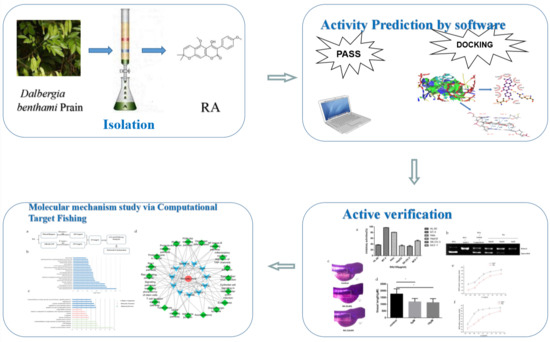
Antitumor Activity and Mechanism of Robustic Acid from Dalbergia benthami Prain via Computational Target Fishing
Abstract
1. Introduction
2. Results
2.1. The Chemical Structure, ADME Evaluation and Bioactivity Prediction of RA
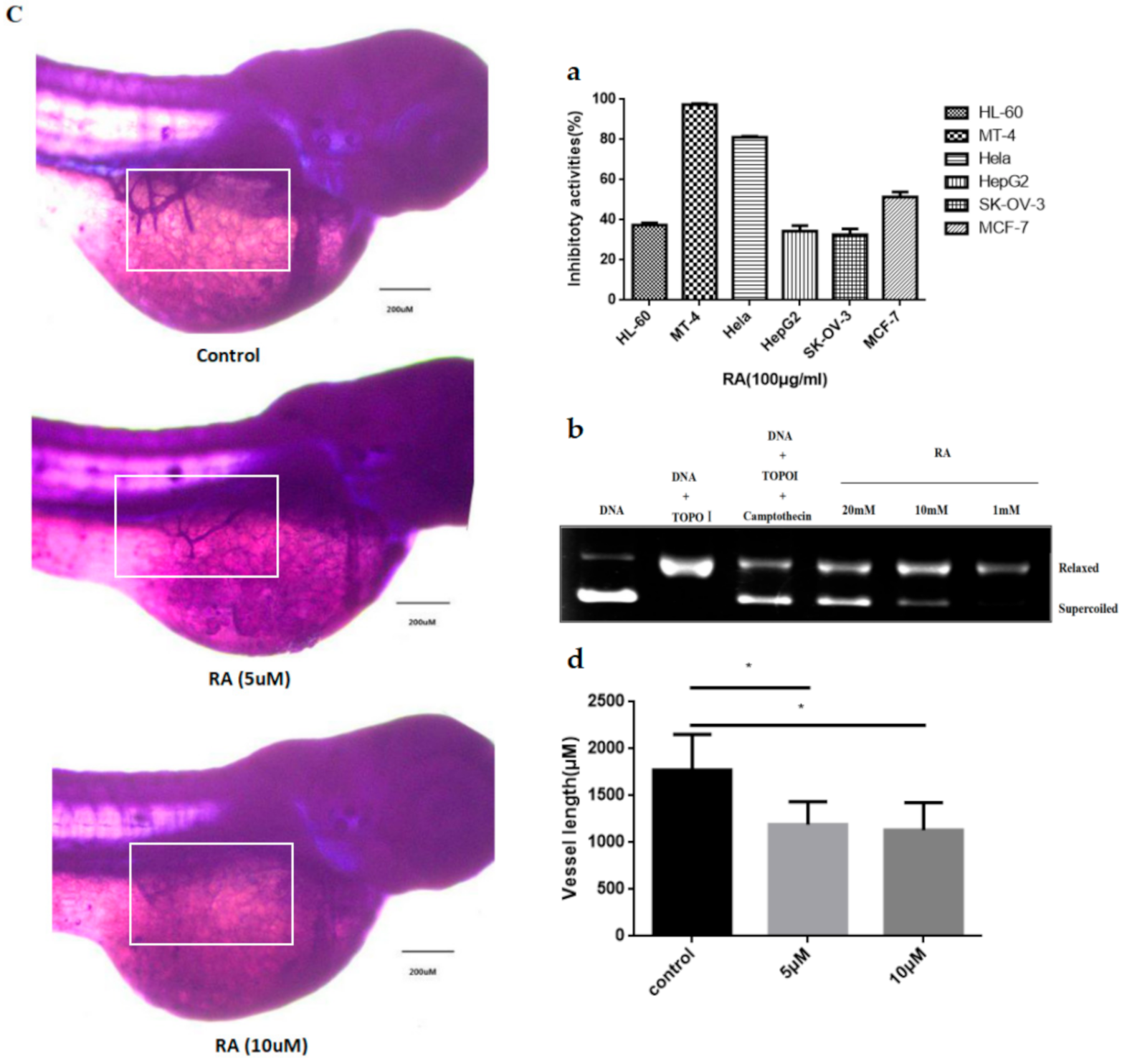
2.2. Biological Activity Characterizations
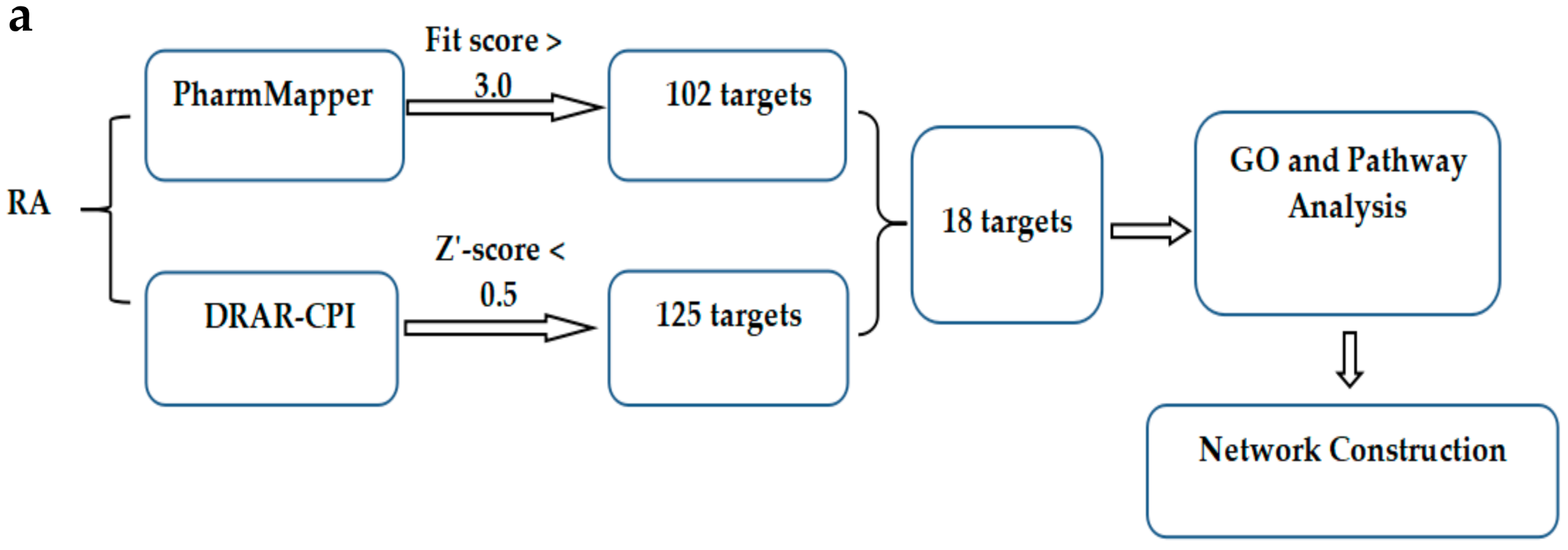
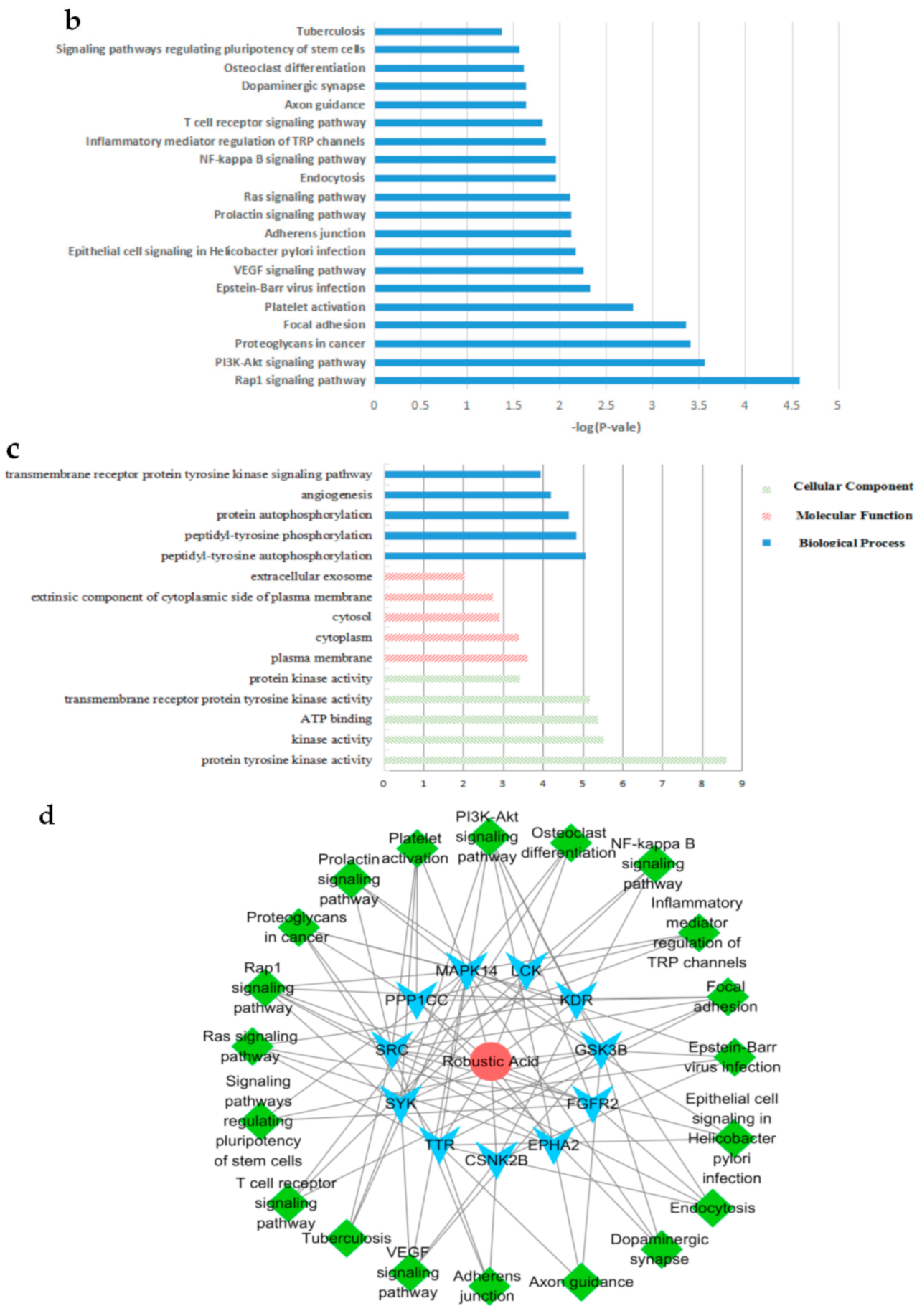
2.3. In Silico Target Fishing for the RA
3. Discussion
4. Materials and Methods
4.1. Sample and Reagents
4.2. Preparation of Plant Extract
4.3. The Isolation of RA
4.4. ADME Evaluation and Bioactivity Prediction
4.5. Bioactivity Study of Rustic Acid
4.5.1. MTS Method
4.5.2. Topo I Inhibitory Activity
4.5.3. Zebrafish Embryos Angiogenesis Assay
4.5.4. Radical-Scavenging Activity
DPPH Radical-Scavenging Activity
ABTS Radical-Scavenging Activity
4.6. Systematic Understanding of the Mechanism of Action of RA via Computational Target Fishing
4.6.1. Computational Target Fishing by PharmMapper and DRAR-CPI
4.6.2. GO and Pathway Analysis, and Network Construction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Editorial Committee of the National Chinese Medicine Administrative Bureau. Chinese Materia Medica, 4th ed.; Shanghai Scientific and Technical Education Publishing House: Shanghai, China, 2005; pp. 432–433. [Google Scholar]
- Liu, R.-H.; Lin, S.; Zhang, P.-Z.; Chen, L.-Y.; Huang, H.-L.; Mei, D.-Y. Neoflavonoids and their pharmacological activities in Dalbergia genus. China J. Chin. Mater. Med. 2017, 42, 4707–4715. [Google Scholar]
- Chen, S.S.; Shi, X.H. Antibacterial and Anti-inflammatory Effect of Crude Extract of Two Dalbergia Plants. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 157–162. [Google Scholar]
