Strong Positive Correlation between TSH and Ghrelin in Euthyroid Non-Growth Hormone-Deficient Children with Short Stature
Abstract
1. Introduction
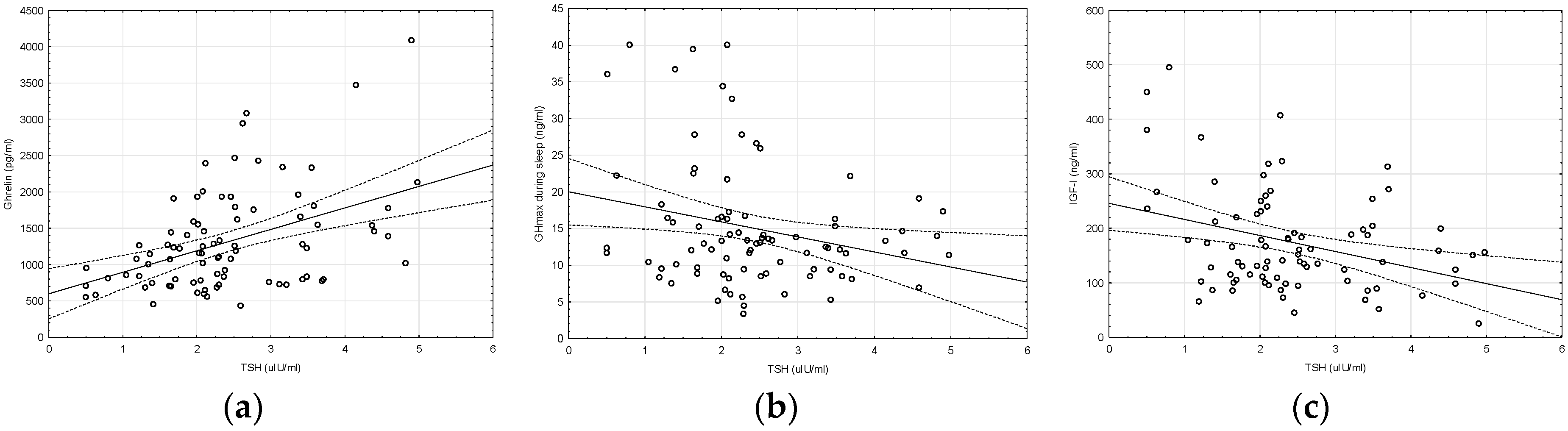
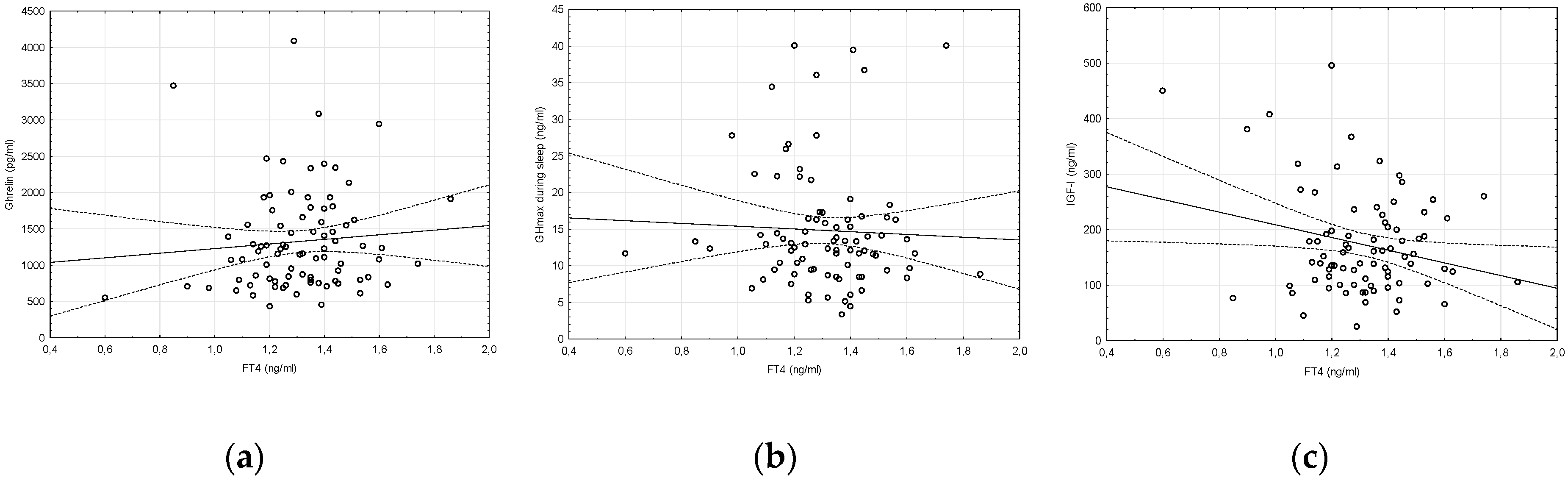
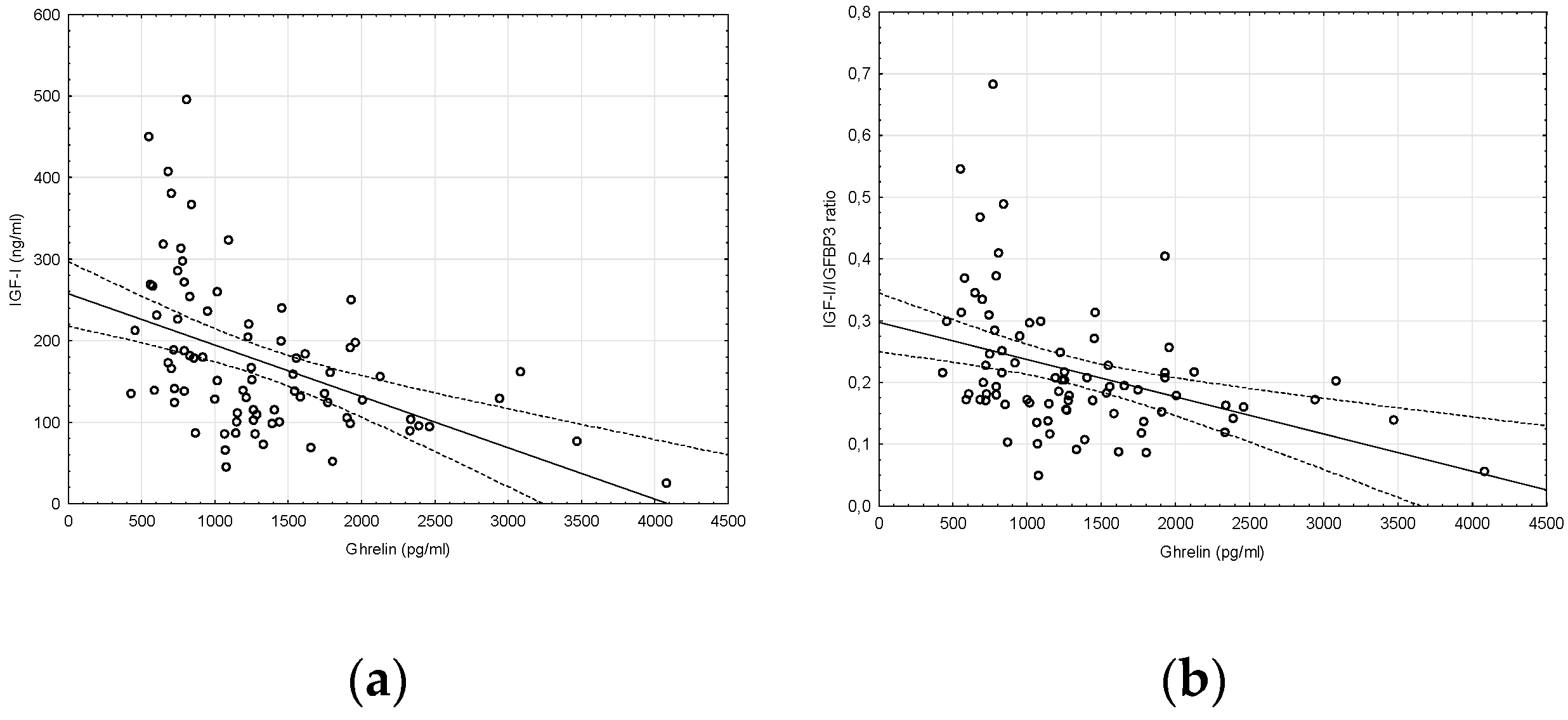
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Álvarez-Nava, F.; Lanes, R. GH/IGF-1 Signaling and current knowledge of epigenetics; a review and considerations on possible therapeutic options. Int. J. Mol. Sci. 2017, 18, 1624. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, J.D.; Bowers, C.Y. Integrating GHS into the ghrelin system. Int. J. Pept. 2010, 2010, 879503. [Google Scholar] [CrossRef] [PubMed]
- Benso, A.; Calvi, E.; Gramaglia, E.; Olivetti, I.; Tomelini, M.; Ghigo, E.; Broglio, F. Other than growth hormone neuroendocrine actions of ghrelin. Endocr. Dev. 2013, 25, 59–68. [Google Scholar] [PubMed]
- Diéguez, C.; Da Boit, K.; Novelle, M.G.; Martínez de Morentin, P.B.; Nogueiras, R.; López, M. New insights in ghrelin orexigenic effect. Front. Horm. Res. 2010, 38, 196–205. [Google Scholar] [PubMed]
- Akin, F.; Yaylali, G.F.; Turgut, S.; Kaptanoglu, B. Growth hormone/insulin-like growth factor axis in patients with subclinical thyroid dysfunction. Growth Horm. IGF Res. 2009, 19, 252–255. [Google Scholar] [CrossRef]
- Rose, S.R. Isolated central hypothyroidism in short stature. Pediatr. Res. 1995, 38, 967–973. [Google Scholar] [CrossRef]
- Jørgensen, J.O.; Møller, J.; Laursen, T.; Orskov, H.; Christiansen, J.S.; Weeke, J. Growth hormone administration stimulates energy expenditure and extrathyroidal conversion of thyroxine to triiodothyronine in a dose-dependent manner and suppresses circadian thyrotrophin levels: Studies in GH-deficient adults. Clin. Endocrinol. 1994, 41, 609–614. [Google Scholar] [CrossRef]
- García, R.J.; Iñiguez, G.; Gaete, X.; Linares, J.; Ocaranza, P.; Avila, A.; Roman, R.; Cassorla, F. Effects of levothyroxine on growth hormone (GH) sensitivity in children with idiopathic short stature. Growth Horm. IGF Res. 2014, 24, 119–122. [Google Scholar] [CrossRef]
