Factors Governing the Chemical Stability and NMR Parameters of Uracil Tautomers and Its 5-Halogen Derivatives
Abstract
1. Introduction
2. Results and Discussion
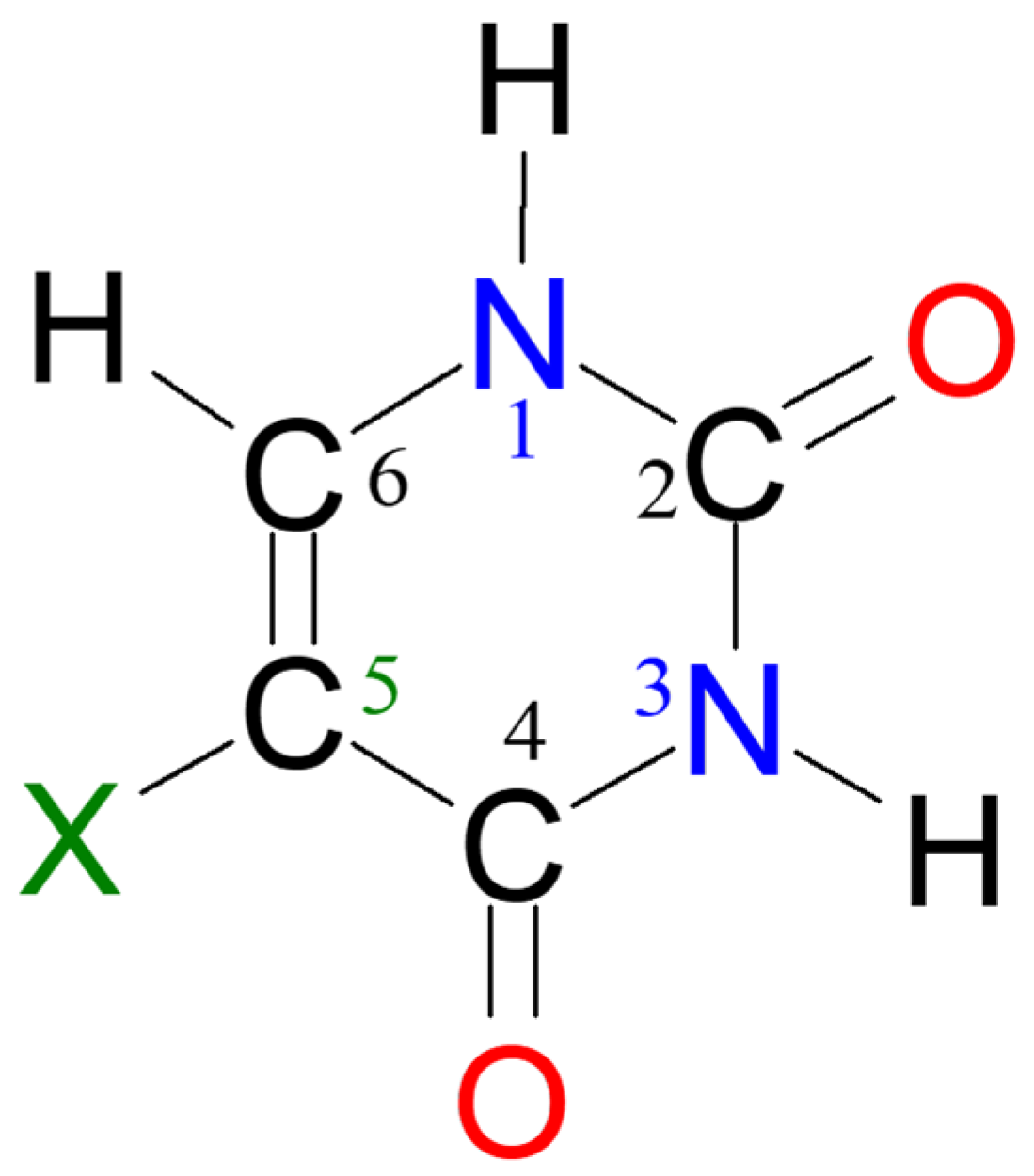
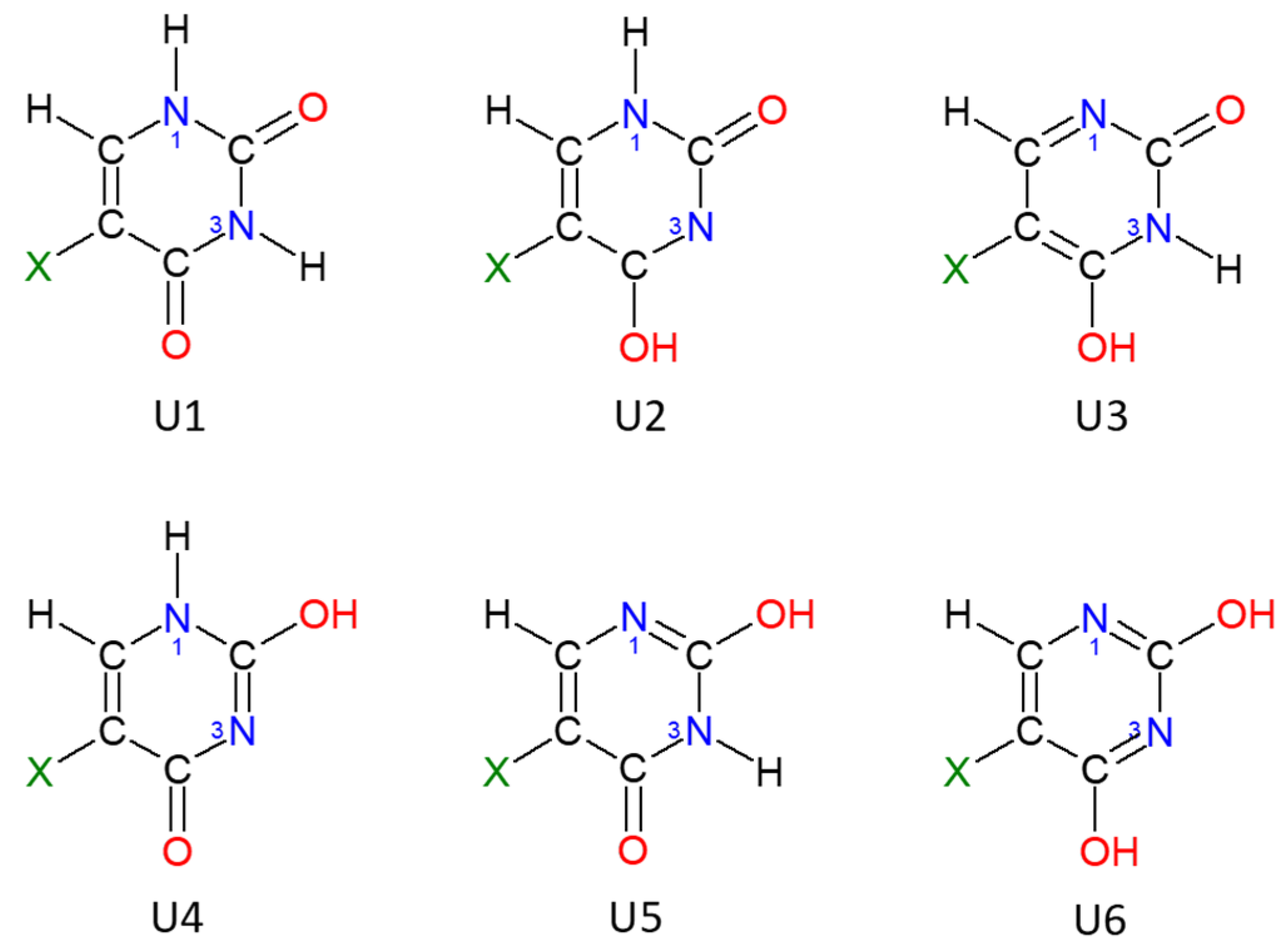
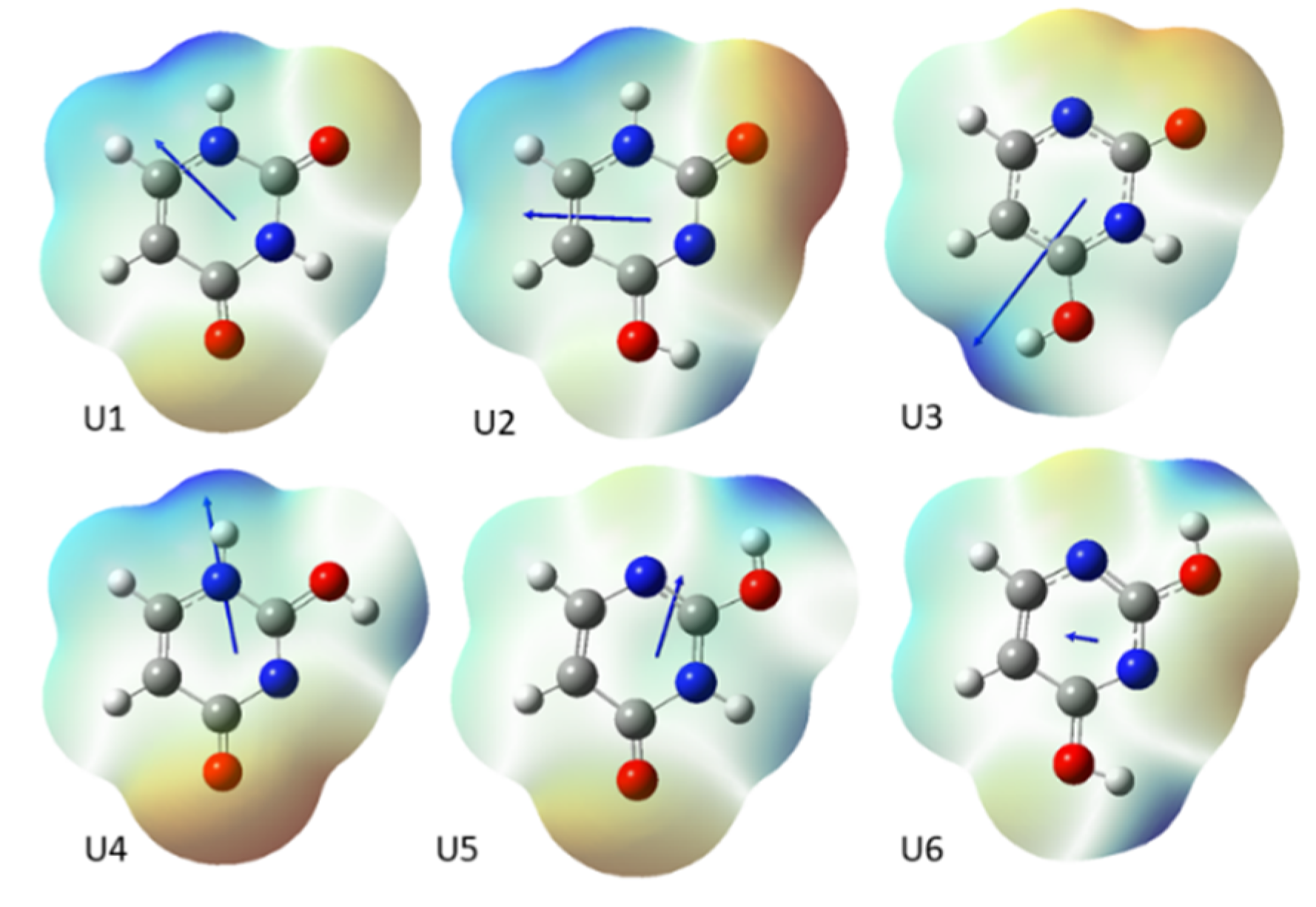
2.1. Energy of Uracil Tautomers and Its Derivatives
2.2. Aromaticity of Uracil, 5XU and Their Tautomers
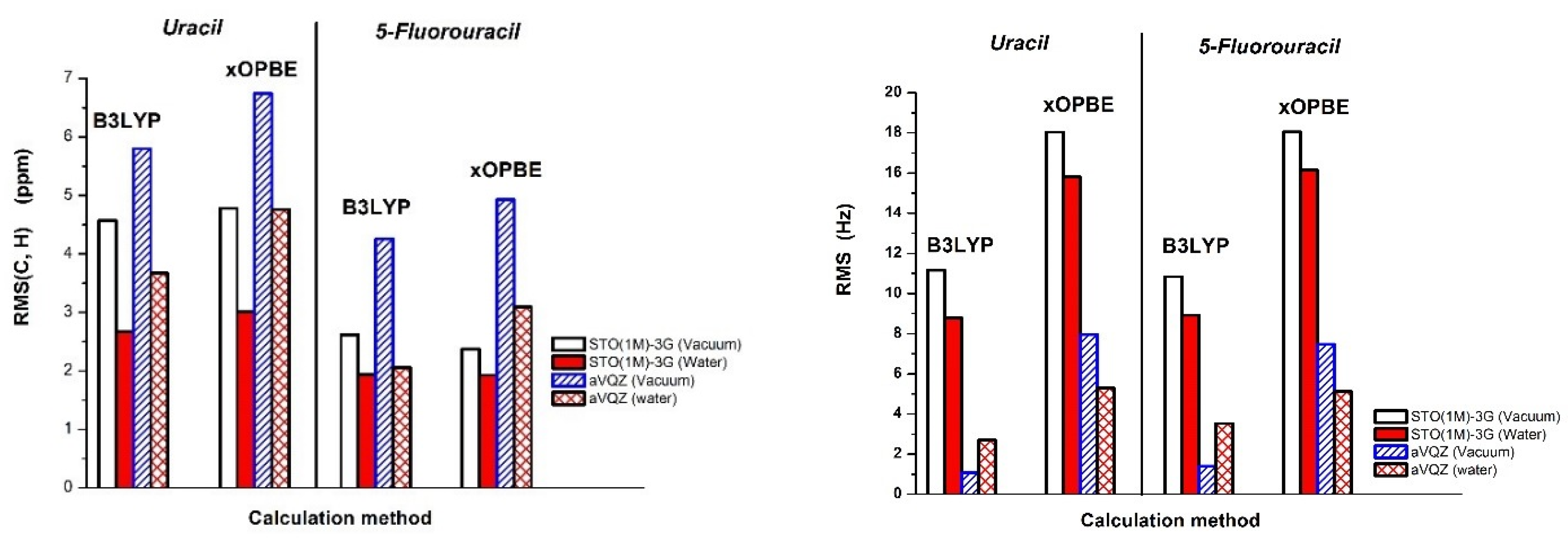
2.3. Chemical Shifts and Indirect Spin-Spin Coupling Constants of Uracil and 5-Fluorouracil
3. Methods
3.1. Computational Methods
3.2. NMR Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ur-Rahman, A.; Choudhary, M.I. Preface. In Applications of NMR Spectroscopy; ur-Rahman, A., Choudhary, M.I., Eds.; Bentham Science Publishers: Potomac, MD, USA, 2015; pp. vii–viii. [Google Scholar]
- Fan, T.W.M.; Lane, A.N. Applications of NMR spectroscopy to systems biochemistry. Prog. Nucl. Magn. Reson. Spectrosc. 2016, 92–93, 18–53. [Google Scholar] [CrossRef] [PubMed]
- Mari, H.S.; Varras, C.P.; Atia tul, W.; Choudhary, M.I.; Siskos, G.M.; Gerothanassis, P.I. Solvent-Dependent Structures of Natural Products Based on the Combined Use of DFT Calculations and 1H-NMR Chemical Shifts. Molecules 2019, 24, 2290. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Piner, R.D.; Stadermann, F.J.; Park, S.; Shaibat, M.A.; Ishii, Y.; Yang, D.; Velamakanni, A.; An, S.J.; Stoller, M.; et al. Synthesis and Solid-State NMR Structural Characterization of 13C–Labeled Graphite Oxide. Science 2008, 321, 1815–1817. [Google Scholar] [CrossRef]
- Breton, R.C.; Reynolds, W.F. Using NMR to identify and characterize natural products. Nat. Prod. Rep. 2013, 30, 501–524. [Google Scholar] [CrossRef] [PubMed]
- Stockman, B.J.; Dalvit, C. NMR screening techniques in drug discovery and drug design. Prog. Nucl. Mag. Res. Sp. 2002, 41, 187–231. [Google Scholar] [CrossRef]
- Pauli, G.F.; Jaki, B.U.; Lankin, D.C. Quantitative 1H NMR: Development and Potential of a Method for Natural Products Analysis. J. Nat. Prod. 2005, 68, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, G.R.; Vervoort, H.C.; Lee, C.M.; Cremin, P.A.; Williams, C.T.; Hart, S.M.; Goering, M.G.; O’Neil-Johnso, M.; Zeng, L. High-Throughput Method for the Production and Analysis of Large Natural Product Libraries for Drug Discovery. Anal. Chem. 2002, 74, 3963–3971. [Google Scholar] [CrossRef]
- Dong, Z. Proton MRS and MRSI of the brain without water suppression. Prog. Nucl. Magn. Reson. Spectrosc. 2015, 86–87, 65–79. [Google Scholar] [CrossRef]
- van der Graaf, M. In vivo magnetic resonance spectroscopy: Basic methodology and clinical applications. Eur. Biophys. J. 2010, 39, 527–540. [Google Scholar] [CrossRef]
- Bundi, A.; Wüthrich, K. 1H-nmr parameters of the common amino acid residues measured in aqueous solutions of the linear tetrapeptides H-Gly-Gly-X-L-Ala-OH. Biopolymers 1979, 18, 285–297. [Google Scholar] [CrossRef]
