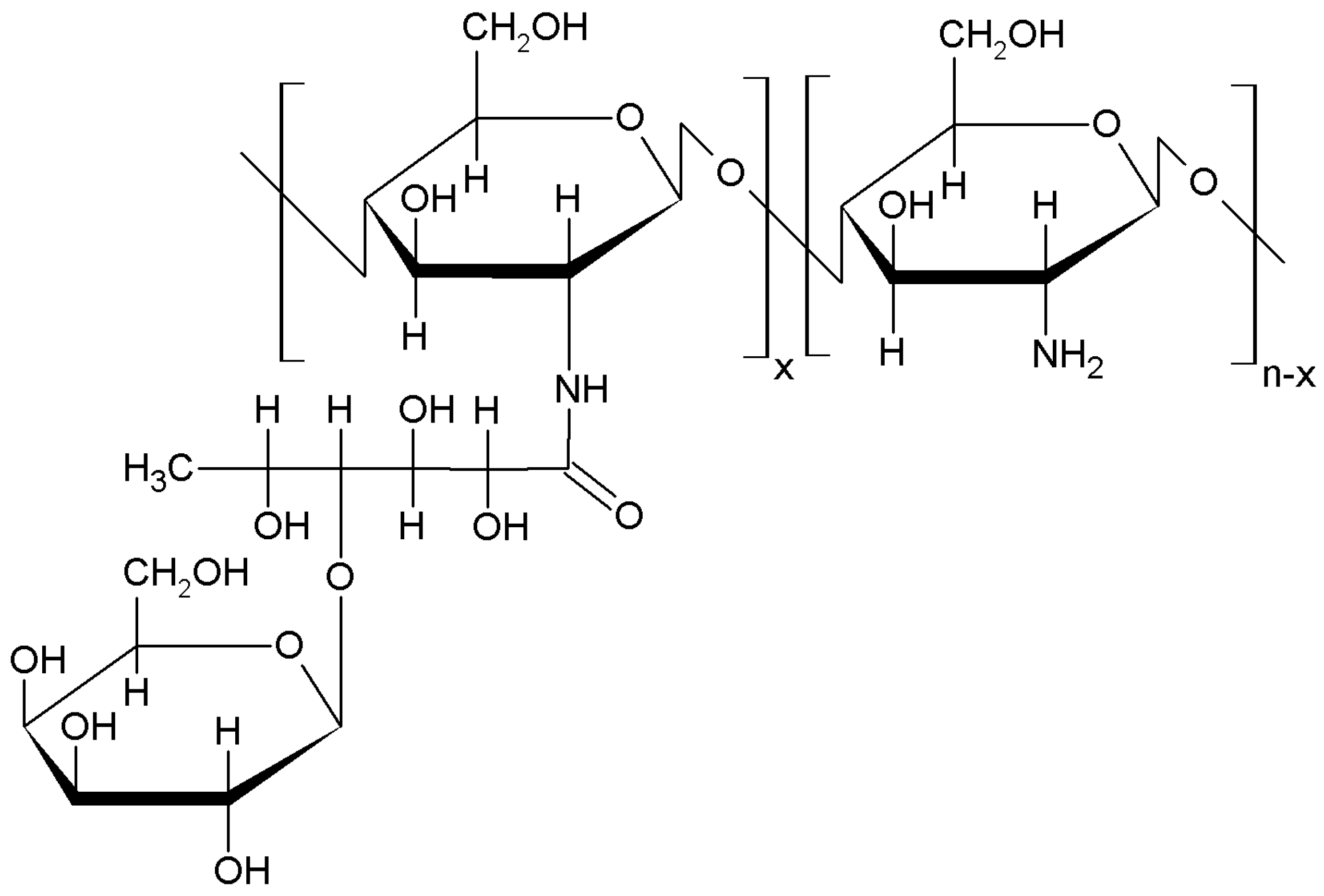
2.1.2. Graft Copolymerization
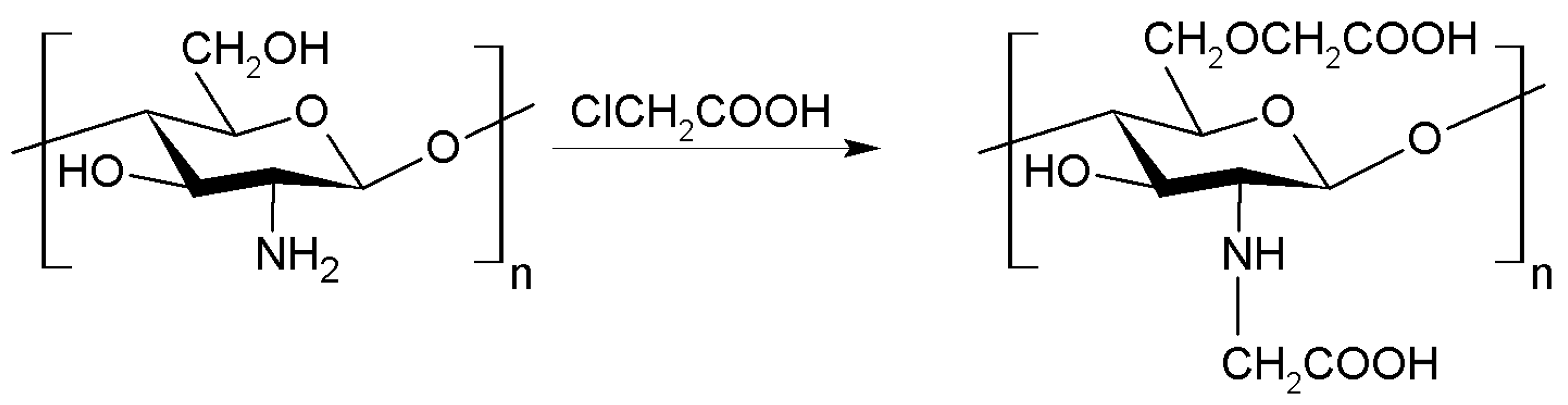
2.1.3. Carboxymethylation
2.1.4. Etherification
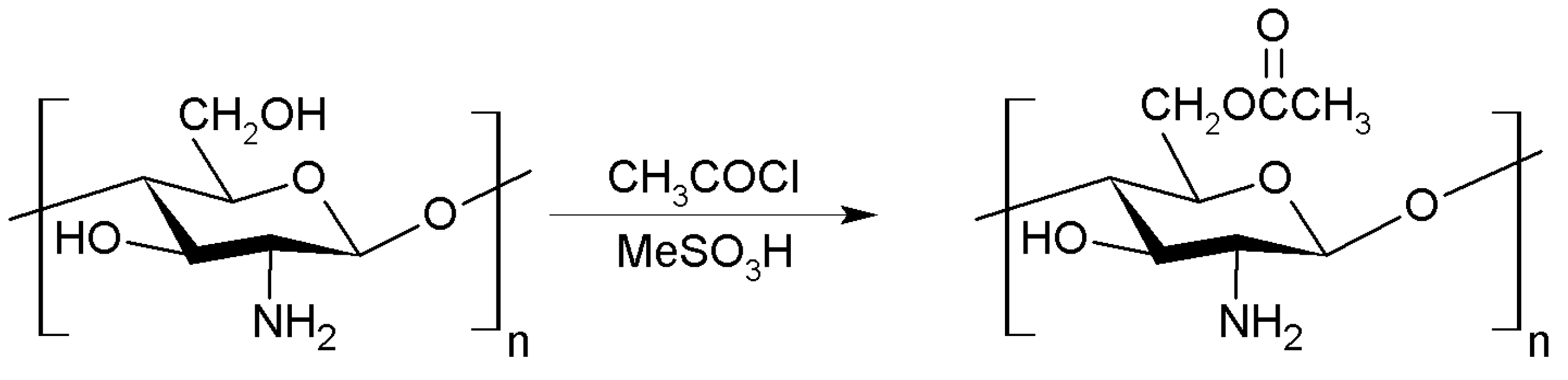
2.1.5. Esterification
2.1.6. Phosphorilation
2.1.7. Sulphatation
2.1.8. Guanidinylation/Biguanidinylation
3. Applications of Chitosan
3.1. Uses in Pharmacy and Medicine
3.2. Biomaterial
3.3. Tissue Engineering
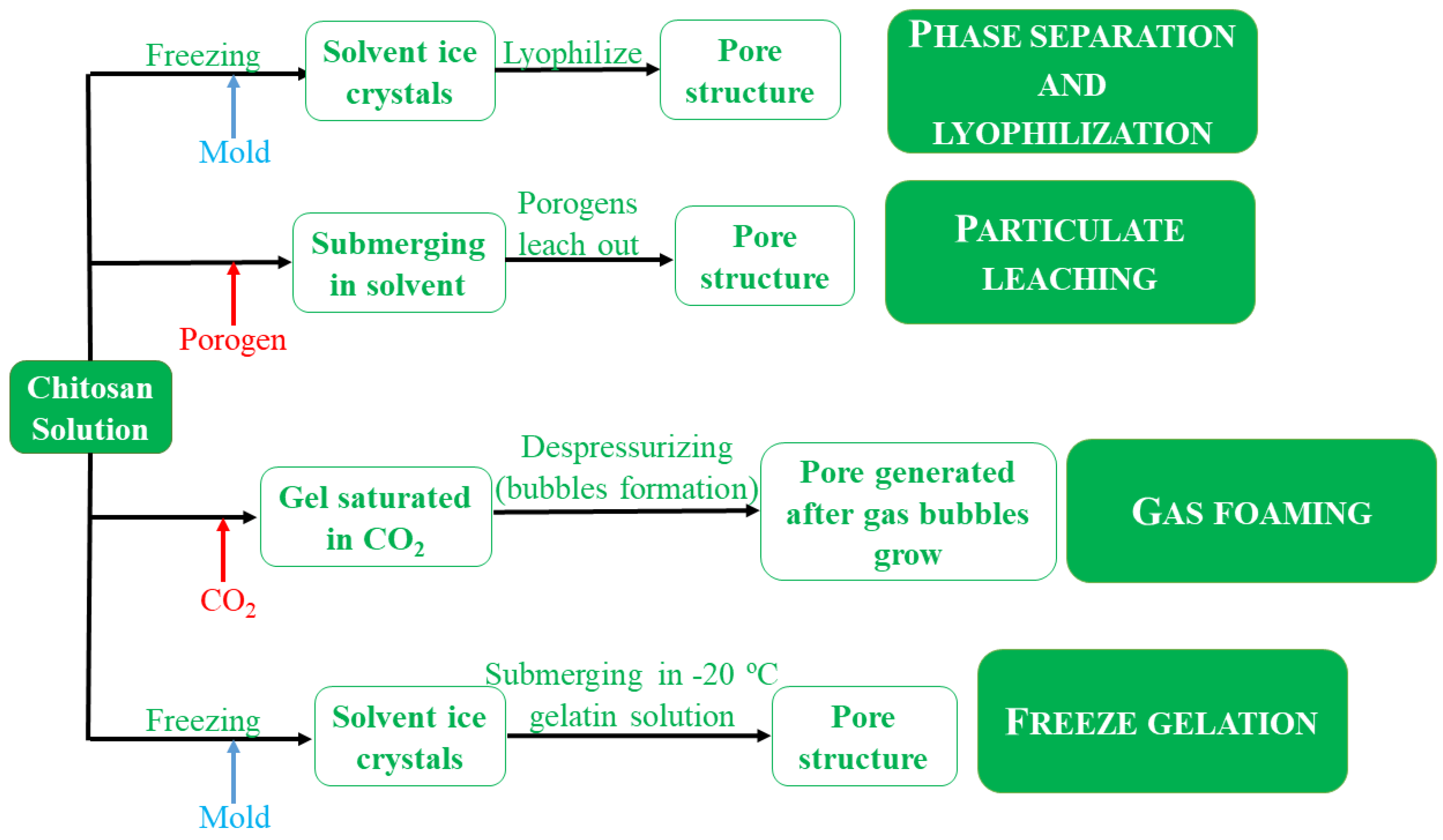
- Phase separation and lyophilization. Firstly, a chitosan solution is introduced into a mold and then a freezing step makes it ready for phase separation with acetic acid as solvent and chitosan acetate salt.
- Particulate leaching techniques. A porogen, usually gelatin, is mixed with a chitosan solution prior to phase separation and lyophilization steps. When is submerged in a solvent, the scaffold is formed through porogen leaching. This fact implies that the obtained scaffolds can have an additional porosity.
- Gas foaming. A chitosan solution contains a cross-linked, mainly glutaraldehyde, which is saturated with CO2 under high pressure, favoring the cross-linking. When the system is depressurized, the thermodymamic instability leads to nucleation and gas bubble growth. The porosity is formed by the bubbled space of the polymer solution).
- Freeze gelation. The obtained scaffolds is placed in a gelation solution of NaOH and ethanol below the chitosan freezing temperature. Then, the gel is air-dried to remove the residual liquid.
3.4. Wounds and Burns
- Homeostasis
- Inflammation
- Migration
- Proliferation
- Maturation
3.5. Drug Delivery
3.6. Artificial Kidney Membrane
3.7. Blood Vessel
3.8. Ophthalmology
3.9. Cosmetics
3.10. Agricultural Applications
- Plant protection against diseases and plagues (pre- and post-harvest).
- Support of beneficial microorganism-plant symbiotic relationships.
- Enhancing biological control and antagonist microorganism action.
- Plant growth development and regulation.
