Isoliquiritigenin Derivatives Inhibit RANKL-Induced Osteoclastogenesis by Regulating p38 and NF-κB Activation in RAW 264.7 Cells
Abstract
1. Introduction
2. Results
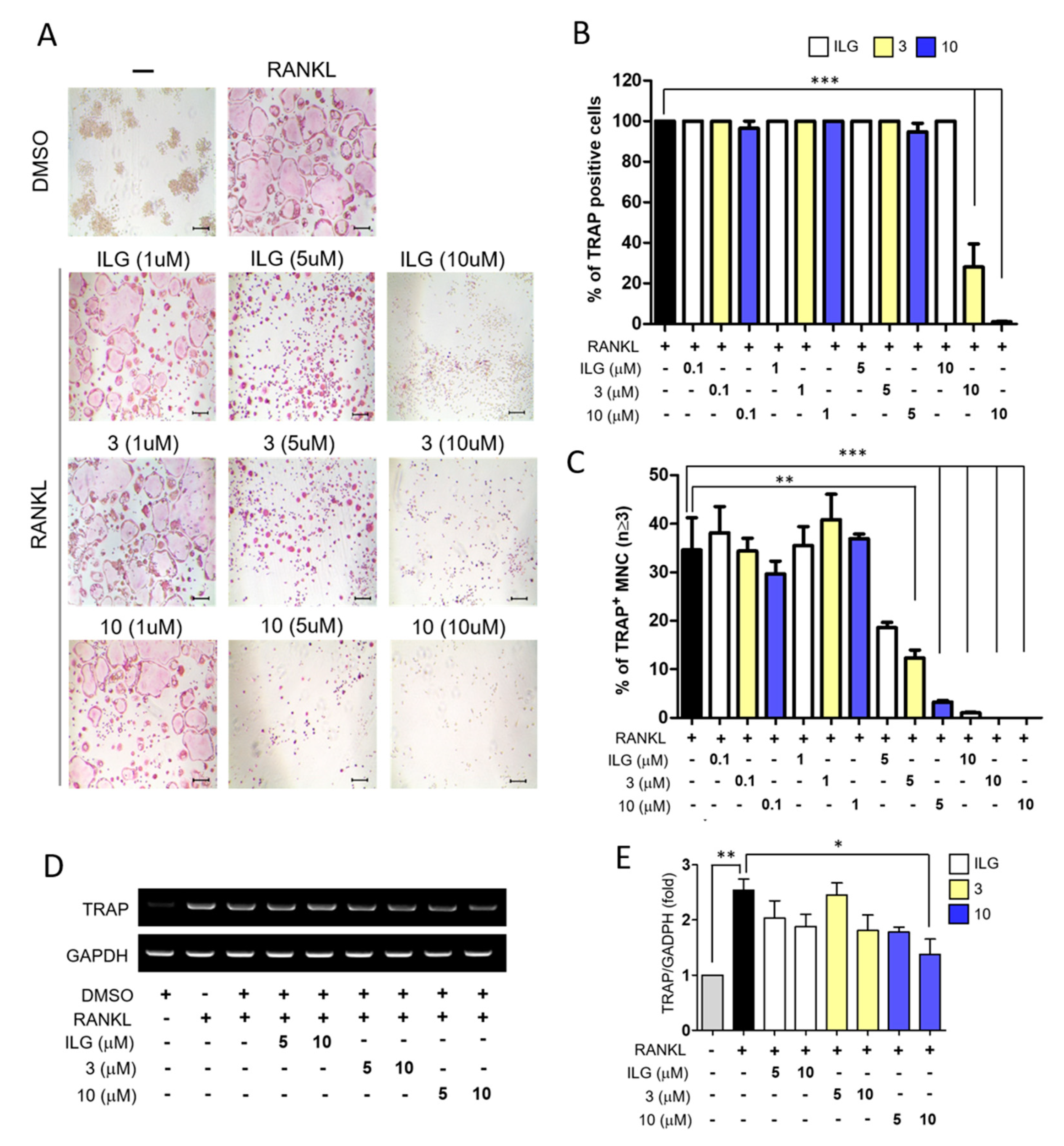
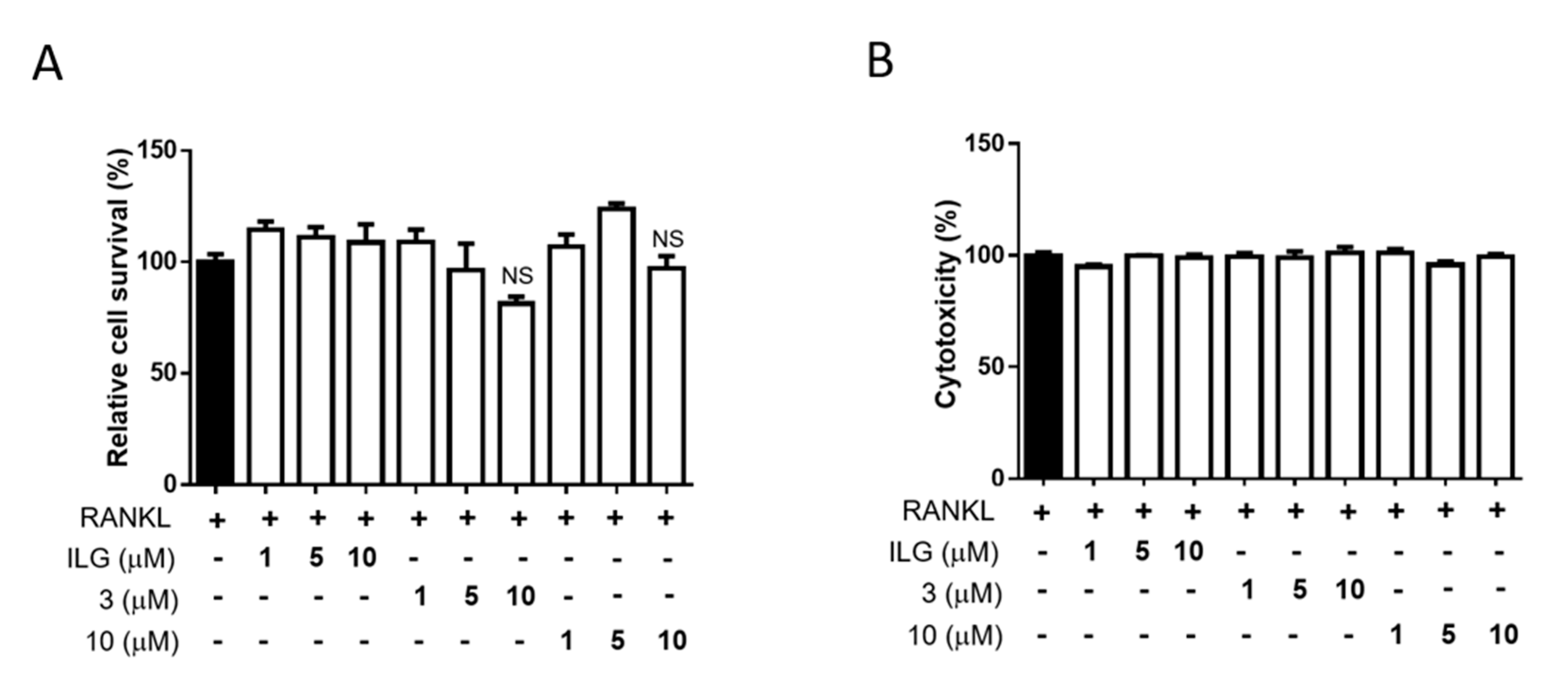
2.1. ILG Derivatives Inhibit RANKL-Induced Osteoclast Differentiation
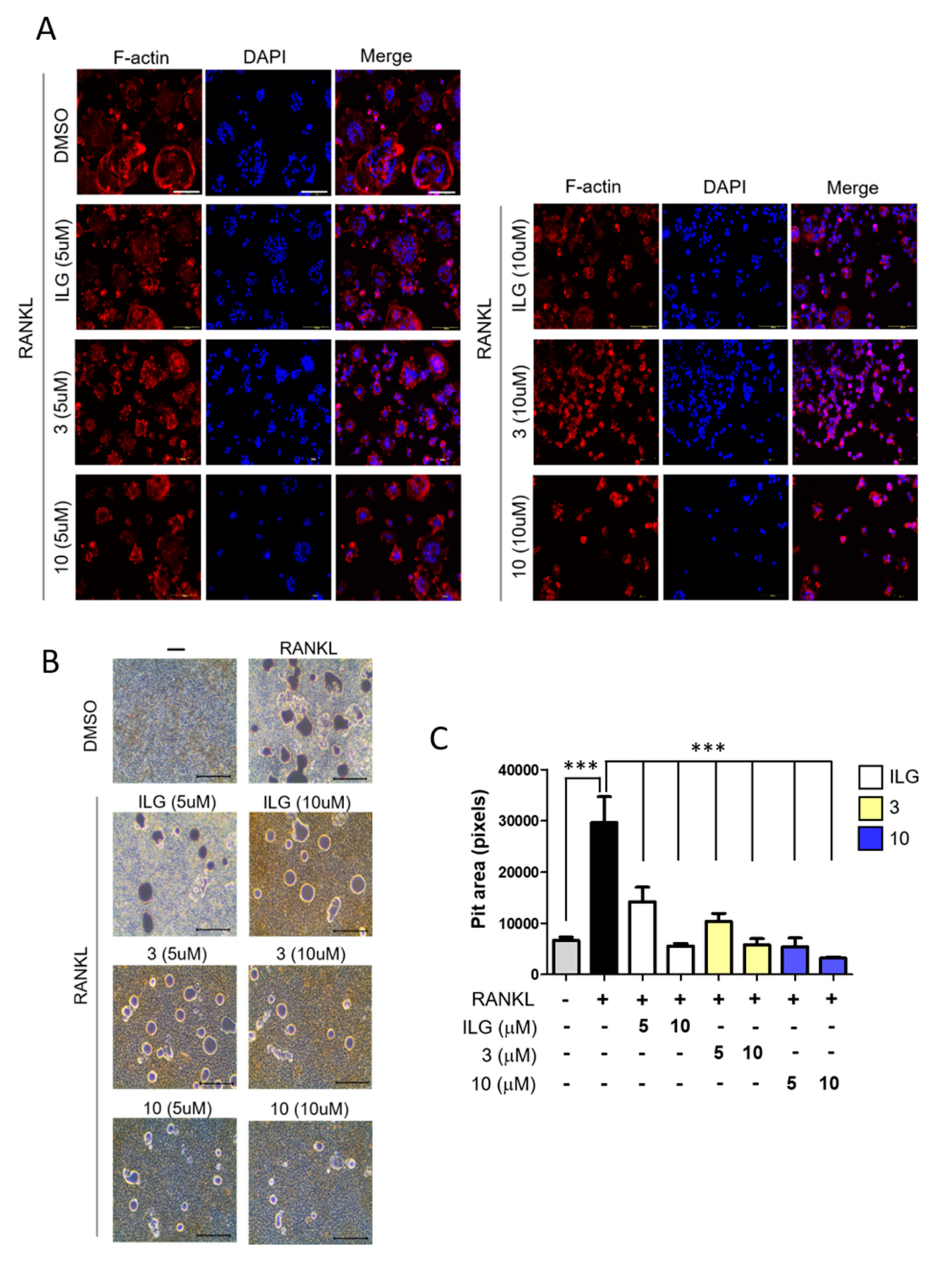
2.2. ILG Derivatives Impaired Bone Resorptive Activity of RANKL-Induced Osteoclasts
2.3. Combinational Treatment of ILG Derivatives Showed a Synergistic Effect on RANKL-Induced Osteoclast Differentiation and Activation
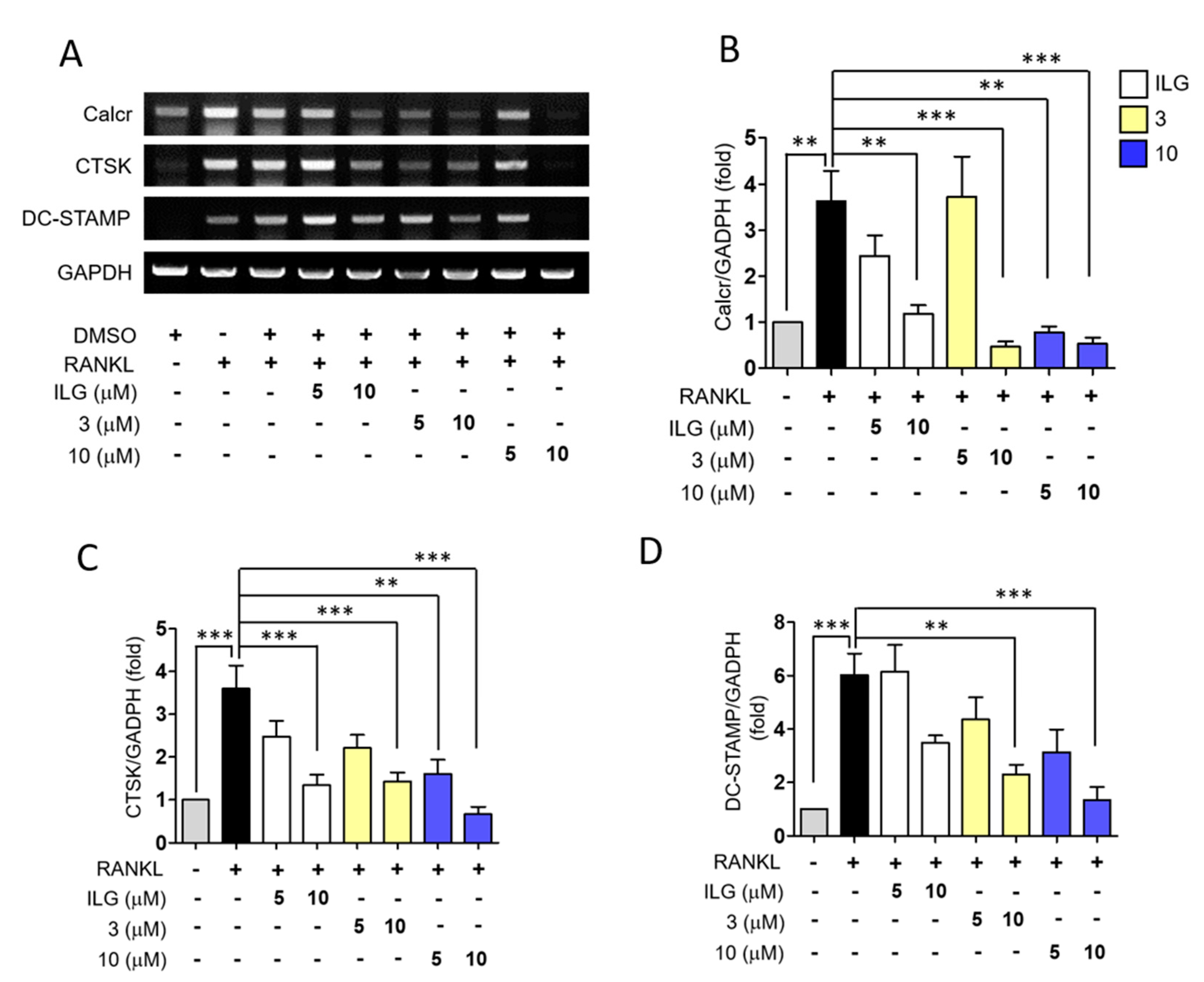
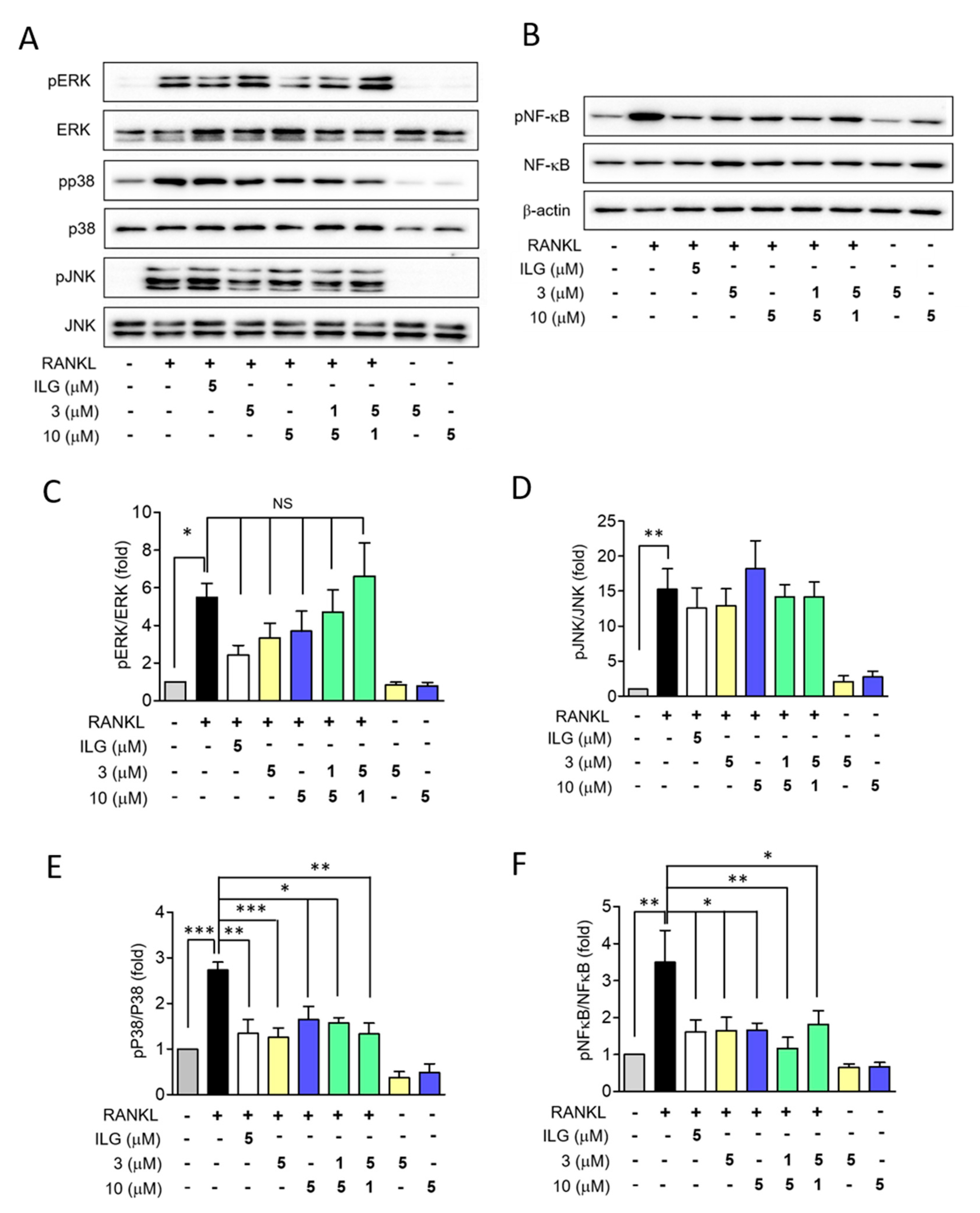
2.4. ILG Derivatives Suppressed RANKL-Induced Osteoclast Differentiation Signaling Pathways
3. Discussion
4. Materials and Methods
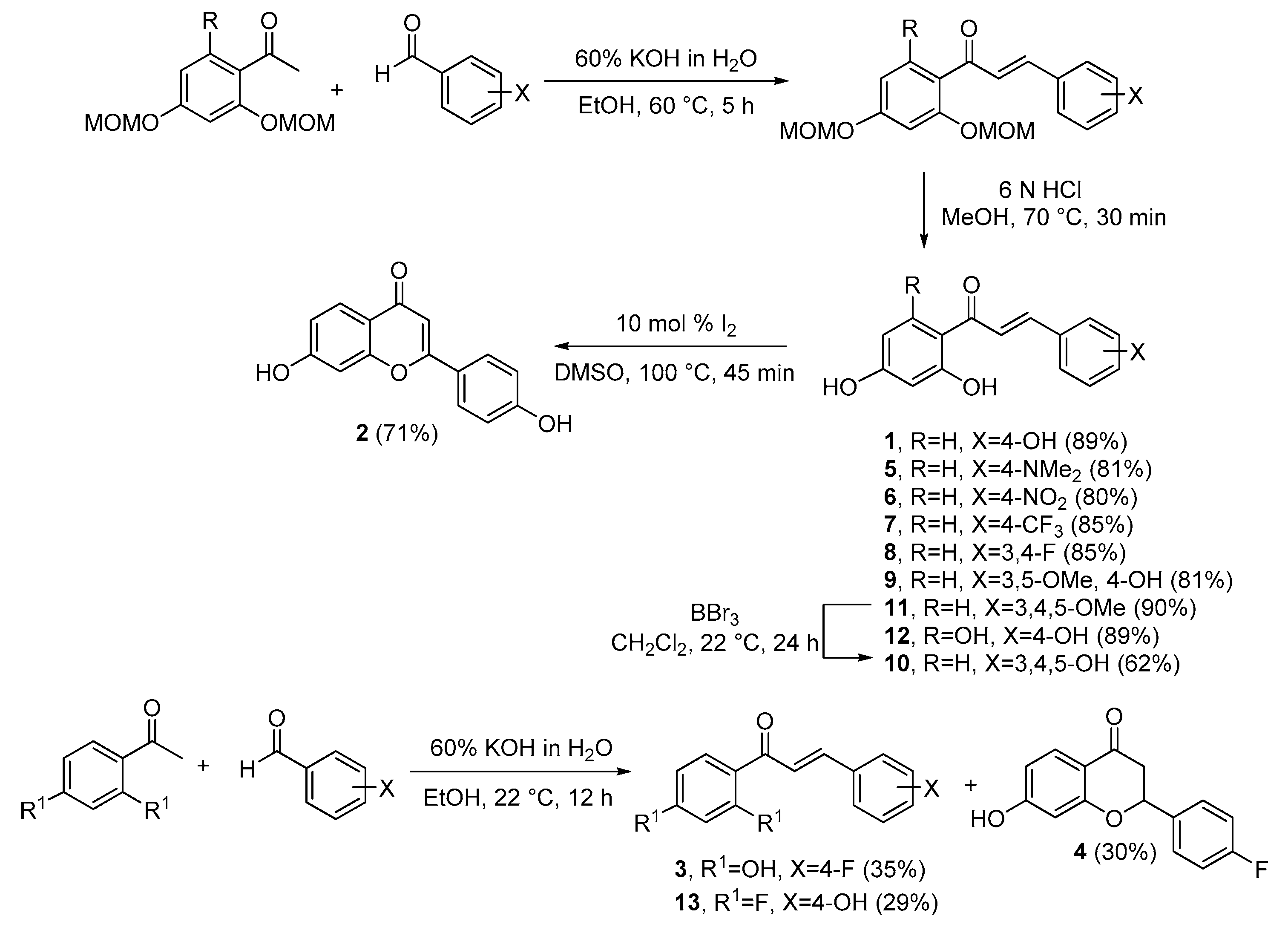
4.1. Synthesis
4.2. Cell Lines
4.3. Cell Viability and Toxicity
4.4. TRAP Staining
4.5. Reverse Transcription PCR
4.6. Bone Resorption Assay
4.7. Western Blot Analysis
4.8. Actin Ring Formation Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Han, Y.; You, X.; Xing, W.; Zhang, Z.; Zou, W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018, 6, 16. [Google Scholar] [CrossRef]