- Huo, L.N.; Liu, H.G. Evaluation of the antioxidant and alpha-Glucosidase inhibitory activities of different extracts of Dalbergia benthami. Jiangsu Agric. Sci. 2014, 42, 242–244. [Google Scholar]
- Wei, J.H.; Tan, H.S. Chemical constituents from Zhuang medicine Dalbergia benthami. Chin. Tradit. Herb. Drugs 2017, 49, 2159–2163. [Google Scholar]
- Khalid, S.A.; Waterman, P.G. Thonningine-A and thonningine-B: Two 3-phenylcoumarins from the seeds of Millettia thonningii. Phytochemistry 1983, 22, 1001–1003. [Google Scholar] [CrossRef]
- Nkengfack, A.E.; Waffo, A.K.; Azebaze, G.A.; Fomum, Z.T.; Meyer, M.; Bodo, B.; Van Heerden, F.R. Indicanine A, a new 3-phenylcoumarin from root bark of Erythrina indica. J. Nat. Prod. 2000, 63, 855–856. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.A.D.; Huang, T. Determination of the Chemical Composition of Tugancao by X-ray Crystal Diffraction. Lishizhen Med. Mater. Med. Res. 2012, 29, 2160–2162. [Google Scholar]
- Donnelly, D.M.X.; Molloy, D.J.; Reilly, J.P.; Finet, J.-P. Aryllead-mediated synthesis of linear 3-arylpyranocoumarins: Synthesis of robustin and robustic acid. J. Chem. Soc. Perkin Trans. 1 1995, 2531. [Google Scholar] [CrossRef]
- qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature. Available online: https://pubchem.ncbi.nlm.nih.gov/bioassay/924 (accessed on 20 December 2007).
- qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature. Available online: https://pubchem.ncbi.nlm.nih.gov/bioassay/902 (accessed on 18 December 2007).
- Khalid, S.A.; Farouk, A.; Geary, T.G.; Jensen, J.B. Potential antimalarial candidates from African plants: An in vitro approach using Plasmodium falciparum. J. Ethnopharmacol. 1986, 15, 201–209. [Google Scholar] [CrossRef]
