Formation and Characterization of β-Lactoglobulin and Gum Arabic Complexes: the Role of pH
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of β-Lg−GA Mixed Solution and β-Lg–GA Complexes
2.3. Turbidity Measurements
2.4. Effect of pH on the Structure of β-Lg−GA Complexes
2.5. Effect of pH on the Particle Size and Zeta-Potential of β-Lg−GA Complexes
2.6. Effect of pH on the Viscosity of β-Lg−GA Complexes
2.7. Differential Scanning Calorimetry (DSC) and Fourier Transform Infrared (FTIR) Spectroscopy
3. Results and discussion
3.1. Effect of pH on Turbidity of β-Lg−GA Complexes
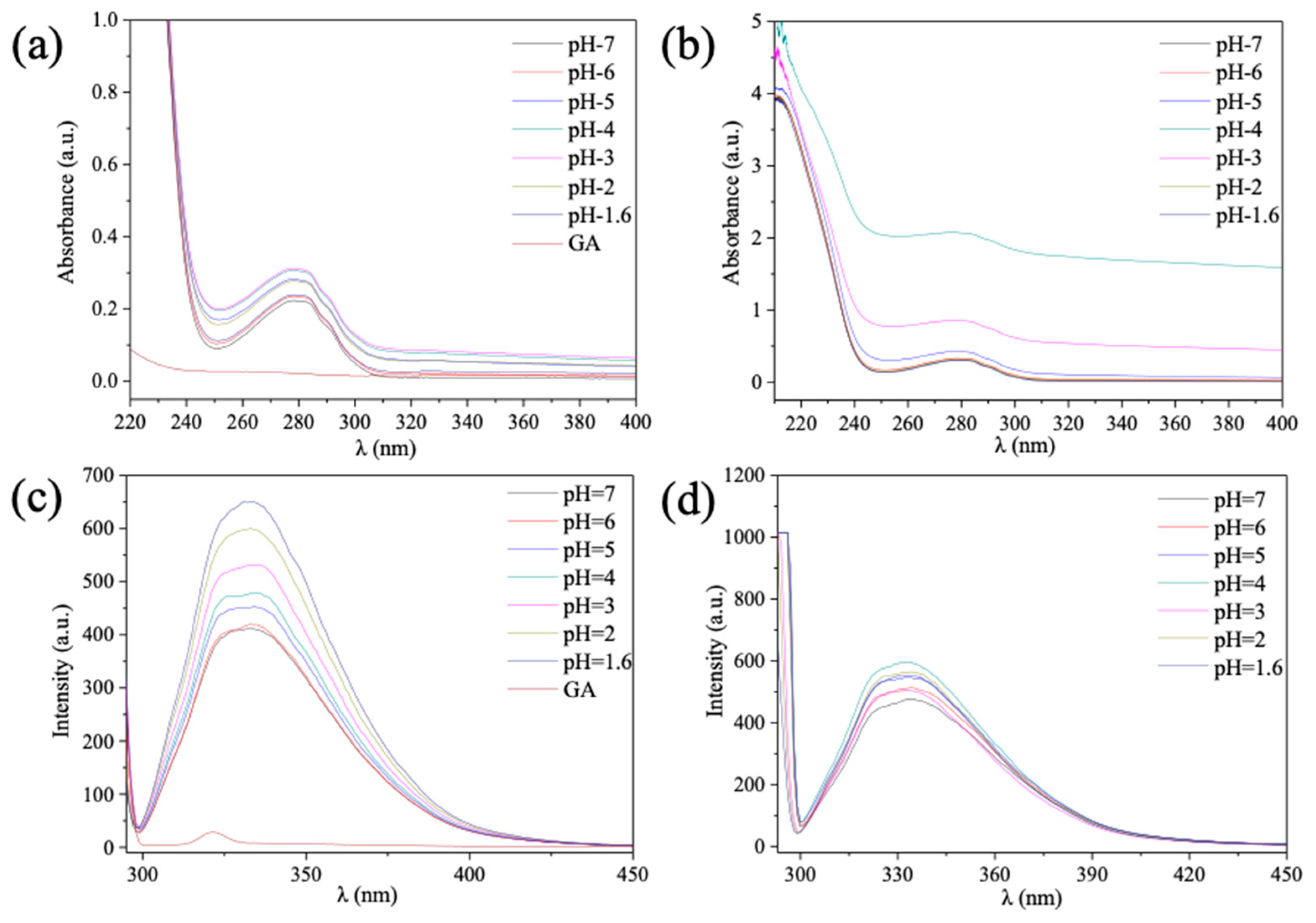
3.2. Effect of pH on the Structures of β-Lg−GA Mixed Solution
3.3. Effect of pH on the Zeta-Potential and Size Distribution of β-Lg−GA Complexes
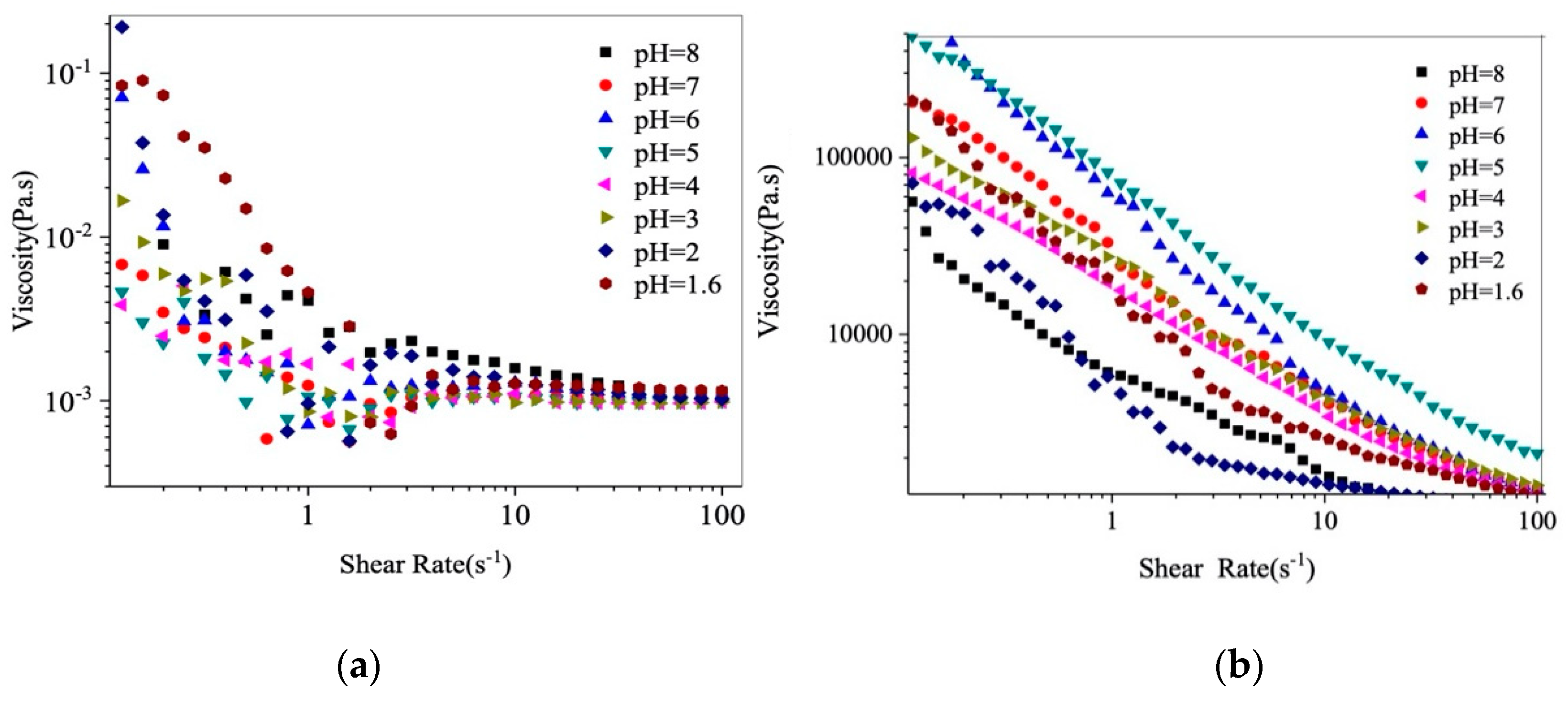
3.4. Effect of pH on the Rheological Properties of β-Lg−GA Complexes
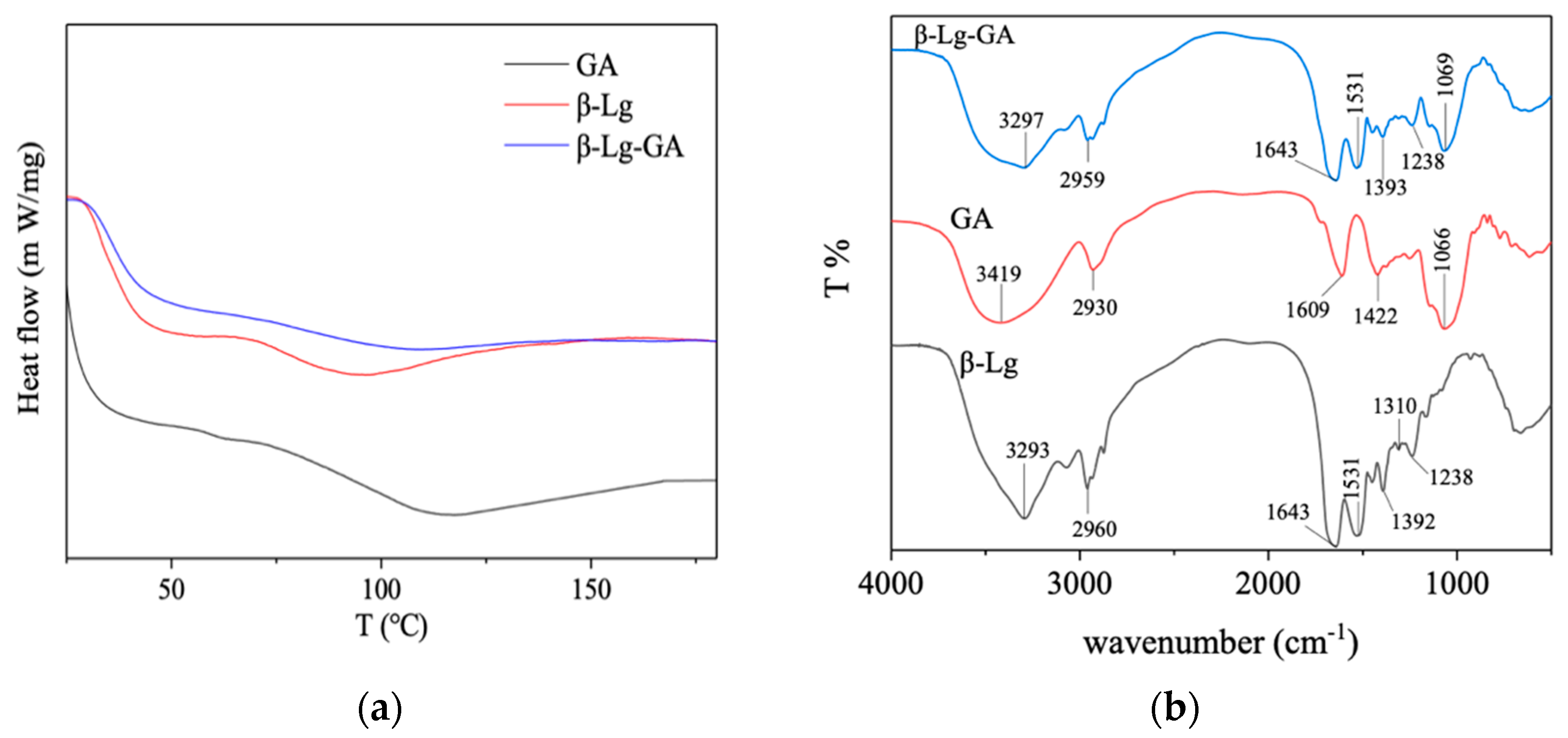
3.5. Thermal Behavior and Fourier Transform Infrared Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wei, Z.H.; Huang, Q.R. Assembly of Protein-Polysaccharide Complexes for Delivery of Bioactive Ingredients: A Perspective Paper. J. Agric. Food Chem. 2019, 67, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; Turgeon, S.L. Protein/polysaccharide complexes and coacervates in food systems. Adv. Colloid Interface Sci. 2011, 167, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.G.; McClements, D.J. Recent progress in biopolymer nanoparticle and microparticle formation by heat-treating electrostatic protein-polysaccharide complexes. Adv. Colloid Interfac. 2011, 167, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jiang, Y.F.; Liu, F.; Chai, Z.; Li, Y.X.; Li, Y.Y.; Leng, X.J. Synergistic effects of whey protein-polysaccharide complexes on the controlled release of lipid-soluble and water-soluble vitamins in W1/O/W2 double emulsion systems. Int. J. Food Sci. Technol. 