3.11. Food and Nutrition Applications
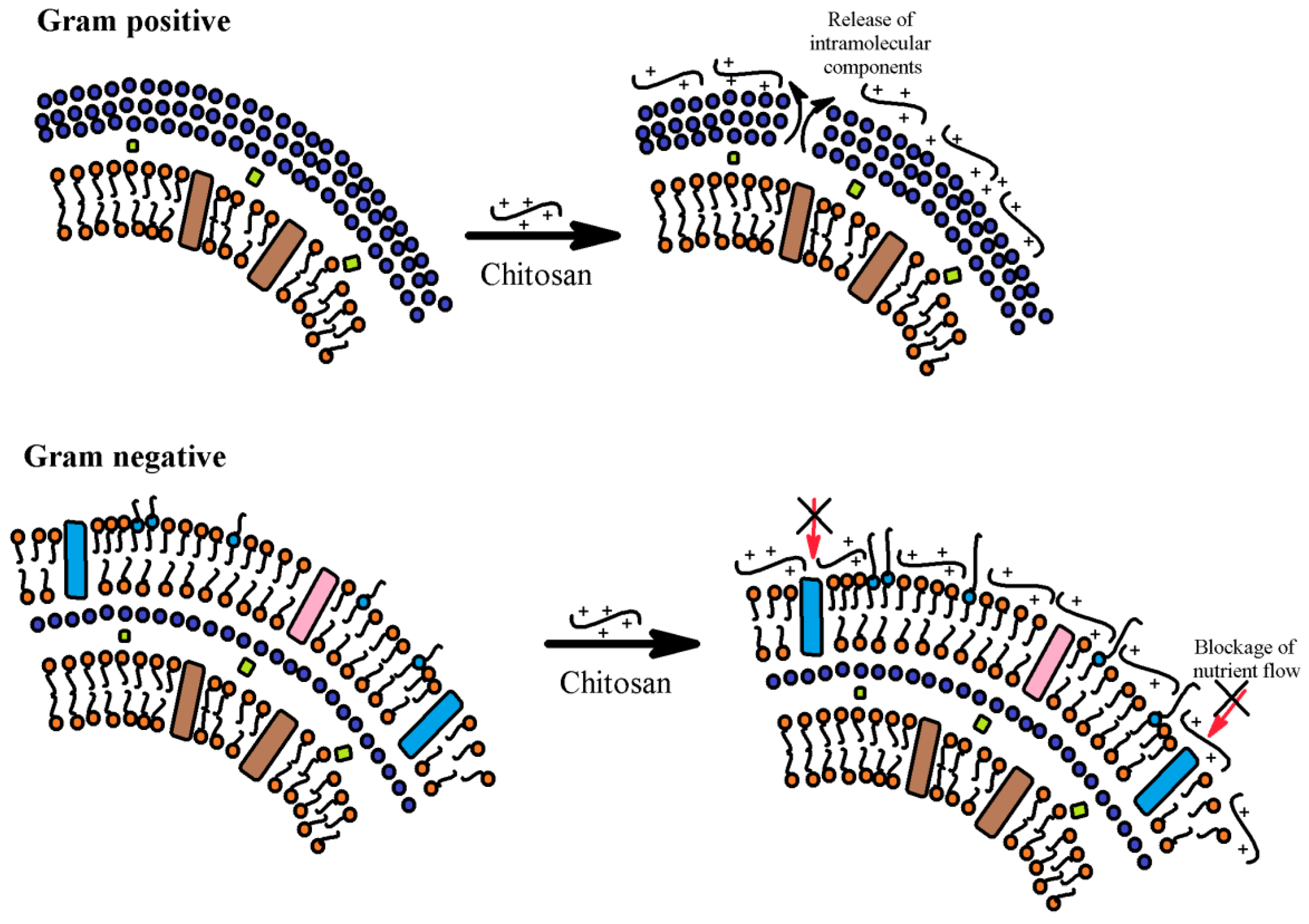
3.12. Antioxidant and Antimicrobial Properties
3.13. Adsorption of Pigments, Dyes and Metals
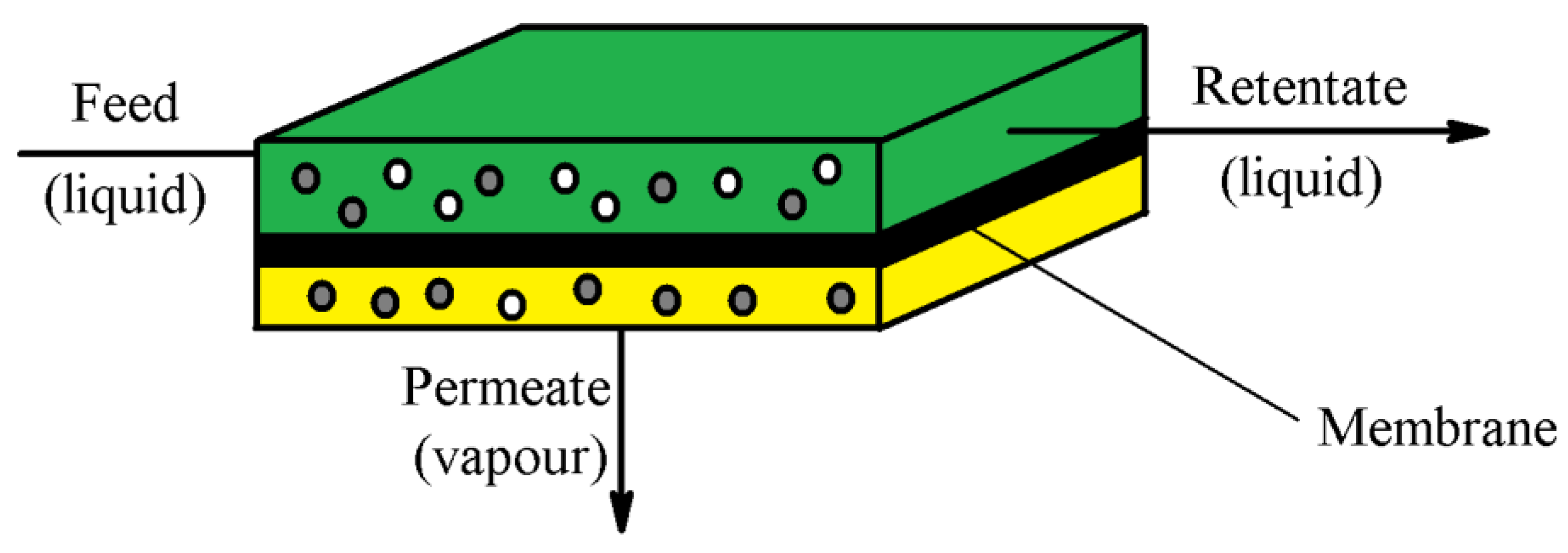
3.14. Pervaporation
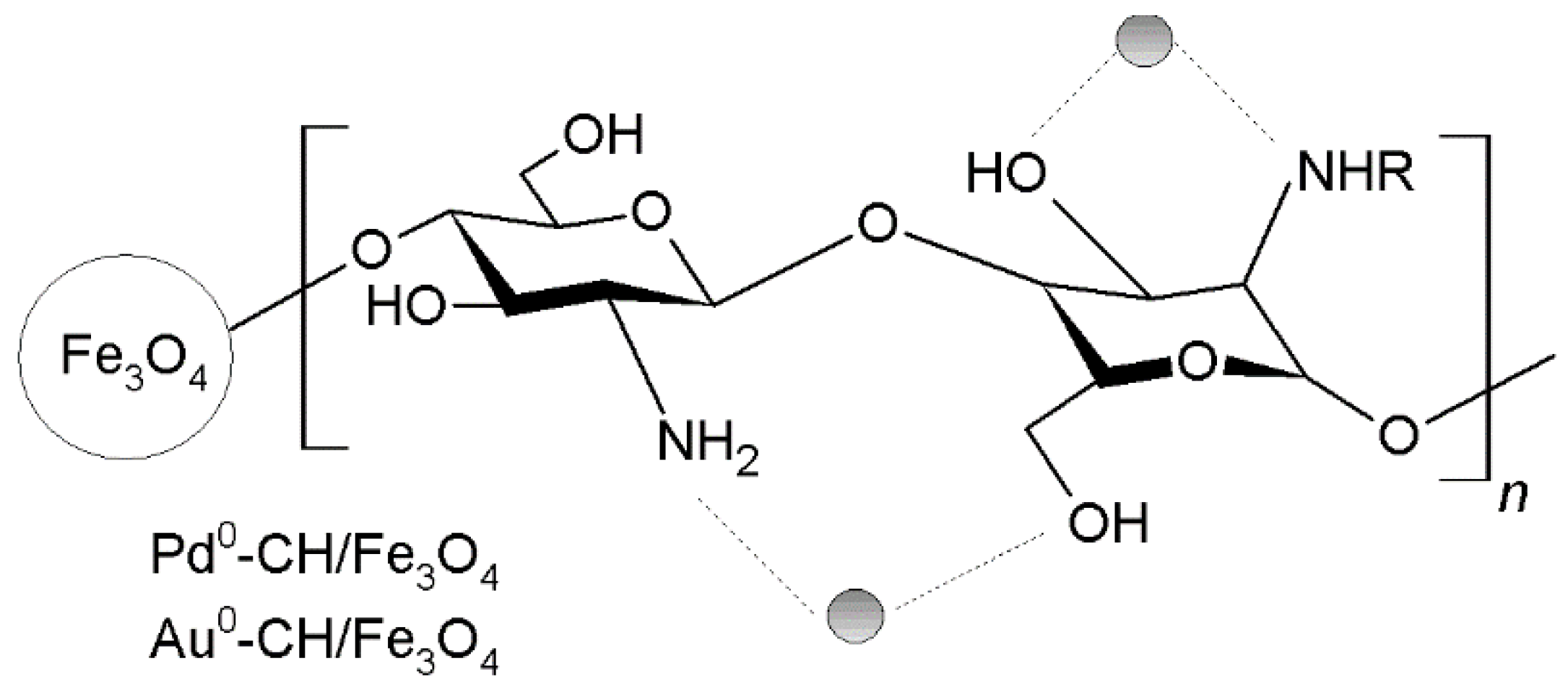
3.15. Catalytic Applications
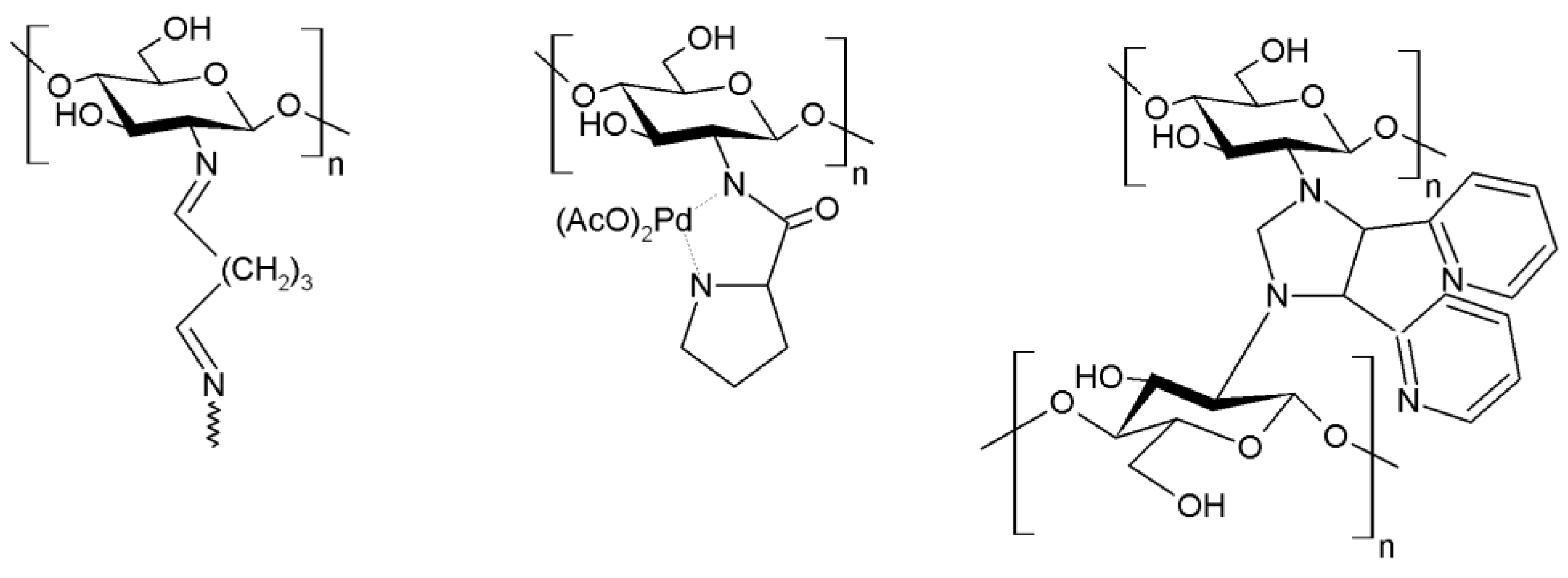
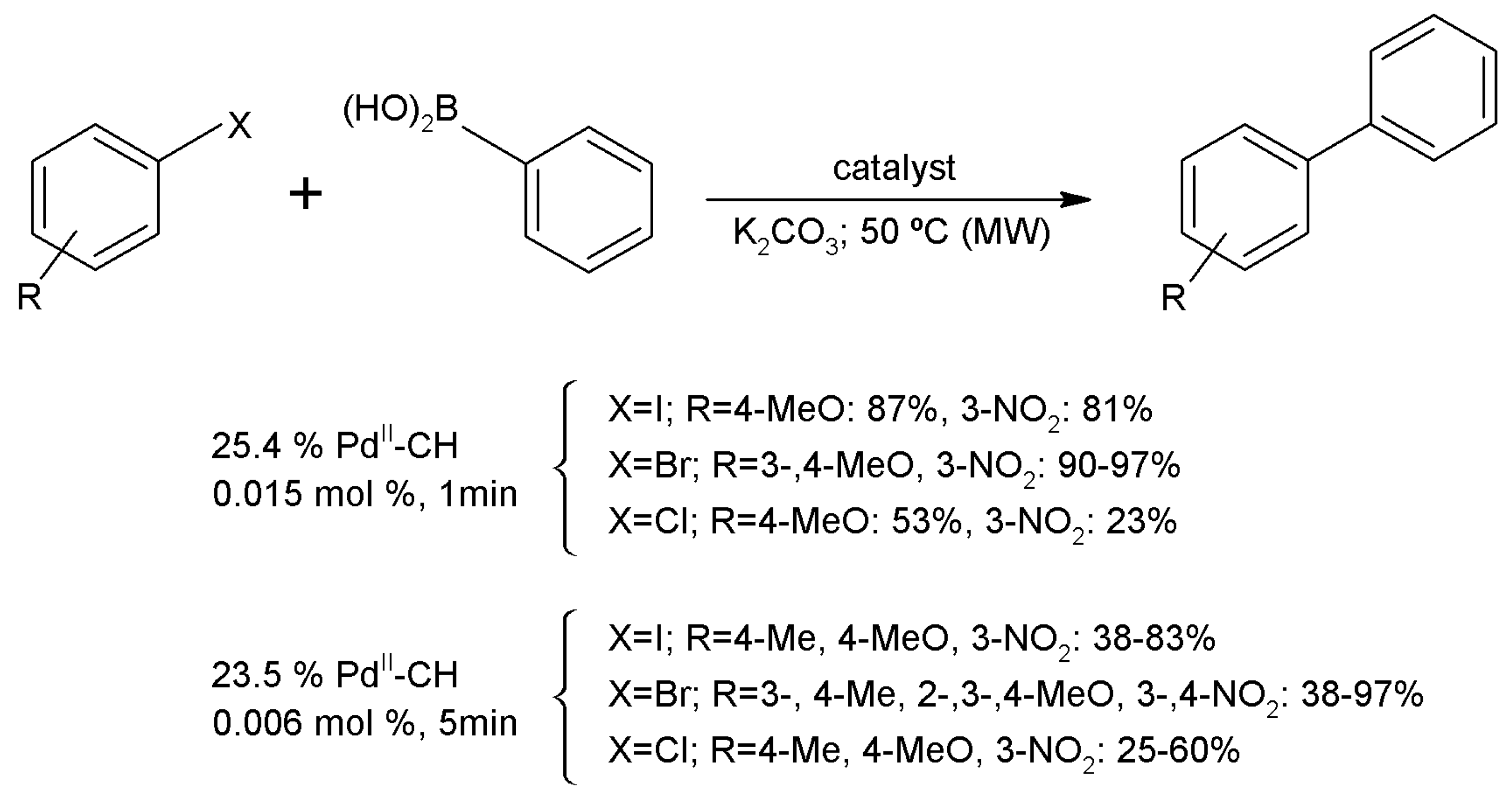
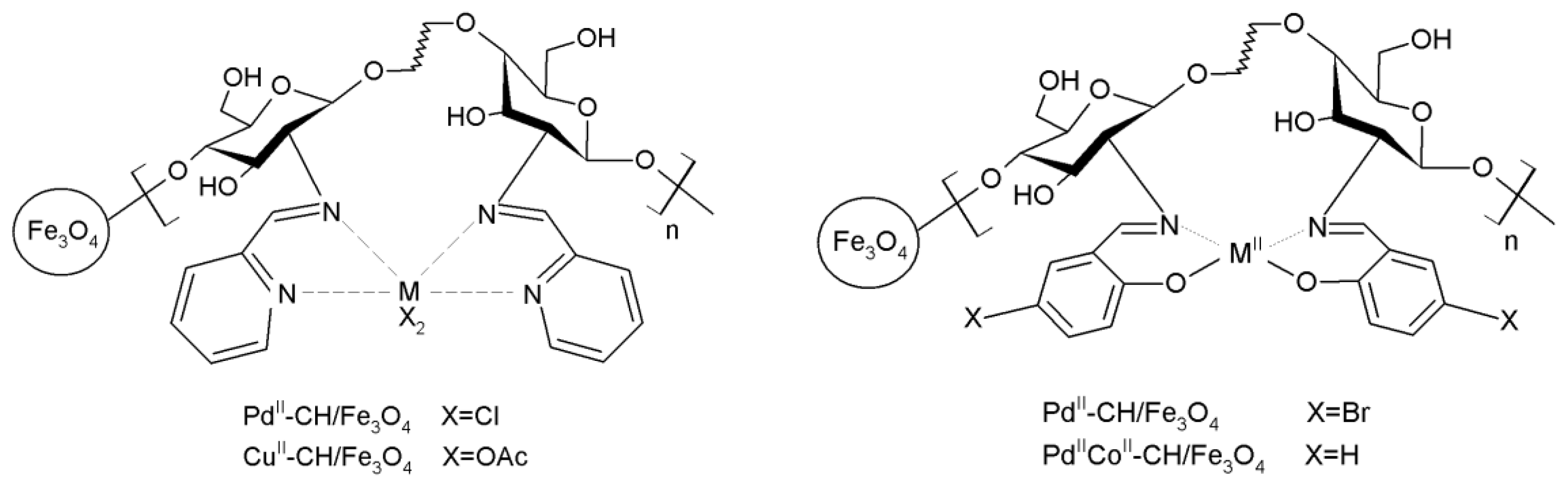
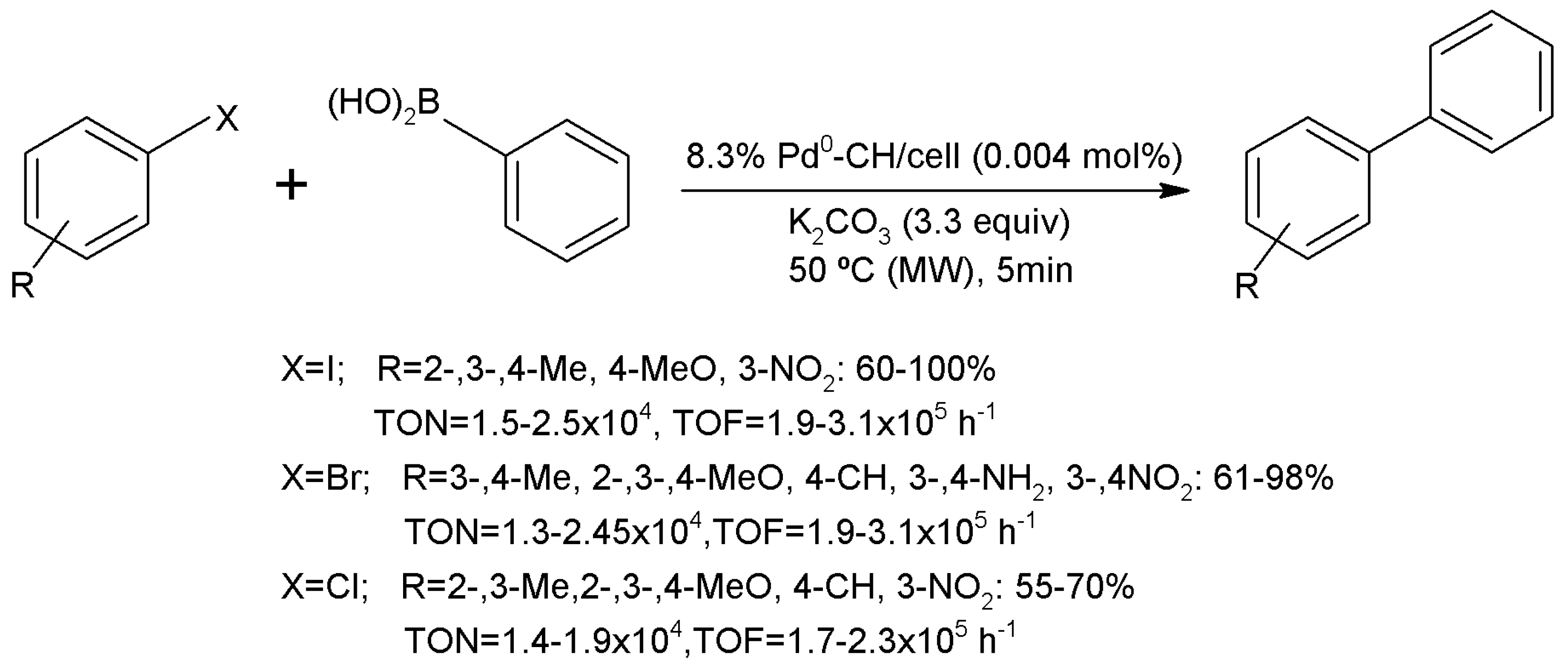
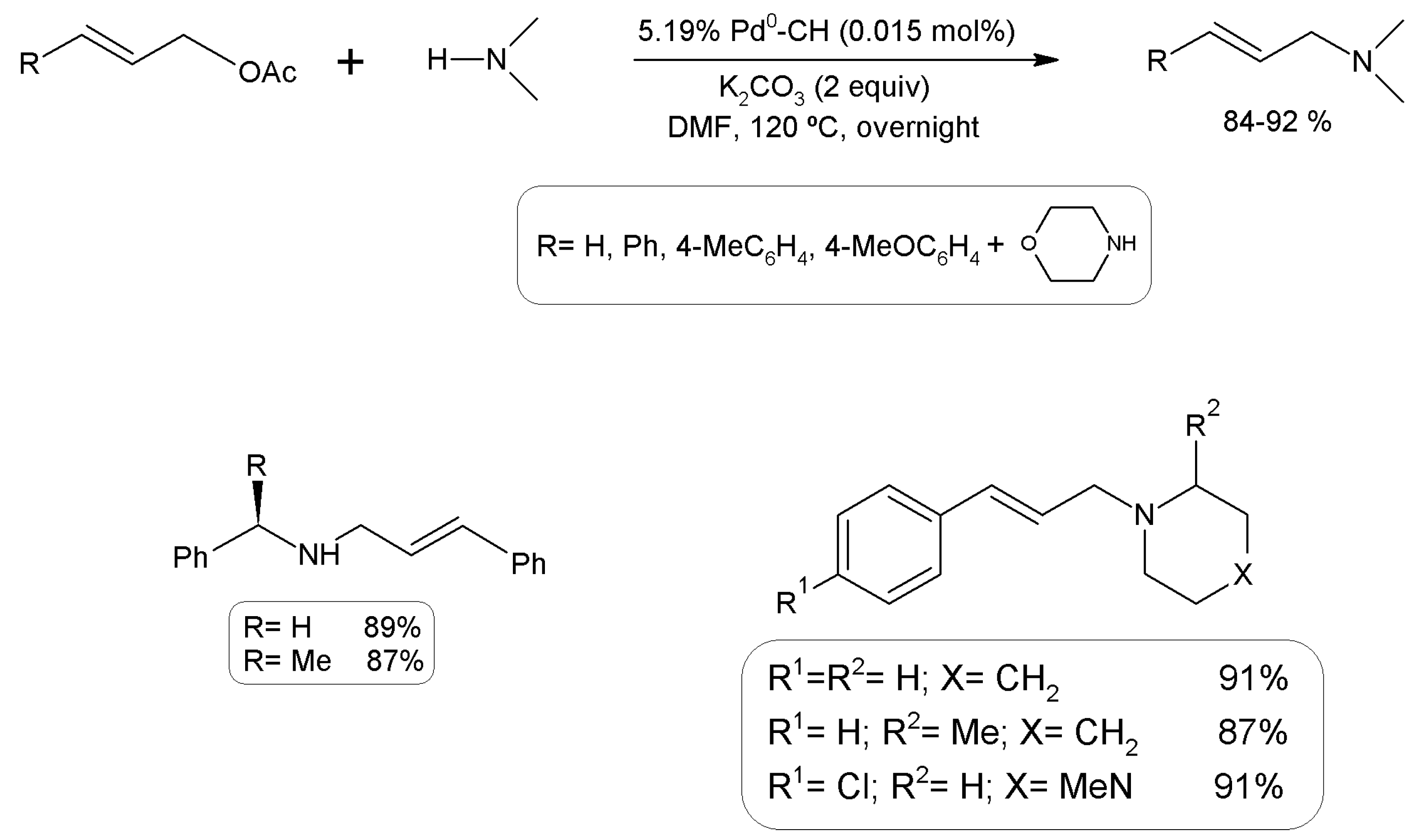
3.15.1. Carbon-Carbon Coupling Reactions
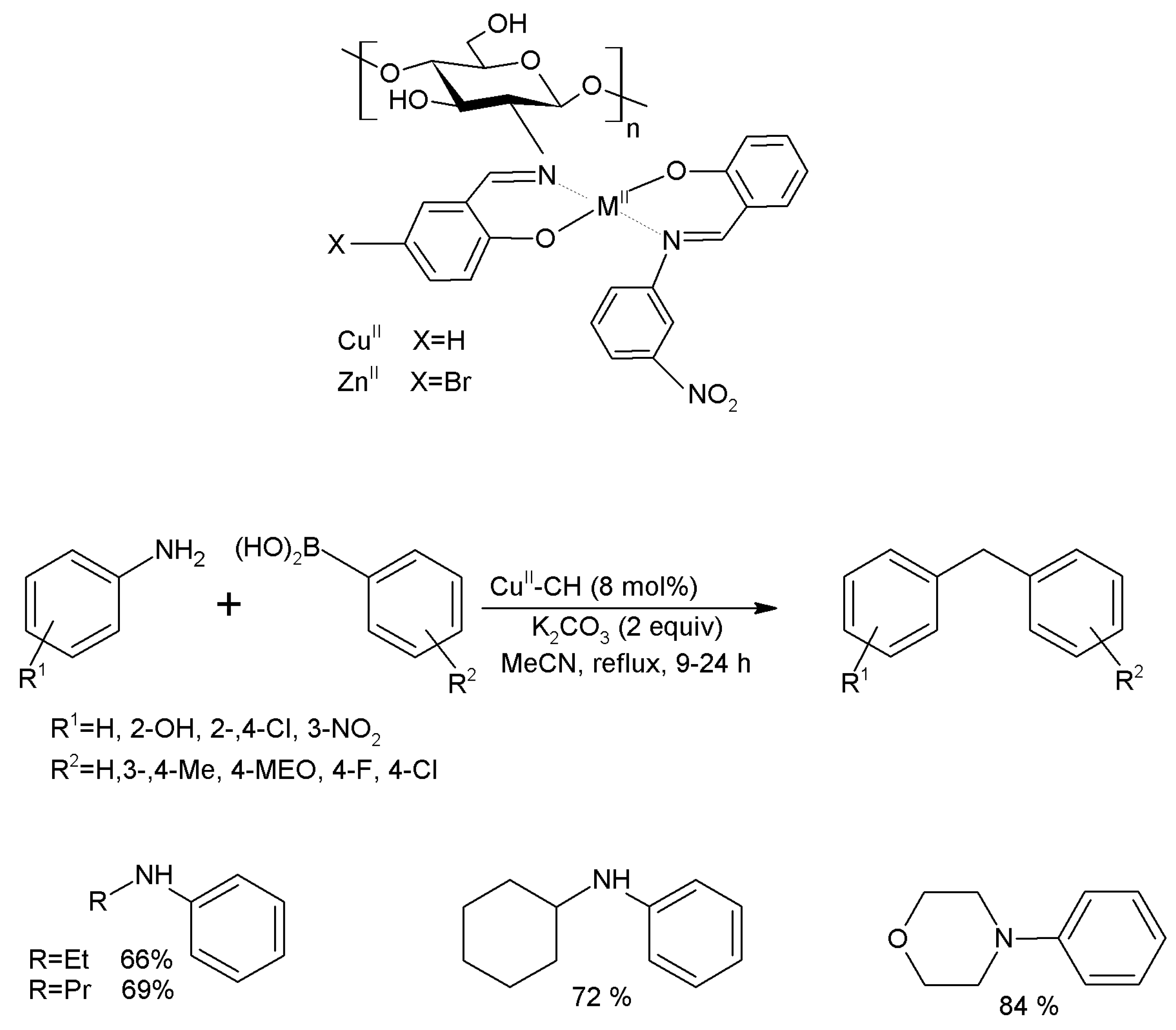
3.15.2. Carbon-Nitrogen Coupling Reactions
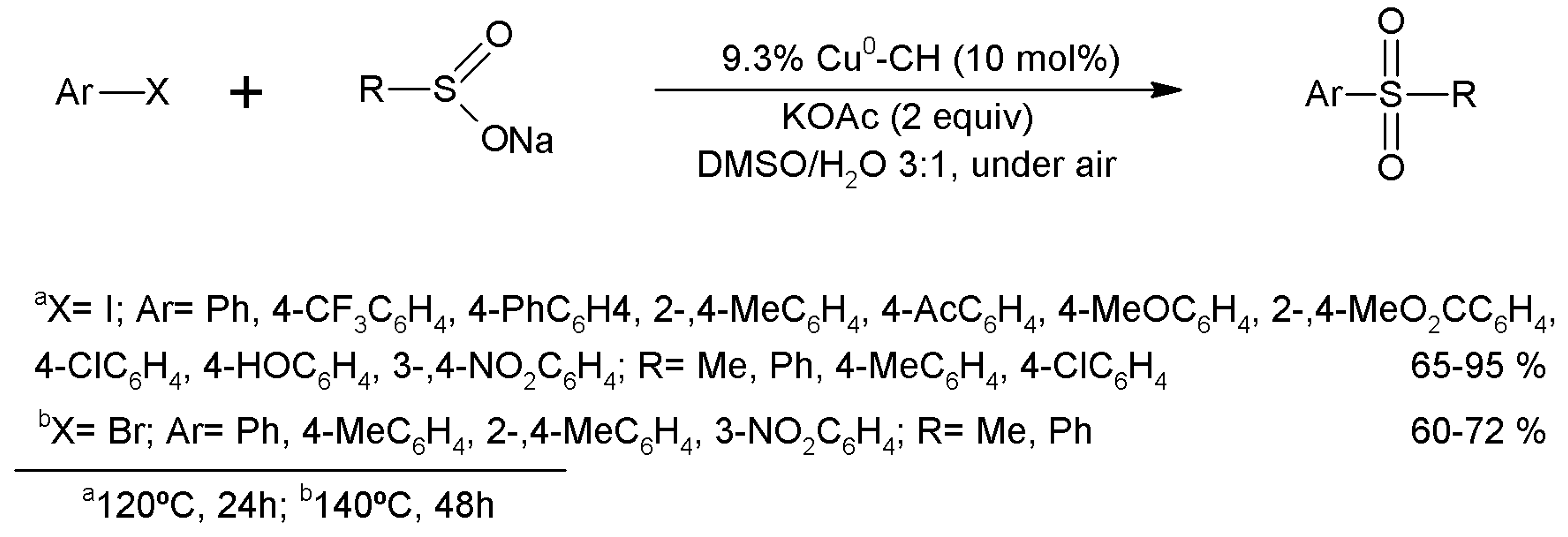
3.15.3. Carbon-Sulfur Coupling Reactions
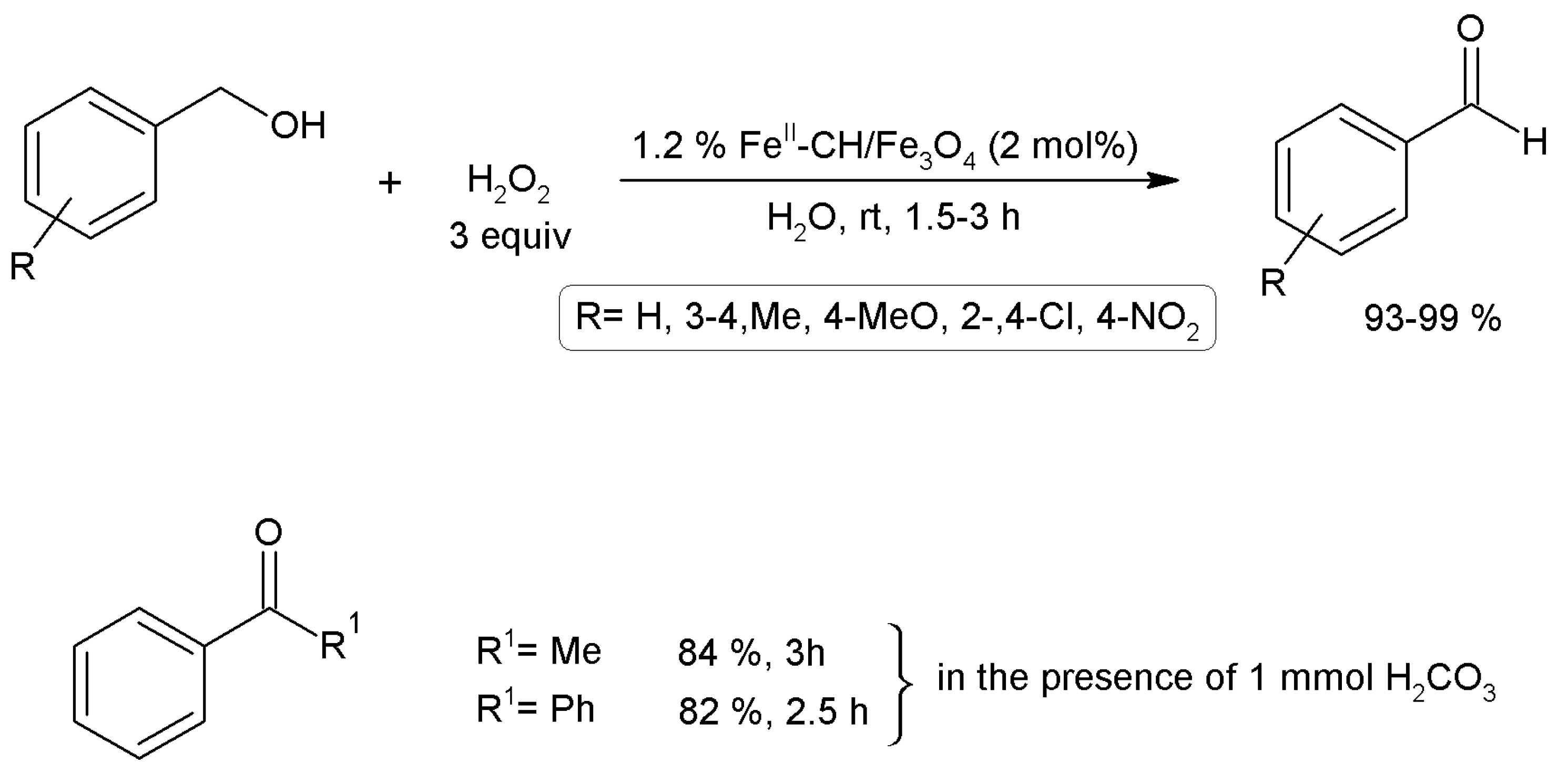
3.15.4. Oxidation Reactions
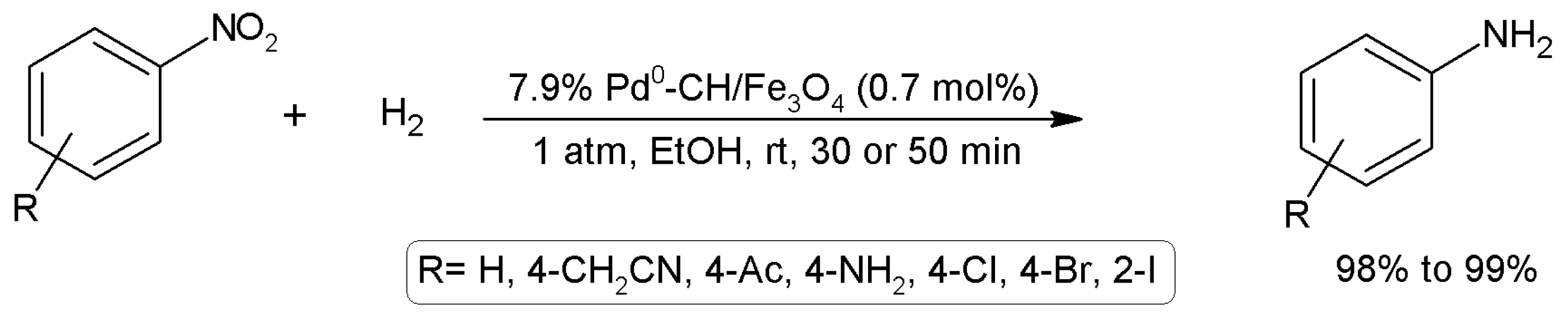
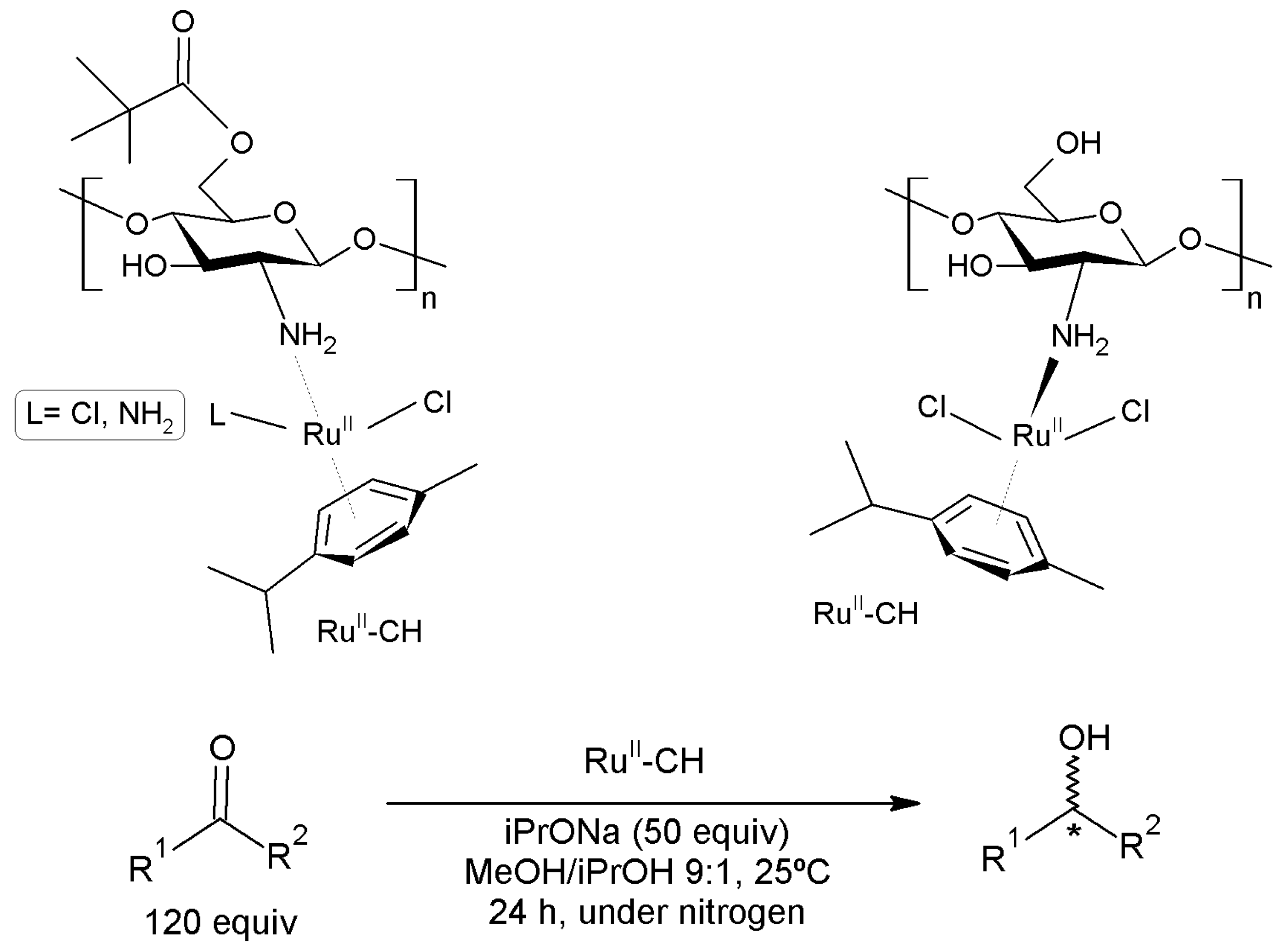
3.15.5. Hydrogenation Reactions
3.15.6. Hydrogenolysis Reactions
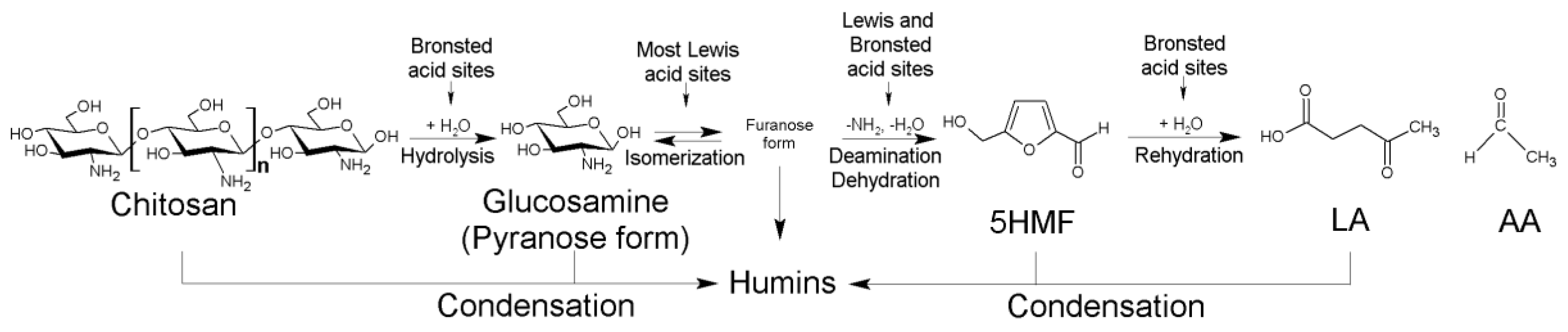
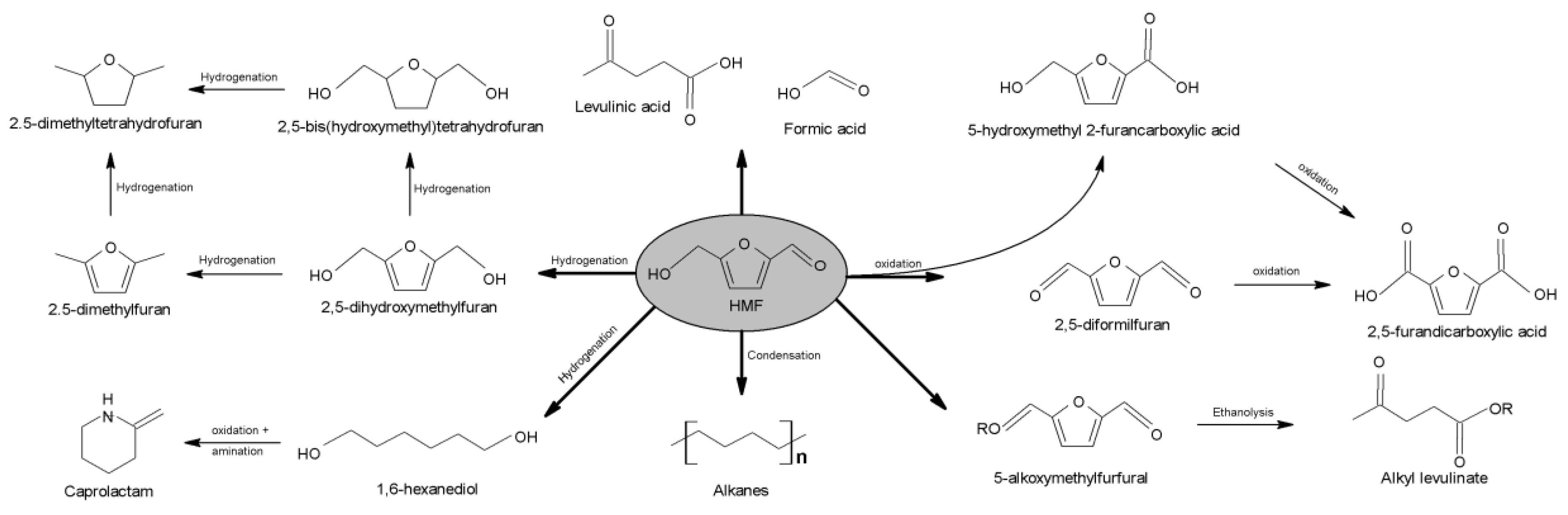
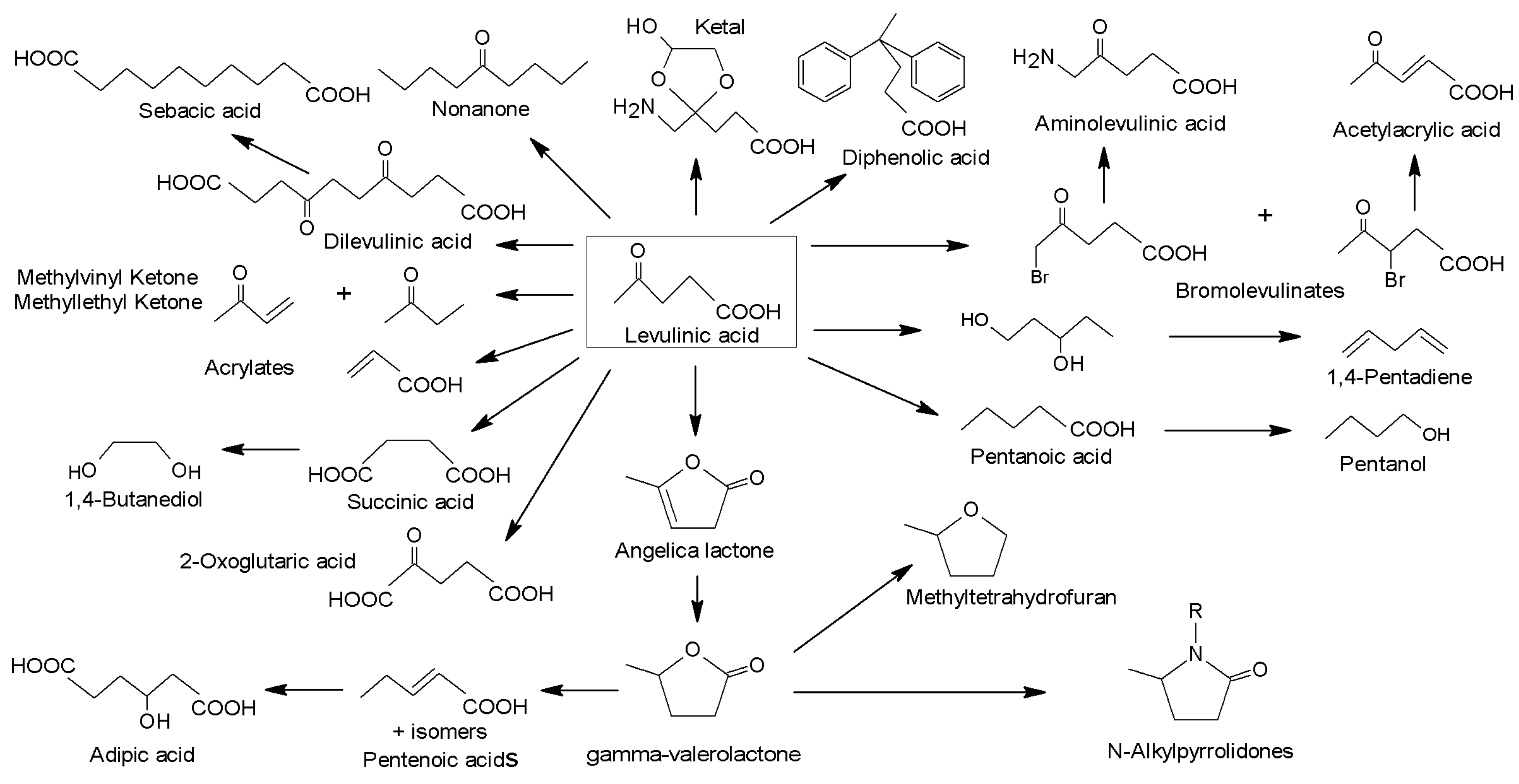
3.16. Catalytic Processes to Valorize Chitosan into Valuable Products
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaur, S.; Dhillon, G.S. The versatile biopolymer chitosan: Potential sources, evaluation of extraction methods and applications. Crit. Rev. Microbiol. 2014, 40, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Khoushab, F.; Yamabhai, M. Chitin research revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.G. Chitin and Chitosan in Fungi; Wiley: Honoken, NJ, USA, 2005. [Google Scholar]
- Shahidi, F.; Abuzaytoun, R. Chitin, chitosan, and co-products: Chemistry, production, applications, and health effects. Adv. Food Nutr. Res. 2005, 49, 93–135. [Google Scholar]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Shahidi, F.; Synowiecki, J. Isolation and Characterization of Nutrients and Value-Added Products from Snow Crab (Chinoecetes Opilio) and Shrimp (Pandalus Borealis) Processing Discards. J. Agric. Food Chem. 1991, 39, 1527–1532. [Google Scholar] [CrossRef]
- Beaney, P.; Lizardi-Mendoza, J.; Healy, M. Comparison of chitins produced by chemical and bioprocessing methods. J. Chem. Technol. Biotechnol. 2005, 80, 145–150. [Google Scholar] [CrossRef]
- Navard, P. The European Polysaccharide Network of Excellence (EPNOE); Springer: Wien, Austria, 2012. [Google Scholar]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Hong, K.; Meyers, S.P. Preparation and characterization of chitin and chitosan—A review. J. Aquat. Food Prod. Technol. 1995, 4, 27–52. [Google Scholar]
- dos Santos, Z.M.; Caroni, A.L.P.F.; Pereira, M.R.; da Silva, D.R.; Fonseca, J.L.C. Determination of deacetylation degree of chitosan: A comparison between conductometric titration and CHN elemental analysis. Carbohydr. Res. 2009, 344, 2591–2595. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, L.; Zhong, W. A new linear potentiometric titration method for the determination of deacetylation degree of chitosan. Carbohydr. Polym. 2003, 54, 457–463. [Google Scholar] [CrossRef]
- Muñoz, G.; Valencia, C.; Valderruten, N.; Ruiz-Durántez, E.; Zuluaga, F. Extraction of chitosan from Aspergillus niger mycelium and synthesis of hydrogels for controlled release of betahistine. React. Funct. Polym. 2015, 91–92, 1–10. [Google Scholar] [CrossRef]
- Sajomsang, W.; Rungsardthong Ruktanonchai, U.; Gonil, P.; Nuchuchua, O. Mucoadhesive property and biocompatibility of methylated N-aryl chitosan derivatives. Carbohydr. Polym. 2009, 78, 945–952. [Google Scholar] [CrossRef]
- Urreaga, J.M.; de la Orden, M.U. Chemical interactions and yellowing in chitosan-treated cellulose. Eur. Polym. J. 2006, 42, 2606–2616. [Google Scholar] [CrossRef]
- Nwe, N.; Stevens, W.F.; Tokura, S.; Tamura, H. Characterization of chitosan and chitosan-glucan complex extracted from the cell wall of fungus Gongronella butleri USDB 0201 by enzymatic method. Enzyme Microb. Technol. 2008, 42, 242–251. [Google Scholar] [CrossRef]
- Kasaai, M.R. A review of several reported procedures to determine the degree of N-acetylation for chitin and chitosan using infrared spectroscopy. Carbohydr. Polym. 2008, 71, 497–508. [Google Scholar] [CrossRef]
- Heux, L.; Brugnerotto, J.; Desbrières, J.; Versali, M.F.; Rinaudo, M. Solid state NMR for determination of degree of acetylation of chitin and chitosan. Biomacromolecules 2000, 1, 746–751. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Lavertu, M.; Xia, Z.; Serreqi, A.N.; Berrada, M.; Rodrigues, A.; Wang, D.; Buschmann, M.D.; Gupta, A. A validated 1H NMR method for the determination of the degree of deacetylation of chitosan. J. Pharm. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kasaai, M.R. Calculation of Mark-Houwink-Sakurada (MHS) equation viscometric constants for chitosan in any solvent-temperature system using experimental reported viscometric constants data. Carbohydr. Polym. 2007, 68, 477–488. [Google Scholar] [CrossRef]
- Zielinska, K.; Shostenko, A.G.; Truszkowski, S. Analysis of chitosan by gel permeation chromatography. High Energy Chem. 2014, 48, 72–75. [Google Scholar] [CrossRef]
- De Benedictis, V.M.; Soloperto, G.; Demitri, C. Correction of MHS viscosimetric constants upon numerical simulation of temperature induced degradation kinetic of chitosan solutions. Polymers (Basel) 2016, 8, 210. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Khor, E. Chitin: Fulfilling a Biomaterials Promise; Elsevier Ltd.: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Thakur, V.K.; Thakur, M.K. Recent advances in graft copolymerization and applications of chitosan: A review. ACS Sustain. Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Yi, H.; Wu, L.Q.; Bentley, W.E.; Ghodssi, R.; Rubloff, G.W.; Culver, J.N.; Payne, G.F. Biofabrication with chitosan. Biomacromolecules 2005, 6, 2881–2894. [Google Scholar] [CrossRef]
- Rafique, A.; Mahmood Zia, K.; Zuber, M.; Tabasum, S.; Rehman, S. Chitosan functionalized poly(vinyl alcohol) for prospects biomedical and industrial applications: A review. Int. J. Biol. Macromol. 2016, 87, 141–154. [Google Scholar] [CrossRef]
- Gil, E.S.; Hudson, S.M. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Dashtimoghadam, E.; Hasani-Sadrabadi, M.M.; Moaddel, H. Structural modification of chitosan biopolymer as a novel polyelectrolyte membrane for green power generation. Polym. Adv. Technol. 2010, 21, 726–734. [Google Scholar] [CrossRef]
- Bodnar, M.; Hartmann, J.F.; Borbely, J. Preparation and characterization of chitosan-based nanoparticles. Biomacromolecules 2005, 6, 2521–2527. [Google Scholar] [CrossRef]
- Valderruten, N.E.; Valverde, J.D.; Zuluaga, F.; Ruiz-Durántez, E. Synthesis and characterization of chitosan hydrogels cross-linked with dicarboxylic acids. React. Funct. Polym. 2014, 84, 21–28. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer (Guildf) 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Xie, W.; Xu, P.; Wang, W.; Liu, Q. Preparation and antibacterial activity of a water-soluble chitosan derivative. Carbohydr. Polym. 2002, 50, 35–40. [Google Scholar] [CrossRef]
- Yang, S.; Tirmizi, S.A.; Burns, A.; Barney, A.A.; Risen, W.M. Chitaline materials: Soluble Chitosan-polyaniline copolymers and their conductive doped forms. Synth. Met. 1989, 32, 191–200. [Google Scholar] [CrossRef]
- Gorochovceva, N.; Makuška, R. Synthesis and study of water-soluble chitosan-O-poly(ethylene glycol) graft copolymers. Eur. Polym. J. 2004, 40, 685–691. [Google Scholar] [CrossRef]
- De Abreu, F.R.; Campana-Filho, S.P. Preparation and characterization of carboxymethylchitosan. Polímeros 2005, 15, 79–83. [Google Scholar] [CrossRef]
- Cao, J.; You, J.; Zhang, L.; Zhou, J. Homogeneous synthesis and characterization of chitosan ethers prepared in aqueous alkali/urea solutions. Carbohydr. Polym. 2018, 185, 138–144. [Google Scholar] [CrossRef]
- Meng, G.; He, J.; Wu, Y.; Wu, F.; Gu, Z. Antibiotic-loaded chitosan hydrogel with superior dual functions: Antibacterial efficacy and osteoblastic cell responses. ACS Appl. Mater. Interfaces 2014, 6, 10005–10013. [Google Scholar]
- Tseng, T.C.; Tao, L.; Hsieh, F.Y.; Wei, Y.; Chiu, I.M.; Hsu, S.H. An injectable, self-healing hydrogel to repair the central nervous system. Adv. Mater. 2015, 27, 3518–3524. [Google Scholar] [CrossRef]
- Liu, H.; Ojha, B.; Morris, C.; Jiang, M.; Wojcikiewicz, E.P.; Rao, P.P.N.; Du, D. Positively Charged Chitosan and N-Trimethyl Chitosan Inhibit Aβ40 Fibrillogenesis. Biomacromolecules 2015, 16, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, N.Y.; Waly, A.I.; Kandile, N.G.; Rushdy, A.A.; El-Sheikh, M.A.; Ibrahim, H.M. Preparation, characterization and antibacterial properties of cyanoethylchitosan/cellulose acetate polymer blended films. Carbohydr. Polym. 2011, 84, 223–230. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I.; Rogge, T.M.; Stevens, C.V.; Smagghe, G.; Steurbaut, W.; Höfte, M. Synthesis and fungicidal activity of new N,O-acyl chitosan derivatives. Biomacromolecules 2004, 5, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; Rabea, E.I.; Rogge, T.M.; Stevens, C.V.; Steurbaut, W.; Höfte, M.; Smagghe, G. Fungicidal and insecticidal activity of O-acyl chitosan derivatives. Polym. Bull. 2005, 54, 279–289. [Google Scholar] [CrossRef]
