Synergistic Effects of Ginkgolide B and Protocatechuic Acid on the Treatment of Parkinson’s Disease
Abstract
1. Introduction
2. Results
2.1. The Construction of Cell Model
2.2. Effects of Drugs on Cell Viability and Cytotoxicity of Rot-Induced PC12 Cells
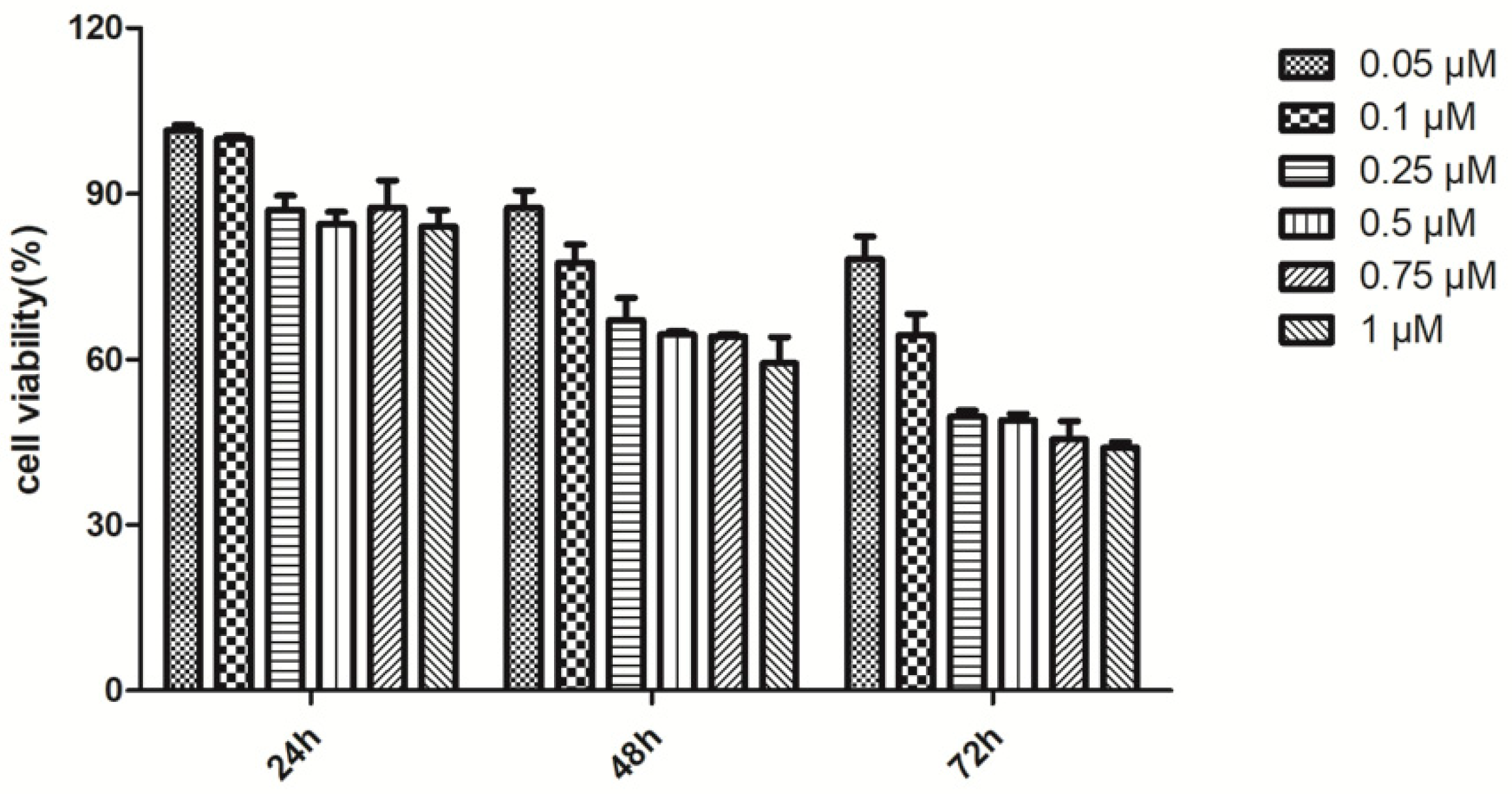
2.2.1. The Effects of GB, PCA and Combination of Both Drugs on the Cell Viability of Rot-Induced PC12 Cells
2.2.2. Combined Effects of GB and PCA on Cell Damage of Rot-Induced PC12 Cells
2.3. Combined Effects of GB and PCA on Oxidative Stress in Rot-Induced PC12 Cells
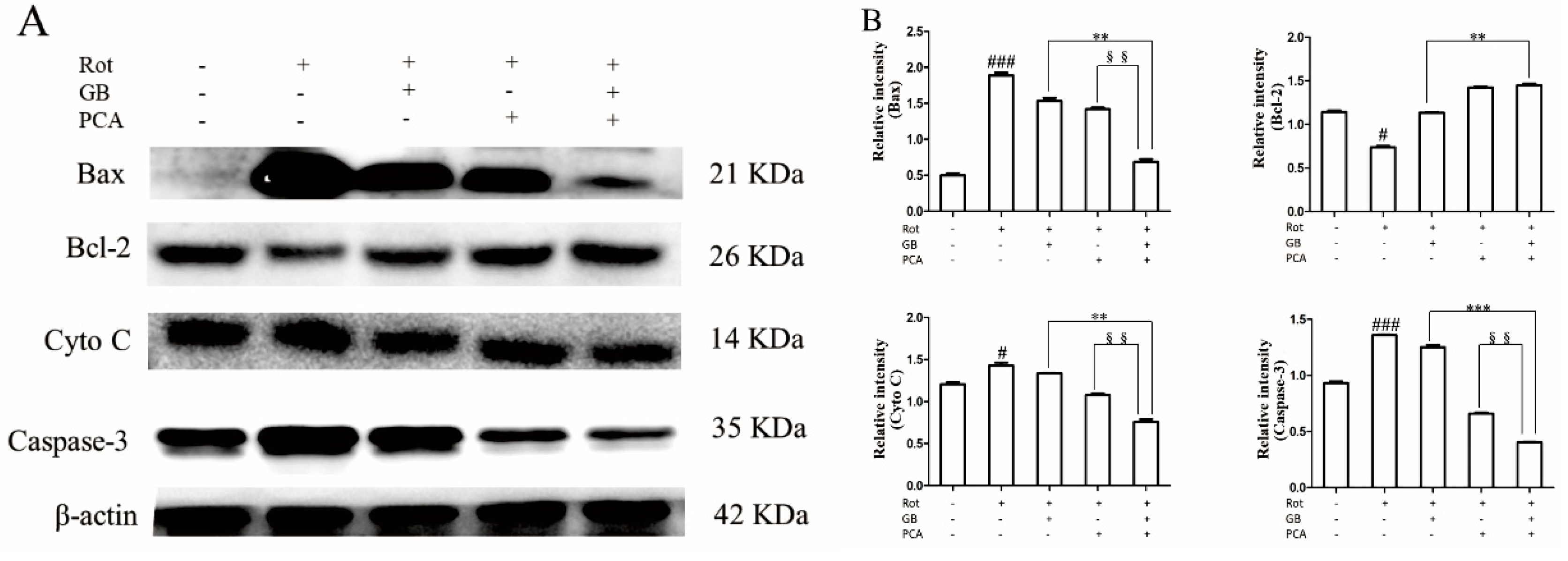
2.4. Combined Effects of GB and PCA on Expression of Mitochondrial Apoptosis Signaling Pathway Related Proteins in Rot-Induced PC12 Cells
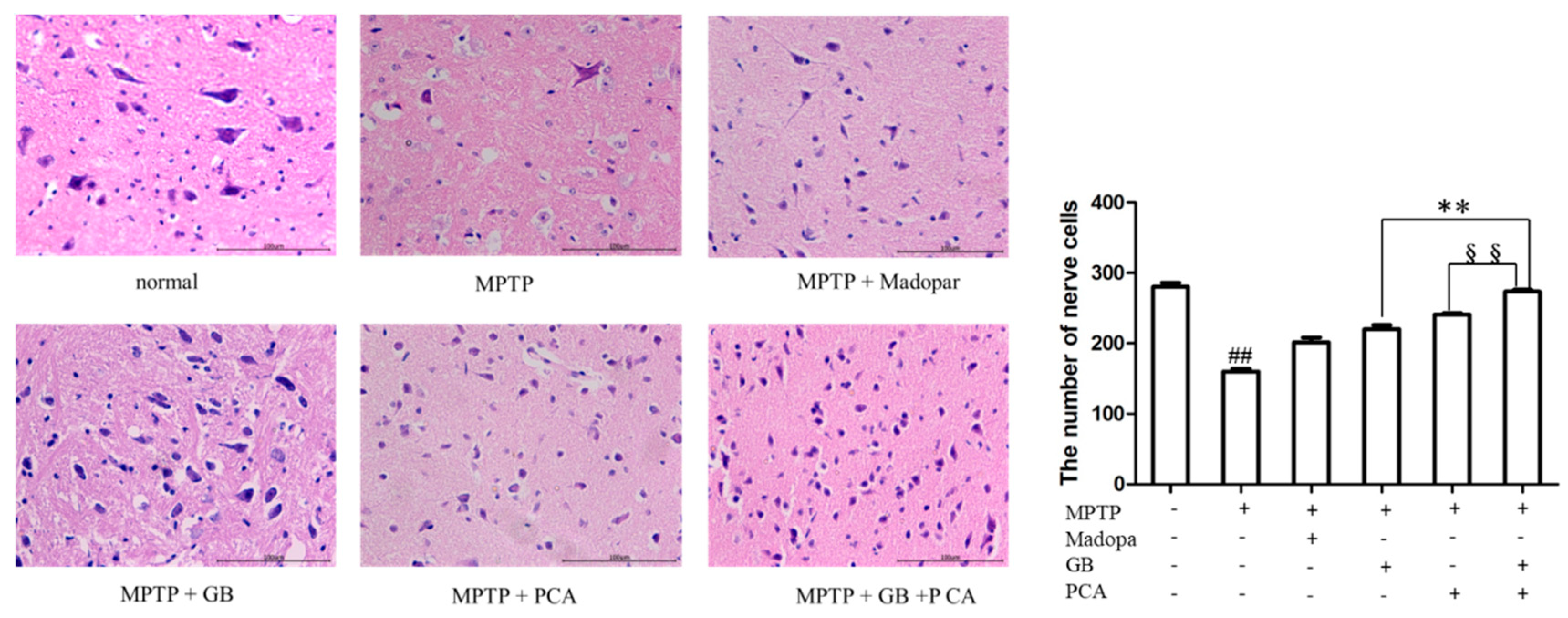
2.5. Combined Effects of GB and PCA on the Behavioural Outcome and Nerve Cell Injury of Parkinson Model Mice
2.6. Combined Effects of GB and PCA on Oxidative Stress Levels in the Midbrain of Parkinson’s Disease Mice
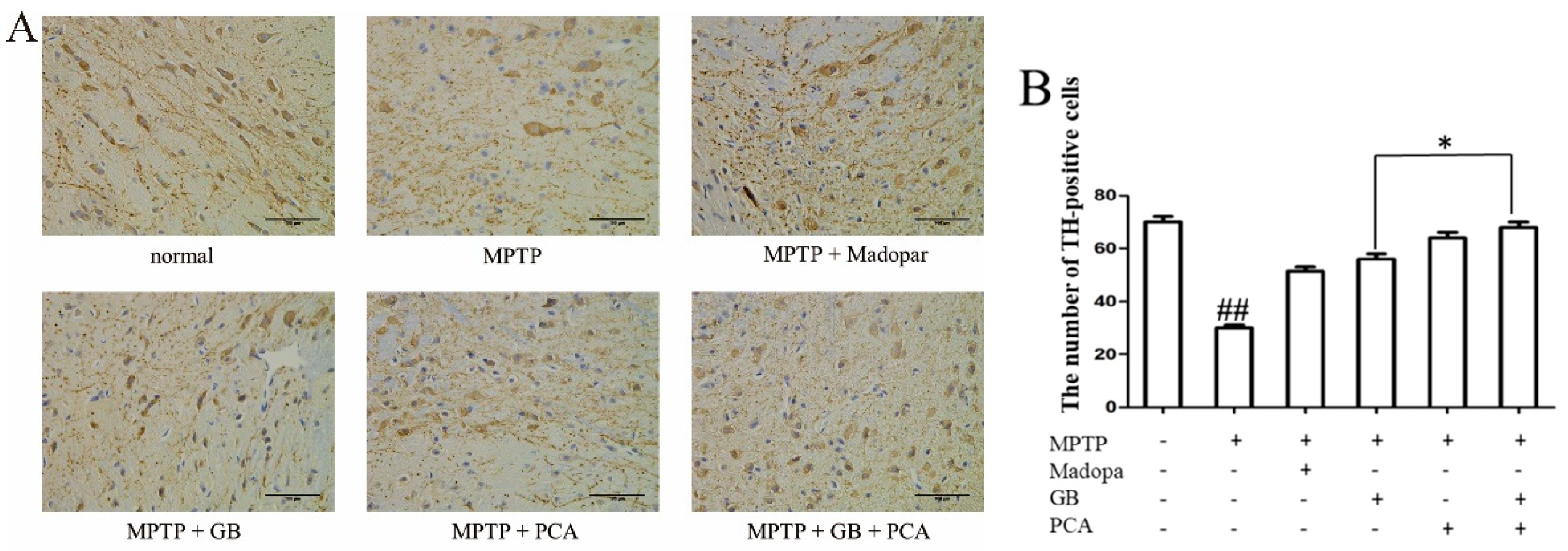
2.7. Combined Effects of GB and PCA on TH Expression in Substantia Nigra of Parkinson′s Disease Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Assay of Cell Viability
4.3. Morphological Analysis of PC12 Cells
4.4. LDH Release
4.5. Detection of Intracellular Reactive Oxygen Species (ROS)
4.6. Determination of Antioxidant Enzyme Activity
4.7. Protein Extraction and Western Blotting
4.8. Animals and Drug Treatment
4.9. Behavioural Test
4.10. Tissue Preparation
4.11. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EGB | Ginkgo biloba extract |
| GB | Ginkgolide B |
| PCA | Protocatechuic acid |