- Smyczyńska, J.; Stawerska, R.; Lewiński, A.; Hilczer, M. Do IGF-I concentrations better reflect growth hormone (GH) action in children with short stature than the results of GH stimulating tests? Evidence from the simultaneous assessment of thyroid function. Thyroid Res. 2011, 4, 6. [Google Scholar] [CrossRef]
- Cohen, P.; Rogol, A.D.; Deal, C.L.; Saenger, P.; Reiter, E.O.; Ross, J.L.; Chernausek, S.D.; Savage, M.O.; Wit, J.M. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: A summary of the Growth Hormone Research Society. the Lawson Wilkins Pediatric Endocrine Society. and the European Society for Paediatric Endocrinology Workshop. J. Clin. Endocrinol. Metab. 2008, 93, 4210–4217. [Google Scholar] [CrossRef]
- Griffin, I.J. Catch-up growth: Basic mechanisms. Nestle Nutr. Inst. Workshop Ser. 2015, 81, 87–97. [Google Scholar] [PubMed]
- Blum, W.F.; Böttcher, C.; Wudy, S.A. Insulin-like growth factors and their binding proteins. In Diagnostics of Endocrine Function in Children and Adolescents, 4th ed.; Ranke, M.B., Mullis, P.E., Eds.; Karger: Basel, Switzerland, 2011; pp. 157–182. [Google Scholar]
- Stawerska, R.; Smyczyńska, J.; Czkwianianc, E.; Pisarek, H.; Hilczer, M.; Lewiński, A. Ghrelin concentration is correlated with IGF-I/IGFBP-3 molar ratio but not with GH secretion in children with short stature. Neuroendocrinol. Lett. 2012, 33, 412–418. [Google Scholar] [PubMed]
- Stawerska, R.; Smyczynska, J.; Czkwianianc, E.; Hilczer, M.; Lewinski, A. High concentration of ghrelin in children with growth hormone deficiency and neurosecretory dysfunction. Neuroendocrinol. Lett. 2012, 33, 331–339. [Google Scholar]
- Gurgul, E.; Ruchała, M.; Kosowicz, J.; Zamysłowska, H.; Wrotkowska, E.; Moczko, J.; Sowiński, J. Ghrelin and obestatin in thyroid dysfunction. Endokrynol. Pol. 2012, 63, 456–462. [Google Scholar] [PubMed]
- Ruchala, M.; Gurgul, E.; Stangierski, A.; Wrotkowska, E.; Moczko, J. Individual plasma ghrelin changes in the same patients in hyperthyroid. hypothyroid and euthyroid state. Peptides 2014, 51, 31–34. [Google Scholar] [CrossRef]