2012, 47, 248–254. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, Y.F.; Du, B.J.; Chai, Z.; Jiao, T.; Zhang, C.Y.; Ren, F.Z.; Leng, X.J. Design and Characterization of Controlled-Release Edible Packaging Films Prepared with Synergistic Whey-Protein Polysaccharide Complexes. J. Agric. Food Chem. 2013, 61, 5824–5833. [Google Scholar] [CrossRef]
- Humblet-Hua, N.P.K.; van der Linden, E.; Sagis, L.M.C. Surface rheological properties of liquid-liquid interfaces stabilized by protein fibrillar aggregates and protein-polysaccharide complexes. Soft Matter 2013, 9, 2154–2165. [Google Scholar] [CrossRef]
- Bouyer, E.; Mekhloufi, G.; Rosilio, V.; Grossiord, J.L.; Agnely, F. Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: Alternatives to synthetic surfactants in the pharmaceutical field? Int. J. Pharmaceut. 2012, 436, 359–378. [Google Scholar] [CrossRef]
- Ganzevles, R.A.; Zinoviadou, K.; van Vliet, T.; Cohen, M.A.; de Jongh, H.H. Modulating surface rheology by electrostatic protein/polysaccharide interactions. Langmuir 2006, 22, 10089–10096. [Google Scholar] [CrossRef]
- Ni, X.; Wang, K.; Wu, K.; Corke, H.; Nishinari, K.; Jiang, F. Stability, microstructure and rheological behavior of konjac glucomannan-zein mixed systems. Carbohydr. Polym. 2018, 188, 260–267. [Google Scholar] [CrossRef]
- Dai, L.; Wei, Y.; Sun, C.X.; Mao, L.K.; McClements, D.J.; Gao, Y.X. Development of protein-polysaccharide-surfactant ternary complex particles as delivery vehicles for curcumin. Food Hydrocolloid 2018, 85, 75–85. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Reichert, C.; Salvia-Trujillo, L.; Zeeb, B.; Martin-Belloso, O.; Weiss, J. Protein/Polysaccharide Complexes to Stabilize Decane-in-Water Nanoemulsions. Food Biophys. 2020, 15, 335–345. [Google Scholar] [CrossRef]