- Bi, H.; Chen, X.; Gao, S.; Yu, X.; Xiao, J.; Zhang, B.; Liu, X.; Dai, M. Key triggers of osteoclast-related diseases and available strategies for targeted therapies: A review. Front. Med. 2017, 4, 234. [Google Scholar] [CrossRef]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of osteoclast differentiation by cytokine networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef]
- Tanaka, S.; Takahashi, N.; Udagawa, N.; Tamura, T.; Akatsu, T.; Stanley, E.R.; Kurokawa, T.; Suda, T. Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J. Clin. Investig. 1993, 91, 257–263. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kadono, Y.; Naito, A.; Matsumoto, K.; Yamamoto, T.; Tanaka, S.; Inoue, J. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001, 20, 1271–1280. [Google Scholar] [CrossRef]
- Abu-Amer, Y. NF-kappaB signaling and bone resorption. Osteoporos. Int. 2013, 24, 2377–2386. [Google Scholar] [CrossRef]
- Cella, M.; Buonsanti, C.; Strader, C.; Kondo, T.; Salmaggi, A.; Colonna, M. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J. Exp. Med. 2003, 198, 645–651. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- McLean, W.; Olsen, B.R. Mouse models of abnormal skeletal development and homeostasis. Trends. Genet. 2001, 17, S38–S43. [Google Scholar] [CrossRef]
- Stefanick, M.L. Estrogens and progestins: Background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am. J. Med. 2005, 118, 64–73. [Google Scholar] [CrossRef]