- Chen, R.; Huang, J.; Jaiswal, Y.; Wei, J.; Huo, L.; Xia, X.; Zhong, J.; Williams, L.; Huang, M.; Liang, Y. Molecular Design, Synthesis and Docking Study of Alkyl and Benzyl Derivatives of Robustic Acid as Topoisomerase I Inhibitors. Chem. Biodivers. 2020, 17. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Meng, L.; Gao, W.-Y.; Song, N.-N.; Jia, W.; Duan, H.-Q. Advances on biological activities of coumarins. China J. Chin. Mater. Med. 2005, 30, 410–414. [Google Scholar]
- Doak, B.C.; Over, B.; Giordanetto, F.; Kihlberg, J. Oral Druggable Space beyond the Rule of 5: Insights from Drugs and Clinical Candidates. Chem. Biol. 2014, 21, 1115–1142. [Google Scholar] [CrossRef] [PubMed]
- Jadaun, A.; Subbarao, N.; Dixit, A. Allosteric inhibition of topoisomerase I by pinostrobin: Molecular docking, spectroscopic and topoisomerase I activity studies. J. Photochem. Photobiol. B Biol. 2017, 167, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Arepalli, S.K.; Lee, C.; Sim, S.; Lee, K.; Jo, H.; Jun, K.-Y.; Kwon, Y.; Kang, J.-S.; Jung, J.-K.; Lee, H. Development of 13H-benzo[f]chromeno[4,3-b][1,7]naphthyridines and their salts as potent cytotoxic agents and topoisomerase I/IIα inhibitors. Bioorg. Med. Chem. 2018, 26, 5181–5193. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.; Essa, E.A.; Zaki, R.M.; Elkordy, A.A. An Overview of the Antioxidant Effects of Ascorbic Acid and Alpha Lipoic Acid (in Liposomal Forms) as Adjuvant in Cancer Treatment. Antioxidants 2020, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Pottier, C.; Fresnais, M.; Gilon, M.; Jerusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers 2020, 12, 731. [Google Scholar] [CrossRef]
- Yu, Y.; Rahmanto, Y.S.; Lee, M.-H.; Wu, P.-H.; Phillip, J.M.; Huang, C.-H.; Vitolo, M.I.; Gaillard, S.; Martin, S.S.; Wirtz, D.; et al. Inhibition of ovarian tumor cell invasiveness by targeting SYK in the tyrosine kinase signaling pathway. Oncogene 2018, 37, 3778–3789. [Google Scholar] [CrossRef]