- Shakibayi Far, J.; Ziglari, A.; Sayadian, M.; Shahriari, S.; Khalilimofrad, M.S.; Malakian, F.; Elsagh, A.; Mollaamin, F. Drug Delivery and NMR Tensors Studies of Methamphetamine and Carbon-Nanotube Binding. J. Comput. Nanosci. 2015, 12, 4158–4165. [Google Scholar] [CrossRef]
- Ramalho, T.C.; Pereira, D.H.; Thiel, W. Thermal and Solvent Effects on NMR Indirect Spin–Spin Coupling Constants of a Prototypical Chagas Disease Drug. J. Phys. Chem. A 2011, 115, 13504–13512. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wei, X.; Liu, X.; Yan, T. Comparison of Different Theory Models and Basis Sets in the Calculations of Structures and 13C NMR Spectra of [Pt(en) (CBDCA−O, O′)], an Analogue of the Antitumor Drug Carboplatin. J. Phys. Chem. B 2010, 114, 4056–4062. [Google Scholar] [CrossRef] [PubMed]
- Duschinsky, R.; Pleven, E.; Heidelberger, C. The synthesis of 5-fluoropyrimidines. J. Am. Chem. Soc. 1957, 79, 4559–4560. [Google Scholar] [CrossRef]
- Heidelberger, C.; Chaudhuri, N.K.; Danneberg, P.; Mooren, D.; Griesbach, L.; Duschinsky, R.; Schnitzer, R.J.; Pleven, E.; Scheiner, J. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature 1957, 179, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla-Veloz, R.I.; Stelzer, T.; López-Mejías, V. Measurement and Correlation of the Solubility of 5-Fluorouracil in Pure and Binary Solvents. J. Chem. Eng. Data 2018, 63, 3809–3817. [Google Scholar] [CrossRef]
- Arakawa, Y.; Nakano, M.; Juni, K.; Arita, T. Physical Properties of Pyrimidine and Purine Antimetabolites. I. The Effects of Salts and Temperature on the Solubility of 5-Fluorouracil, 1-(2-Tetrahydrofuryl)-5-fluorouracil, 6-Mercaptopurine, and Thioinosine. Chem. Pharm. Bull. 1976, 24, 1654–1657. [Google Scholar] [CrossRef]
- Chabner, B.A.; Longo, D.L. Harrison’s Manual of Oncology, 2nd ed.; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Krenitsky, T.A.; Freeman, G.A.; Shaver, S.R.; Beacham, L.M.; Hurlbert, S.; Cohn, N.K.; Elwell, L.P.; Selway, J.W.T. 3’-Amino-2’,3’-dideoxyribonucleosides of some pyrimidines: Synthesis and biological activities. J. Med. Chem. 1983, 26, 891–895. [Google Scholar] [CrossRef]
- Patra, A.; Harp, J.; Pallan, P.S.; Zhao, L.; Abramov, M.; Herdewijn, P.; Egli, M. Structure, stability and function of 5-chlorouracil modified A: U and G: U base pairs. Nucleic Acids Res. 2013, 41, 2689–2697. [Google Scholar] [CrossRef]
- Colasurdo, D.D.; Pila, M.N.; Iglesias, D.A.; Laurella, S.L.; Ruiz, D.L. Tautomerism of uracil and related compounds: A mass spectrometry study. Eur. J. Mass Spectrom. 2017, 24, 214–224. [Google Scholar] [CrossRef]
- Tian, S.X.; Zhang, C.F.; Zhang, Z.J.; Chen, X.J.; Xu, K.Z. How many uracil tautomers there are? Density functional studies of stability ordering of tautomers. Chem. Phys. 1999, 242, 217–225. [Google Scholar] [CrossRef]
- Zhang, R.; Ceulemans, A.; Nguyen, M.T. A theoretical study of uracil and its tautomers in their lowest-lying triplet state. Mol. Phys. 2005, 103, 983–994. [Google Scholar] [CrossRef]
- Kua, J. Exploring Free Energy Profiles of Uracil and Cytosine Reactions with Formaldehyde. J. Phys. Chem. A 2019, 123, 3840–3850. [Google Scholar] [CrossRef] [PubMed]
