Synthesis, In Silico and In Vitro Evaluation for Acetylcholinesterase and BACE-1 Inhibitory Activity of Some N-Substituted-4-Phenothiazine-Chalcones
Abstract
1. Introduction
2. Results and Discussion
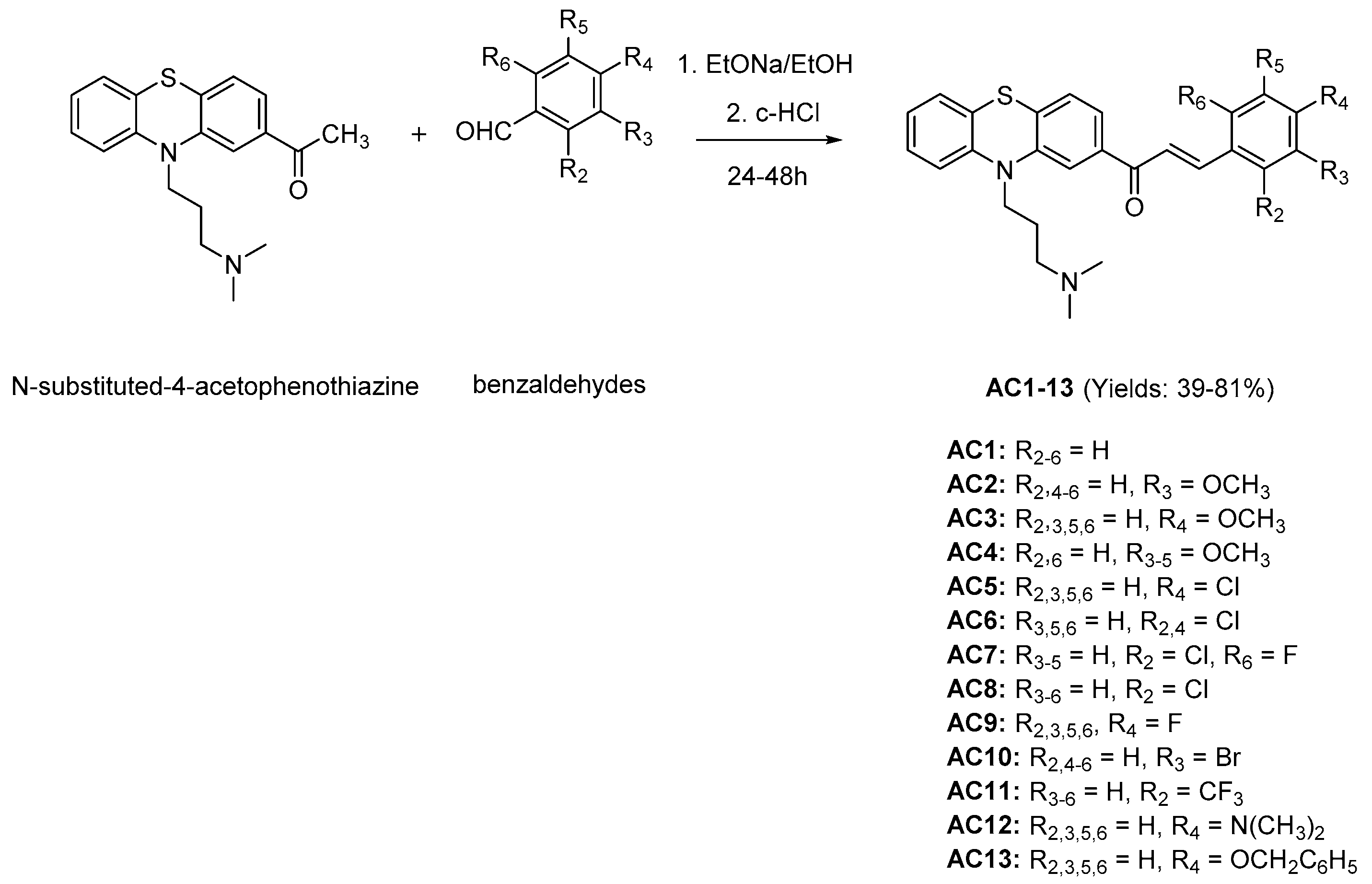
2.1. Chemistry
2.2. In Vitro Assays
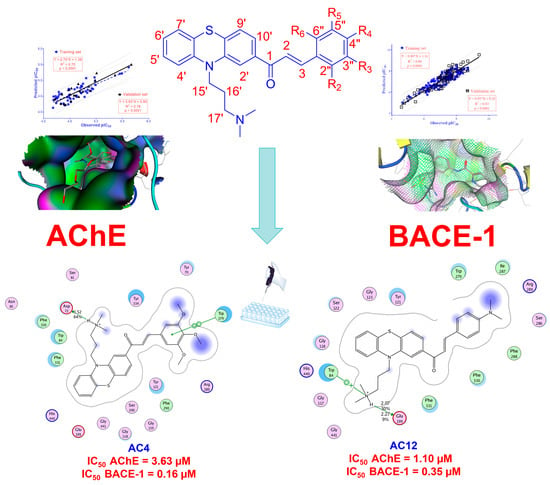
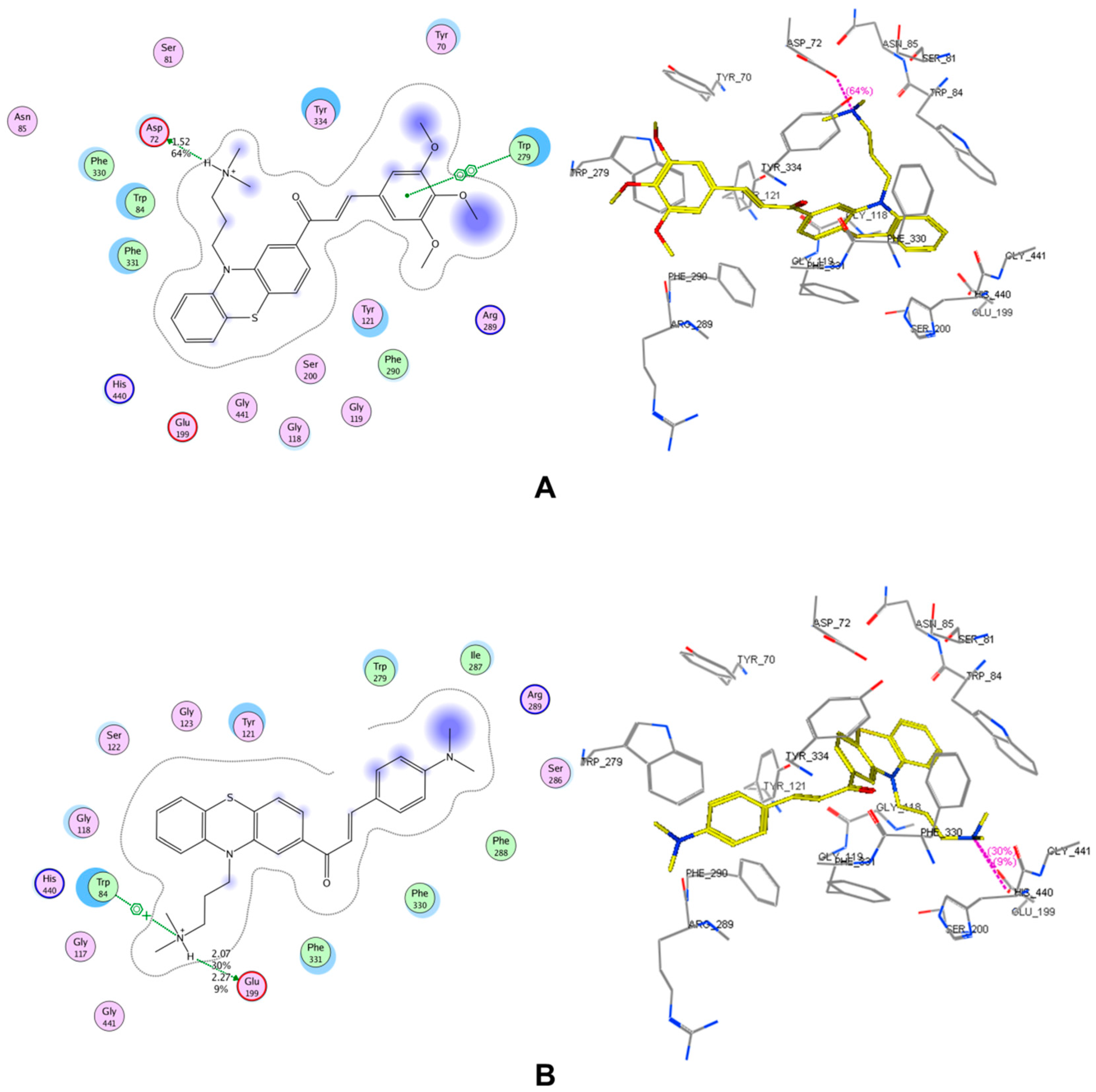
2.3. Molecular Docking
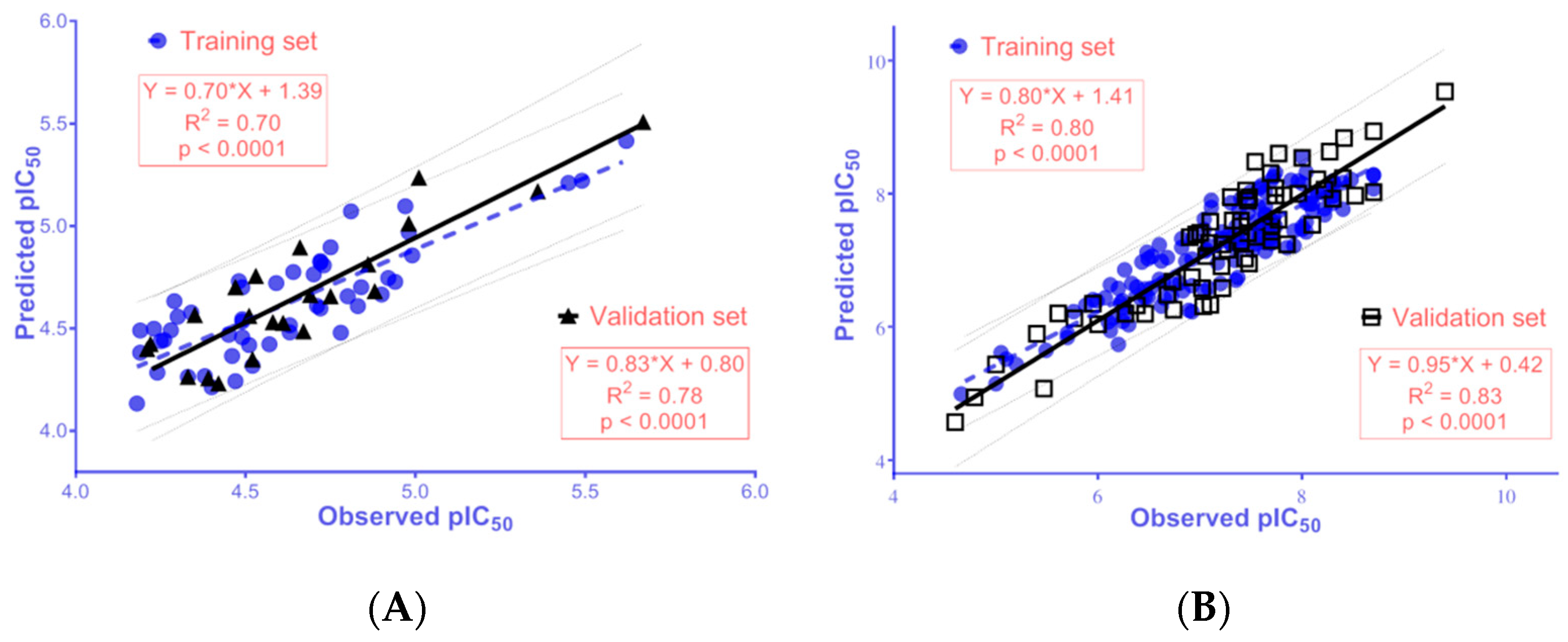
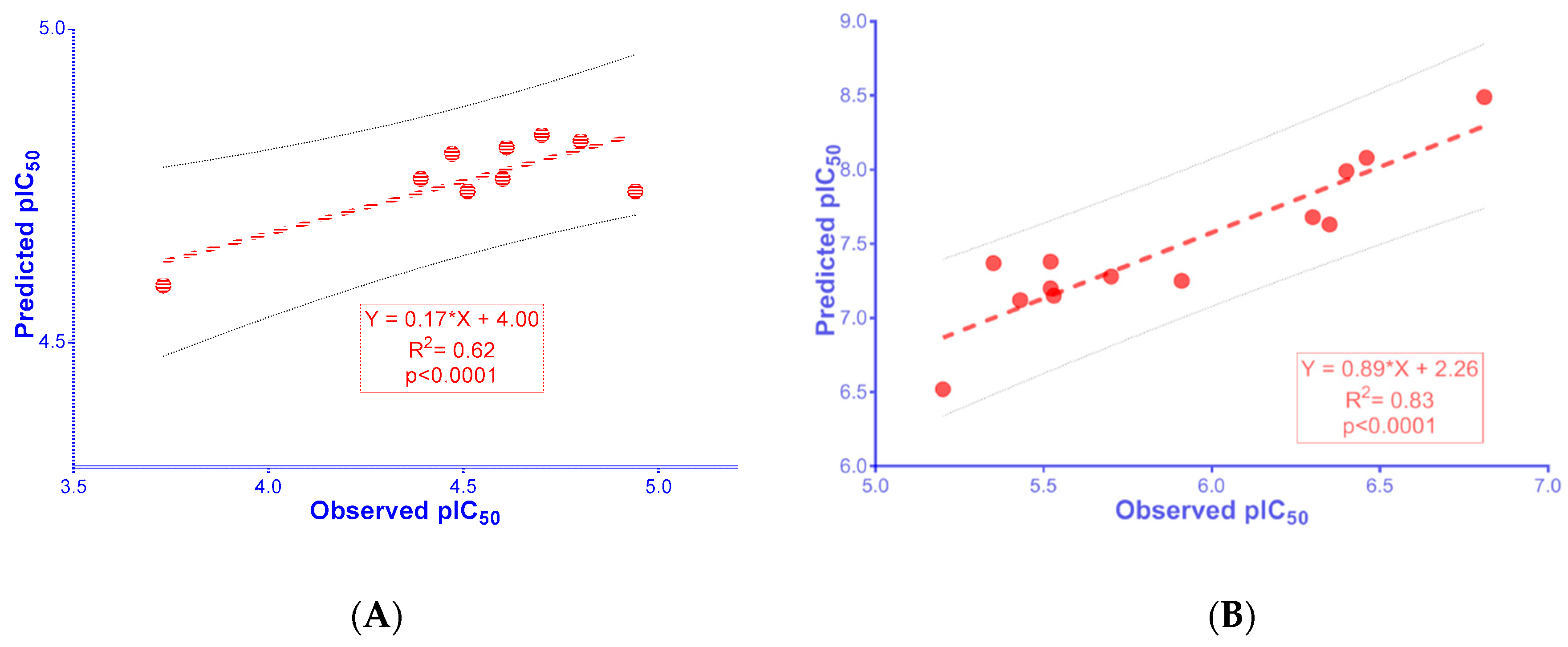
2.4. 2D-QSAR Models
3. Materials and Methods
3.1. Material and Instruments
3.2. Chemistry
3.3. Acetylcholinesterase Inhibitory Activity Assay
3.4. β-secretase Inhibitory Activity Assay
3.5. Building 2D-QSAR Model
3.5.1. Data Collection and Ligand Preparation
3.5.2. Molecular Descriptors Calculation and Processing
3.5.3. Database Division into Training Set and Validation Set
3.5.4. Model Building and Validation
3.6. Molecular Docking Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kim, M.; Park, H.E.; Lee, S.-H.; Han, K.; Lee, J.H. Increased risk of Alzheimer’s disease in patients with psoriasis: A nationwide population-based cohort study. Sci. Rep. 2020, 10, 6454. [Google Scholar] [CrossRef]
- Ringman, J.M. Update on Alzheimer’s and the Dementias: Introduction. Neurol. Clin. 2017, 35, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Graham, W.V.; Bonito-Oliva, A.; Sakmar, T.P. Update on Alzheimer’s Disease Therapy and Prevention Strategies. Annu. Rev. Med. 2017, 68, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Getsios, D.; Revankar, N.; Xu, P.; Thompson, G.; Bobula, J.; Lacey, L.; Gaudig, M. Evaluating disease-modifying agents: A simulation framework for Alzheimer’s disease. Pharmacoeconomics 2014, 32, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association 2015. Alzheimer’s disease facts and figures. Alzheimers Dement. 2015, 11, 332–384. [Google Scholar]
- Lalut, J.; Payan, H.; Davis, A.; Lecoutey, C.; Legay, R.; Santos, J.S.-D.O.; Claeysen, S.; Dallemagne, P.; Rochais, C. Rational design of novel benzisoxazole derivatives with acetylcholinesterase inhibitory and serotoninergic 5-HT4 receptors activities for the treatment of Alzheimer’s disease. Sci. Rep. 2020, 10, 3014. [Google Scholar] [CrossRef]
- Blazer, L.L.; Neubig, R.R. Small molecule protein-protein interaction inhibitors as CNS therapeutic agents: Current progress and future hurdles. Neuropsychopharmacology 2009, 34, 126–141. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef]
- Tumiatti, V.; Minarini, A.; Bolognesi, M.L.; Milelli, A.; Rosini, M.; Melchiorre, C. Tacrine derivatives and Alzheimer’s disease. Curr. Med. Chem. 2010, 17, 1825–1838. [Google Scholar] [CrossRef]
- Gong, C.X.; Liu, F.; Iqbal, K. Multifactorial Hypothesis and Multi-Targets for Alzheimer’s Disease. J. Alzheimers Dis. 2018, 64, S107–S117. [Google Scholar] [CrossRef]
- Rees, T.M.; Brimijoin, S. The role of acetylcholinesterase in the pathogenesis of Alzheimer’s disease. Drugs Today 2003, 39, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R. BACE1: The beta-secretase enzyme in Alzheimer’s disease. J. Mol. Neurosci. 2004, 23, 105–114. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, M.; Kumar, A.; Singh, S.K. Chalcone and curcumin derivatives: A way ahead for malarial treatment. Mini Rev. Med. Chem. 2013, 13, 2116–2133. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Anand, A.; Kumar, V. Recent developments in biological activities of chalcones: A mini review. Eur. J. Med. Chem. 2014, 85, 758–777. [Google Scholar] [CrossRef]