- Guo, A.; Lu, P.; Coffey, G.; Conley, P.; Pandey, A.; Wang, Y.L. Dual SYK/JAK inhibition overcomes ibrutinib resistance in chronic lymphocytic leukemia: Cerdulatinib, but not ibrutinib, induces apoptosis of tumor cells protected by the microenvironment. Oncotarget 2017, 8, 12953–12967. [Google Scholar] [CrossRef]
- Jiang, Q.H.; Cheng, N.N. Advances in research on antitumor based on drugs tyrosine kinase signal pathway. Chin. J. Clin. Pharm. 2011, 20, 379–383. [Google Scholar]
- Weinberg, R.A. The Biology of Cancer, 1st ed.; Garland Science, Taylor & Francis Group, LLC: New York, NY, USA, 2007; pp. 757–759. [Google Scholar]
- Carnevale, J.; Ross, L.; Puissant, A.; Banerji, V.; Stone, R.M.; DeAngelo, D.J.; Ross, K.N.; Stegmaier, K. SYK regulates mTOR signaling in AML. Leukemia 2013, 27, 2118–2128. [Google Scholar] [CrossRef]
- Poon, C.L.; Brumby, A.M.; Richardson, H. Src Cooperates with Oncogenic Ras in Tumourigenesis via the JNK and PI3K Pathways in Drosophila epithelial Tissue. Int. J. Mol. Sci. 2018, 19, 1585. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.H.; Huo, L.N.; Huang, M.C.; Chen, R.; Feng, X. 3-Phenylcoumarin Robustic Acid as well as Extraction Method and Application Thereof. ZL 201410522224.9, 24 August 2016. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Lohidakshan, K.; Rajan, M.; Ganesh, A.; Paul, M.; Jerin, J. Pass and Swiss ADME collaborated in silico docking approach to the synthesis of certain pyrazoline spacer compounds for dihydrofolate reductase inhibition and antimalarial activity. Bangladesh J. Pharmacol. 2018, 13, 23. [Google Scholar] [CrossRef]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: Prediction of activity spectra for biologically active substances. Bioinformatics 2000, 16, 747–748. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Liu, X.; Ouyang, S.; Yu, B.; Liu, Y.; Huang, K.; Gong, J.; Zheng, S.; Li, Z.; Li, H.; Jiang, H. PharmMapper server: A web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res. 2010, 38, W609–W614. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Chen, J.; Shi, L.; Mikailov, M.; Zhu, H.; Wang, K.; He, L.; Yang, L. DRAR-CPI: A server for identifying drug repositioning potential and adverse drug reactions via the chemical–protein interactome. Nucleic Acids Res. 2011, 39, W492–W498. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2008, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the compound RA are available from the authors. |


| Name | MW (g/mol) | Hdon | Hacc | Rbon | TPSA (Å) | LogP | LogS | Log Kp (cm/s) |
|---|---|---|---|---|---|---|---|---|
| RA | 380.39 | 1 | 6 | 3 | 78.13 | 3.72 | −4.80 | −5.94 |
| Pa | Pi | Activity Name |
|---|---|---|
| 0.784 | 0.014 | Antineoplastic |
| 0.699 | 0.007 | Chemopreventive |
| 0.655 | 0.005 | Free radical scavenger |
| 0.615 | 0.008 | Antiparasitic |
| 0.614 | 0.012 | Antiinfective |
| 0.594 | 0.009 | Antileukemic |
| 0.583 | 0.004 | Antihelmintic |
| 0.570 | 0.022 | Antifungal |
| 0.554 | 0.042 | Antiinflammatory |
| Compound | Total Score | Crash | Polar | D Score | PMF Score | G Score | CHEM SCORE | C SCORE |
|---|---|---|---|---|---|---|---|---|
| Camptothecin | 10.33 | −0.70 | 1.15 | −176.36 | −164.18 | −301.14 | −13.21 | 4 |
| RA | 7.80 | −1.80 | 0.78 | −173.03 | −163.80 | −249.97 | −15.70 | 4 |
| Rank | PDB ID | Name | Target Gene |
|---|---|---|---|
| 1 | 1AXN | Annexin A3 | ANXA3 |
| 2 | 1YOL | Proto-oncogene tyrosine-protein kinase Src | SRC |
| 3 | 2PVY | Fibroblast growth factor receptor 2 | FGFR2 |
| 4 | 1J1B | Glycogen synthase kinase-3 beta | GSK3B |
| 5 | 1JWH | Casein kinase II subunit alpha | CSNK2B |
| 6 | 1Q11 | Tyrosyl-tRNA synthetase, cytoplasmic | YARS |