- El Gawad, S.S.; El Kenawy, F.; Mousa, A.A.; Omar, A.A. Plasma levels of resistin and ghrelin before and after treatment in patients with hyperthyroidism. Endocr Pract 2012, 18, 376–381. [Google Scholar] [CrossRef]
- Chang, Y.J.; Hwu, C.M.; Yeh, C.C.; Wang, P.S.; Wang, S.W. Effects of subacute hypothyroidism on metabolism and growth-related molecules. Molecules 2014, 19, 11178–11195. [Google Scholar] [CrossRef]
- Stawerska, R.; Czkwianianc, E.; Smyczyńska, J.; Hilczer, M.; Lewiński, A. Nutritional status in short stature children is related to both ghrelin and insulin-like growth factor I concentrations. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 812–817. [Google Scholar] [CrossRef]
- Sawicka, B.; Bossowski, A.; Urban, M.; Szalecki, M.; Wysocka, J.; Koput, A.; Zelazowska-Rutkowska, B.; Tobolczyk, J.; Skrzydło, M.; Rogowski, F.; et al. Analysis of serum levels of ghrelin and obestatin in children and adolescents with autoimmune thyroid diseases. Pediatr. Endocrinol. Diabetes Metab. 2009, 15, 20–27. (In Polish) [Google Scholar]
- Reichenbach, A.; Steyn, F.J.; Sleeman, M.W.; Andrews, Z.B. Ghrelin receptor expression and colocalization with anterior pituitary hormones using a GHSR-GFP mouse line. Endocrinology 2012, 153, 5452–5466. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Leproult, R.; Scherberg, N.; Van Cauter, E. Twenty-four-hour profiles of acylated and total ghrelin: Relationship with glucose levels and impact of time of day and sleep. J. Clin. Endocrinol. Metab. 2011, 96, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Sangiao-Alvarellos, S.; Cordido, F. Effect of ghrelin on glucose-insulin homeostasis: Therapeutic implications. Int. J. Pept. 2010, 2010, 234709. [Google Scholar] [CrossRef] [PubMed]
- Otto, B.; Cuntz, U.; Fruehauf, E.; Wawarta, R.; Folwaczny, C.; Riepl, R.L.; Heiman, M.L.; Lehnert, P.; Fichter, M.; Tschöp, M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur. J. Endocrinol. 2001, 145, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Haqq, A.M.; Farooqi, I.S.; O’Rahilly, S.; Stadler, D.D.; Rosenfeld, R.G.; Pratt, K.L.; LaFranchi, S.H.; Purnell, J.Q. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 174–178. [Google Scholar] [CrossRef]