- Lunardi, F.; Guzela, M.; Rodrigues, A.T.; Corrêa, R.; Eger-Mangrich, I.; Steindel, M.; Grisard, E.C.; Assreuy, J.; Calixto, J.B.; Santos, A.R.S. Trypanocidal and Leishmanicidal Properties of Substitution-Containing Chalcones. Antimicrob. Agents Chemother. 2003, 47, 1449–1451. [Google Scholar] [CrossRef]
- De Mello, T.F.; Cardoso, B.M.; Lopes, S.N.; Bitencourt, H.R.; Voltarelli, E.M.; Hernandes, L.; Aristides, S.M.; Lonardoni, M.V.; Silveira, T.G. Activity of synthetic chalcones in hamsters experimentally infected with Leishmania (Viannia) braziliensis. Parasitol. Res. 2015, 114, 3587–3600. [Google Scholar] [CrossRef]
- Passalacqua, T.G.; Dutra, L.A.; de Almeida, L.; Velasquez, A.M.; Torres, F.A.; Yamasaki, P.R.; dos Santos, M.B.; Regasini, L.O.; Michels, P.A.; Bolzani, V.D.S.; et al. Synthesis and evaluation of novel prenylated chalcone derivatives as anti-leishmanial and anti-trypanosomal compounds. Bioorg. Med. Chem. Lett. 2015, 25, 3342–3345. [Google Scholar] [CrossRef]
- Shakhatreh, M.A.; Al-Smadi, M.L.; Khabour, O.F.; Shuaibu, F.A.; Hussein, E.I.; Alzoubi, K.H. Study of the antibacterial and antifungal activities of synthetic benzyl bromides, ketones, and corresponding chalcone derivatives. Drug Des. Dev. Ther. 2016, 10, 3653–3660. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Chi, K.Q.; Yu, Z.K.; Liu, H.Y.; Sun, L.P.; Zheng, C.J.; Piao, H.R. Synthesis and biological evaluation of chalcone derivatives containing aminoguanidine or acylhydrazone moieties. Bioorg. Med. Chem. Lett. 2016, 26, 5920–5925. [Google Scholar] [CrossRef]
- Evranos-Aksoz, B.; Onurdag, F.K.; Ozgacar, S.O. Antibacterial, antifungal and antimycobacterial activities of some pyrazoline, hydrazone and chalcone derivatives. Zeitschrift für Naturforschung C 2015, 70, 183–189. [Google Scholar] [CrossRef]
- Tran, T.D.; Do, T.H.; Tran, N.C.; Ngo, T.D.; Huynh, T.N.; Tran, C.D.; Thai, K.M. Synthesis and anti Methicillin resistant Staphylococcus aureus activity of substituted chalcones alone and in combination with non-beta-lactam antibiotics. Bioorg. Med. Chem. Lett. 2012, 22, 4555–4560. [Google Scholar] [CrossRef]
- Yin, B.T.; Yan, C.Y.; Peng, X.M.; Zhang, S.L.; Rasheed, S.; Geng, R.X.; Zhou, C.H. Synthesis and biological evaluation of alpha-triazolyl chalcones as a new type of potential antimicrobial agents and their interaction with calf thymus DNA and human serum albumin. Eur. J. Med. Chem. 2014, 71, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Dong, H.H.; Zhao, F.; Wang, J.; Yan, F.; Jiang, Y.Y.; Jin, Y.S. The synthesis and synergistic antifungal effects of chalcones against drug resistant Candida albicans. Bioorg. Med. Chem. Lett. 2016, 26, 3098–3102. [Google Scholar] [CrossRef] [PubMed]