- Ar, W.J.; Fang, Y.P.; Xiang, S.P.; Yao, X.L.; Nihinari, K.; Phillips, G.O. Protein/Polysaccharide Electrostatic Complexes and Their Applications in Stabilizing Oil-in-Water Emulsions. J. Nutr. Sci. Vitaminol 2015, 61, S168–S169. [Google Scholar] [CrossRef]
- Weinbreck, F.; de Vries, R.; Schrooyen, P.; de Kruif, C.G. Complex coacervation of whey proteins and gum arabic. Biomacromolecules 2003, 4, 293–303. [Google Scholar] [CrossRef]
- Murillo-Martinez, M.M.; Pedroza-Islas, R.; Lobato-Calleros, C.; Martinez-Ferez, A.; Vernon-Carter, E.J. Designing W-1/O/W-2 double emulsions stabilized by protein-polysaccharide complexes for producing edible films: Rheological, mechanical and water vapour properties. Food Hydrocolloid 2011, 25, 577–585. [Google Scholar] [CrossRef]
- Schmitt, C.; Bovay, C.; Frossard, P. Kinetics of formation and functional properties of conjugates prepared by dry-state incubation of beta-lactoglobulin/acacia gum electrostatic complexes. J. Agric. Food Chem. 2005, 53, 9089–9099. [Google Scholar] [CrossRef] [PubMed]
- Aberkane, L.; Jasniewski, J.; Gaiani, C.; Scher, J.; Sanchez, C. Thermodynamic characterization of acacia gum-beta-lactoglobulin complex coacervation. Langmuir 2010, 26, 12523–12533. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.A.M.; van Miltenburg, J.C.; van de Eerden, J.P.; van Mil, P.J.J.; de Kruif, C.G. Isothermal and Scanning Calorimetry Measurements on β-Lactoglobulin. J. Phys. Chem. B 1997, 101, 6988–6994. [Google Scholar] [CrossRef]
- Hoffmann, M.A.M.; van Mil, P.J.J. Heat-Induced Aggregation of β-Lactoglobulin: Role of the Free Thiol Group and Disulfide Bonds. J. Agric. Food Chem. 1997, 45, 2942–2948. [Google Scholar] [CrossRef]
- Xu, A.Y.; Melton, L.D.; Jameson, G.B.; Williams, M.A.; McGillivray, D.J. Structural mechanism of complex assemblies: Characterisation of beta-lactoglobulin and pectin interactions. Soft Matter 2015, 11, 6790–6799. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef]