- Barington, M.; Brorson, M.M.; Hofman-Bang, J.; Rasmussen, Å.K.; Holst, B.; Feldt-Rasmussen, U. Ghrelin-mediated inhibition of the TSH-stimulated function of differentiated human thyrocytes ex vivo. PLoS ONE 2017, 12, e0184992. [Google Scholar] [CrossRef]
- Boulanger, L.; Andersen, P.H.; Gaudreau, P. Development of a site-directed polyclonal antibody against the pituitary growth hormone-releasing hormone receptor and its use to estimate GHRH receptor concentration in normal and hypothyroid rats. Neuroendocrinology 1999, 70, 117–127. [Google Scholar] [CrossRef]
- Palczewska, I.; Niedźwiecka, Z. Indices of somatic development of Warsaw children and adolescents. Medycyna Wieku Rozwojowego 2001, 5 (Suppl. S1), 2. (In Polish) [Google Scholar]
- Tanner, J.M.; Whitehouse, R.H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch. Dis. Child. 1976, 51, 170–179. [Google Scholar] [CrossRef]
- Grimberg, A.; DiVall, S.A.; Polychronakos, C.; Allen, D.B.; Cohen, L.E.; Quintos, J.B.; Rossi, W.C.; Feudtner, C.; Murad, M.H. Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: Growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm. Res. Paediatr. 2016, 86, 361–397. [Google Scholar] [CrossRef]
- GH Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone deficiency in childhood and adolescence: Summary statement of the GH Research Society. J. Clin. Endocrinol. Metab. 2000, 85, 3990–3993. [Google Scholar]
- Cianfarani, S.; Liguori, A.; Germani, D. IGF-I and IGFBP-3 assessment in the management of childhood onset growth hormone deficiency. Endocr. Dev. 2005, 9, 66–75. [Google Scholar]
- Juul, A.; Dalgaard, P.; Blum, W.F.; Bang, P.; Hall, K.; Michaelsen, K.F.; Müller, J.; Skakkebaek, N. Serum levels of insulin-like growth factor (IGF) binding protein 3 (IGFBP-3) in healthy infants, children and adolescents: The relation to IGF-I, IGF-II, IGFBP-1, IGFBP-2, age, sex, body mass index, and pubertal maturation. J. Clin. Endocrinol. Metab. 1995, 80, 2534–2542. [Google Scholar] [PubMed]