- Lahtchev, K.L.; Batovska, D.I.; Parushev, S.P.; Ubiyvovk, V.M.; Sibirny, A.A. Antifungal activity of chalcones: A mechanistic study using various yeast strains. Eur. J. Med. Chem. 2008, 43, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Mateeva, N.; Eyunni, S.V.K.; Redda, K.K.; Ononuju, U.; Hansberry, T.D., II; Aikens, C.; Nag, A. Functional evaluation of synthetic flavonoids and chalcones for potential antiviral and anticancer properties. Bioorg. Med. Chem. Lett. 2017, 27, 2350–2356. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Patil, V.; Patil, R.; Beaman, K.; Patil, S.A. Identification of novel 5,6-dimethoxyindan-1-one derivatives as antiviral agents. Med. Chem. Med. Chem. 2017, 13, 787–795. [Google Scholar] [CrossRef]
- Lee, J.S.; Bukhari, S.N.; Fauzi, N.M. Effects of chalcone derivatives on players of the immune system. Drug Des. Dev. Ther. 2015, 9, 4761–4778. [Google Scholar] [CrossRef]
- Sivakumar, P.M.; Prabhakar, P.K.; Doble, M. Synthesis, antioxidant evaluation, and quantitative structure–activity relationship studies of chalcones. Med. Chem. Res. 2011, 20, 482–492. [Google Scholar] [CrossRef]
- Yamali, C.; Gul, H.I.; Ozgun, D.O.; Hiroshi, S.S.; Umemura, N.; Kazaz, C.; Gul, M. Synthesis and Cytotoxic Activities of Difluoro-Dimethoxy Chalcones. Anticancer Agents Med. Chem. 2017, 17. [Google Scholar] [CrossRef]
- Sakagami, H.; Masuda, Y.; Tomomura, M.; Yokose, S.; Uesawa, Y.; Ikezoe, N.; Asahara, D.; Takao, K.; Kanamoto, T.; Terakubo, S.; et al. Quantitative Structure-Cytotoxicity Relationship of Chalcones. Anticancer Res. 2017, 37, 1091–1098. [Google Scholar] [CrossRef]
- Echeverria, C.; Santibanez, J.F.; Donoso-Tauda, O.; Escobar, C.A.; Ramirez-Tagle, R. Structural antitumoral activity relationships of synthetic chalcones. Int. J. Mol. Sci. 2009, 10, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, A.; Altintop, M.D.; Turan-Zitouni, G.; Ciftci, G.A.; Ertorun, I.; Alatas, O.; Kaplancikli, Z.A. Synthesis and evaluation of new indole-based chalcones as potential antiinflammatory agents. Eur. J. Med. Chem. 2015, 89, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Bukhari, S.N.A.; Adekoya, O.A.; Sylte, I. Studies of synthetic chalcone derivatives as potential inhibitors of secretory phospholipase A(2), cyclooxygenases, lipoxygenase and pro-inflammatory cytokines. Drug Des. Dev. Ther. 2014, 8, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, K.R.; Elshemy, H.A.; Salama, S.A.; Omar, H.A. Synthesis, characterization and biological evaluation of novel 4’-fluoro-2’-hydroxy-chalcone derivatives as antioxidant, anti-inflammatory and analgesic agents. J. Enzym. Inhib. Med. Chem. 2015, 30, 484–491. [Google Scholar] [CrossRef][Green Version]
- Heidari, M.R.; Foroumadi, A.; Noroozi, H.; Samzadeh-Kermani, A.; Azimzadeh, B.S. Study of the anti-inflammatory and analgesic effects of novel rigid benzofuran-3, 4-dihydroxy chalcone by formalin, hot-plate and carrageenan tests in mice. Pak. J. Pharm Sci. 2009, 22, 395–401. [Google Scholar]
- Ismail, N.I.; Ming-Tatt, L.; Lajis, N.; Akhtar, M.N.; Akira, A.; Perimal, E.K.; Israf, D.A.; Sulaiman, M.R. Antinociceptive Effect of 3-(2,3-Dimethoxyphenyl)-1-(5-methylfuran-2-yl)prop-2-en-1-one in Mice Models of Induced Nociception. Molecules 2016, 21, 1077. [Google Scholar] [CrossRef]