- O’Chiu, E.; Vardhanabhuti, B. Utilizing whey protein isolate and polysaccharide complexes to stabilize aerated dairy gels. J. Dairy Sci. 2017, 100, 3404–3412. [Google Scholar] [CrossRef]
- Li, M.; He, Z.; Li, G.; Zeng, Q.; Su, D.; Zhang, J.; Wang, Q.; Yuan, Y.; He, S. The formation and characterization of antioxidant pickering emulsions: Effect of the interactions between gliadin and chitosan. Food Hydrocolloid 2019, 90, 482–489. [Google Scholar] [CrossRef]
- Niu, F.; Zhang, Y.; Chang, C.; Pan, W.; Sun, W.; Su, Y.; Yang, Y. Influence of the preparation method on the structure formed by ovalbumin/gum Arabic to observe the stability of oil-in-water emulsion. Food Hydrocolloids 2017, 63, 602–610. [Google Scholar] [CrossRef]
- Yildiz, G.; Ding, J.Z.; Andrade, J.; Engeseth, N.J.; Feng, H. Effect of plant protein-polysaccharide complexes produced by mano-thermo-sonication and pH-shifting on the structure and stability of oil-in-water emulsions. Innov. Food Sci. Emerg. 2018, 47, 317–325. [Google Scholar] [CrossRef]
- Hernandez-Marin, N.Y.; Lobato-Calleros, C.; Vernon-Carter, E.J. Stability and rheology of water-in-oil-in-water multiple emulsions made with protein-polysaccharide soluble complexes. J. Food Eng. 2013, 119, 181–187. [Google Scholar] [CrossRef]
- Schmitt, C.; Sanchez, C.; Despond, S.; Renard, D.; Thomas, F.; Hardy, J. Effect of protein aggregates on the complex coacervation between β-lactoglobulin and acacia gum at pH 4.2. Food Hydrocolloid 2020, 14, 403–413. [Google Scholar] [CrossRef]
- Xie, H.; Xiang, C.; Li, Y.; Wang, L.; Zhang, Y.; Song, Z.; Ma, X.; Lu, X.; Lei, Q.; Fang, W. Fabrication of ovalbumin/κ-carrageenan complex nanoparticles as a novel carrier for curcumin delivery. Food Hydrocolloid 2019, 89, 111–121. [Google Scholar] [CrossRef]