- Jayakumar, R.; Selvamurugan, N.; Nair, S.V.; Tokura, S.; Tamura, H. Preparative methods of phosphorylated chitin and chitosan-An overview. Int. J. Biol. Macromol. 2008, 43, 221–225. [Google Scholar] [CrossRef]
- Heras, A.; Rodríguez, N.M.; Ramos, V.M.; Agulló, E. N-methylene phosphonic chitosan: A novel soluble derivative. Carbohydr. Polym. 2001, 44, 1–8. [Google Scholar] [CrossRef]
- Ramos, V.M.; Rodríguez, N.M.; Rodríguez, M.S.; Heras, A.; Agulló, E. Modified chitosan carrying phosphonic and alkyl groups. Carbohydr. Polym. 2003, 51, 425–429. [Google Scholar] [CrossRef]
- Ramos, V.M.; Rodríguez, N.M.; Henning, I.; Díaz, M.F.; Monachesi, M.P.; Rodríguez, M.S.; Abarrategi, A.; Correas-Magaña, V.; López-Lacomba, J.L.; Agulló, E. Poly(ethylene glycol)-crosslinked N-methylene phosphonic chitosan. Preparation and characterization. Carbohydr. Polym. 2006, 64, 328–336. [Google Scholar] [CrossRef]
- Terbojevich, M.; Carraro, C.; Cosani, A.; Focher, B.; Naggi, A.M.; Torri, G. Solution studies of chitosan 6-O-sulfate. Die Makromol. Chemie 1989, 190, 2847–2855. [Google Scholar] [CrossRef]
- Holme, K.R.; Perlin, A.S. Chitosan N-sulfate. A water-soluble polyelectrolyte. Carbohydr. Res. 1997, 302, 7–12. [Google Scholar] [CrossRef]
- Fan, L.; Wu, P.; Zhang, J.; Gao, S.; Wang, L.; Li, M.; Sha, M.; Xie, W.; Nie, M. Synthesis and anticoagulant activity of the quaternary ammonium chitosan sulfates. Int. J. Biol. Macromol. 2012, 50, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Subhapradha, N.; Ramasamy, P.; Srinivasan, A.; Madeswaran, P.; Shanmugam, V.; Shanmugam, A. Sulfation of β-chitosan and evaluation of biological activity from gladius of Sepioteuthis lessoniana. Int. J. Biol. Macromol. 2013, 62, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Sun, P.; Luo, Y.; Ma, C.; Xu, J.; Liu, W. Guanidinylation: A Simple Way to Fabricate Cell Penetrating Peptide Analogue-Modified Chitosan Vector for Enhanced Gene Delivery. J. Appl. Polym. Sci. 2011, 121, 3569–3578. [Google Scholar] [CrossRef]
- Hu, Y.; Du, Y.; Yang, J.; Kennedy, J.F.; Wang, X.; Wang, L. Synthesis, characterization and antibacterial activity of guanidinylated chitosan. Carbohydr. Polym. 2007, 67, 66–72. [Google Scholar] [CrossRef]
- Sahariah, P.; Óskarsson, B.M.; Hjálmarsdóttir, M.; Másson, M. Synthesis of guanidinylated chitosan with the aid of multiple protecting groups and investigation of antibacterial activity. Carbohydr. Polym. 2015, 127, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ye, Y.; Xiao, H.; He, B.; Qian, L. Microwave assisted preparation of antimicrobial chitosan with guanidine oligomers and its application in hygiene paper products. Polymers (Basel) 2017, 9, 633. [Google Scholar] [CrossRef]
- Zhao, X.; Qiao, Z.Z.; He, J.X. Preparation of chitosan biguanidine hydrochloride and application in antimicrobial finish of wool fabric. J. Eng. Fibers Fabr. 2010, 5, 16–24. [Google Scholar] [CrossRef]
- Pantaleone, D.; Yalpani, M.; Scollar, M. Unusual susceptibility of chitosan to enzymic hydrolysis. Carbohydr. Res. 1992, 237, 325–332. [Google Scholar] [CrossRef]
- Yeh, H.Y.; Lin, J.C. Surface characterization and in vitro platelet compatibility study of surface sulfonated chitosan membrane with amino group protection-deprotection strategy. J. Biomater. Sci. Polym. Ed. 2008, 19, 291–310. [Google Scholar] [CrossRef]
- Yang, H.; Luan, S.; Zhao, J.; Shi, H.; Li, X.; Song, L.; Jin, J.; Shi, Q.; Yin, J.; Shi, D.; et al. Improving hemocompatibility of styrene-b-(ethylene-co-butylene)-b-styrene elastomer via N-vinyl pyrrolidone-assisted grafting of poly(ethylene glycol) methacrylate. Polymer (Guildf) 2012, 53, 1675–1683. [Google Scholar] [CrossRef]
- Peniche, C.; Argüelles-Monal, W.; Peniche, H.; Acosta, N. Chitosan: An Attractive Biocompatible Polymer for Microencapsulation. Macromol. Biosci. 2003, 3, 511–520. [Google Scholar] [CrossRef]
- Reis, R.L.; Román, J.S. (Eds.) Biodegradable Systems in Tissue Engineering and Regenerative Medicine, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Peña, J.; Izquierdo-Barba, I.; Martínez, A.; Vallet-Regí, M. New method to obtain chitosan/apatite materials at room temperature. Solid State Sci. 2006, 8, 513–519. [Google Scholar] [CrossRef]
- Singla, A.K.; Chawla, M. Chitosan: Some pharmaceutical and biological aspects—An update. J. Pharm. Pharmacol. 2001, 53, 1047–1067. [Google Scholar] [CrossRef] [PubMed]
- Francis Suh, J.K.; Matthew, H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [CrossRef]
- Venkatesan, J.; Vinodhini, P.A.; Sudha, P.N.; Kim, S.K. Chitin and chitosan composites for bone tissue regeneration. Adv. Food Nutr. Res. 2014, 73, 59–81. [Google Scholar] [PubMed]
- Jayakumar, R.; Jayakumar, S.V.; Jayakumar, T.; Murat, H. Perspectives of Chitin and Chitosan Nanofibrous Scaffolds in Tissue Engineering; Intech: Rijeka, Croatia, 2010. [Google Scholar]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Cheng, M.; Deng, J.; Yang, F.; Gong, Y.; Zhao, N.; Zhang, X. Study on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials 2003, 24, 2871–2880. [Google Scholar] [CrossRef]
- Zhao, F.; Yin, Y.; Lu, W.W.; Leong, J.C.; Zhang, W.; Zhang, J.; Zhang, M.; Yao, K. Preparation and histological evaluation of biomimetic three-dimensional hydroxyapatite/chitosan-gelatin network composite scaffolds. Biomaterials 2002, 23, 3227–3234. [Google Scholar] [CrossRef]
- Risbud, M.; Endres, M.; Ringe, J.; Bhonde, R.; Sittinger, M. Biocompatible hydrogel supports the growth of respiratory epithelial cells: Possibilities in tracheal tissue engineering. J. Biomed. Mater. Res. 2001, 56, 120–127. [Google Scholar] [CrossRef]
- Fiench, L.L.; Wilson, J. Surface-Active Biomaterials. Science 1984, 226, 630–636. [Google Scholar]
- Kim, I.Y.; Seo, S.J.; Moon, H.S.; Yoo, M.K.; Park, I.Y.; Kim, B.C.; Cho, C.S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Grande, D.A.; Halberstadt, C.; Naughton, G.; Schwartz, R.; Manji, R. Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. J. Biomed. Mater. Res. 1997, 34, 211–220. [Google Scholar] [CrossRef]
- Sechriest, V.F.; Miao, Y.J.; Niyibizi, C.; Westerhausen-Larson, A.; Matthew, H.W.; Evans, C.H.; Fu, F.H.; Suh, J.K. GAG-augmented polysaccharide hydrogel: A novel biocompatible and biodegradable material to support chondrogenesis. J. Biomed. Mater. Res. 2000, 49, 534–541. [Google Scholar] [CrossRef]
- Shahabeddin, L.; Berthod, F.; Damour, O.; Collombel, C. Characterization of Skin Reconstructed on a Chitosan-Cross-Linked Collagen-Glycosaminoglycan Matrix. Ski. Pharmacol. 1990, 3, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Heath, C.A.; Rutkowski, G.E. The development of bioartificial nerve grafts for peripheral-nerve regeneration. Trends Biotechnol. 1998, 16, 163–168. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Haipeng, G.; Yinghui, Z.; Jianchun, L.; Yandao, G.; Nanming, Z.; Xiufang, Z. Studies on nerve cell affinity of chitosan-derived materials. J. Biomed. Mater. Res. 2000, 52, 285–295. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, P.; Yang, Y.; Wang, X.; Gu, X. The interaction of Schwann cells with chitosan membranes and fibers in vitro. Biomaterials 2004, 25, 4273–4278. [Google Scholar] [CrossRef]
- Azuma, K.; Izumi, R.; Osaki, T.; Ifuku, S.; Morimoto, M.; Saimoto, H.; Minami, S.; Okamoto, Y. Chitin, Chitosan, and Its Derivatives for Wound Healing: Old and New Materials. J. Funct. Biomater. 2015, 6, 104–142. [Google Scholar] [CrossRef]
- Silva, D.J.B.; Zuluaga, F.; Carlos, H. Evaluation of Biocompatibility of Chitosan Films from the Mycelium of Aspergillus niger in Connective Tissue of Rattus norvegicus. J. Mol. Genet. Med. 2015, 09, 1–8. [Google Scholar]
- Gopal, A.; Kant, V.; Gopalakrishnan, A.; Tandan, S.K.; Kumar, D. Chitosan-based copper nanocomposite accelerates healing in excision wound model in rats. Eur. J. Pharmacol. 2014, 731, 8–19. [Google Scholar] [CrossRef]
- Sudheesh Kumar, P.T.; Lakshmanan, V.K.; Anilkumar, T.V.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.G.; Nair, S.V.; Jayakumar, R. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Blažević, F.; Milekić, T.; Romić, M.D.; Juretić, M.; Pepić, I.; Filipović-Grčić, J.; Lovrić, J.; Hafner, A. Nanoparticle-mediated interplay of chitosan and melatonin for improved wound epithelialisation. Carbohydr. Polym. 2016, 146, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. In vivo evaluation of chitosan-PVP-titanium dioxide nanocomposite as wound dressing material. Carbohydr. Polym. 2013, 95, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Shigemasa, Y.; Minami, S. Applications of chitin and chitosan for biomaterials. Biotechnol. Genet. Eng. Rev. 1996, 13, 383–420. [Google Scholar] [CrossRef]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299. [Google Scholar] [CrossRef]
- Kim, K.Y.; Min, D.S. Wound covering materials from polyelectrolyte complexes of chitosan with sulphonated chitosan. Trans. Soc. Biomater. 1988, 11, 558. [Google Scholar]
- Mao, J.; Zhao, L.; De Yao, K.; Shang, Q.; Yang, G.; Cao, Y. Study of novel chitosan-gelatin artificial skin in vitro. J. Biomed. Mater. Res. Part A 2003, 64, 301–308. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Yao, K.D.; Yin, Y.J.; Xu, M.X.; Wang, Y.F. Investigation of pH-sensitive drug delivery system of chitosan/gelatin hybrid polymer network. Polym. Int. 1995, 38, 77–82. [Google Scholar] [CrossRef]