| Rot | Rotrnone |
| MPTP | 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine |
| PD | Parkinson’s disease |
| LDH | lactate dehydrogenase |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| TH | Tyrosine hydroxylase |
References
- Van Beek, T.A. Chemical analysis of Ginkgo biloba leaves and extracts. J. Chromatogr. A 2002, 967, 21–55. [Google Scholar] [CrossRef]
- Priyanka, A.; Nisha, V.M.; Anusree, S.S. Bilobalide attenuates hypoxia induced oxidative stress, inflammation, and mitochondrial dysfunctions in 3T3-L1 adipocytes via its antioxidant potential. Free Radic Res. 2014, 48, 1206–1217. [Google Scholar] [CrossRef]
- Joyeux, M.; Lobstein, A.; Anton, R. Comparative Antilipoperoxidant, Antinecrotic and Scavanging Properties of Terpenes and Biflavones from Ginkgo and some Flavonoids. Planta Med. 1995, 61, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Maclennan, K.M.; Darlington, C.L.; Smith, P.F. The CNS effects of Ginkgo biloba extracts and ginkgolide B. Prog. Neurobiol. 2002, 67, 235–257. [Google Scholar] [CrossRef]
- Mohammad, N.S.; Habtemariam, S.; Daglia, M. Neuroprotective effects of ginkgolide B against ischemic stroke: A review of current literature. Curr. Top. Med. Chem. 2015, 15, 2222–2232. [Google Scholar]
- Wang, X.J.; Chen, S.D.; Ma, G.Z. Involvement of proinflammatory factors, apoptosis, caspase-3 activation and Ca2+ disturbance in microglia activation-mediated dopaminergic cell degeneration. Mech. Ageing Dev. 2005, 126, 1241–1254. [Google Scholar] [CrossRef]
- Scholtyssek, H.; Damerau, W.; Wessel, R. Antioxidative activity of ginkgolides against superoxide in an aprotic environment. Chem. Biol. Interact. 1997, 106, 183–190. [Google Scholar] [CrossRef]