- Hanus, M.; Kabeláč, M.; Nachtigallová, D.; Hobza, P. Mutagenic Properties of 5-Halogenuracils: Correlated Quantum Chemical ab Initio Study. Biochemistry 2005, 44, 1701–1707. [Google Scholar] [CrossRef]
- Alcolea Palafox, M.; Tardajos, G.; Guerrero-Martínez, A.; Vats, J.K.; Joe, H.; Rastogi, V.K. Relationships observed in the structure and spectra of uracil and its 5-substituted derivatives. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 75, 1261–1269. [Google Scholar] [CrossRef]
- Muñoz Freán, S.; Alcolea Palafox, M.; Rastogi, V.K. Effect of the microhydration on the tautomerism in the anticarcinogenic drug 5-fluorouracil and relationships with other 5-haloderivatives. J. Mol. Struct. 2013, 1054–1055, 32–45. [Google Scholar]
- Ortiz, S.; Alvarez-Ros, M.C.; Alcolea Palafox, M.; Rastogi, V.K.; Balachandran, V.; Rathor, S.K. FT-IR and FT-Raman spectra of 6-chlorouracil: Molecular structure, tautomerism and solid state simulation. A comparison between 5-chlorouracil and 6-chlorouracil. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 653–668. [Google Scholar] [CrossRef]
- Rastogi, V.K.; Palafox, M.A. Vibrational spectra, tautomerism and thermodynamics of anticarcinogenic drug: 5-Fluorouracil. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2011, 79, 970–977. [Google Scholar] [CrossRef]
- Rastogi, V.K.; Palafox, M.A.; Mittal, L.; Peica, N.; Kiefer, W.; Lang, K.; Ojha, S.P. FTIR and FT-Raman spectra and density functional computations of the vibrational spectra, molecular geometry and atomic charges of the biomolecule: 5-bromouracil. J. Raman Spectrosc. 2007, 38, 1227–1241. [Google Scholar] [CrossRef]
- Almeida, M.O.; Barros, D.A.S.; Araujo, S.C.; Faria, S.H.D.M.; Maltarollo, V.G.; Honorio, K.M. Study on molecular structure, spectroscopic properties (FTIR and UV–Vis), NBO, QTAIM, HOMO-LUMO energies and docking studies of 5-fluorouracil, a substance used to treat cancer. Spectrochim. Acta—Part A 2017, 84, 169–176. [Google Scholar] [CrossRef]
- Abdrakhimova, G.S.; Ovchinnikov, M.Y.; Lobov, A.N.; Spirikhin, L.V.; Ivanov, S.P.; Khursan, S.L. 5-Fluorouracil solutions: NMR study of acid–base equilibrium in water and DMSO. J. Phys. Org. Chem. 2014, 27, 876–883. [Google Scholar] [CrossRef]
- Alagona, G.; Ghio, C.; Monti, S. Ab initio modeling of competitive drug–drug interactions: 5-fluorouracil dimers in the gas phase and in solution. Int. J. Quantum Chem. 2001, 83, 128–142. [Google Scholar] [CrossRef]
- Shishkin, O.V.; Gorb, L.; Luzanov, A.V.; Elstner, M.; Suhai, S.; Leszczynski, J. Structure and conformational flexibility of uracil: A comprehensive study of performance of the MP2, B3LYP and SCC-DFTB methods. J. Mol. Struct. 2003, 625, 295–303. [Google Scholar] [CrossRef]
- Leszczynski, J. Tautomerism of uracil: The final chapter? Fourth-order electron correlation contributions to the relative energies of tautomers. J. Phys. Chem. 1992, 96, 1649–1653. [Google Scholar] [CrossRef]