- Chandy, T.; Rao, G.H.R.; Wilson, R.F.; Das, G.S. Delivery of LMW heparin via surface coated chitosan/peg-alginate microspheres prevents thrombosis. Drug Deliv. J. Deliv. Target. Ther. Agents 2002, 9, 87–96. [Google Scholar] [CrossRef]
- Chellat, F.; Tabrizian, M.; Dumitriu, S.; Chornet, E.; Rivard, C.H.; Yahia, L. Study of biodegradation behavior of Chitosan-Xanthan microspheres in simulated physiological media. J. Biomed. Mater. Res. 2000, 53, 592–599. [Google Scholar] [CrossRef]
- Stolnik, S.; Illum, L.; Davis, S.S. Long circulating microparticulate drug carriers. Adv. Drug Deliv. Rev. 2012, 64, 290–301. [Google Scholar] [CrossRef]
- Kim, S.-K. Chitin, Chitosan, Oligosaccharides and Their Derivatives: Biological Activie and Applications; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Mathew, M.E.; Mohan, J.C.; Manzoor, K.; Nair, S.V.; Tamura, H.; Jayakumar, R. Folate conjugated carboxymethyl chitosan-manganese doped zinc sulphide nanoparticles for targeted drug delivery and imaging of cancer cells. Carbohydr. Polym. 2010, 80, 442–448. [Google Scholar] [CrossRef]
- Hasegawa, M.; Yagi, K.; Iwakawa, S.; Hirai, M. Chitosan induces apoptosis via caspase-3 activation in bladder tumor cells. Jpn. J. Cancer Res. 2001, 92, 459–466. [Google Scholar] [CrossRef]
- Chen, W.R.; Adams, R.L.; Carubelli, R.; Nordquist, R.E. Laser-photosensitizer assisted immunotherapy: A novel modality for cancer treatment. Cancer Lett. 1997, 115, 25–30. [Google Scholar] [CrossRef]
- Nishimura, I.C.; Nishimura, S.; Nishi, N.; Saiki, I.; Tokura, S.; Azuma, I. Immunological Activity of Chitin. Vaccine 1984, 2, 93–99. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Chen, M. In vitro and in vivo suppression of hepatocellular carcinoma growth by chitosan nanoparticles. Eur. J. Cancer 2007, 43, 184–193. [Google Scholar] [CrossRef]
- Gumińska, M.; Ignacak, J.; Wojcik, E. In vitro inhibitory effect of chitosan and its degradation products on energy metabolism in Ehrlich ascites tumour cells (EAT). Polish J. Pharmacol. 1996, 48, 495–501. [Google Scholar]
- Singh, D.K.; Ray, A.R. Biomedical applications of chitin, chitosan, and their derivatives. J. Macromol. Sci. Polym. Rev. 2000, 40, 69–83. [Google Scholar] [CrossRef]
- Blair, H.S.; Guthrie, J.; Law, T.-K.; Turkington, P. Chitosan and modified chitosan membranes I. Preparation and characterisation. J. Appl. Polym. Sci. 1987, 33, 641–656. [Google Scholar] [CrossRef]
- Kubota, N.; Kikuchi, Y.; Mizuhara, Y.; Ishihara, T.; Takita, Y. Solid-phase modification of chitosan hydrogel membranes and permeability properties of modified chitosan membranes. J. Appl. Polym. Sci. 1993, 50, 1665–1670. [Google Scholar] [CrossRef]
- Hirano, S.; Tobetto, K.; Hasegawa, M.; Matsuda, N. Permeability properties of gels and membranes derived from chitosan. J. Biomed. Mater. Res. 1980, 14, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Greisler, H.P. Biomaterials in the development and future of vascular grafts. J. Vasc. Surg. 2003, 37, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Chupa, J.M.; Foster, A.M.; Sumner, S.R.; Madihally, S.V.; Matthew, H.W. Vascular cell responses to polysaccharide materials. Biomaterials 2000, 21, 2315–2322. [Google Scholar] [CrossRef]
- Madihally, S.V.; Matthew, H.W.T. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Irvine, S.A.; Yun, X.; Venkatraman, S. Anti-platelet and tissue engineering approaches to biomaterial blood compatibilization: How well have these been translated into the clinic? Drug Deliv. Transl. Res. 2012, 2, 384–397. [Google Scholar] [CrossRef]
- Chiellini, E.; Giusti, P. Heparine-Like Substance and Blood Compatible Polymers Obtained from Chitin and Chitosan; Springer: Boston, MA, USA, 1983. [Google Scholar]
- Hudson, S.M.; Jenkins, D.W. Chitin and Chitosan; John Wiley & Sons: New York, NY, USA, 2002; ISBN 9780444519672. [Google Scholar]
- Mark, H.F.; Kroschwitz, J.I. Encyclopedia of Polymer Science and Engineering; Willey: New York, NY, USA, 1989. [Google Scholar]
- Gooday, G.W. Advances in Microbial Ecology; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Schisler, D.A.; Slininger, P.J.; Behle, R.W.; Jackson, M.A. Formulation of Bacillus spp. for biological control of plant diseases. Phytopathology 2004, 94, 1267–1271. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polimeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure-Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Geisberger, G.; Gyenge, E.B.; Hinger, D.; Käch, A.; Maake, C.; Patzke, G.R. Chitosan-thioglycolic acid as a versatile antimicrobial agent. Biomacromolecules 2013, 14, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Cakmak, Y.S.; Baran, T.; Asan-Ozusaglam, M.; Mentes, A.; Tozak, K.O. New chitin, chitosan, and O-carboxymethyl chitosan sources from resting eggs of Daphnia longispina (Crustacea); with physicochemical characterization, and antimicrobial and antioxidant activities. Biotechnol. Bioprocess Eng. 2014, 19, 58–69. [Google Scholar] [CrossRef]
- Hu, L.; Meng, X.; Xing, R.; Liu, S.; Chen, X.; Qin, Y.; Yu, H.; Li, P. Design, synthesis and antimicrobial activity of 6-N-substituted chitosan derivatives. Bioorgan. Med. Chem. Lett. 2016, 26, 4548–4551. [Google Scholar] [CrossRef]
- Sano, H.; Shibasaki, K.I.; Matsukubo, T.; Takaesu, Y. Effect of chitosan rinsing on reduction of dental plaque formation. Bull. Tokyo Dent. Coll. 2003, 44, 9–16. [Google Scholar] [CrossRef]
- Coma, V.; Deschamps, A.; Martial-Gros, A. Bioactive Packaging Materials from Edible Chitosan Polymer—Antimicrobial Activity Assessment on Dairy-Related Contaminants. J. Food Sci. 2003, 68, 2788–2792. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I. A Biopolymer Chitosan and Its Derivatives as Promising Antimicrobial Agents against Plant Pathogens and Their Applications in Crop Protection. Int. J. Carbohydr. Chem. 2011, 2011, 1–29. [Google Scholar] [CrossRef]
- Ong, S.Y.; Wu, J.; Moochhala, S.M.; Tan, M.H.; Lu, J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 2008, 29, 4323–4332. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti. Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef]
- Xiao, Z.; Luo, Y.; Luo, Y.; Wang, Q. Combined effects of sodium chlorite dip treatment and chitosan coatings on the quality of fresh-cut d’Anjou pears. Postharvest Biol. Technol. 2011, 62, 319–326. [Google Scholar] [CrossRef]
- Dragostin, O.M.; Samal, S.K.; Dash, M.; Lupascu, F.; Pânzariu, A.; Tuchilus, C.; Ghetu, N.; Danciu, M.; Dubruel, P.; Pieptu, D.; et al. New antimicrobial chitosan derivatives for wound dressing applications. Carbohydr. Polym. 2016, 141, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Dragostin, O.M.; Sama, S.K.; Lupascu, F.; Pânzariu, A.; Dubruel, P.; Lupascu, D.; Tuchilus, C.; Vasile, C.; Profire, L. Development and characterization of novel films based on sulfonamide-chitosan derivatives for potential wound dressing. Int. J. Mol. Sci. 2015, 16, 29843–29855. [Google Scholar] [CrossRef] [PubMed]
- Stawski, D.; Sahariah, P.; Hjálmarsdóttir, M.; Wojciechowska, D.; Puchalski, M.; Másson, M. N,N,N-trimethyl chitosan as an efficient antibacterial agent for polypropylene and polylactide nonwovens. J. Text. Inst. 2017, 108, 1041–1049. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Sang, W.; Tang, Z.; He, M.Y.; Hua, Y.P.; Xu, Q. Preparation, characterization and application of the novel chitosan derivative modified with phenylalanine. Fibers Polym. 2015, 16, 991–996. [Google Scholar] [CrossRef]
- Ignatova, M.; Manolova, N.; Markova, N.; Rashkov, I. Electrospun non-woven nanofibrous hybrid mats based on chitosan and PLA for wound-dressing applications. Macromol. Biosci. 2009, 9, 102–111. [Google Scholar] [CrossRef]
- Ji, Q.X.; Zhong, D.Y.; Lü, R.; Zhang, W.Q.; Deng, J.; Chen, X.G. In vitro evaluation of the biomedical properties of chitosan and quaternized chitosan for dental applications. Carbohydr. Res. 2009, 344, 1297–1302. [Google Scholar] [CrossRef]
- Sano, H.; Shibasaki, K.; Matsukubo, T.; Takaesu, Y. Comparison of the activity of four chitosan derivatives in reducing initial adherence of oral bacteria onto tooth surfaces. Bull. Tokyo Dent. Coll. 2001, 42, 243–249. [Google Scholar] [CrossRef][Green Version]
- Nechita, P.; Bobu, E.; Parfene, G.; Dinicə, R.M.; Bəlan, T. Antimicrobial coatings based on chitosan derivatives and quaternary ammonium salts for packaging paper applications. Cellul. Chem. Technol. 2015, 49, 625–632. [Google Scholar]
- Gupta, D.; Haile, A. Multifunctional properties of cotton fabric treated with chitosan and carboxymethyl chitosan. Carbohydr. Polym. 2007, 69, 164–171. [Google Scholar] [CrossRef]
- Ye, W.; Leung, M.F.; Xin, J.; Kwong, T.L.; Lee, D.K.L.; Li, P. Novel core-shell particles with poly(n-butyl acrylate) cores and chitosan shells as an antibacterial coating for textiles. Polymer (Guildf) 2005, 46, 10538–10543. [Google Scholar] [CrossRef]
- Nigmatullin, R.; Konovalova, V.; Pobigay, G. Development of Antimicrobial Membranes via the Surface Tethering of Chitosan. J. Appl. Polym. Sci. 2009, 111, 1697–1705. [Google Scholar] [CrossRef]