- Oseni, T.; Patel, R.; Pyle, J.; Jordan, V.C. Selective estrogen receptor modulators and phytoestrogens. Planta Med. 2008, 74, 1656–1665. [Google Scholar] [CrossRef]
- Miller, P.D. Non-vertebral fracture risk reduction with oral bisphosphonates: Challenges with interpreting clinical trial data. Curr. Med. Res. Opin. 2008, 24, 107–119. [Google Scholar] [CrossRef]
- Gruber, H.E.; Ivey, J.L.; Baylink, D.J.; Matthews, M.; Nelp, W.B.; Sisom, K.; Chesnut, C.H., III. Long-term calcitonin therapy in postmenopausal osteoporosis. Metabolism 1984, 33, 295–303. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef]
- Ettinger, B.; Black, D.M.; Mitlak, B.H.; Knickerbocker, R.K.; Nickelsen, T.; Genant, H.K.; Christiansen, C.; Delmas, P.D.; Zanchetta, J.R.; Stakkestad, J.; et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999, 282, 637–645. [Google Scholar] [CrossRef]
- Chesnut, C.H., III; Silverman, S.; Andriano, K.; Genant, H.; Gimona, A.; Harris, S.; Kiel, D.; LeBoff, M.; Maricic, M.; Miller, P.; et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: The prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am. J. Med. 2000, 109, 267–276. [Google Scholar] [CrossRef]
- Shane, E.; Burr, D.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; Dempster, D.W.; et al. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American Society for Bone and Mineral Research. J. Bone. Miner. Res. 2014, 29, 1–23. [Google Scholar] [CrossRef]