- Shishkin, O.V.; Gorb, L.; Leszczynski, J. Modeling of the Hydration Shell of Uracil and Thymine. Int. J. Mol. Sci. 2000, 1, 17–27. [Google Scholar] [CrossRef]
- Leszczynśki, J. Structure and properties of uracil and its sulfur analogs: A systematic study of basis set effects in Ab InitioSCF calculations. Int. J. Quantum Chem. 1991, 40, 9–21. [Google Scholar] [CrossRef]
- Bednarek, E.; Dobrowolski, J.C.; Dobrosz-Teperek, K.; Kozerski, L.; Lewandowski, W.; Mazurek, A.P. Theoretical and experimental 1H, 13C, 15N, and 17O NMR chemical shifts for 5-halogenouracils. J. Mol. Struct. 2000, 554, 233–243. [Google Scholar] [CrossRef]
- Bednarek, E.; Dobrowolski, J.C.; Dobrosz-Teperek, K.; Sitkowski, J.; Kozerski, L.; Lewandowski, W.; Mazurek, A.P. Theoretical and experimental 1H, 13C, 15N, and 17O NMR spectra of 5-nitro, 5-amino, and 5-carboxy uracils. J. Mol. Struct. 1999, 482–483, 333–337. [Google Scholar] [CrossRef]
- Blicharska, B.; Kupka, T. Theoretical DFT and experimental NMR studies on uracil and 5-fluorouracil. J. Mol. Struct. 2002, 613, 153–166. [Google Scholar] [CrossRef]
- Kokko, J.P.; Mandell, L.; Goldstein, J.H. An, N. m. r. Investigation of Proton Mobility in Substituted Uracils. J. Am. Chem. Soc. 1962, 84, 1042–1047. [Google Scholar] [CrossRef]
- Kokko, J.P.; Goldstein, J.H.; Mandell, L. A Nuclear Magnetic Resonance Investigation of Tautomerism and Substituent Effects in Some Pyrimidines and Related Nucleosides. J. Am. Chem. Soc. 1961, 83, 2909–2911. [Google Scholar] [CrossRef]
- Jardetzky, C.D.; Jardetzky, O. Investigation of the Structure of Purines, Pyrimidines, Ribose Nucleosides and Nucleotides by Proton Magnetic Resonance. II1. J. Am. Chem. Soc. 1960, 82, 222–229. [Google Scholar] [CrossRef]
- Dobrowolski, J.C.; Rode, J.E.; Kołos, R.; Jamróz, M.H.; Bajdor, K.; Mazurek, A.P. Ar-Matrix IR Spectra of 5-Halouracils Interpreted by Means of DFT Calculations. J. Phys. Chem. A 2005, 109, 2167–2182. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, L.F.; Bendich, A. The Ultraviolet Absorption Spectra of Pyrimidines and Purines. J. Am. Chem. Soc. 1950, 72, 2587–2594. [Google Scholar] [CrossRef]
- Iza, N.; Gil, M.; Morcillo, J. Identification of ionic and tautomeric species of uracil by second derivative UV absorption spectroscopy. J. Mol. Struct. 1988, 175, 31–36. [Google Scholar] [CrossRef]
- Ivanov, A.Y.; Leontiev, V.S.; Belous, L.F.; Rubin, Y.V.; Karachevtsev, V.A. Infrared spectra of 5-fluorouracil molecules isolated in inert Ar matrices, and their films on graphene oxide at 6 K. Low Temp. Phys. 2017, 43, 400–408. [Google Scholar] [CrossRef]
- Ostakhov, S.S.; Ovchinnikov, M.Y.; Masyagutova, G.A.; Khursan, S.L. Luminescent and DFT Study of Keto–Enol Tautomers of 5-Fluorouracil and Its Derivatives in Aqueous Solutions. J. Phys. Chem. A 2019, 123, 7956–7964. [Google Scholar] [CrossRef]
- Singh, V.; Fedeles, B.I.; Essigmann, J.M. Role of tautomerism in RNA biochemistry. RNA 2015, 21, 1–13. [Google Scholar]
