Biased versus Partial Agonism in the Search for Safer Opioid Analgesics
Abstract
1. Introduction
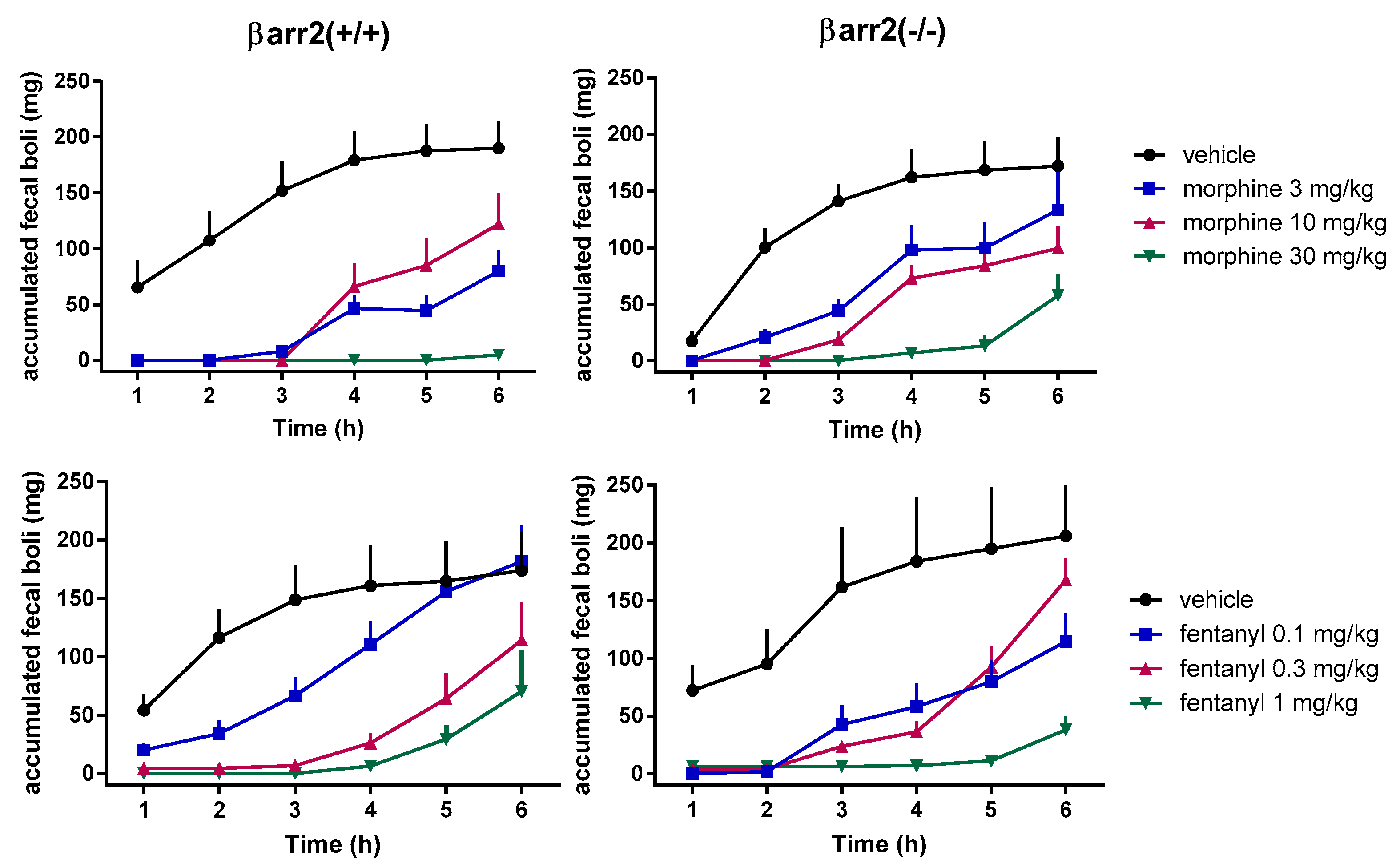
2. Genetic Studies
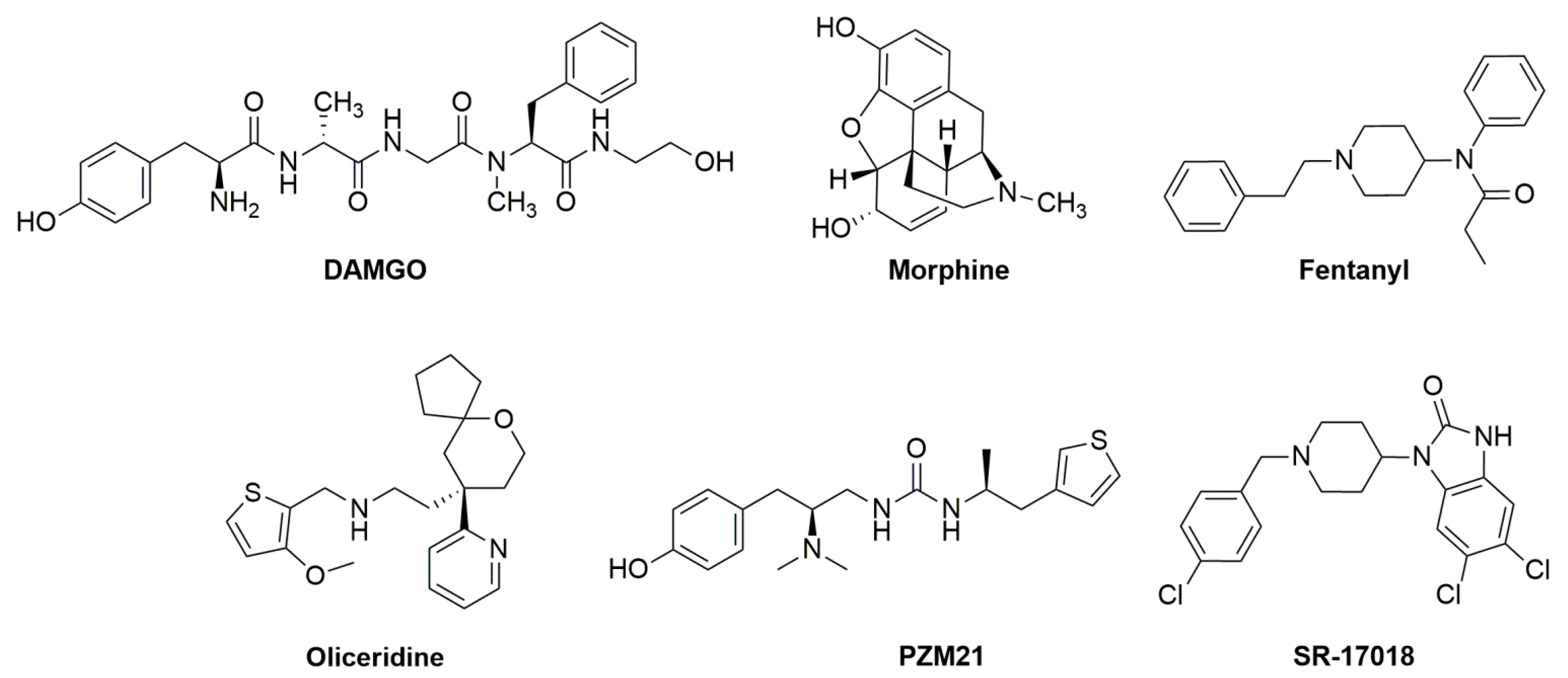
3. Pharmacological Studies—Are Mu-Receptor Agonists Biased Toward G Proteins Safer Analgesics?
4. Pharmacological Studies—Are Mu Receptors Partial Agonists Safer Analgesics?
5. Conclusions
Funding
Conflicts of Interest
References
- Leone, R.; Magro, L. In a Pharmaco-Vigillance.eu, Opioids: The Real Concern is That They are Not Used in Pain. 2015. Available online: https://www.farmacovigilanza.eu/content/oppioidi-la-vera-preoccupazione-%C3%A8-che-non-si-usano-nel-dolore (accessed on 22 July 2020).