- Xu, X.; Zhuang, X.; Cheng, B.; Xu, J.; Long, G.; Zhang, H. Manufacture and properties of cellulose/O-hydroxyethyl chitosan blend fibers. Carbohydr. Polym. 2010, 81, 541–544. [Google Scholar] [CrossRef]
- Inta, O.; Yoksan, R.; Limtrakul, J. Hydrophobically modified chitosan: A bio-based material for antimicrobial active film. Mater. Sci. Eng. C 2014, 42, 569–577. [Google Scholar] [CrossRef]
- Sridhari, T.R.; Dutta, P.K. Synthesis and characterization of maleilated chitosan for dye house effluent. Indian J. Chem. Technol. 2000, 7, 198–201. [Google Scholar]
- El Mouzdahir, Y.; Elmchaouri, A.; Mahboub, R.; Gil, A.; Korili, S.A. Equilibrium modeling for the adsorption of methylene blue from aqueous solutions on activated clay minerals. Desalination 2010, 250, 335–338. [Google Scholar] [CrossRef]
- Chang, M.Y.; Juang, R.S. Adsorption of tannic acid, humic acid, and dyes from water using the composite of chitosan and activated clay. J. Colloid Interface Sci. 2004, 278, 18–25. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Ariff, N.F.M.; Hanafiah, M.A.K.M. Preparation, characterization, and environmental application of crosslinked chitosan-coated bentonite for tartrazine adsorption from aqueous solutions. Water. Air. Soil Pollut. 2010, 206, 225–236. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Ariff, N.F.M.; Hashim, A.; Hanafiah, M.A.K.M. Malachite green adsorption onto chitosan coated bentonite beads: Isotherms, kinetics and mechanism. Clean Soil Air Water 2010, 38, 394–400. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Jiang, R.; Xiao, L. Adsorption of an anionic azo dye by chitosan/kaolin/γ-Fe2O3 composites. Appl. Clay Sci. 2010, 48, 522–526. [Google Scholar] [CrossRef]
- Wang, L.; Wang, A. Adsorption characteristics of Congo Red onto the chitosan/montmorillonite nanocomposite. J. Hazard. Mater. 2007, 147, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Ahmad, A.L.; Hameed, B.H. Adsorption of reactive dye onto cross-linked chitosan/oil palm ash composite beads. Chem. Eng. J. 2008, 136, 164–172. [Google Scholar] [CrossRef]
- Lee, H.C.; Jeong, Y.G.; Min, B.G.; Lyoo, W.S.; Lee, S.C. Preparation and acid dye adsorption behavior of polyurethane/chitosan composite foams. Fibers Polym. 2009, 10, 636–642. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Sumathi, S.; Hameed, B.H. Chitosan: A natural biopolymer for the adsorption of residue oil from oily wastewater. Adsorpt. Sci. Technol. 2004, 22, 75–88. [Google Scholar] [CrossRef]
- Yang, T.; Zall, R.R. Chitosan Membranes for Reverse Osmosis Application. J. Food Sci. 1984, 49, 91–93. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Monier, M. Adsorption of Hg2+, Cu2+ and Zn2+ ions from aqueous solution using formaldehyde cross-linked modified chitosan-thioglyceraldehyde Schiff’s base. Int. J. Biol. Macromol. 2012, 50, 773–781. [Google Scholar] [CrossRef]
- Lü, H.; An, H.; Xie, Z. Ion-imprinted carboxymethyl chitosan-silica hybrid sorbent for extraction of cadmium from water samples. Int. J. Biol. Macromol. 2013, 56, 89–93. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Fatinathan, S. Adsorption of Cu(II) ions in aqueous solution using chitosan beads, chitosan-GLA beads and chitosan-alginate beads. Chem. Eng. J. 2008, 143, 62–72. [Google Scholar] [CrossRef]
- Vijaya, Y.; Popuri, S.R.; Boddu, V.M.; Krishnaiah, A. Modified chitosan and calcium alginate biopolymer sorbents for removal of nickel (II) through adsorption. Carbohydr. Polym. 2008, 72, 261–271. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, Z.; Zhang, C.; Yuan, S.; Zhu, Y.; Wang, J. PVAm–PIP/PS composite membrane with high performance for CO2/N2 separation. AIChE J. 2012, 59, 215–228. [Google Scholar] [CrossRef]
- Boddu, V.M.; Abburi, K.; Talbott, J.L.; Smith, E.D.; Haasch, R. Removal of arsenic (III) and arsenic (V) from aqueous medium using chitosan-coated biosorbent. Water Res. 2008, 42, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Boddu, V.M.; Abburi, K.; Randolph, A.J.; Smith, E.D. Removal of copper (II) and nickel (II) ions from aqueous solutions by a composite chitosan biosorbent. Sep. Sci. Technol. 2008, 43, 1365–1381. [Google Scholar] [CrossRef]
- Boddu, V.M.; Abburi, K.; Talbott, J.L.; Smith, E.D. Removal of hexavalent chromium from wastewater using a new composite chitosan biosorbent. Environ. Sci. Technol. 2003, 37, 4449–4456. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V.; Timpu, D. Preparation and characterization of novel composites based on chitosan and clinoptilolite with enhanced adsorption properties for Cu2+. Bioresour. Technol. 2010, 101, 812–817. [Google Scholar] [CrossRef]
- Dinu, M.V.; Dragan, E.S. Evaluation of Cu2+, Co2+ and Ni2+ ions removal from aqueous solution using a novel chitosan/clinoptilolite composite: Kinetics and isotherms. Chem. Eng. J. 2010, 160, 157–163. [Google Scholar] [CrossRef]
- Qu, R.; Sun, C.; Ma, F.; Zhang, Y.; Ji, C.; Xu, Q.; Wang, C.; Chen, H. Removal and recovery of Hg(II) from aqueous solution using chitosan-coated cotton fibers. J. Hazard. Mater. 2009, 167, 717–727. [Google Scholar] [CrossRef]
- Qu, R.; Sun, C.; Wang, M.; Ji, C.; Xu, Q.; Zhang, Y.; Wang, C.; Chen, H.; Yin, P. Adsorption of Au(III) from aqueous solution using cotton fiber/chitosan composite adsorbents. Hydrometallurgy 2009, 100, 65–71. [Google Scholar] [CrossRef]
- Zhang, G.; Qu, R.; Sun, C.; Ji, C.; Chen, H.; Wang, C.; Niu, Y. Adsorption for Metal Ions of Chitosan Coated Cotton Fiber. J. Appl. Polym. Sci. 2008, 110, 2321–2327. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, H.; Shi, J.X.; Langrish, T.A.G. Adsorption of chromium(VI) from aqueous solutions using cross-linked magnetic chitosan beads. Ind. Eng. Chem. Res. 2009, 48, 2646–2651. [Google Scholar] [CrossRef]
- Tran, H.V.; Tran, L.D.; Nguyen, T.N. Preparation of chitosan/magnetite composite beads and their application for removal of Pb(II) and Ni(II) from aqueous solution. Mater. Sci. Eng. C 2010, 30, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, S.; Priya, J.A.; Rao, P.S.; Krishnaiah, A. Removal of copper and nickel from aqueous solutions using chitosan coated on perlite as biosorbent. Sep. Sci. Technol. 2005, 40, 1483–1495. [Google Scholar] [CrossRef]
- Hasan, S.; Krishnaiah, A.; Ghosh, T.K.; Viswanath, D.S.; Boddu, V.M.; Smith, E.D. Adsorption of divalent cadmium (Cd(II)) from aqueous solutions onto chitosan-coated perlite beads. Ind. Eng. Chem. Res. 2006, 45, 5066–5077. [Google Scholar] [CrossRef]
- Hasan, S.; Krishnaiah, A.; Ghosh, T.K.; Viswanath, D.S.; Boddu, V.M.; Smith, E.D. Adsorption of chromium(VI) on chitosan-coated perlite. Sep. Sci. Technol. 2003, 38, 3775–3793. [Google Scholar] [CrossRef]
- Hasan, S.; Ghosh, T.K.; Viswanath, D.S.; Boddu, V.M. Dispersion of chitosan on perlite for enhancement of copper(II) adsorption capacity. J. Hazard. Mater. 2008, 152, 826–837. [Google Scholar] [CrossRef]
- Kumar, M.; Tripathi, B.P.; Shahi, V.K. Crosslinked chitosan/polyvinyl alcohol blend beads for removal and recovery of Cd(II) from wastewater. J. Hazard. Mater. 2009, 172, 1041–1048. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Kamari, A.; Koay, Y.J. Equilibrium and kinetics studies of adsorption of copper (II) on chitosan and chitosan/PVA beads. Int. J. Biol. Macromol. 2004, 34, 155–161. [Google Scholar] [CrossRef]
- Popuri, S.R.; Vijaya, Y.; Boddu, V.M.; Abburi, K. Adsorptive removal of copper and nickel ions from water using chitosan coated PVC beads. Bioresour. Technol. 2009, 100, 194–199. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, Y.; Wu, H. Preparation of CS/GPTMS hybrid molecularly imprinted membrane for efficient chiral resolution of phenylalanine isomers. J. Membr. Sci. 2006, 280, 876–882. [Google Scholar] [CrossRef]
- Jiraratananon, R.; Chanachai, A.; Huang, R.Y.M.; Uttapap, D. Pervaporation dehydration of ethanol-water mixtures with chitosan/hydroxyethylcellulose (CS/HEC) composite membranes I. Effect of operating conditions. J. Membr. Sci. 2002, 195, 143–151. [Google Scholar] [CrossRef]
- Mochizuki, A.; Amiya, S.; Sato, Y.; Ogawara, H.; Yamashita, S. Pervaporation separation of water/ethanol mixtures through polysaccharide membranes. III. The Permselectivity of the Neutralized Chitosan Membrane and the Relationships between Its Permselectivity and Solid State Structure. J. Appl. Polym. Sci. 1989, 37, 3385–3398. [Google Scholar] [CrossRef]
- Goto, M.; Shiosaki, A.; Hirose, T. Separation of Water/Ethanol Vapor Mixtures through Chitosan and Crosslinked Chitosan Membranes. Sep. Sci. Technol. 1994, 29, 1915–1923. [Google Scholar] [CrossRef]
- Shieh, J.J.; Huang, R.Y.M. Preparation of N-methylol nylon-6 membranes for pervaporation of ethanol-water mixtures. J. Appl. Polym. Sci. 1997, 64, 855–863. [Google Scholar] [CrossRef]
- Chen, X.; Yang, H.; Gu, Z.; Shao, Z. Preparation and characterization of HY zeolite-filled chitosan membranes for pervaporation separation. J. Appl. Polym. Sci. 2001, 79, 1144–1149. [Google Scholar] [CrossRef]
- Uragami, T.; Tanaka, Y.; Nishida, S. Permeation and separation under high temperature and high pressure for ethanol/water vapors through cross-linked quaternized chitosan composite membranes. Desalination 2002, 147, 449–454. [Google Scholar] [CrossRef]