- Khosla, S.; Burr, D.; Cauley, J.; Dempster, D.W.; Ebeling, P.R.; Felsenberg, D.; Gagel, R.F.; Gilsanz, V.; Guise, T.; Koka, S.; et al. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the American Society for Bone and Mineral Research. J. Bone. Miner. Res. 2007, 22, 1479–1491. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, H.; Wu, Y.; Yang, S.; Xiao, L.; Zhang, J.; Peng, B. Licorice isoliquiritigenin suppresses RANKL-induced osteoclastogenesis in vitro and prevents inflammatory bone loss in vivo. Int. J. Biochem. Cell. Biol. 2012, 44, 1139–1152. [Google Scholar] [CrossRef]
- Ji, B.; Zhang, Z.; Guo, W.; Ma, H.; Xu, B.; Mu, W.; Amat, A.; Cao, L. Isoliquiritigenin blunts osteoarthritis by inhibition of bone resorption and angiogenesis in subchondral bone. Sci. Rep. 2018, 8, 1721. [Google Scholar] [CrossRef]
- Carnovali, M.; Banfi, G.; Mariotti, M. Liquiritigenin reduces osteoclast activity in zebrafish model of glucocorticoid-induced osteoporosis. J. Pharmacol. Sci. 2020, 143, 300–306. [Google Scholar] [CrossRef]
- Yerra, V.G.; Kalvala, A.K.; Kumar, A. Isoliquiritigenin reduces oxidative damage and alleviates mitochondrial impairment by SIRT1 activation in experimental diabetic neuropathy. J. Nutr. Biochem. 2017, 47, 41–52. [Google Scholar] [CrossRef]