- Dhiyaaldeen, S.M.; Amin, Z.A.; Darvish, P.H.; Mustafa, I.F.; Jamil, M.M.; Rouhollahi, E.; Abdulla, M.A. Protective effects of (1-(4-hydroxy-phenyl)-3-m-tolyl-propenone chalcone in indomethacin-induced gastric erosive damage in rats. BMC Vet. Res. 2014, 10, 961. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Avula, S.R.; Mishra, V.; Palnati, G.R.; Singh, L.R.; Singh, N.; Chhonker, Y.S.; Swami, P.; Bhatta, R.S.; Palit, G. Identification of quinoline-chalcone hybrids as potential antiulcer agents. Eur. J. Med. Chem. 2015, 89, 638–653. [Google Scholar] [CrossRef]
- Ansari, F.L.; Umbreen, S.; Hussain, L.; Makhmoor, T.; Nawaz, S.A.; Lodhi, M.A.; Khan, S.N.; Shaheen, F.; Choudhary, M.I. Syntheses and biological activities of chalcone and 1,5-benzothiazepine derivatives: Promising new free-radical scavengers, and esterase, urease, and alpha-glucosidase inhibitors. Chem. Biodivers. 2005, 2, 487–496. [Google Scholar] [CrossRef]
- Seo, W.D.; Kim, J.H.; Kang, J.E.; Ryu, H.W.; Curtis-Long, M.J.; Lee, H.S.; Yang, M.S.; Park, K.H. Sulfonamide chalcone as a new class of alpha-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 5514–5516. [Google Scholar] [CrossRef]
- Hasan, A.; Khan, K.M.; Sher, M.; Maharvi, G.M.; Nawaz, S.A.; Choudhary, M.I.; Atta ur, R.; Supuran, C.T. Synthesis and inhibitory potential towards acetylcholinesterase, butyrylcholinesterase and lipoxygenase of some variably substituted chalcones. J. Enzym. Inhib. Med. Chem. 2005, 20, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Najafian, M.; Ebrahim-Habibi, A.; Hezareh, N.; Yaghmaei, P.; Parivar, K.; Larijani, B. Trans-chalcone: A novel small molecule inhibitor of mammalian alpha-amylase. Mol. Biol. Rep. 2011, 38, 1617–1620. [Google Scholar] [CrossRef] [PubMed]
- Kashani-Amin, E.; Larijani, B.; Ebrahim-Habibi, A. Neohesperidin dihydrochalcone: Presentation of a small molecule activator of mammalian alpha-amylase as an allosteric effector. FEBS Lett. 2013, 587, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, E.; Webster, J.; Do, T.; Kline, R.; Snider, L.; Hauser, Q.; Higginbottom, G.; Campbell, A.; Ma, L.; Paula, S. Hydroxylated chalcones with dual properties: Xanthine oxidase inhibitors and radical scavengers. Bioorg. Med. Chem. 2016, 24, 578–587. [Google Scholar] [CrossRef]
- Mathew, B.; Mathew, G.E.; Ucar, G.; Joy, M.; Nafna, E.K.; Suresh, J. Monoamine oxidase inhibitory activity of methoxy-substituted chalcones. Int. J. Boil. Macromol. 2017, 104, 1321–1329. [Google Scholar] [CrossRef]
- Mathew, B.; Mathew, G.E.; Ucar, G.; Baysal, I.; Suresh, J.; Mathew, S.; Haridas, A.; Jayaprakash, V. Potent and Selective Monoamine Oxidase-B Inhibitory Activity: Fluoro- vs. Trifluoromethyl-4-hydroxylated Chalcone Derivatives. Chem. Biodivers. 2016, 13, 1046–1052. [Google Scholar] [CrossRef]
- Minders, C.; Petzer, J.P.; Petzer, A.; Lourens, A.C. Monoamine oxidase inhibitory activities of heterocyclic chalcones. Bioorg. Med. Chem. Lett. 2015, 25, 5270–5276. [Google Scholar] [CrossRef]
- Liu, H.R.; Liu, X.J.; Fan, H.Q.; Tang, J.J.; Gao, X.H.; Liu, W.K. Design, synthesis and pharmacological evaluation of chalcone derivatives as acetylcholinesterase inhibitors. Bioorg. Med. Chem. 2014, 22, 6124–6133. [Google Scholar] [CrossRef]