- Chen, Z.; Wannere, C.S.; Corminboeuf, C.; Puchta, R.; Schleyer, P.v.R. Nucleus-Independent Chemical Shifts (NICS) as an Aromaticity Criterion. Chem. Rev. 2005, 105, 3842–3888. [Google Scholar] [CrossRef]
- Schleyer, P.v.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; Hommes, N.J.R.v.E. Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef]
- Kruszewski, J.; Krygowski, T.M. Definition of aromaticity basing on the harmonic oscillator model. Tetrahedron Lett. 1972, 13, 3839–3842. [Google Scholar] [CrossRef]
- Frizzo, C.; Martins, M. Aromaticity in heterocycles: New HOMA index parametrization. Struct. Chem. 2011, 23, 375–380. [Google Scholar] [CrossRef]
- Walesa, R.; Kupka, T.; Broda, M.A. Density functional theory (DFT) prediction of structural and spectroscopic parameters of cytosine using harmonic and anharmonic approximations. Struct. Chem. 2015, 26, 1083–1093. [Google Scholar] [CrossRef]
- Kupka, T.; Mnich, A.; Broda, M.A. Performance of revised STO(1M)−3G basis set for prediction of 5-fluorocytosine chemical shifts. Magn. Reson. Chem. 2019, 57, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Lukmanov, T.; Ivanov, S.P.; Khamitov, E.M.; Khursan, S.L. Relative stability of keto-enol tautomers in 5,6-substituted uracils: Ab initio, DFT and PCM study. Comput. Chem. 2013, 1023, 38–45. [Google Scholar] [CrossRef]
- McMurry, J. Organic Chemistry, 7th ed; Brooks-Cole: Pacific Grove, CA, USA, 2007. [Google Scholar]
- Cysewski, P. An ab initio study on nucleic acid bases aromaticities. J. Mol. Struct. 2005, 714, 29–34. [Google Scholar] [CrossRef]
- Udagawa, T. Theoretical analysis on the aromaticity of uracil: Important electronic configurations and solvent effect on the aromaticity. Chem. Phys. Let. 2015, 637, 115–119. [Google Scholar] [CrossRef]
- Galvão, T.L.P.; Rocha, I.M.; Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V. Is Uracil Aromatic? The Enthalpies of Hydrogenation in the Gaseous and Crystalline Phases, and in Aqueous Solution, as Tools to Obtain an Answer. J. Phys. Chem. A 2013, 117, 5826–5836. [Google Scholar] [CrossRef]
- Makulski, W.; Wilczek, M.; Jackowski, K. 17O and 1H NMR spectral parameters in isolated water molecules. Phys. Chem. Chem. Phys. 2018, 20, 22468–22476. [Google Scholar] [CrossRef]
- Wisconsin. In Biological Magnetic Resonance Data Bank. A Repository for Data from NMR Spectroscopy on Proteins, Peptides, Nucleic Acids, and Other Biomolecules. Available online: http://www.bmrb.wisc.edu/metabolomics/mol_summary/show_data.php?id=bmse000940 (accessed on 10 July 2020).
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation-energy density functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, S. A consistent and accurate ab initio parametrization of density functional dispersion correction (dft-d) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, Q.; Yin, C.; Wu, A.-a.; Xu, X. xOPBE: A Specialized Functional for Accurate Prediction of 13C Chemical Shifts. J. Phys. Chem. A 2020, 124, 5824–5831. [Google Scholar] [CrossRef]