- Volkow, N.D.; Blanco, C. The changing opioid crisis: Development, challenges and opportunities. Mol. Psychiatry 2020. [Google Scholar] [CrossRef]
- Raffa, R.B.; Pergolizzi, J.V.; Taylor, R.; Ossipov, M.H. Indirect-acting strategy of opioid action instead of direct receptor activation: Dual-acting enkephalinase inhibitors (DENKIs). J. Clin. Pharm. Ther. 2018, 43, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Roques, B.P.; Fournié-Zaluski, M.C.; Wurm, M. Inhibiting the breakdown of endogenous opioids and cannabinoids to alleviate pain. Nat. Rev. Drug Discov. 2012, 11, 292–310. [Google Scholar] [CrossRef] [PubMed]
- Remesic, M.; Hruby, V.J.; Porreca, F.; Lee, Y.S. Recent Advances in the Realm of Allosteric Modulators for Opioid Receptors for Future Therapeutics. ACS Chem. Neurosci. 2017, 8, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Livingston, K.E.; Traynor, J.R. Allostery at opioid receptors: Modulation with small molecule ligands. Br. J. Pharmacol. 2018, 175, 2846–2856. [Google Scholar] [CrossRef]
- Sehgal, N.; Smith, H.; Manchikanti, L. Narrative Review Peripherally Acting Opioids and Clinical Implications for Pain Control. Pain Physician 2011, 14, 249–258. [Google Scholar]
- Spahn, V.; Del Vecchio, G.; Rodriguez-Gaztelumendi, A.; Temp, J.; Labuz, D.; Kloner, M.; Reidelbach, M.; Machelska, H.; Weber, M.; Stein, C. Opioid receptor signaling, analgesic and side effects induced by a computationally designed pH-dependent agonist. Sci. Rep. 2018, 8, 8965. [Google Scholar] [CrossRef]
- Rodriguez-Gaztelumendi, A.; Spahn, V.; Labuz, D.; MacHelska, H.; Stein, C. Analgesic effects of a novel pH-dependent m-opioid receptor agonist in models of neuropathic and abdominal pain. Pain 2018, 159, 2277–2284. [Google Scholar] [CrossRef]
- Dietis, N.; Guerrini, R.; Calo, G.; Salvadori, S.; Rowbotham, D.; Lambert, D.G. Simultaneous targeting of multiple opioid receptors: A strategy to improve side-effect profil. Br. J. Anaesth. 2009, 103, 38–49. [Google Scholar] [CrossRef]
- Azzam, A.A.H.; McDonald, J.; Lambert, D.G. Hot topics in opioid pharmacology: Mixed and biased opioids. Br. J. Anaesth. 2019, 122, e136–e145. [Google Scholar] [CrossRef]
- Kiguchi, N.; Ding, H.; Ko, M.C. Therapeutic potentials of NOP and MOP receptor coactivation for the treatment of pain and opioid abuse. J. Neurosci. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Linz, K.; Christoph, T.; Tzschentke, T.M.; Koch, T.; Schiene, K.; Gautrois, M.; Schröder, W.; Kögel, B.Y.; Beier, H.; Englberger, W.; et al. Cebranopadol: A novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. J. Pharmacol. Exp. Ther. 2014, 349, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Tzschentke, T.M.; Linz, K.; Koch, T.; Christoph, T. Cebranopadol: A novel first-in-class potent analgesic acting via NOP and opioid receptors. In Handbook of Experimental Pharmacology; Springer: New York, NY, USA, 2019; Volume 254, pp. 367–398. [Google Scholar] [CrossRef]
- Calo, G.; Lambert, D.G. Nociceptin/orphanin FQ receptor ligands and translational challenges: Focus on cebranopadol as an innovative analgesic. Br. J. Anaesth. 2018, 121, 1105–1114. [Google Scholar] [CrossRef]
- Kenakin, T. Biased receptor signaling in drug discovery. Pharmacol. Rev. 2019, 71, 267–315. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yan, W.; McCorvy, J.D.; Cheng, J. Biased Ligands of G Protein-Coupled Receptors (GPCRs): Structure-Functional Selectivity Relationships (SFSRs) and Therapeutic Potential. J. Med. Chem. 2018, 61, 9841–9878. [Google Scholar] [CrossRef] [PubMed]
- Bohn, L.M.; Lefkowitz, R.J.; Gainetdinov, R.R.; Peppel, K.; Caron, M.G.; Lin, F.T. Enhanced morphine analgesia in mice lacking β-arrestin 2. Science 1999, 286, 2495–2498. [Google Scholar] [CrossRef]
- Bohn, L.M.; Gainetdinov, R.R.; Lin, F.T.; Lefkowitz, R.J.; Caron, M.G. μ-opioid receptor desensitization by β-arrestin-2 determines morphine tolerance but not dependence. Nature 2000, 408, 720–723. [Google Scholar] [CrossRef]