- Liu, H.R.; Huang, X.Q.; Lou, D.H.; Liu, X.J.; Liu, W.K.; Wang, Q.A. Synthesis and acetylcholinesterase inhibitory activity of Mannich base derivatives flavokawain B. Bioorg. Med. Chem. Lett. 2014, 24, 4749–4753. [Google Scholar] [CrossRef]
- Shah, M.S.; Khan, S.U.; Ejaz, S.A.; Afridi, S.; Rizvi, S.U.; Najam-Ul-Haq, M.; Iqbal, J. Cholinesterases inhibition and molecular modeling studies of piperidyl-thienyl and 2-pyrazoline derivatives of chalcones. Biochem. Biophys. Res. Commun. 2017, 482, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Ghosh, S.; Tulsan, R.; Sood, A.; Zhou, W.; Schifone, C.; Foster, M.; LeVine, H., III; Torok, B.; Torok, M. Design, synthesis and biological activity of multifunctional alpha,beta-unsaturated carbonyl scaffolds for Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2013, 23, 2614–2618. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fan, H.; Gao, X.; Huang, X.; Liu, X.; Liu, L.; Zhou, C.; Tang, J.; Wang, Q.; Liu, W. Design, synthesis and preliminary structure-activity relationship investigation of nitrogen-containing chalcone derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors: A further study based on Flavokawain B Mannich base derivatives. J. Enzym. Inhib. Med. Chem. 2016, 31, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.E.; Cho, J.K.; Curtis-Long, M.J.; Ryu, H.W.; Kim, J.H.; Kim, H.J.; Yuk, H.J.; Kim, D.W.; Park, K.H. Inhibitory evaluation of sulfonamide chalcones on β-secretase and acylcholinesterase. Molecules 2012, 18, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, Z.; Li, C.; Zhu, Z.; Shen, X.; Hu, L. Design, synthesis and SAR study of hydroxychalcone inhibitors of human beta-secretase (BACE1). J. Enzym. Inhib. Med. Chem. 2011, 26, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Comprehensive Organic Name Reactions and Reagents; John Wiley & Sons: New York, NY, USA, 2010. [Google Scholar]
- Balkrishna, A.; Pokhrel, S.; Tomer, M.; Verma, S.; Kumar, A.; Nain, P.; Gupta, A.; Varshney, A. Anti-Acetylcholinesterase Activities of Mono-Herbal Extracts and Exhibited Synergistic Effects of the Phytoconstituents: A Biochemical and Computational Study. Molecules 2019, 24, 4175. [Google Scholar] [CrossRef]
- Paudel, P.; Seong, S.H.; Zhou, Y.; Ha, M.T.; Min, B.S.; Jung, H.A.; Choi, J.S. Arylbenzofurans from the Root Bark of Morus alba as Triple Inhibitors of Cholinesterase, β-Site Amyloid Precursor Protein Cleaving Enzyme 1, and Glycogen Synthase Kinase-3β: Relevance to Alzheimer’s Disease. ACS Omega 2019, 4, 6283–6294. [Google Scholar] [CrossRef]
- Jannat, S.; Balupuri, A.; Hong, S.S.; Choi, C.; Choi, Y.-H.; Ku, J.-M.; Kim, W.; Leem, J.; Kim, J.; Shrestha, A.; et al. Inhibition of β-site amyloid precursor protein cleaving enzyme 1 and cholinesterases by pterosins via a specific structure−activity relationship with a strong BBB permeability. Exp. Mol. Med. 2019, 51. [Google Scholar] [CrossRef]
- Sussman, J.L.; Silman, I. Acetylcholinesterase: Structure and use as a model for specific cation-protein interactions. Curr. Opin. Struct. Biol. 1992, 2, 721–729. [Google Scholar] [CrossRef]
- ChEMBL Database. Available online: https://www.ebi.ac.uk/chembl/ (accessed on 20 May 2019).