- Guan, S.; Ge, D.; Liu, T.Q. Protocatechuic acid promotes cell proliferation and reduces basal apoptosis in cultured neural stem cells. Toxicol. Vitr. 2009, 23, 201–208. [Google Scholar] [CrossRef]
- Sipos, I.; Tretter, L.; Adam-Vizi, V. Quantitative relationship between inhibition of respiratory complexes and formation of reactive oxygen species in isolated nerve terminals. J. Neurochem. 2003, 84, 112–118. [Google Scholar] [CrossRef]
- Tabner, B.J.; Oma, E.A.; German, M.J. Protein aggregation, metals and oxidative stress in neurodegenerative diseases. Biochem. Soc. Trans. 2007, 33, 1082–1086. [Google Scholar] [CrossRef]
- Li, N.; Ragheb, K.E.; Lawler, G.; Sturgis, J.; Rajwa, B.; Melendez, J.A.; Robinson, J.P. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2002, 278, 8516–8525. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Q.G.; Lai, L.Y. Neuroprotective Effect of Ginkgolide B on Bupivacaine-Induced Apoptosis in SH-SY5Y Cells. Oxidative Med. Cell. Longev. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Jiang, B.; Bao, Y.M. Protocatechuic acid inhibits apoptosis by mitochondrial dysfunction in rotenone-induced PC12 cells. Toxicol. Vitr. 2008, 22, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F.; Boveris, A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem. J. 1980, 191, 421–427. [Google Scholar] [CrossRef]
- Wang, G.; Qi, C.; Fan, G.H.; Zhou, H.Y.; Chen, S.D. PACAP protects neuronal differentiated PC12 cells against the neurotoxicity induced by a mitochondrial complex I inhibitor, rotenone. FEBS Lett. 2005, 579, 4005–4011. [Google Scholar] [CrossRef]