| 7 | 1QPC | Proto-oncogene tyrosine-protein kinase LCK | LCK |
| 8 | 1MQB | Ephrin type-A receptor 2 | EPHA2 |
| 9 | 1XBA | Tyrosine-protein kinase SYK | SYK |
| 10 | 3E92 | Mitogen-activated protein kinase 14 | MAPK14 |
| 11 | 1S0X | Nuclear receptor ROR-alpha | RORA |
| 12 | 1CBS | Cellular retinoic acid-binding protein 2 | CRABP2 |
| 13 | 1IT6 | Serine/threonine-protein phosphatase PP1-gamma catalytic subunit | PPP1CC |
| 14 | 1BOA | Methionine aminopeptidase 2 | METAP2 |
| 15 | 1R1H | Neprilysin | MME |
| 16 | 1RLB | Transthyretin | TTR |
| 17 | 3F82 | Hepatocyte growth factor receptor | MET |
| 18 | 3cp9 | Vascular endothelial growth factor receptor 2 | KDR |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Liang, Y.; Tian, W.; Ma, J.; Huang, L.; Li, B.; Chen, R.; Li, D. Antitumor Activity and Mechanism of Robustic Acid from Dalbergia benthami Prain via Computational Target Fishing. Molecules 2020, 25, 3919. https://doi.org/10.3390/molecules25173919
Huang J, Liang Y, Tian W, Ma J, Huang L, Li B, Chen R, Li D. Antitumor Activity and Mechanism of Robustic Acid from Dalbergia benthami Prain via Computational Target Fishing. Molecules. 2020; 25(17):3919. https://doi.org/10.3390/molecules25173919
Chicago/Turabian StyleHuang, Juanjuan, Ying Liang, Wenyu Tian, Jing Ma, Ling Huang, Benjie Li, Rui Chen, and Dianpeng Li. 2020. "Antitumor Activity and Mechanism of Robustic Acid from Dalbergia benthami Prain via Computational Target Fishing" Molecules 25, no. 17: 3919. https://doi.org/10.3390/molecules25173919
APA StyleHuang, J., Liang, Y., Tian, W., Ma, J., Huang, L., Li, B., Chen, R., & Li, D. (2020). Antitumor Activity and Mechanism of Robustic Acid from Dalbergia benthami Prain via Computational Target Fishing. Molecules, 25(17), 3919. https://doi.org/10.3390/molecules25173919