- Lau, C.H.; Muniandy, S. Novel adiponectin-resistin (AR) and insulin resistance (IRAR) indexes are useful integrated diagnostic biomarkers for insulin resistance, type 2 diabetes and metabolic syndrome: A case control study. Cardiovasc. Diabetol. 2011, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, F.; Iannello, S.; Volpicelli, G. Insulin sensitivity indices calculated from basal and OGTT-induced insulin, glucose and FFA levels. Mol. Gen. Metab. 1998, 63, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Stawerska, R.; Zawodniak-Szalapska, M.; Cypryk, K.; Lukamowicz, J.; Lewiński, A. Glucose and insulin concentrations during oral glucose tolerance test in healthy children-application of insulin resistance index according to Belfiore in the developmental age. Endokrynol. Diabetol. Chor. Przemiany. Materii. Wieku. Rozw. 2006, 12, 251–256. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| Lower Normal TSH TSH < 2.29 µIU/mL | Higher Normal TSH TSH ≥ 2.29 µIU/mL | p | |
|---|---|---|---|
| N (f/m) | 43 (22/21) | 42 (14/28) | |
| CA (years) | 9.43 ± 2.98 | 8.85 ± 2.87 | 0.1147 |
| Height SDS | −2.65 ± 0.92 | −2.43 ± 0.69 | 0.2135 |
| BMI SDS | −0.30 ± 1.09 | −0.39 ± 0.71 | 0.6395 |
| TSH (µIU/mL) | 1.65 ± 0.51 * | 3.22 ± 0.82 * | 0.0000 |
| FT4 (ng/mL) | 1.30 ± 0.22 | 1.32 ± 0.16 | 0.6020 |
| FT3 (pg/dL) | 3.89 ± 0.56 | 4.16 ± 0.37 | 0.1993 |
| FT4/FT3 ratio | 0.34 ± 0.05 | 0.34 ± 0.04 | 0.7459 |
| GHmax after clonidine (ng/mL) | 19.56 ± 10.33 | 16.67 ± 7.91 | 0.1548 |
| GHmax after glucagone (ng/mL) | 12.15 ± 7.65 | 10.31 ± 7.09 | 0.2757 |
| GHmax during sleep (ng/mL) | 17.56 ± 10.31 * | 12.46 ± 4.91 * | 0.0048 |
| IGF-I (ng/mL) | 198.40 ± 107.33 * | 148.13 ± 67.42 * | 0.0144 |
| IGFBP-3 (µg/dL) | 4.59 ± 0.98 | 4.40 ± 1.58 | 0.5041 |
| IGF-I/IGFBP-3 ratio | 0.24 ± 0.11 | 0.20 ± 0.10 | 0.0944 |
| Ghrelin (pg/mL) | 1062.36 ± 443.43 * | 1578.23 ± 807.37 * | 0.0004 |
| Leptin (ng/mL) | 5.73 ± 8.09 | 3.50 ± 5.39 | 0.1583 |
| Adiponectin (ng/mL) | 18.10 ± 8.41 | 16.72 ± 7.27 | 0.4416 |