- Comert, F.; Azarikia, F.; Dubin, P.L. Polysaccharide zeta-potentials and protein-affinity. Phys. Chem. Chem. Phys. 2017, 19, 21090–21094. [Google Scholar] [CrossRef]
- Crowther, J.M.; Jameson, G.B.; Hodgkinson, A.J.; Dobson, R.C.J. Structure, Oligomerisation and Interactions of β-Lactoglobulin, Milk Proteins-From Structure to Biological Properties and Health Aspects; IntechOpen Limited: London, UK, 2016. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, X.; Wang, H.; Sun, X.; Guo, M. Interactions between β-Lactoglobulin and 3,3′-Diindolylmethane in model system molecules. Molecules 2019, 24, 2151. [Google Scholar] [CrossRef]
- Lii, C.-y.; Liaw, S.C.; Lai, V.M.-F.; Tomasik, P. Xanthan gum-gelatin complexes. Eur. Polym. J. 2002, 38, 1377–1381. [Google Scholar] [CrossRef]
- Tolstoguzov, V. Some thermodynamic considerations in food formulation. Food Hydrocolloid 2003, 17, 1–23. [Google Scholar] [CrossRef]
- de Kruif, C.G.; Tuinier, R. Polysaccharide protein interactions. Food Hydrocolloid 2001, 15, 555–563. [Google Scholar] [CrossRef]
- Schmitt, C.; Sanchez, C.; Desobry-Banon, S.; Hardy, J. Structure and technofunctional properties of protein-polysaccharide complexes: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 689–753. [Google Scholar] [CrossRef] [PubMed]
- Shaddel, R.; Hesari, J.; Azadmard-Damirchi, S.; Hamishehkar, H.; Fathi-Achachlouei, B.; Huang, Q. Use of gelatin and gum Arabic for encapsulation of black raspberry anthocyanins by complex coacervation. Int. J. Biol. Macromol. 2018, 107, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, V.Y.; Burova, T.V.; Haertle, T.; Tolstoguzov, V.B. Interpretation of DSC data on protein denaturation complicated by kinetic and irreversible effects. J. Biotechnol. 2000, 79, 269–280. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ray, S.; Jin, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Liu, D.; Quek, S.Y. Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein-gallic acid system. Food Chem. 2013, 136, 1013–1021. [Google Scholar] [CrossRef]