- Voronkov, E.; Rossikhin, V.; Okovytyy, S.; Shatckih, A.; Bolshakov, V.; Leszczynski, J. Novel physically adapted STO ##−3G basis sets. Efficiency for prediction of second-order electric and magnetic properties of aromatic hydrocarbons. Int. J. Quantum Chem. 2012, 112, 2444–2449. [Google Scholar]
- Kapusta, K.; Voronkov, E.; Okovytyy, S.; Korobov, V.; Leszczynski, J. Reconstruction of STO−3G Family Basis Set for the Accurate Calculation of Magnetic Properties. Russ. J. Phys. Chem. A 2018, 92, 2827–2834. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J. GIAO Calculations of Chemical Shifts in Heterocyclic Compounds. Struct. Chem. 2003, 14, 377–389. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |
| ΔE | μ | |||||
|---|---|---|---|---|---|---|
| Tautomer | Vacuum | PCM | SMD | Vacuum | PCM | SMD |
| U1 a | 0.00 | 0.00 | 0.00 | 4.46 | 6.12 | 6.93 |
| U2 | 12.07 | 11.42 | 9.54 | 4.88 | 6.95 | 7.84 |
| U3 | 21.19 | 18.27 | 15.24 | 7.17 | 10.26 | 11.62 |
| U4 | 19.63 | 16.76 | 14.08 | 6.56 | 9.56 | 10.83 |
| U5 | 11.61 | 14.32 | 12.95 | 3.31 | 4.63 | 5.45 |
| U6 | 13.73 | 18.86 | 17.14 | 1.19 | 1.68 | 1.82 |
| 5FU1 b | 0.00 | 0.00 | 0.00 | 4.10 | 5.74 | 6.49 |
| 5FU2 | 12.90 | 12.61 | 10.57 | 3.60 | 5.27 | 6.01 |
| 5FU3 | 20.46 | 19.64 | 16.83 | 5.85 | 8.54 | 9.76 |
| 5FU4 | 17.06 | 14.27 | 11.69 | 7.02 | 10.22 | 11.57 |
| 5FU5 | 9.64 | 12.30 | 10.96 | 4.33 | 6.02 | 6.88 |
| 5FU6 | 12.46 | 17.93 | 16.04 | 0.60 | 0.63 | 0.68 |
| 5ClU1 c | 0.00 | 0.00 | 0.00 | 4.02 | 5.75 | 6.49 |
| 5ClU2 | 12.53 | 12.32 | 10.43 | 3.58 | 5.22 | 5.87 |
| 5ClU3 | 18.32 | 18.10 | 16.37 | 5.71 | 8.40 | 9.45 |
| 5ClU4 | 17.86 | 15.05 | 12.57 | 6.87 | 10.17 | 11.50 |
| 5ClU5 | 10.08 | 12.57 | 11.20 | 4.28 | 6.12 | 7.03 |
| 5ClU6 | 12.54 | 17.95 | 16.17 | 0.61 | 0.73 | 0.84 |
| 5BrU1 d | 0.00 | 0.00 | 0.00 | 3.97 | 5.74 | 6.32 |
| 5BrU2 | 12.44 | 12.22 | 10.08 | 3.62 | 5.32 | 5.86 |
| 5BrU3 | 18.02 | 17.90 | 17.05 | 5.77 | 8.51 | 9.22 |
| 5BrU4 | 17.97 | 15.17 | 12.73 | 6.77 | 10.10 | 11.22 |
| 5BrU5 | 10.18 | 12.65 | 11.20 | 4.20 | 6.04 | 6.80 |
| 5BrU6 | 12.58 | 17.95 | 15.80 | 0.55 | 0.67 | 0.72 |
| 5IU1 e | 0.00 | 0.00 | 0.00 | 3.91 | 5.36 | 6.11 |
| 5IU2 | 13.89 | 13.41 | 11.30 | 3.42 | 4.73 | 5.75 |
| 5IU3 | 20.22 | 20.00 | 16.33 | 5.69 | 7.95 | 9.44 |
| 5IU4 | 20.17 | 17.12 | 14.60 | 6.68 | 9.40 | 10.49 |
| 5IU5 | 11.04 | 12.99 | 11.83 | 4.29 | 5.87 | 6.35 |
| 5IU6 | 14.28 | 18.52 | 16.57 | 0.75 | 0.86 | 0.69 |
| Molecule | Vacuum | Water | ||||||
|---|---|---|---|---|---|---|---|---|
| NICS (0) | NICS (1) | NICS (1)zz | HOMA | NICS (0) | NICS (1) | NICS (1) zz | HOMA | |
| U1 | −0.449 | −1.141 | −2.082 | 0.545 | −0.852 | −1.596 | −3.298 | 0.644 |
| 5FU1 | −2.354 | −1.680 | −2.150 | 0.526 | −2.763 | −2.101 | −3.213 | 0.603 |
| 5ClU1 | −1.324 | −1.435 | −1.773 | 0.469 | −1.661 | −1.821 | −2.776 | 0.602 |
| 5BrU1 | −1.071 | −1.360 | −1.515 | 0.472 | −1.400 | −1.744 | −2.518 | 0.604 |
| 5IU1 a | −0.732 | −1.269 | −1.287 | 0.504 | −1.053 | −1.653 | −2.303 | 0.609 |