| Resistin (ng/mL) | 10.02 ± 3.89 | 10.27 ± 4.22 | 0.7856 |
| AR index | 0.76 ± 0.27 | 0.79 ± 0.25 | 0.5408 |
| Triglycerides (mg/dL) | 71.03 ± 26.54 | 64.57 ± 29.19 | 0.3729 |
| Cholesterol (mg/dL) | 159.73 ± 40.60 | 162.27 ± 29.72 | 0.7761 |
| LDL-cholesterol (mg/dL) | 92.30 ± 36.32 | 89.93 ± 25.52 | 0.7737 |
| HDL-cholesterol (mg/dL) | 57.83 ± 15.32 | 57.39 ± 16.87 | 0.9162 |
| OGTT, n = 28 (f/m) | 12 (6/6) | 16 (8/8) | |
| Glucose 0′ (mg/dL) | 82.32 ± 9.19 | 83.66 ± 9.55 | 0.5481 |
| Glucose 60′ (mg/dL) | 115.25 ± 36.51 | 137.31 ± 49.29 | 0.2039 |
| Glucose 120′ (mg/dL) | 91.00 ± 20.60 * | 120.69 ± 28.73 * | 0.0053 |
| Insulin 0′ (µIU/mL) | 5.72 ± 3.93 * | 3.56 ± 1.90 * | 0.0096 |
| Insulin 60′ (µIU/mL) | 33.34 ± 20.80 | 34.15 ± 16.24 | 0.9081 |
| Insulin 120′ (µIU/mL) | 20.15 ± 12.21 * | 31.04 ± 15.12 * | 0.0481 |
| IRI HOMA | 1.20 ± 0.84 * | 0.76 ± 0.44 * | 0.0158 |
| IRI Belfiore | 0.93 ± 0.37 | 1.05 ± 0.43 | 0.4570 |
| Lower Normal FT4 | Higher Normal FT4 | p | |
|---|---|---|---|
| FT4 < 1.1 ng/mL | FT4 ≥ 1.1 ng/mL | ||
| N | 21 | 64 | |
| CA (years) | 9.80 ± 3.27 | 9.54 ± 2.94 | 0.7382 |
| Height SDS | −2.74 ± 0.52 | −2.49 ± 0.88 | 0.2364 |
| BMI SDS | −0.44 ± 0.93 | −0.31 ± 0.92 | 0.5978 |
| TSH (µIU/mL) | 2.16 ± 1.11 | 2.52 ± 1.02 | 0.1792 |
| FT4 (ng/mL) | 1.07 ± 0.15 | 1.3 ± 0.13 | 0.0000 |
| FT3 (pg/dL) | 3.50 ± 0.71 | 4.13 ± 0.36 | 0.0147 |
| FT4/FT3 ratio | 0.34 ± 0.06 | 0.34 ± 0.04 | 0.9778 |
| GHmax after clonidine (ng/mL) | 15.76 ± 7.63 | 18.84 ± 9.75 | 0.2007 |
| GHmax after glucagone (ng/mL) | 11.04 ± 6.54 | 11.34 ± 7.79 | 0.8744 |
| GHmax during sleep (ng/mL) | 15.97 ± 7.76 | 14.47 ± 8.46 | 0.4825 |
| IGF-I (ng/mL) | 191.05 ± 118.11 | 167.01 ± 83.72 | 0.3214 |
| IGFBP-3 (µg/dL) | 4.49 ± 0.90 | 4.50 ± 1.42 | 0.9817 |
| IGF-I/IGFBP-3 ratio | 0.23 ± 0.13 | 0.21 ± 0.10 | 0.4081 |
| Ghrelin (pg/mL) | 1224.93 ± 712.56 | 1357.95 ± 691.69 | 0.4581 |
| Leptin (ng/mL) | 5.12 ± 8.60 | 4.54 ± 6.55 | 0.7633 |
| Adiponectin (ng/mL) | 14.98±6.38 | 18.07 ± 8.19 | 0.1548 |
| Resistin (ng/mL) | 9.49 ± 4.13 | 10.27 ± 4.03 | 0.4841 |