- Meng, H.; Li, C.; Feng, L. Effects of Ginkgolide B on 6-OHDA-induced apoptosis and calcium over load in cultured PC12. Int. J. Dev. Neurosci. 2007, 25, 510–514. [Google Scholar] [CrossRef]
- Chio, A.; Defazio, G. A new scale for prognostication in Parkinson disease: Of animals and men. Neurology 2016, 86, 982–983. [Google Scholar] [CrossRef]
- Ketharanathan, T.; Hanwella, R.; Weerasundera, R. Major depressive disorder in Parkinson’s disease: A cross-sectional study from Sri Lanka. BMC Psychiatry 2014, 14, 278. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
- Cassarino, D.S.; Parks, J.K.; Parker, J.R.; Bennett, J.P. The Parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim. Biophys. Acta 1999, 1453, 49–62. [Google Scholar] [CrossRef]
- Mythri, R.B.; Venkateshappa, C.; Harish, G. Evaluation of markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson’s disease brains. Neurochem. Res. 2011, 36, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Mini review: Glutathione-ascorbic acid antioxidant system in animals. J. Biol. Chem. 1994, 269, 9397–9400. [Google Scholar] [PubMed]
- Kroemer, G.; Reed, J.G. Mitochondrial control of cell death. Nat. Med. 2000, 6, 513–519. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Jenner, P.; Daniel, S.E. Conjugates of catecholamines with cysteine and GSH in Parkinson’s disease: Possible mechanisms of formation involving reactive oxygen species. J. Neurochem. 2010, 71, 2112–2122. [Google Scholar] [CrossRef]
- KIuck, R.M.; Bossy-Wetzel, E.; Green, D.R. the release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science. 1997, 275, 1132–1136. [Google Scholar]