- Beswick, P.; Charrier, N.; Clarke, B.; Demont, E.; Dingwall, C.; Dunsdon, R.; Faller, A.; Gleave, R.; Hawkins, J.; Hussain, I.; et al. BACE-1 inhibitors part 3: Identification of hydroxy ethylamines (HEAs) with nanomolar potency in cells. Bioorg. Med. Chem. Lett. 2008, 18, 1022–1026. [Google Scholar] [CrossRef]
- Charrier, N.; Clarke, B.; Cutler, L.; Demont, E.; Dingwall, C.; Dunsdon, R.; Hawkins, J.; Howes, C.; Hubbard, J.; Hussain, I.; et al. Second generation of BACE-1 inhibitors. Part 1: The need for improved pharmacokinetics. Bioorg. Med. Chem. Lett. 2009, 19, 3664–3668. [Google Scholar] [CrossRef]
- Charrier, N.; Clarke, B.; Cutler, L.; Demont, E.; Dingwall, C.; Dunsdon, R.; Hawkins, J.; Howes, C.; Hubbard, J.; Hussain, I.; et al. Second generation of BACE-1 inhibitors part 3: Towards non hydroxyethylamine transition state mimetics. Bioorg. Med. Chem. Lett. 2009, 19, 3674–3678. [Google Scholar] [CrossRef]
- Chen, S.H.; Lamar, J.; Guo, D.; Kohn, T.; Yang, H.C.; McGee, J.; Timm, D.; Erickson, J.; Yip, Y.; May, P.; et al. P3 cap modified Phe*-Ala series BACE inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.; Demont, E.; Dingwall, C.; Dunsdon, R.; Faller, A.; Hawkins, J.; Hussain, I.; MacPherson, D.; Maile, G.; Matico, R.; et al. BACE-1 inhibitors part 2: Identification of hydroxy ethylamines (HEAs) with reduced peptidic character. Bioorg. Med. Chem. Lett. 2008, 18, 1017–1021. [Google Scholar] [CrossRef]
- Ginman, T.; Viklund, J.; Malmstrom, J.; Blid, J.; Emond, R.; Forsblom, R.; Johansson, A.; Kers, A.; Lake, F.; Sehgelmeble, F.; et al. Core refinement toward permeable beta-secretase (BACE-1) inhibitors with low hERG activity. J. Med. Chem. 2013, 56, 4181–4205. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Kiso, Y. Advances in the identification of beta-secretase inhibitors. Expert Opin. Drug Discov. 2013, 8, 709–731. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.A.; Sun, M.; Bowers, S.; Hom, R.K.; Probst, G.D.; John, V.; Fang, L.Y.; Maillard, M.; Gailunas, A.; Brogley, L.; et al. Design and synthesis of hydroxyethylamine (HEA) BACE-1 inhibitors: Prime side chromane-containing inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 4674–4679. [Google Scholar] [CrossRef]
- Oehlrich, D.; Prokopcova, H.; Gijsen, H.J. The evolution of amidine-based brain penetrant BACE1 inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 2033–2045. [Google Scholar] [CrossRef]
- Weiss, M.M.; Williamson, T.; Babu-Khan, S.; Bartberger, M.D.; Brown, J.; Chen, K.; Cheng, Y.; Citron, M.; Croghan, M.D.; Dineen, T.A.; et al. Design and preparation of a potent series of hydroxyethylamine containing beta-secretase inhibitors that demonstrate robust reduction of central beta-amyloid. J. Med. Chem. 2012, 55, 9009–9024. [Google Scholar] [CrossRef]
- Woltering, T.J.; Wostl, W.; Hilpert, H.; Rogers-Evans, M.; Pinard, E.; Mayweg, A.; Gobel, M.; Banner, D.W.; Benz, J.; Travagli, M.; et al. BACE1 inhibitors: A head group scan on a series of amides. Bioorg. Med. Chem. Lett. 2013, 23, 4239–4243. [Google Scholar] [CrossRef]
- Charrier, N.; Clarke, B.; Demont, E.; Dingwall, C.; Dunsdon, R.; Hawkins, J.; Hubbard, J.; Hussain, I.; Maile, G.; Matico, R.; et al. Second generation of BACE-1 inhibitors, Part 2: Optimisation of the non-prime side substituent. Bioorg. Med. Chem. Lett. 2009, 19, 3669–3673. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Jiménez, G.; Gonzalez-Ponce, K.; Castillo-Pazos, D.J.; Madariaga-Mazon, A.; Barroso-Flores, J.; Cortes-Guzman, F.; Martinez-Mayorga, K. The OECD Principles for (Q)SAR Models in the Context of Knowledge Discovery in Databases (KDD). Adv. Protein Chem. Struct. Biol. 2018, 113, 85–117. [Google Scholar] [CrossRef] [PubMed]
- Ojha, P.K.; Mitra, I.; Das, R.N.; Roy, K. Further exploring rm2 metrics for validation of QSPR models. Chemom. Intell. Lab. Syst. 2011, 107, 194–205. [Google Scholar] [CrossRef]
- Thai, K.-M.; Bui, Q.-H.; Tran, T.-D.; Huynh, T.-N.-P. QSAR modeling on benzo[c]phenanthridine analogues as topoisomerase I inhibitors and anti-cancer agents. Molecules 2012, 17, 5690–5712. [Google Scholar] [CrossRef] [PubMed]
- Chirico, N.; Gramatica, P. Real external predictivity of QSAR models: How to evaluate it? Comparison of different validation criteria and proposal of using the concordance correlation coefficient. J. Chem. Inf. Model. 2011, 51, 2320–2335. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Ambure, P.; Kar, S. How Precise Are Our Quantitative Structure–Activity Relationship Derived Predictions for New Query Chemicals? ACS Omega 2018, 3, 11392–11406. [Google Scholar] [CrossRef] [PubMed]
- Niraj, R.R.; Saini, V.; Kumar, A. QSAR analyses of organophosphates for insecticidal activity and its in-silico validation using molecular docking study. Environ. Toxicol. Pharmacol. 2015, 40, 886–894. [Google Scholar] [CrossRef]
- Ambure, P.; Roy, K. Understanding the structural requirements of cyclic sulfone hydroxyethylamines as hBACE1 inhibitors against Aβ plaques in Alzheimer’s disease: A predictive QSAR approach. RSC Adv. 2016, 6, 28171–28186. [Google Scholar] [CrossRef]
- Hossain, T.; Islam, M.A.; Pal, R.; Saha, A. Exploring structural requirement and binding interactions of β-amyloid cleavage enzyme inhibitors using molecular modeling techniques. Med. Chem. Res. 2013, 22, 4766–4774. [Google Scholar] [CrossRef]
- Tran, T.-D.N.; Nguyen, V.; Nguyen, N.-S.; Nguyen, D.-M.; Nguyen, T.-T.-H.; Le, M.-T.; Thai, K.-M. Synthesis of Novel Chalcones as Acetylcholinesterase Inhibitors. Appl. Sci. 2016, 6, 198. [Google Scholar] [CrossRef]
- Nuthakki, V.K.; Sharma, A.; Kumar, A.; Bharate, S.B. Identification of embelin, a 3-undecyl-1,4-benzoquinone from Embelia ribes as a multitargeted anti-Alzheimer agent. Drug Dev. Res. 2019, 80, 655–665. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chemical characterization, antioxidant properties, cholinesterase inhibitory and anti-amyloidogenic activities of sulfated polysaccharides from some seaweeds. Bioact. Carbohydr. Diet. Fibre 2019, 18, 100182. [Google Scholar] [CrossRef]
- Chemical Computing Group. MOE 2008.10 edition. Available online: https://www.chemcomp.com/ (accessed on 20 May 2019).