| AR index | 0.80 ± 0.20 | 0.77 ± 0.28 | 0.6998 |
| Triglycerides (mg/dL) | 77.13 ± 36.27 | 64.41 ± 23.69 | 0.1186 |
| Cholesterol (mg/dL) | 172.33 ± 47.79 | 156.65 ± 29.93 | 0.1137 |
| LDL-cholesterol (mg/dL) | 103.13 ± 44.56 | 86.67 ± 23.73 | 0.0718 |
| HDL-cholesterol (mg/dL) | 56.25 ± 13.22 | 58.12 ± 16.98 | 0.6922 |
| OGTT, n = 28 (f/m) | 9 (5/7) | 19 (9/7) | |
| Glucose 0′ (mg/dL) | 84.56 ± 6.96 | 82.28 ± 10.01 | 0.3763 |
| Glucose 60′ (mg/dL) | 139.57 ± 48.49 | 123.95 ± 44.19 | 0.4358 |
| Glucose 120′ (mg/dL) | 110.29 ± 35.53 | 107.19 ± 27.81 | 0.8136 |
| Insulin 0′ (µIU/mL) | 4.30 ± 3.61 | 4.51 ± 2.90 | 0.8200 |
| Insulin 60′ (µIU/mL) | 34.85 ± 24.59 | 33.41 ± 16.20 | 0.8597 |
| Insulin 120′ (µIU/mL) | 26.15 ± 19.81 | 25.93±13.15 | 0.9741 |
| IRI HOMA | 0.93 ± 0.86 | 0.94 ± 0.58 | 0.9614 |
| IRI Belfiore | 0.83 ± 0.55 | 1.05 ± 0.33 | 0.2136 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczewska, K.; Adamczewski, Z.; Łupińska, A.; Lewiński, A.; Stawerska, R. Strong Positive Correlation between TSH and Ghrelin in Euthyroid Non-Growth Hormone-Deficient Children with Short Stature. Molecules 2020, 25, 3912. https://doi.org/10.3390/molecules25173912
Adamczewska K, Adamczewski Z, Łupińska A, Lewiński A, Stawerska R. Strong Positive Correlation between TSH and Ghrelin in Euthyroid Non-Growth Hormone-Deficient Children with Short Stature. Molecules. 2020; 25(17):3912. https://doi.org/10.3390/molecules25173912
Chicago/Turabian StyleAdamczewska, Katarzyna, Zbigniew Adamczewski, Anna Łupińska, Andrzej Lewiński, and Renata Stawerska. 2020. "Strong Positive Correlation between TSH and Ghrelin in Euthyroid Non-Growth Hormone-Deficient Children with Short Stature" Molecules 25, no. 17: 3912. https://doi.org/10.3390/molecules25173912
APA StyleAdamczewska, K., Adamczewski, Z., Łupińska, A., Lewiński, A., & Stawerska, R. (2020). Strong Positive Correlation between TSH and Ghrelin in Euthyroid Non-Growth Hormone-Deficient Children with Short Stature. Molecules, 25(17), 3912. https://doi.org/10.3390/molecules25173912