| 1,2-diazine | −4.924 | −10.269 | −29.170 | 0.975 | −4.895 | −10.231 | −29.117 | 0.969 |
| pyrimidine | −5.281 | −9.781 | −28.236 | 0.992 | −5.253 | −9.780 | −28.252 | 0.991 |
| 1,4-diazine | −5.001 | −10.088 | −29.374 | 0.997 | −4.962 | −10.077 | −29.353 | 0.997 |
| pyridine | −6.579 | −10.007 | −29.470 | 0.993 | −6.546 | −9.999 | −29.472 | 0.993 |
| benzene | −7.828 | −10.014 | −30.041 | 0.991 | −7.774 | −10.000 | −30.016 | 0.994 |
| B3LYP | xOPBE | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| STO(1M)−3G | aug-cc-pVQZ | STO(1M)−3G | aug-cc-pVQZ | ||||||
| Signal | Exp. | Vacuum | Water | Vacuum | Water | Vacuum | Water | Vacuum | Water |
| C2 | 155.93 b | −4.61 | −2.96 | −7.26 | −5.01 | −4.86 | −3.48 | −8.95 | −7.02 |
| C4 | 170.30 b | −7.12 | −4.01 | −8.81 | −4.89 | −8.15 | −5.45 | −11.15 | −7.71 |
| C5 | 103.79 b | −1.12 | −2.99 | −2.96 | −4.80 | −0.07 | −1.93 | −2.16 | −4.02 |
| C6 | 146.26 b | −7.12 | −2.83 | −7.83 | −2.82 | −6.75 | −2.76 | −7.89 | −3.23 |
| H5 | 5.79 b | −0.94 | −1.00 | −0.62 | −0.64 | −1.04 | −1.10 | −0.66 | −0.68 |
| H6 | 7.53 b | −0.62 | −0.33 | −0.93 | −0.62 | −0.67 | −0.38 | −1.00 | −0.70 |
| N1 | −248.81 c | 17.27 | 24.11 | 19.60 | 27.89 | 8.65 | 15.21 | 11.27 | 19.18 |
| N3 | −221.35 c | 22.98 | 24.57 | 26.71 | 29.62 | 13.09 | 14.62 | 16.67 | 19.49 |
| O2 | 252.5 c | 12.36 | −6.83 | 34.50 | 12.78 | −6.22 | −23.02 | 21.21 | 2.22 |
| O4 | 334 c | 20.71 | −17.07 | 53.71 | 10.36 | −2.45 | −36.48 | 36.81 | −1.95 |
| RMS (C) | 5.56 | 3.23 | 7.08 | 4.47 | 5.82 | 3.65 | 8.24 | 5.82 | |
| RMS (C, H) | 4.57 | 2.67 | 5.80 | 3.67 | 4.78 | 3.01 | 6.74 | 4.76 | |
| RMS (N, O) | 18.76 | 19.52 | 35.96 | 21.94 | 8.53 | 24.01 | 23.50 | 13.75 | |
| B3LYP | xOPBE | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| STO(1M)−3G | aug-cc-pVQZ | STO(1M)−3G | aug-cc-pVQZ | ||||||
| Signal | Exp. | Vacuum | Water | Vacuum | Water | Vacuum | Water | Vacuum | Water |
| C2 | 152.19 b | −1.97 | −0.52 | −5.08 | −3.11 | −2.24 | −1.06 | −6.77 | −5.11 |
| C4 | 160.98 b | −2.65 | −0.25 | −5.50 | −2.41 | −3.19 | −1.17 | −7.23 | −4.58 |
| C5 | 141.30 b | 4.57 | 3.17 | 3.48 | 2.24 | 3.47 | 2.03 | 1.46 | 0.17 |
| C6 | 127.54 b | −1.41 | 2.88 | −4.64 | 0.39 | −0.53 | 3.43 | −4.44 | 0.22 |
| H6 | 7.65 b | −0.72 | −0.38 | −1.06 | −0.69 | −0.83 | −0.50 | −1.19 | −0.84 |
| N1 | −261.06 c | 16.12 | 24.61 | 17.19 | 27.30 | 8.13 | 16.19 | 9.48 | 19.03 |
| N3 | −221.55 c | 22.23 | 23.77 | 26.01 | 28.92 | 12.56 | 14.02 | 16.27 | 19.09 |
| O2 | 250 c | 12.91 | −4.42 | 34.00 | 14.28 | −5.98 | −21.16 | 20.30 | 3.04 |
| O4 | 321.3 c | 23.08 | −15.25 | 56.15 | 12.76 | 0.22 | −34.53 | 39.51 | 0.46 |
| F | −169.31 d | 3.76 | −2.51 | −14.60 | −21.87 | 11.59 | 5.39 | −2.49 | −9.59 |
| RMS (C) | 2.90 | 2.16 | 4.74 | 2.27 | 2.62 | 2.14 | 5.48 | 3.43 | |
| RMS (C, H) | 2.62 | 1.94 | 4.26 | 2.06 | 2.37 | 1.93 | 4.93 | 3.09 | |
| RMS (N, O, F) | 17.13 | 16.91 | 33.15 | 22.03 | 8.88 | 20.63 | 21.60 | 12.87 | |
| B3LYP | xOPBE | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| STO(1M)−3G | aug-cc-pVQZ | STO(1M)−3G | aug-cc-pVQZ | ||||||