- Sybyl X 2.0. Available online: https://sybyl-x.software.informer.com/2.0/ (accessed on 20 May 2019).
- Demel, M.; Janecek, A.; Thai, K.-M.; Ecker, G.; Gansterer, W. Predictive QSAR Models for Polyspecific Drug Targets: The Importance of Feature Selection. Curr. Comput. Aided Drug Des. 2008, 4, 91–110. [Google Scholar] [CrossRef]
- RapidMiner 5.3.013. Available online: https://rapidminer.com/ (accessed on 20 May 2019).
- Weka Software 3.8. Available online: https://waikato.github.io/weka-wiki/ (accessed on 20 May 2019).
- Ngo, T.D.; Tran, T.D.; Le, M.T.; Thai, K.M. Computational predictive models for P-glycoprotein inhibition of in-house chalcone derivatives and drug-bank compounds. Mol. Divers. 2016, 20, 945–961. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Madi, A.M.; Ali, H.I. Molecular Docking and Prediction of Pharmacokinetic Properties of Dual Mechanism Drugs that Block MAO-B and Adenosine A(2A) Receptors for the Treatment of Parkinson’s Disease. J. Young Pharm. 2012, 4, 184–192. [Google Scholar] [CrossRef]
- Protein Data Bank. Available online: https://www.rcsb.org/ (accessed on 20 May 2019).
- LeadIT 2.0.2. Available online: https://www.biosolveit.de/LeadIT/ (accessed on 20 May 2019).
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef]
- Pagadala, N.S.; Syed, K.; Tuszynski, J. Software for molecular docking: A review. Biophys. Rev. 2017, 9, 91–102. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| AChE | BACE-1 | |||||
|---|---|---|---|---|---|---|
| Compound | IC50 (µM) * | pIC50 | IC50 (µM) * | pIC50 | ||
| Observed * | Predicted | Observed * | Predicted | |||
| AC1 | 33.88 ± 1.45 | 4.47 ± 0.02 | 4.80 | 6.34 ± 0.46 | 5.20 ± 0.03 | 6.52 |
| AC2 | 30.90 ± 2.10 | 4.51 ± 0.03 | 4.74 | 3.00 ± 0.00 | 5.52 ± 0.00 | 7.38 |
| AC3 | 11.48 ± 1.29 | 4.94 ± 0.05 | 4.74 | 4.48 ± 0.40 | 5.35 ± 0.04 | 7.37 |
| AC4 | 3.63± 0.61 | 5.44± 0.08 | 4.71 | 0.16± 0.03 | 6.81± 0.09 | 8.49 |
| AC5 | 60.26 ± 1.84 | 4.22 ± 0.01 | 4.82 | 1.24 ± 0.12 | 5.91 ± 0.04 | 7.25 |
| AC6 | 19.95 ± 1.57 | 4.70 ± 0.03 | 4.83 | 0.50 ± 0.00 | 6.30 ± 0.00 | 7.68 |
| AC7 | 40.74 ± 2.48 | 4.39 ± 0.03 | 4.76 | 0.45 ± 0.04 | 6.35 ± 0.04 | 7.63 |
| AC8 | 15.85 ± 1.08 | 4.80 ± 0.03 | 4.82 | 2.97 ± 0.05 | 5.53 ± 0.01 | 7.15 |
| AC9 | 25.12 ± 0.83 | 4.60 ± 0.01 | 4.76 | 3.72 ± 0.21 | 5.43 ± 0.02 | 7.12 |
| AC10 | 24.55 ± 1.09 | 4.61 ± 0.02 | 4.81 | 1.99 ± 0.21 | 5.70 ± 0.05 | 7.28 |
| AC11 | 186.21 ± 4.52 | 3.73 ± 0.01 | 4.59 | 0.40 ± 0.00 | 6.40 ± 0.00 | 7.99 |
| AC12 | 1.10± 0.24 | 5.96± 0.10 | 4.81 | 0.35± 0.04 | 6.46 ± 0.05 | 8.08 |
| AC13 | 11.75 ± 0.63 | 4.93 ± 0.02 | 4.63 | 3.03 ± 0.21 | 5.52 ± 0.03 | 7.20 |
| Galanthamine | 1.26 ± 0.12 | 5.90 ± 0.04 | 5.14 | - | - | - |
| Quercetin | - | - | - | 9.55 ± 0.37 | 5.02 ± 0.02 | 5.24 |
| Comp. | pIC50 (AChE) | pIC50 (BACE-1) | Docking Score (kJ·mol−1) (AChE: 1DX6) | Docking Score (kJ·mol−1) (BACE-1: 5HU1 chain A, B) | ||
|---|---|---|---|---|---|---|
| Obs. * | Pred. | Obs. * | Pred. | |||
| AC1 | 4.47 ± 0.02 | 4.80 | 5.20 ± 0.03 | 6.52 | −25.83 | −17.82; −17.69 |
| AC2 | 4.51 ± 0.03 | 4.74 | 5.52 ± 0.00 | 7.38 | −26.03 | −17.22; −17.81 |
| AC3 | 4.94 ± 0.05 | 4.74 | 5.35 ± 0.04 | 7.37 | −27.67 | −16.28; −15.37 |
| AC4 | 5.44 ± 0.08 | 4.71 | 6.81 ± 0.09 | 8.49 | −24.49 | −16.71; −13.92 |
| AC5 | 4.22 ± 0.01 | 4.82 | 5.91 ± 0.04 | 7.25 | −17.71 | −20.77; −16.87 |
| AC6 | 4.70 ± 0.03 | 4.83 | 6.30 ± 0.00 | 7.68 | −25.45 | −18.79; −16.73 |
| AC7 | 4.39 ± 0.03 | 4.76 | 6.35 ± 0.04 | 7.63 | −17.94 | −19.51; −16.85 |
| AC8 | 4.80 ± 0.03 | 4.82 | 5.53 ± 0.01 | 7.15 | −26.27 | −20.36; −16.81 |
| AC9 | 4.60 ± 0.01 | 4.76 | 5.43 ± 0.02 | 7.12 | −27.80 | −20.97; −18.06 |
| AC10 | 4.61 ± 0.02 | 4.81 | 5.70 ± 0.05 | 7.28 | −27.30 | −22.51; −20.81 |
| AC11 | 3.73 ± 0.01 | 4.59 | 6.40 ± 0.00 | 7.99 | −25.33 | −19.35; −16.41 |