- Nawawi, M.G.M.; Huang, R.Y.M. Pervaporation dehydration of isopropanol with chitosan membranes. J. Membr. Sci. 1997, 124, 53–62. [Google Scholar]
- Chanachai, A.; Jiraratananon, R.; Uttapap, D.; Moon, G.Y.; Anderson, W.A.; Huang, R.Y.M. Pervaporation with chitosan/hydroxyethylcellulose (CS/HEC) blended membranes. J. Membr. Sci. 2000, 166, 271–280. [Google Scholar] [CrossRef]
- Devi, D.A.; Smitha, B.; Sridhar, S.; Aminabhavi, T.M. Pervaporation separation of isopropanol/water mixtures through crosslinked chitosan membranes. J. Membr. Sci. 2005, 262, 91–99. [Google Scholar] [CrossRef]
- Kittur, A.A.; Kulkarni, S.S.; Aralaguppi, M.I.; Kariduraganavar, M.Y. Preparation and characterization of novel pervaporation membranes for the separation of water-isopropanol mixtures using chitosan and NaY zeolite. J. Membr. Sci. 2005, 247, 75–86. [Google Scholar] [CrossRef]
- Svang-Ariyaskul, A.; Huang, R.Y.M.; Douglas, P.L.; Pal, R.; Feng, X.; Chen, P.; Liu, L. Blended chitosan and polyvinyl alcohol membranes for the pervaporation dehydration of isopropanol. J. Membr. Sci. 2006, 280, 815–823. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yu, C.H.; Lee, K.R.; Lai, J.Y. Chitosan/poly(tetrafluoroethylene) composite membranes using in pervaporation dehydration processes. J. Membr. Sci. 2007, 287, 230–236. [Google Scholar] [CrossRef]
- Chen, J.H.; Liu, Q.L.; Fang, J.; Zhu, A.M.; Zhang, Q.G. Composite hybrid membrane of chitosan-silica in pervaporation separation of MeOH/DMC mixtures. J. Colloid Interface Sci. 2007, 316, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, S.K.; Kittur, A.A.; Kulkarni, S.S.; Kariduraganavar, M.Y. Development of novel blocked diisocyanate crosslinked chitosan membranes for pervaporation separation of water-isopropanol mixtures. J. Membr. Sci. 2007, 302, 197–206. [Google Scholar] [CrossRef]
- Tsai, H.A.; Chen, W.H.; Kuo, C.Y.; Lee, K.R.; Lai, J.Y. Study on the pervaporation performance and long-term stability of aqueous iso-propanol solution through chitosan/polyacrylonitrile hollow fiber membrane. J. Membr. Sci. 2008, 309, 146–155. [Google Scholar] [CrossRef]
- Rachipudi, P.S.; Kittur, A.A.; Choudhari, S.K.; Varghese, J.G.; Kariduraganavar, M.Y. Development of polyelectrolyte complexes of chitosan and phosphotungstic acid as pervaporation membranes for dehydration of isopropanol. Eur. Polym. J. 2009, 45, 3116–3126. [Google Scholar] [CrossRef]
- Varghese, J.G.; Kittur, A.A.; Rachipudi, P.S.; Kariduraganavar, M.Y. Synthesis, characterization and pervaporation performance of chitosan-g-polyaniline membranes for the dehydration of isopropanol. J. Membr. Sci. 2010, 364, 111–121. [Google Scholar] [CrossRef]
- Sajjan, A.M.; Jeevan Kumar, B.K.; Kittur, A.A.; Kariduraganavar, M.Y. Novel approach for the development of pervaporation membranes using sodium alginate and chitosan-wrapped multiwalled carbon nanotubes for the dehydration of isopropanol. J. Membr. Sci. 2013, 425–426, 77–88. [Google Scholar] [CrossRef]
- Yong Nam, S.; Moo Lee, Y. Pervaporation of ethylene glycol-water mixtures. I. Pervaporation performance of surface crosslinked chitosan membranes. J. Membr. Sci. 1999, 153, 155–162. [Google Scholar] [CrossRef]
- Hu, C.; Guo, R.; Li, B.; Ma, X.; Wu, H.; Jiang, Z. Development of novel mordenite-filled chitosan-poly(acrylic acid) polyelectrolyte complex membranes for pervaporation dehydration of ethylene glycol aqueous solution. J. Membr. Sci. 2007, 293, 142–150. [Google Scholar] [CrossRef]
- Du, J.R.; Chakma, A.; Feng, X. Dehydration of ethylene glycol by pervaporation using poly(N,N-dimethylaminoethyl methacrylate)/polysulfone composite membranes. Sep. Purif. Technol. 2008, 64, 63–70. [Google Scholar] [CrossRef]
- Hyder, M.N.; Chen, P. Pervaporation dehydration of ethylene glycol with chitosan-poly(vinyl alcohol) blend membranes: Effect of CS-PVA blending ratios. J. Membr. Sci. 2009, 340, 171–180. [Google Scholar] [CrossRef]
- Cao, S.; Shi, Y.; Chen, G. Properties and pervaporation characteristics of chitosan-poly(N-vinyl-2-pyrrolidone) blend membranes for MeOH-MTBE. J. Appl. Polym. Sci. 1999, 74, 1452–1458. [Google Scholar] [CrossRef]
- Yong Nam, S.; Moo Lee, Y. Pervaporation separation of methanol/methyl t-butyl ether through chitosan composite membrane modified with surfactants. J. Membr. Sci. 1999, 157, 63–71. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Moon, G.Y.; Pal, R. Chitosan/anionic surfactant complex membranes for the pervaporation separation of methanol/MTBE and characterization of the polymer/surfactant system. J. Membr. Sci. 2001, 184, 1–15. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Moon, G.Y.; Pal, R. N-acetylated chitosan membranes for the pervaporation separation of alcohol/toluene mixtures. J. Membr. Sci. 2000, 176, 223–231. [Google Scholar] [CrossRef]
- Patil, M.B.; Aminabhavi, T.M. Pervaporation separation of toluene/alcohol mixtures using silicalite zeolite embedded chitosan mixed matrix membranes. Sep. Purif. Technol. 2008, 62, 128–136. [Google Scholar] [CrossRef]
- Won, W.; Feng, X.; Lawless, D. Pervaporation with chitosan membranes: Separation of dimethyl carbonate/methanol/water mixtures. J. Membr. Sci. 2002, 209, 493–508. [Google Scholar] [CrossRef]
- Liu, B.; Cao, Y.; Wang, T.; Yuan, Q. Preparation of Novel ZSM-5 Zeolite-Filled Chitosan Membranes for Pervaporation Separation of Dimethyl Carbonate/Methanol Mixtures. J. Appl. Polym. Sci. 2007, 116, 2117–2125. [Google Scholar] [CrossRef]
- Lu, L.; Peng, F.; Jiang, Z.; Wang, J. Poly(vinyl alcohol)/chitosan blend membranes for pervaporation of benzene/cyclohexane mixtures. J. Appl. Polym. Sci. 2006, 101, 167–173. [Google Scholar] [CrossRef]
- Shen, J.; Chu, Y.; Ruan, H.; Wu, L.; Gao, C.; Van der Bruggen, B. Pervaporation of benzene/cyclohexane mixtures through mixed matrix membranes of chitosan and Ag+/carbon nanotubes. J. Membr. Sci. 2014, 462, 160–169. [Google Scholar] [CrossRef]
- Anjali Devi, D.; Smitha, B.; Sridhar, S.; Aminabhavi, T.M. Dehydration of 1,4-dioxane through blend membranes of poly(vinyl alcohol) and chitosan by pervaporation. J. Membr. Sci. 2006, 280, 138–147. [Google Scholar] [CrossRef]
- Smitha, B.; Dhanuja, G.; Sridhar, S. Dehydration of 1,4-dioxane by pervaporation using modified blend membranes of chitosan and nylon 66. Carbohydr. Polym. 2006, 66, 463–472. [Google Scholar] [CrossRef]
- Reddy, A.S.; Kalyani, S.; Kumar, N.S.; Boddu, V.M.; Krishnaiah, A. Dehydration of 1,4-dioxane by pervaporation using crosslinked calcium alginate-chitosan blend membranes. Polym. Bull. 2008, 61, 779–790. [Google Scholar] [CrossRef]
- Ong, Y.T.; Ahmad, A.L.; Zein, S.H.S.; Sudesh, K.; Tan, S.H. Poly(3-hydroxybutyrate)-functionalised multi-walled carbon nanotubes/chitosan green nanocomposite membranes and their application in pervaporation. Sep. Purif. Technol. 2011, 76, 419–427. [Google Scholar] [CrossRef]
- Li, Q.; Yu, P.; Zhu, T.; Zhang, L.; Li, Q.; Luo, Y. Pervaporation performance of crosslinked PVA and chitosan membranes for dehydration of caprolactam solution. Desalin. Water Treat. 2010, 16, 304–312. [Google Scholar] [CrossRef]
- Lin, W.; Li, Q.; Zhu, T. New chitosan/Konjac glucomannan blending membrane for application in pervaporation dehydration of caprolactam solution. J. Ind. Eng. Chem. 2012, 18, 934–940. [Google Scholar] [CrossRef]
- Li, Q.; Lin, W.; Zhu, T.; Luo, Y.; Yu, P. New chitosan/poly (acrylic acid) composite membrane for application in pervaporation dehydration of caprolactam solution. Desalin. Water Treat. 2012, 43, 43–51. [Google Scholar] [CrossRef]
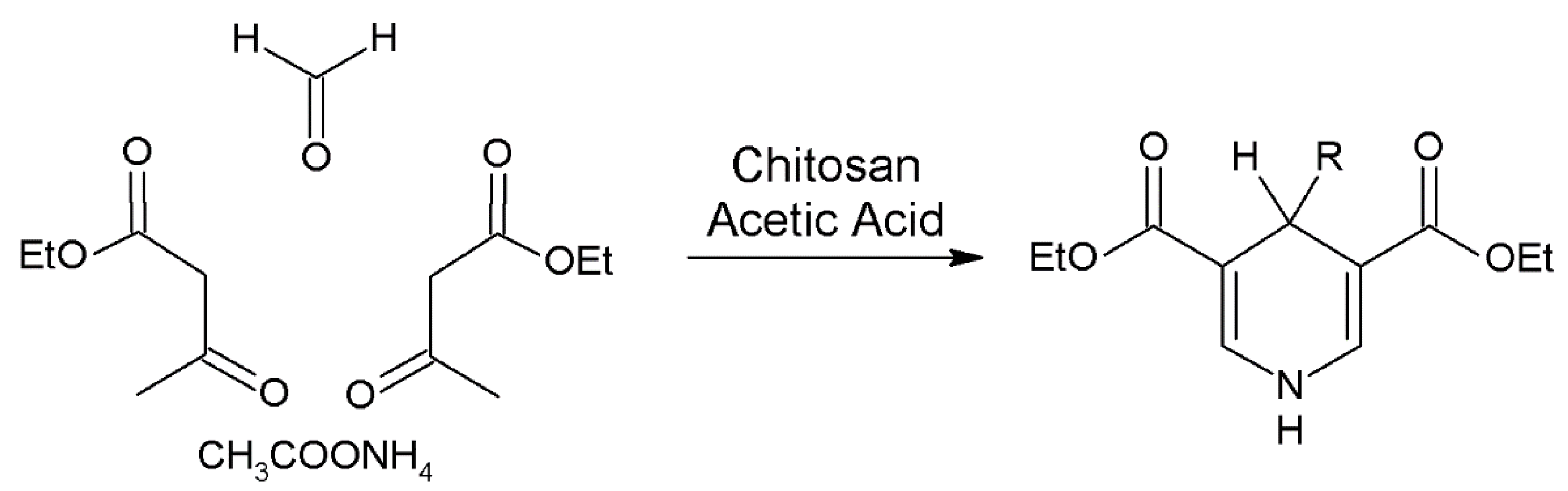
- Sahu, P.K.; Sahu, P.K.; Gupta, S.K.; Agarwal, D.D. Chitosan: An efficient, reusable, and biodegradable catalyst for green synthesis of heterocycles. Ind. Eng. Chem. Res. 2014, 53, 2085–2091. [Google Scholar] [CrossRef]
- Safari, J.; Zarnegar, Z.; Sadeghi, M.; Azizi, F. Chitosan-SO3H: An efficient and biodegradable catalyst for the green syntheses of 1,4-dihydropyridines. Curr. Org. Chem. 2016, 20, 2926–2932. [Google Scholar] [CrossRef]
- Guibal, E.; Vincent, T.; Navarro, R. Metal ion biosorption on chitosan for the synthesis of advanced materials. J. Mater. Sci. 2014, 49, 5505–5518. [Google Scholar] [CrossRef]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The good, the bad and the ugly” of chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Zhang, M. O-carboxymethyl chitosan supported heterogeneous palladium and Ni catalysts for heck reaction. Molecules 2017, 22, 150. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, M.; Zou, J.; Byrne, L.; Swaminathan Iyer, K.; Stewart, S.G.; Raston, C.L. Pd(ii) conjugated chitosan nanofibre mats for application in Heck cross-coupling reactions. Chem. Commun. 2011, 47, 12292–12294. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, A.R.; Boostani, E.; Mohammadsaleh, F. Proline-functionalized chitosan-palladium(ii) complex, a novel nanocatalyst for C-C bond formation in water. RSC Adv. 2015, 5, 24742–24748. [Google Scholar] [CrossRef]
- Baran, T.; Sargin, I.; Menteş, A.; Kaya, M. Exceptionally high turnover frequencies recorded for a new chitosan-based palladium(II) catalyst. Appl. Catal. A Gen. 2016, 523, 12–20. [Google Scholar] [CrossRef]