- Wu, C.H.; Chen, H.Y.; Wang, C.W.; Shieh, T.M.; Huang, T.C.; Lin, L.C.; Wang, K.L.; Hsia, S.M. Isoliquiritigenin induces apoptosis and autophagy and inhibits endometrial cancer growth in mice. Oncotarget 2016, 7, 73432–73447. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nagai, Y.; Honda, H.; Okamoto, N.; Yamamoto, S.; Hamashima, T.; Ishii, Y.; Tanaka, M.; Suganami, T.; Sasahara, M.; et al. Isoliquiritigenin attenuates adipose tissue inflammation in vitro and adipose tissue fibrosis through inhibition of innate immune responses in mice. Sci. Rep. 2016, 6, 23097. [Google Scholar] [CrossRef]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 202, 345–351. [Google Scholar] [CrossRef]
- Soe, K.; Delaisse, J.M. Time-lapse reveals that osteoclasts can move across the bone surface while resorbing. J. Cell. Sci. 2017, 130, 2026–2035. [Google Scholar] [CrossRef]
- Chellaiah, M.A. Regulation of actin ring formation by rho GTPases in osteoclasts. J. Biol. Chem. 2005, 280, 32930–32943. [Google Scholar] [CrossRef]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends. Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef]
- McCormack, J.P.; Allan, G.M.; Virani, A.S. Is bigger better? An argument for very low starting doses. CMAJ 2011, 183, 65–69. [Google Scholar] [CrossRef]
- Rowe, J.W. Natural Products of Woody Plants: Chemicals Extraneous to the Lignocellulosic Cell Wall; Springer: Berlin, Germany; New York, NY, USA, 1989. [Google Scholar]