| SSCC | Exp. | Vacuum | Water | Vacuum | Water | Vacuum | Water | Vacuum | Water |
| 1J (C5H5) | 177.83 | −21.71 | −21.00 | 7.21 | 8.06 | −40.33 | −39.74 | −13.44 | −12.76 |
| 1J (C6H6) | 183.82 | −29.47 | −23.13 | −0.92 | 6.65 | −47.55 | −41.64 | −20.78 | −13.74 |
| 2J (C5H6) | 2.96 | −0.82 | −0.43 | 0.05 | 0.48 | −2.14 | −1.73 | −1.97 | −1.50 |
| 3J (C2H6) | 9.42 | −1.72 | −1.46 | −0.13 | 0.16 | −2.23 | −1.94 | −0.75 | −0.38 |
| 3J (C4H6) | 10.54 | −1.22 | −1.24 | 0.57 | 0.56 | −1.21 | −1.26 | 0.67 | 0.61 |
| 3J (H5H6) | 7.69 | 1.01 | 0.95 | 1.65 | 1.59 | 0.62 | 0.53 | 1.37 | 1.27 |
| 2J (H5C6) | 3.64 | 0.77 | 0.45 | 2.07 | 1.64 | −1.13 | −1.40 | −0.62 | −0.94 |
| 2J (H5C4) | 1.79 | −0.21 | 0.23 | 0.29 | 0.82 | −2.02 | −1.58 | −2.04 | −1.50 |
| RMS | 12.97 | 11.07 | 2.75 | 3.80 | 22.09 | 20.39 | 8.83 | 6.70 | |
| RMS c | 11.17 | 8.78 | 1.09 | 2.69 | 18.04 | 15.80 | 7.96 | 5.30 | |
| B3LYP | xOPBE | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| STO(1M)−3G | aug-cc-pVQZ | STO(1M)−3G | aug-cc-pVQZ | ||||||
| SSCC | Exp. | Vacuum | Water | Vacuum | Water | Vacuum | Water | Vacuum | Water |
| 1J (C5F5) | 227.0 | 8.74 | −4.57 | 86.09 | 69.64 | 10.58 | −1.12 | 88.60 | 73.41 |
| 1J (C6H6) | 182.0 | −26.54 | −20.91 | 2.37 | 8.86 | −45.06 | −39.80 | −18.13 | −11.88 |
| 2J (C5H6) | 4.1 | 0.28 | 0.16 | 0.79 | 0.65 | 1.63 | 1.48 | 2.69 | 2.41 |
| 3J (C2H6) | 10.1 | −2.33 | −2.12 | −0.74 | −0.47 | −2.86 | −2.62 | −1.39 | −1.08 |
| 3J (C4H6) | 7.3 | −1.25 | −1.30 | −0.03 | 0.01 | −0.97 | −1.05 | 0.34 | 0.25 |
| 3J (F5H6) | 6.0 | 2.09 | 0.87 | −1.90 | −0.53 | 4.30 | 3.25 | −6.22 | −4.86 |
| 2J (F5C6) | 31.1 | 7.49 | 6.79 | 1.74 | 2.28 | 11.82 | 11.06 | −3.01 | −0.47 |
| 2J (F5C4) | 25.6 | 7.12 | 8.18 | −0.22 | −1.66 | 9.02 | 9.95 | −2.23 | −3.50 |
| RMS | 10.60 | 8.50 | 30.46 | 24.84 | 17.30 | 15.11 | 32.09 | 26.39 | |
| RMS c | 10.84 | 8.92 | 1.39 | 3.53 | 18.05 | 16.15 | 7.47 | 5.13 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzepiela, K.; Buczek, A.; Kupka, T.; Broda, M.A. Factors Governing the Chemical Stability and NMR Parameters of Uracil Tautomers and Its 5-Halogen Derivatives. Molecules 2020, 25, 3931. https://doi.org/10.3390/molecules25173931
Rzepiela K, Buczek A, Kupka T, Broda MA. Factors Governing the Chemical Stability and NMR Parameters of Uracil Tautomers and Its 5-Halogen Derivatives. Molecules. 2020; 25(17):3931. https://doi.org/10.3390/molecules25173931
Chicago/Turabian StyleRzepiela, Kacper, Aneta Buczek, Teobald Kupka, and Małgorzata A. Broda. 2020. "Factors Governing the Chemical Stability and NMR Parameters of Uracil Tautomers and Its 5-Halogen Derivatives" Molecules 25, no. 17: 3931. https://doi.org/10.3390/molecules25173931
APA StyleRzepiela, K., Buczek, A., Kupka, T., & Broda, M. A. (2020). Factors Governing the Chemical Stability and NMR Parameters of Uracil Tautomers and Its 5-Halogen Derivatives. Molecules, 25(17), 3931. https://doi.org/10.3390/molecules25173931