| AC12 | 5.96 ± 0.10 | 4.81 | 6.46 ± 0.05 | 8.08 | −22.15 | −18.25; −16.18 |
| AC13 | 4.93 ± 0.02 | 4.63 | 5.52 ± 0.03 | 7.20 | −23.30 | −11.50; −14.09 |
| Galantamine | 5.90 ± 0.04 | 5.14 | - | - | −28.53 | - |
| Verubecestat | - | - | - | 7.66 | - | −24.95; −22.43 |
| Quercetin | - | - | 5.02 ± 0.02 | 5.24 | - | −22.23; −23.95 |
| AChE | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pIC50 = −0.928 + (2.348 × BCUT_SLOGP_3) − (0.150 × reactive) − (0.004 × PEOE_VSA + 1) − (0.005 × PEOE_VSA−3) − (0.002 × SlogP_VSA2) − (0.004 × SMR_VSA2) | ||||||||||||
| Internal Validation | External Validation | |||||||||||
| N | RMSE | R2 | RMSELOO | Q2LOO | N | RMSE | R2 | R2(PRED) | CCC | |||
| 50 | 0.18 | 0.70 | 0.22 | 0.57 | 22 | 0.16 | 0.78 | 0.78 | 0.64 | 0.69 | 0.11 | 0.88 |
| BACE-1 | ||||||||||||
| pIC50 = 1.268 + (0.870 × petitjean) + (6.370 × BCUT_PEOE_1) + (3.305 × a_ICM) − (0.478 × chiral_u) + (0.085 × rings) + (0.157 × a_Nn) + (0.006 × PEOE_VSA − 0) + (0.022 × PEOE_VSA − 6) − (0.260 × logS) + (0.009 × SlogP_VSA3) + (0.009 × SlogP_VSA5) | ||||||||||||
| Internal Validation | External Validation | |||||||||||
| N | RMSE | R2 | RMSELOO | Q2LOO | N | RMSE | R2 | R2(PRED) | CCC | |||
| 150 | 0.37 | 0.80 | 0.40 | 0.77 | 65 | 0.41 | 0.83 | 0.81 | 0.79 | 0.76 | 0.05 | 0.91 |
| Source | Model | Training Set | Validation Set | |||
|---|---|---|---|---|---|---|
| N | R2 | Q2 | N | R2PRED | ||
| This study | PLS | 55 | 0.70 | 0.57 | 22 | 0.78 |
| Roy et al. 2018 [77] | MLR | 284 | 0.52–0.74 | 0.50–0.71 | 142 | 0.50–0.63 |
| Niraj et al. 2015 [78] | PLS | 24 | 0.78 | 0.70 | 11 | 0.66 |
| Source | Model | Training Set | Validation Set | |||
|---|---|---|---|---|---|---|
| N | R2 | Q2 | n | R2PRED | ||
| This study | PLS | 150 | 0.80 | 0.77 | 65 | 0.81 |
| Ambure et al. 2016 [79] | PLS | 52 | 0.83 | 0.76 | 22 | 0.81 |
| Ambure et al. 2016 [79] | MLR | 51 | 0.83 | 0.76 | 22 | 0.80 |
| Hossain et al. 2013 [80] | CoMFA | 71 | 1.00 | 0.77 | 35 | 0.77 |
| Hossain et al. 2013 [80] | CoMSIA | 71 | 1.00 | 0.73 | 35 | 0.71 |
| Hossain et al. 2013 [80] | PLS | 71 | 0.94 | 0.79 | 35 | 0.71 |
| Roy et al. 2018 [77] | MLR | 51 | 0.76–0.83 | 0.71–0.76 | 23 | 0.75–0.91 |
| Samples | ATCI | DTNB | Buffer | Chalcone | AChE |
|---|---|---|---|---|---|
| Control blank sample with enzyme (A0E) | + | + | + | − | + |
| Blank sample (A0) | + | + | + | − | − |
| Tested sample (AC) | + | + | + | + | + |
| Blank test sample (A0C) | + | + | + | + | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.-S.; Le, M.-T.; Nguyen, T.-C.-V.; Tran, T.-H.; Tran, T.-D.; Thai, K.-M. Synthesis, In Silico and In Vitro Evaluation for Acetylcholinesterase and BACE-1 Inhibitory Activity of Some N-Substituted-4-Phenothiazine-Chalcones. Molecules 2020, 25, 3916. https://doi.org/10.3390/molecules25173916
Tran T-S, Le M-T, Nguyen T-C-V, Tran T-H, Tran T-D, Thai K-M. Synthesis, In Silico and In Vitro Evaluation for Acetylcholinesterase and BACE-1 Inhibitory Activity of Some N-Substituted-4-Phenothiazine-Chalcones. Molecules. 2020; 25(17):3916. https://doi.org/10.3390/molecules25173916
Chicago/Turabian StyleTran, Thai-Son, Minh-Tri Le, Thi-Cam-Vi Nguyen, The-Huan Tran, Thanh-Dao Tran, and Khac-Minh Thai. 2020. "Synthesis, In Silico and In Vitro Evaluation for Acetylcholinesterase and BACE-1 Inhibitory Activity of Some N-Substituted-4-Phenothiazine-Chalcones" Molecules 25, no. 17: 3916. https://doi.org/10.3390/molecules25173916
APA StyleTran, T.-S., Le, M.-T., Nguyen, T.-C.-V., Tran, T.-H., Tran, T.-D., & Thai, K.-M. (2020). Synthesis, In Silico and In Vitro Evaluation for Acetylcholinesterase and BACE-1 Inhibitory Activity of Some N-Substituted-4-Phenothiazine-Chalcones. Molecules, 25(17), 3916. https://doi.org/10.3390/molecules25173916