- Shaik, A.; Bhandare, R.R.; Palleapati, K.; Nissankararao, S.; Kancharlapalli, V.; Shaik, S. Antimicrobial, antioxidant, and anticancer activities of some novel isoxazole ring containing chalcone and dihydropyrazole derivatives. Molecules 2020, 25, 1047. [Google Scholar] [CrossRef]
- Razmi, A.; Zarghi, A.; Arfaee, S.; Naderi, N.; Faizi, M. Evaluation of anti-nociceptive and anti-inflammatory activities of novel chalcone derivatives. Iran J. Pharm. Res. 2013, 12, 153–159. [Google Scholar]
- Lin, P.H.; Chiang, Y.F.; Shieh, T.M.; Chen, H.Y.; Shih, C.K.; Wang, T.H.; Wang, K.L.; Huang, T.C.; Hong, Y.H.; Li, S.C.; et al. Dietary compound isoliquiritigenin, an antioxidant from licorice, suppresses triple-negative breast tumor growth via apoptotic death program activation in cell and xenograft animal models. Antioxidants 2020, 9, 228. [Google Scholar] [CrossRef]
- Maggiolini, M.; Statti, G.; Vivacqua, A.; Gabriele, S.; Rago, V.; Loizzo, M.; Menichini, F.; Amdo, S. Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells. J. Steroid. Biochem. Mol. Biol. 2002, 82, 315–322. [Google Scholar] [CrossRef]
- Mersereau, J.E.; Levy, N.; Staub, R.E.; Baggett, S.; Zogovic, T.; Chow, S.; Ricke, W.A.; Tagliaferri, M.; Cohen, I.; Bjeldanes, L.F.; et al. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Mol. Cell. Endocrinol. 2008, 283, 49–57. [Google Scholar] [CrossRef]