- Capitani, C.; Perez, O.E.; Pacheco, B.; Teresa, M.; Pilosof, A.M. Influence of complexing carboxymethylcellulose on the thermostability and gelation of a-lactalbumin and b-lactoglobulin. Food Hydrocolloid 2007, 21, 1344–1352. [Google Scholar] [CrossRef]
- Miquelim, J.N.; Lannes, S.C.S.; Mezzenga, R. pH Influence on the stability of foams with protein-polysaccharide complexes at their interfaces. Food Hydrocolloid 2010, 24, 398–405. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
Sample Availability: Samples of β-lactoglobulin and gum arabic complexes are available from the authors. |


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Liu, J.; Gao, J.; Cao, M.; Ren, G.; Xie, H.; Yao, M. Formation and Characterization of β-Lactoglobulin and Gum Arabic Complexes: the Role of pH. Molecules 2020, 25, 3871. https://doi.org/10.3390/molecules25173871
Wang Z, Liu J, Gao J, Cao M, Ren G, Xie H, Yao M. Formation and Characterization of β-Lactoglobulin and Gum Arabic Complexes: the Role of pH. Molecules. 2020; 25(17):3871. https://doi.org/10.3390/molecules25173871
Chicago/Turabian StyleWang, Ziyuan, Jie Liu, Jian Gao, Mengna Cao, Gerui Ren, Hunjun Xie, and Mingfei Yao. 2020. "Formation and Characterization of β-Lactoglobulin and Gum Arabic Complexes: the Role of pH" Molecules 25, no. 17: 3871. https://doi.org/10.3390/molecules25173871
APA StyleWang, Z., Liu, J., Gao, J., Cao, M., Ren, G., Xie, H., & Yao, M. (2020). Formation and Characterization of β-Lactoglobulin and Gum Arabic Complexes: the Role of pH. Molecules, 25(17), 3871. https://doi.org/10.3390/molecules25173871