- Baran, T.; Menteş, A. Microwave assisted synthesis of biarlys by C-C coupling reactions with a new chitosan supported Pd(II) catalyst. J. Mol. Struct. 2016, 1122, 111–116. [Google Scholar] [CrossRef]
- Zeng, M.; Sun, X.; Qi, C.; Zhang, X.M. Preparation of a novel CuI/PCMS heterogeneous catalyst for heck-type cross-coupling reactions. Kinet. Catal. 2013, 54, 716–723. [Google Scholar] [CrossRef]
- Rafiee, F.; Hosseini, S.A. CNC pincer palladium complex supported on magnetic chitosan as highly efficient and recyclable nanocatalyst in C─C coupling reactions. Appl. Organomet. Chem. 2018, 32, 1–10. [Google Scholar] [CrossRef]
- Fakhri, A.; Naghipour, A. Organometallic polymer-functionalized Fe3O4 nanoparticles as a highly efficient and eco-friendly nanocatalyst for C–C bond formation. Transit. Met. Chem. 2018, 43, 463–472. [Google Scholar] [CrossRef]
- Jadhav, S.; Kumbhar, A.; Salunkhe, R. Palladium supported on silica-chitosan hybrid material (Pd-CS@SiO2) for Suzuki-Miyaura and Mizoroki-Heck cross-coupling reactions. Appl. Organomet. Chem. 2015, 29, 339–345. [Google Scholar] [CrossRef]
- Zeng, M.; Yuan, X.; Zuo, S.; Qi, C. Novel chitosan-based/montmorillonite/palladium hybrid microspheres as heterogeneous catalyst for Sonogashira reactions. RSC Adv. 2015, 5, 37995–38000. [Google Scholar] [CrossRef]
- Affrose, A.; Suresh, P.; Azath, I.A.; Pitchumani, K. Palladium nanoparticles embedded on thiourea-modified chitosan: A green and sustainable heterogeneous catalyst for the Suzuki reaction in water. RSC Adv. 2015, 5, 27533–27539. [Google Scholar] [CrossRef]
- Veisi, H.; Ghadermazi, M.; Naderi, A. Biguanidine-functionalized chitosan to immobilize palladium nanoparticles as a novel, efficient and recyclable heterogeneous nanocatalyst for Suzuki-Miyaura coupling reactions. Appl. Organomet. Chem. 2016, 30, 341–345. [Google Scholar] [CrossRef]
- Sophiphun, O.; Wittayakun, J.; Dhital, R.N.; Haesuwannakij, S.; Murugadoss, A.; Sakurai, H. Gold/palladium bimetallic alloy nanoclusters stabilized by chitosan as highly efficient and selective catalysts for homocoupling of arylboronic acid. Aust. J. Chem. 2012, 65, 1238–1243. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Abolfathi, P. Novel triazole-modified chitosan@nickel nanoparticles: Efficient and recoverable catalysts for Suzuki reaction. New J. Chem. 2017, 41, 2386–2391. [Google Scholar] [CrossRef]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of Palladium-Catalyzed C-N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef]
- Wang, L.-X.; Wang, Z.-W.; Wang, G.-S.; Lin, X.-D.; Ren, J.-G. Catalytic performance of chitosan-Schiff base supported Pd/Co bimetallic catalyst for acrylamide with phenyl halide. Polym. Adv. Technol. 2010, 21, 244–249. [Google Scholar]
- Anuradha; Kumari, S.; Pathak, D.D. Synthesis and development of Chitosan anchored copper(II) Schiff base complexes as heterogeneous catalysts for N-arylation of amines. Tetrahedron Lett. 2015, 56, 4135–4142. [Google Scholar] [CrossRef]
- Bodhak, C.; Kundu, A.; Pramanik, A. An efficient and recyclable chitosan supported copper(II) heterogeneous catalyst for C-N cross coupling between aryl halides and aliphatic diamines. Tetrahedron Lett. 2015, 56, 419–424. [Google Scholar] [CrossRef]
- Nasir Baig, R.B.; Vaddula, B.R.; Gonzalez, M.A.; Varma, R.S. N-Allylation of amines with allyl acetates using chitosan-immobilized palladium. RSC Adv. 2014, 4, 9103–9106. [Google Scholar] [CrossRef]
- Shen, C.; Xu, J.; Yu, W.; Zhang, P. A highly active and easily recoverable chitosan@copper catalyst for the C-S coupling and its application in the synthesis of zolimidine. Green Chem. 2014, 16, 3007–3012. [Google Scholar] [CrossRef]
- Frindy, S.; El Kadib, A.; Lahcini, M.; Primo, A.; García, H. Copper Nanoparticles Stabilized in a Porous Chitosan Aerogel as a Heterogeneous Catalyst for C-S Cross-coupling. ChemCatChem 2015, 7, 3307–3315. [Google Scholar] [CrossRef]
- Chiessi, E.; Pispisa, B. Polymer-supported catalysis: Oxidation of catecholamines by Fe(III) and Cu(II) complexes immobilized to chitosan. J. Mol. Catal. 1994, 87, 177–193. [Google Scholar] [CrossRef]
- Huang, G.; Guo, C.C.; Tang, S.S. Catalysis of cyclohexane oxidation with air using various chitosan-supported metallotetraphenylporphyrin complexes. J. Mol. Catal. A Chem. 2007, 261, 125–130. [Google Scholar] [CrossRef]
- Huang, G.; Luo, J.; Cai, C.C.; Guo, Y.A.; Luo, G.W. The influence of chitosan on the performances of mono and μ-oxo dimeric iron tetraphenylporphyrins catalysts for aerobic oxidation of toluene. Catal. Commun. 2008, 9, 1882–1885. [Google Scholar] [CrossRef]
- Di Giuseppe, A.; Crucianelli, M.; Passacantando, M.; Nisi, S.; Saladino, R. Chitin- and chitosan-anchored methyltrioxorhenium: An innovative approach for selective heterogeneous catalytic epoxidations of olefins. J. Catal. 2010, 276, 412–422. [Google Scholar] [CrossRef]
- Hatefi Ardakani, M.; Moghadam, M.; Saeednia, S.; Pakdin-Parizi, Z. Epoxidation of alkenes with NaIO4 catalyzed by an efficient and reusable natural polymer-supported ruthenium(III) salophen catalyst. J. Iran. Chem. Soc. 2016, 13, 631–636. [Google Scholar] [CrossRef]
- Ghiaci, M.; Dorostkar, N.; Martínez-Huerta, M.V.; Fierro, J.L.G.; Moshiri, P. Synthesis and characterization of gold nanoparticles supported on thiol functionalized chitosan for solvent-free oxidation of cyclohexene with molecular oxygen. J. Mol. Catal. A Chem. 2013, 379, 340–349. [Google Scholar] [CrossRef]
- Shaabani, A.; Boroujeni, M.B.; Sangachin, M.H. Cobalt-chitosan: Magnetic and biodegradable heterogeneous catalyst for selective aerobic oxidation of alkyl arenes and alcohols. J. Chem. Sci. 2015, 127, 1927–1935. [Google Scholar] [CrossRef]
- Murugadoss, A.; Sakurai, H. Chitosan-stabilized gold, gold-palladium, and gold-platinum nanoclusters as efficient catalysts for aerobic oxidation of alcohols. J. Mol. Catal. A Chem. 2011, 341, 1–6. [Google Scholar] [CrossRef]
- Shen, C.; Qiao, J.; Zhao, L.; Zheng, K.; Jin, J.; Zhang, P. An efficient silica supported Chitosan@vanadium catalyst for asymmetric sulfoxidation and its application in the synthesis of esomeprazole. Catal. Commun. 2017, 92, 114–118. [Google Scholar] [CrossRef]
- Barskiy, D.A.; Kovtunov, K.V.; Primo, A.; Corma, A.; Kaptein, R.; Koptyug, I.V. Selective Hydrogenation of 1,3-Butadiene and 1-Butyne over a Rh/Chitosan Catalyst Investigated by using Parahydrogen-Induced Polarization. ChemCatChem 2012, 4, 2031–2035. [Google Scholar] [CrossRef]
- Wu, L.H.; Nordin, M.R.; Yaakob, Z.; Liew, K.Y.; Li, J.L. Effects of Fe on catalytic hydrogenation of palm oil in aqueous solution. Chem. Pap. 2017, 71, 119–126. [Google Scholar] [CrossRef]
- Adlim, M.; Bakar, M.A. The properties of Pd/Au bimetallic colloidal catalysts stabilized by chitosan and prepared by simultaneous and stepwise chemical reduction of the precursor ions. Kinet. Catal. 2013, 54, 586–596. [Google Scholar] [CrossRef]
- Zhou, J.; Dong, Z.; Yang, H.; Shi, Z.; Zhou, X.; Li, R. Pd immobilized on magnetic chitosan as a heterogeneous catalyst for acetalization and hydrogenation reactions. Appl. Surf. Sci. 2013, 279, 360–366. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Li, Y.; Wang, J. Asymmetric transfer hydrogenation of acetophenone promoted by chitosan ester ruthenium complex. Chin. J. Org. Chem. 2014, 34, 2554–2558. [Google Scholar] [CrossRef]
- Szőllősi, G.; Kolcsár, V.J. Highly Enantioselective Transfer Hydrogenation of Prochiral Ketones Using Ru(II)-Chitosan Catalyst in Aqueous Media. ChemCatChem 2019, 11, 820–830. [Google Scholar] [CrossRef]
- Viswanadhan, M.; Potdar, A.; Divakaran, A.; Badiger, M.; Rode, C. Product distribution in hydrogenation of styrene oxide over Pd/chitosan catalyst. Res. Chem. Intermed. 2016, 42, 7581–7595. [Google Scholar] [CrossRef]
- Ríos-Caloch, G.; Santes, V.; Escobar, J.; Pérez-Romo, P.; Díaz, L.; Lartundo-Rojas, L. Effect of Chitosan on the Performance of NiMoP-Supported Catalysts for the Hydrodesulfurization of Dibenzothiophene. J. Nanomater. 2016, 2016, 13. [Google Scholar] [CrossRef]
- Lee, S.B.; Jeong, G.T. Catalytic Conversion of Chitosan to 5-Hydroxymethylfurfural Under Low Temperature Hydrothermal Process. Appl. Biochem. Biotechnol. 2015, 176, 1151–1161. [Google Scholar] [CrossRef]
- Li, M.; Zang, H.; Feng, J.; Yan, Q.; Yu, N.; Shi, X.; Cheng, B. Efficient conversion of chitosan into 5-hydroxymethylfurfural via hydrothermal synthesis in ionic liquids aqueous solution. Polym. Degrad. Stab. 2015, 121, 331–339. [Google Scholar] [CrossRef]
- Wang, Y.; Pedersen, C.M.; Deng, T.; Qiao, Y.; Hou, X. Direct conversion of chitin biomass to 5-hydroxymethylfurfural in concentrated ZnCl2 aqueous solution. Bioresour. Technol. 2013, 143, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, H.; Yan, N. Shell Biorefinery: Dream or Reality? Chem. Eur. J. 2016, 22, 13402–13421. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.J.; Holladay, J.E.; Johnson, D.; White, J.F. Top Value Added Chemicals from Biomass Volume II: Results of Screening for Potential Candidates from Biorefinery Lignin Produced; 2007. Available online: https://www.osti.gov/biblio/1216434 (accessed on 1 September 2020).