- Mokbel, K. The evolving role of aromatase inhibitors in breast cancer. Int. J. Clin. Oncol. 2002, 7, 279–283. [Google Scholar] [CrossRef]
- Calliste, C.A.; Le Bail, J.C.; Trouillas, P.; Pouget, C.; Habrioux, G.; Chulia, A.J.; Duroux, J.L. Chalcones: Structural requirements for antioxidant, estrogenic and antiproliferative activities. Anticancer Res. 2001, 21, 3949–3956. [Google Scholar]
- Liu, X.; Zhu, Q.; Zhang, M.; Yin, T.; Xu, R.; Xiao, W.; Wu, J.; Deng, B.; Gao, X.; Gong, W.; et al. Isoliquiritigenin Ameliorates Acute Pancreatitis in Mice via Inhibition of Oxidative Stress and Modulation of the Nrf2/HO-1 Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 7161592. [Google Scholar] [CrossRef]
- Feng, X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Curr. Chem. Biol. 2009, 3, 189–196. [Google Scholar] [CrossRef]
- Blair, H.C.; Teitelbaum, S.L.; Ghiselli, R.; Gluck, S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science 1989, 245, 855–857. [Google Scholar] [CrossRef]
- Holliday, L.S.; Lu, M.; Lee, B.S.; Nelson, R.D.; Solivan, S.; Zhang, L.; Gluck, S.L. The amino-terminal domain of the B subunit of vacuolar H+-ATPase contains a filamentous actin binding site. J. Biol. Chem. 2000, 275, 32331–32337. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds IGL and Robtein are available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Lee, S.; Kim, K.; Lee, Y.; Lee, J.; Oh, S.; Choi, J.-W.; Kim, S.W.; Hwang, K.-C.; Lim, S. Isoliquiritigenin Derivatives Inhibit RANKL-Induced Osteoclastogenesis by Regulating p38 and NF-κB Activation in RAW 264.7 Cells. Molecules 2020, 25, 3908. https://doi.org/10.3390/molecules25173908
Jeong S, Lee S, Kim K, Lee Y, Lee J, Oh S, Choi J-W, Kim SW, Hwang K-C, Lim S. Isoliquiritigenin Derivatives Inhibit RANKL-Induced Osteoclastogenesis by Regulating p38 and NF-κB Activation in RAW 264.7 Cells. Molecules. 2020; 25(17):3908. https://doi.org/10.3390/molecules25173908
Chicago/Turabian StyleJeong, Seongtae, Seahyoung Lee, Kundo Kim, Yunmi Lee, Jiyun Lee, Sena Oh, Jung-Won Choi, Sang Woo Kim, Ki-Chul Hwang, and Soyeon Lim. 2020. "Isoliquiritigenin Derivatives Inhibit RANKL-Induced Osteoclastogenesis by Regulating p38 and NF-κB Activation in RAW 264.7 Cells" Molecules 25, no. 17: 3908. https://doi.org/10.3390/molecules25173908
APA StyleJeong, S., Lee, S., Kim, K., Lee, Y., Lee, J., Oh, S., Choi, J.-W., Kim, S. W., Hwang, K.-C., & Lim, S. (2020). Isoliquiritigenin Derivatives Inhibit RANKL-Induced Osteoclastogenesis by Regulating p38 and NF-κB Activation in RAW 264.7 Cells. Molecules, 25(17), 3908. https://doi.org/10.3390/molecules25173908