- Song, W.; Guan, H.J. Protective effect of bilobalide against nitric oxide-induced neurotoxicity in PC12 cells. Acta Pharmacol. Sin. 2000, 5, 33–38. [Google Scholar]
- Tamatani, M.; Ogawa, S.; Tohyama, M. Roles of Bcl-2 and caspases in hypoxia-induced neuronal cell death: A possible neuroprotective mechanism of peptide growth factors. Mol. Brain Res. 1998, 58, 27–39. [Google Scholar] [CrossRef]
- Gross, A.; McDonnell, J.M.; Korsmeyer, S.J. Bcl-2 family members and the mitochondria in apoptosis. Gene Dev. 1999, 13, 1899–1911. [Google Scholar] [CrossRef]
- Korsmeyer, S.J. Bcl-2 initiates a new category of oncogenes: Regulators of cell death. Blood 1992, 80, 879–886. [Google Scholar] [CrossRef]
- Li, M.M. Influence of Ginsenoside Rg1 on Expressions of Neuregulin1 and ErbB4 in Substantia Nigra of Mice Model with Parkinson’s Disease and its Significance. Master’s Thesis, Xinxiang Medical University, Xinxiang, China, 2016. [Google Scholar]
- Goldberg, M.S.; Fleming, S.M.; Palacino, J.J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J. Biol. Chem. 2003, 278, 43628–43635. [Google Scholar] [CrossRef]
- Hu, B.L. Protective effects of paeoniflorin against rotenone-induced cell apoptosis and mitochondrial damage in SH-SY5Y cells. Chin. J. Clin. Pharmacol. 2018, 10, 1187–1190. [Google Scholar]
- Li, J.F.; Kuang, S.S. Protective effect of ginkgo biloba extract on mouse with Parkinson’s disease induced by MPTP. Chin. J. Comp. Med. 2016, 1, 46–53. [Google Scholar]