- van Putten, R.-J.; de Vries, J.G.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J. Hydroxymethylfurfural, A Versatile Platform Chemical Made from. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Szabolcs, Á.; Molnár, M.; Dibó, G.; Mika, L.T. Microwave-assisted conversion of carbohydrates to levulinic acid: An essential step in biomass conversion. Green Chem. 2013, 15, 439–445. [Google Scholar] [CrossRef]
- Omari, K.W.; Besaw, J.E.; Kerton, F.M. Hydrolysis of chitosan to yield levulinic acid and 5-hydroxymethylfurfural in water under microwave irradiation. Green Chem. 2012, 14, 1480–1487. [Google Scholar] [CrossRef]
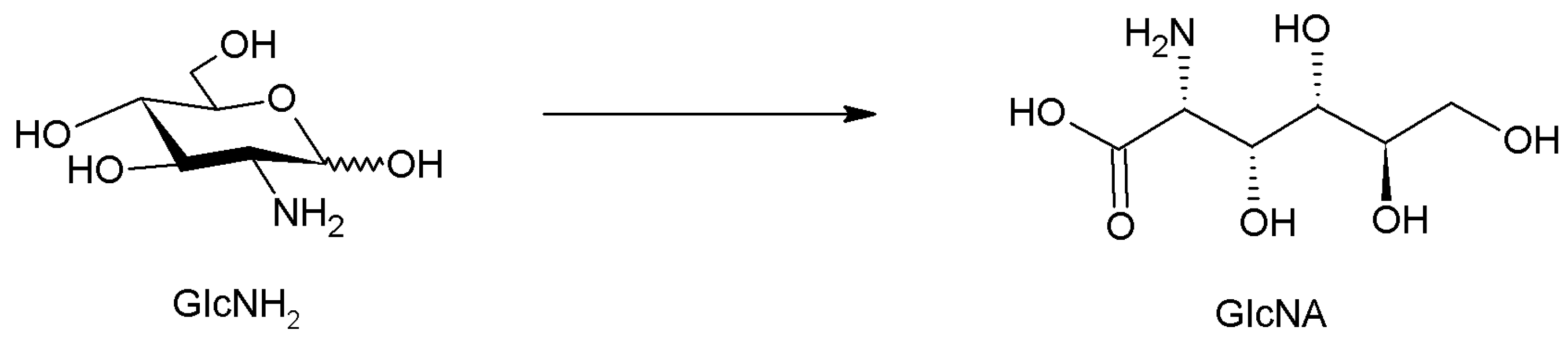
- Jeong, G.T. Production of levulinic acid from glucosamine by dilute-acid catalyzed hydrothermal process. Ind. Crop. Prod. 2014, 62, 77–83. [Google Scholar] [CrossRef]
- Ohmi, Y.; Nishimura, S.; Ebitani, K. Synthesis of α-amino acids from glucosamine-HCl and its derivatives by aerobic oxidation in water catalyzed by au nanoparticles on basic supports. ChemSusChem 2013, 6, 2259–2262. [Google Scholar] [CrossRef]



| Sea Animals | Insects | Microorganisms |
|---|---|---|
| Crustaceans | Scorpions | Green algae |
| Coelenterata | Brachiopods | Yeast (β-type) |
| Annelida | Cockroaches | Fungi (cell walls) |
| Mollusca | Spiders | Mycelia penicillium |
| Lobster | Beetles | Brown algae |
| Shrimp | Ants | Chytridiaceae |
| Prawn | - | Ascomydes |
| Krill | - | Blastocladiacease |
| Crab | - | Spores |
| Characterization Methods | Chitosan Property | Ref. |
|---|---|---|
| Potenciometric titration | Deacetylation degree | [12,13] |
| Elemental analysis | [14] | |
| Fourier transform infrared (FTIR) | [15,16,17,18] | |
| Nuclear magnetic resonance (NMR) | [19,20] | |
| Viscosimetry | Molecular weight | [21,22] |
| Gel permeation chromatography | [23,24,25] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ● Treating major burns |
| ● Preparation of artificial skin |
| ● Surgical sutures |
| ● Contact lenses |
| ● Blood dialysis membranes |
| ● Artificial blood vessels |
| ● Antitumor |
| ● Blood anticoagulant |
| ● Antigastritis |
| ● Haemostatic |
| ● Hypochlesterolaemic agent |
| ● Antithrombogeic agent |
| ● Drug and gene-delivery systems |
| ● Dental therapy |
| ● Cell growth and proliferation in tracheal cartilage, nerve |
| ● Bone tissue repair and regeneration materials for cartilage repair |
| ● Porous 3-D scaffold of chitosan-hydroxyapatite composites for bone regeneration |
| ● Chitosan-chondroitin sulfate sponges in bone regeneration |
| ● Chitosan-calcium alginate capsules to develop artificial pancreas for diabetes mellitus treatment |
| Drug | Dosage Form |
|---|---|
| Aspirin | Wet granulation formulation |
| Chlorpheniramine maleate | Tablet |
| Dapsone | Gel |
| Oxyphenbutazone | Coated tablet |
| Prednisolone | Granules |
| Pullulan | Film |
| Chitosan Application | Example |
|---|---|
| Additive | Clarification and deacilification of fruits and beverages |
| Color stabilization | |
| Emusifying agent | |
| Food mimetic | |
| Natural flavor extender | |
| Texture controlling agent | |
| Thickening and stabilizing agent | |
| Antimicrobial agent | Bactericidal |
| Fungicidal | |
| Measure of mold contamination in agricultural commodities | |
| Edible film industry | Controlled release of antimicrobial substances |
| Controlled release of antioxidants | |
| Controlled release of nutrients, flavors and drugs | |
| Controlled moisture transfer between foo and surrounding environment | |
| Nutritional quality | Antigastritis agent |
| Dietary fiber | |
| Hypocholesterolemic effect | |
| Infant feed ingredient | |
| Livestock and fish feed additive | |
| Production of single cell protein |
| Chitosan/Chitosan Derivative | Microbial Strain | Application | Ref. |
|---|---|---|---|
| Chitosan | Streptococcus | Dental materials | [127] |
| Listeria monocytogenes, Pseudomonas aeruginosa and S. aureus | Dairy food packaging | [128] | |
| F. acuminatum, Cylindrocladium floridanum, Aspergillus flavus, Magnaporthe grisea, Bipolaris sorokiniana, F. graminearum, Phytophthora parasitica, Sclerotinia sclerotiorum | Plant protection | [129] | |
| Chitosan-polyphosphate-silver | P. aeruginosa and S. aureus | Wound dressing | [130] |
| Chitosan acetate | P. aeruginosa, Proteus mirabilis and S. aureus | Wound dressing | [131] |
| Carboxymethyl chitosan | E. coli | Fruit preservation | [132] |
| Chitosan-sulfonamide derivatives | Staphylococcus aureus, Sarcina lutea, Bacillus cereus, Bacillus subtilis, Escherichia coli, Candida albicans, Candida glabrata and Candida sake | Wound dressing and wound healing | [133,134] |
| N,N,N-trimethylchitosan polylactide/polypropylene fibers | S. aureus | Wound dressing | [135] |
| N-(Carboxymethyl) chitosan | F. solani and C. lindemuthianum | Plant protection | [129] |
| N,N,N-dimethylalkyl chitosans | A. tumefaciens, E. carotovora, fungi B. cinerea, F. oxysporum, and P. debaryanum | Crop protection | [136] |
| N-(o,p-Diethoxybenzyl)chitosan | F. oxysporum and P. debaryanum | Crop protection | [136] |
| N-(o,o-Dichlorobenzyl) chitosan, N-(o,o-dichloro-benzyl) chitosan, N,O-(p-chlorobutyryl) chitosan, N,O-decanoyl chitosan, N,O-cinnamoyl chitosan and N,O-(p-methoxy-benzoyl) chitosan | B. cinerea | Crop protection | [136] |
| N-Phenylalanine-O-carboxymethyl chitosan | S. aureus and E. coli | Food preservative coating | [137] |
| Chitosan/quaternary chitosan-polylactide | S. aureus and E. coli | Wound healing | [138] |
| Chitosan, chitosan-hydroxyapatite, N-[1-hydroxy-3-(trimethylammonium) propyl]chitosan chloride, carboxymethyl chitosan | Streptococcus | Dental care | [139,140] |
| N-[1-Hydroxy-3-(trimethylammonium) propyl]chitosan | Bacillus subtilis | Paper packaging | [141] |
| Carboxymethyl chitosan | E. coli and S. aureus | Cotton fabric | [142] |
| Poly(n-butyl acrylate)-chitosan | S. aureus | Cotton fabric | [143] |
| Chitosan-cellulose | E. coli and S. aureus | Membranes | [144] |
| O-Hydroxyethylchitosan-cellulose | E. coli | Textile | [145] |
| Chitosan-lauric acid-starch | B. subtilis and E. coli | Antimicrobial film | [123] |
| Dodecenyl succinylated phthaloyl chitosan | E. coli, S. aureus and B. subtilis | Antimicrobial film | [146] |
| Adsorbent | Dye | Adsorption Capacity (mg g−1) | pH | Temperature (°C) | Ref. |
|---|---|---|---|---|---|
| Chitosan/activated clay | Methylene blue | 330 | 7.1 | 30 | [149] |
| Reactive dye RR222 | 1912 | 6.5 | 30 | ||
| Chitosan/bentonite | Tartrazine | 294 | 2.5 | 47 | [150] |
| Malachite green | 435.0 | 6.0 | 37 | [151] | |
| Chitosan/kaolin/γ-Fe2O3 | Methyl orange | - | 6.0 | - | [152] |
| Chitosan/montmorillonite | Congo red | 53 | 7.0 | 30 | [153] |
| Chitosan/oil palm | Reactive Blue 19 | 909 | 6.0 | 50 | [154] |
| Chitosan/polyurethane | Acid violet 48 | 30 | 7.0 | 30 | [155] |
| Adsorbent | Adsorbate | Maximum Adsorption Capacity (mg g−1) | pH | Temperature (°C) | Ref. |
|---|---|---|---|---|---|
| Chitosan/alginate | Cu2+ | 68 | 4.5 | - | [161] |
| Chitosan/calcium arginate | Ni2+ | 222 | 5 | - | [162] |
| Chitosan/cellulose | Cu2+ | 26 | - | 25 | [163] |
| Zn2+ | 20 | ||||
| Cr6+ | 13 | ||||
| Ni2+ | 13 | ||||
| Pb2+ | 26 | ||||
| Chitosan/ceramic alumina | As3+ | 56 | 4.0 | - | [164] |
| As5+ | 96 | 4.0 | 25 | ||
| Cu2+ | 86 | -0- | - | [165] | |
| Ni2+ | 78 | 4 | 25 | ||
| Cr6+ | 154 | 4 | 25 | [166] | |
| Chitosan/clinoptilolite | Cu2+ | 574 | 5.0 | - | [167] |
| Cu2+ | 719 | 5.0 | 25 | [168] | |
| Co2+ | 468 | ||||
| Ni2+ | 247 | ||||
| Chitosan/cotton fibers (via C-N single bond) | Hg2+ | 96 | 5.0 | 25 | [169] |
| Au3+ | 89 | 3.0 | 25 | [170] | |
| Chitosan/cotton fibers (via Schiff base bon) | Hg2+ | 104 | 5.0 | 35 | [169] |
| Au3+ | 77 | 3.0 | 25 | [170] | |
| Cu2+ | 25 | 6.5 | 25 | [171] | |
| Ni2+ | 8 | ||||
| Pd2+ | 102 | ||||
| Cd2+ | 16 | ||||
| Chitosan/magnetite | Cr6+ | 69 | 4.0 | - | [172] |
| Pb2+ | 63 | 6.0 | - | [173] | |
| Ni2+ | 53 | ||||
| Chitosan/perlite | Cu2+ | 196 | 5.0 | - | [174] |
| Ni2+ | 115 | ||||
| Cd2+ | 179 | 6.0 | 25 | [175] | |
| Cr6+ | 154 | 4.0 | 25 | [176] | |
| Cu2+ | 104 | 4.5 | 25 | [177] | |
| Chitosan/polyvinyl alcohol | Cd2+ | 143 | 6.0 | 50 | [178] |
| Cu2+ | 48 | 6.0 | - | [179] | |
| Chitosan/polyvinyl chloride | Cu2+ | 88 | 4.0 | - | [180] |
| Ni2+ | 120 | 5.0 | |||
| Chitosan/silica | Ni2+ | 254 | 5.0 | - | [161] |
| Application | Membrane | Ref. |
|---|---|---|
| Water/ethanol mixture separation | Chitosan salt | [183] |
| Crosslinked chitosan | [184] | |
| Chitosan/N-methyol nylon 6 blend | [185] | |
| HY zeolite-filled chitosan | [186] | |
| Crossline quaternized chitosan composite | [187] | |
| Chitosan-hydroxyethylcellulose composite | [182] | |
| Isopropanol-water separation | Chitosan | [188] |
| Chitosan-hydroxyethylcellulose blended | [189] | |
| Crosslinked chitosan | [190] | |
| Chitosan/NaY zeolite composite | [191] | |
| Blended chitosan/polyvinyl alcohol | [192] | |
| Chitosan-poly(tetrafluoroethylene) composite | [193] | |
| Crosslinked carboxymethyl chitosan-PSF-hollow-fiber composite | [194] | |
| Diisocyanate crosslinked chitosan | [195] | |
| Chitosan-polyacrylonitrile hollow fiber | [196] | |
| Poyelectrolyte complexes of chitosan and phosphotungstic acid | [197] | |
| Chitosan g-polyaniline | [198] | |
| Sodium alginate and chitosan-wrapped MWCNT | [199] | |
| Ethylene glycol/H2O separation | Surface crosslinked chitosan | [200] |
| Chitosan-poly(acrylic acid) polyelectrolyte complex | [201] | |
| Chitosan polysulfone composite | [202] | |
| Chitosan poly(vinyl alcohol) blend | [203] | |
| Separation methanol/methyl t-butyl ether | Chitosan-poly (N.vinyl-2-pyrrolidone) blend | [204] |
| Chitosan composite (modified with surfactants) | [205] | |
| Chitosan-anionic surfactant complex | [206] | |
| Separation alcohol-toluene | N-acetylated chitosan | [207] |
| Silicate zeolite embedded chitosan mixed matrix | [208] | |
| Separation dimethyl carbonate-methanol | Chitosan | [209] |
| ZSM-5 zeolite-filled chitosan | [210] | |
| Separation benzene-cyclohexane | Poly(vinyl alcohol) chitosan blend | [211] |
| Chitosan/Ag+-carbon nanotubes | [212] | |
| Dehydration of 1,4-dioxane | Poly(vinyl alcohol)/chitosan | [213] |
| Chitosan/nylon 66 | [214] | |
| Crosslinked calcium alchinate-chitosan blend | [215] | |
| Poly(3-hydroxybutyrate)-functionalized multiwalled carbon nanotubes-chitosan composite | [216] | |
| Dehydration of caprolactam | Crosslinked PVA/chitosan | [217] |
| Chitosan-konjac glucomannan blending | [218] | |
| Chitosan-poly(acrylic acid) composite | [219] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. https://doi.org/10.3390/molecules25173981
Jiménez-Gómez CP, Cecilia JA. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules. 2020; 25(17):3981. https://doi.org/10.3390/molecules25173981
Chicago/Turabian StyleJiménez-Gómez, Carmen P., and Juan Antonio Cecilia. 2020. "Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications" Molecules 25, no. 17: 3981. https://doi.org/10.3390/molecules25173981
APA StyleJiménez-Gómez, C. P., & Cecilia, J. A. (2020). Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules, 25(17), 3981. https://doi.org/10.3390/molecules25173981