- Hyman, B.T.; Van Hoesen, G.W.; Damasio, A.R.; Barnes, C.L. Alzheimer’s disease: Cell specific pathology isolates the hippocampal formation. Science 1984, 225, 1168–1170. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Sun, W. Synergistic effects of rmhTRAIL and 17-AAG on the proliferation and apoptosis of multiple myeloma cells. Hematology 2018, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds Ginkgolide B and Protocatechuic Acid are available from the authors. |



| Group | Climbing Time/s | Score of Suspension Test/Score |
|---|---|---|
| Normal | 9.33 ± 0.54 | 2.5 ± 0.04 |
| MPTP | 14.05 ± 1.52 ### | 1.95 ± 0.09 ### |
| MPTP + Madopar | 9.95 ± 0.74 | 2.28 ± 0.22 |
| MPTP + GB | 10.69 ± 1.59 | 2.19 ± 0.04 |
| MPTP + PCA | 11.17 ± 1.88 | 2.11 ± 0.04 |
| MPTP + GB + PCA | 10.33 ± 1.20 | 2.25 ± 0.07 *,§§§ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Fang, X.; Xu, J.; Jiang, Y.; Cao, F.; Zhao, L. Synergistic Effects of Ginkgolide B and Protocatechuic Acid on the Treatment of Parkinson’s Disease. Molecules 2020, 25, 3976. https://doi.org/10.3390/molecules25173976
Wu T, Fang X, Xu J, Jiang Y, Cao F, Zhao L. Synergistic Effects of Ginkgolide B and Protocatechuic Acid on the Treatment of Parkinson’s Disease. Molecules. 2020; 25(17):3976. https://doi.org/10.3390/molecules25173976
Chicago/Turabian StyleWu, Tingting, Xianying Fang, Jiahui Xu, Yan Jiang, Fuliang Cao, and Linguo Zhao. 2020. "Synergistic Effects of Ginkgolide B and Protocatechuic Acid on the Treatment of Parkinson’s Disease" Molecules 25, no. 17: 3976. https://doi.org/10.3390/molecules25173976
APA StyleWu, T., Fang, X., Xu, J., Jiang, Y., Cao, F., & Zhao, L. (2020). Synergistic Effects of Ginkgolide B and Protocatechuic Acid on the Treatment of Parkinson’s Disease. Molecules, 25(17), 3976. https://doi.org/10.3390/molecules25173976