- Bohn, L.M.; Gainetdinov, R.R.; Sotnikova, T.D.; Medvedev, I.O.; Lefkowitz, R.J.; Dykstra, L.A.; Caron, M.G. Enhanced Rewarding Properties of Morphine, but not Cocaine, in βarrestin-2 Knock-Out Mice. J. Neurosci. 2003, 23, 10265–10273. [Google Scholar] [CrossRef]
- Farmer, A.D.; Drewes, A.M.; Chiarioni, G.; De Giorgio, R.; O’Brien, T.; Morlion, B.; Tack, J. Pathophysiology and management of opioid-induced constipation: European expert consensus statement. United Eur. Gastroenterol. J. 2019, 7, 7–20. [Google Scholar] [CrossRef]
- Algera, M.H.; Kamp, J.; van der Schrier, R.; van Velzen, M.; Niesters, M.; Aarts, L.; Dahan, A.; Olofsen, E. Opioid-induced respiratory depression in humans: A review of pharmacokinetic–pharmacodynamic modelling of reversal. Br. J. Anaesth. 2019, 122, e168–e179. [Google Scholar] [CrossRef]
- Kiyatkin, E.A. Respiratory depression and brain hypoxia induced by opioid drugs: Morphine, oxycodone, heroin, and fentanyl. Neuropharmacology 2019, 151, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Raehal, K.M.; Walker, J.K.L.; Bohn, L.M. Morphine side effects in β-arrestin 2 knockout mice. J. Pharmacol. Exp. Ther. 2005, 314, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Montandon, G.; Ren, J.; Victoria, N.C.; Liu, H.; Wickman, K.; Greer, J.J.; Horner, R.L. G-protein-gated inwardly rectifying potassium channels modulate respiratory depression by Opioids. Anesthesiology 2016, 124, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Levitt, E.S.; Abdala, A.P.; Paton, J.F.R.; Bissonnette, J.M.; Williams, J.T. μ opioid receptor activation hyperpolarizes respiratory-controlling Kölliker-Fuse neurons and suppresses post-inspiratory drive. J. Physiol. 2015, 593, 4453–4469. [Google Scholar] [CrossRef]
- Madariaga-Mazón, A.; Marmolejo-Valencia, A.F.; Li, Y.; Toll, L.; Houghten, R.A.; Martinez-Mayorga, K. Mu-Opioid receptor biased ligands: A safer and painless discovery of analgesics? Drug Discov. Today 2017, 22, 1719–1729. [Google Scholar] [CrossRef]
- Grim, T.W.; Acevedo-Canabal, A.; Bohn, L.M. Toward Directing Opioid Receptor Signaling to Refine Opioid Therapeutics. Biol. Psychiatry 2020, 87, 15–21. [Google Scholar] [CrossRef]
- Kliewer, A.; Gillis, A.; Hill, R.; Schmiedel, F.; Bailey, C.; Kelly, E.; Henderson, G.; Christie, M.J.; Schulz, S. Morphine-induced respiratory depression is independent of β-arrestin2 signalling. Br. J. Pharmacol. 2020, 177, 2923–2931. [Google Scholar] [CrossRef]
- Kliewer, A.; Schmiedel, F.; Sianati, S.; Bailey, A.; Bateman, J.T.; Levitt, E.S.; Williams, J.T.; Christie, M.J.; Schulz, S. Phosphorylation-deficient G-protein-biased μ-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nat. Commun. 2019, 10, 367. [Google Scholar] [CrossRef]
- Gurevich, V.V.; Gurevich, E.V. GPCR signaling regulation: The role of GRKs and arrestins. Front. Pharmacol. 2019, 10, 125. [Google Scholar] [CrossRef]
- Rizzi, A.; Cerlesi, M.C.; Ruzza, C.; Malfacini, D.; Ferrari, F.; Bianco, S.; Costa, T.; Guerrini, R.; Trapella, C.; Calo’, G. Pharmacological characterization of cebranopadol a novel analgesic acting as mixed nociceptin/orphanin FQ and opioid receptor agonist. Pharmacol. Res. Perspect. 2016, 4, e00247. [Google Scholar] [CrossRef]
- Gan, T.J.; Wase, L. Oliceridine, a G protein-selective ligand at the μ-opioid receptor, for the management of moderate to severe acute pain. Drugs Today 2020, 56, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.T.; Pitis, P.; Liu, G.; Yuan, C.; Gotchev, D.; Cowan, C.L.; Rominger, D.H.; Koblish, M.; Dewire, S.M.; Crombie, A.L.; et al. Structure-activity relationships and discovery of a g protein biased μ opioid receptor ligand, [(3-methoxythiophen-2-yl)methyl]({2-[(9 r)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]decan-9-yl]ethyl})amine (TRV130), for the treatment of acute severe pain. J. Med. Chem. 2013, 56, 8019–8031. [Google Scholar] [CrossRef]
- DeWire, S.M.; Yamashita, D.S.; Rominger, D.H.; Liu, G.; Cowan, C.L.; Graczyk, T.M.; Chen, X.T.; Pitis, P.M.; Gotchev, D.; Yuan, C.; et al. A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphines. J. Pharmacol. Exp. Ther. 2013, 344, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Kuzumaki, N.; Arima, T.; Narita, M.M.; Tateishi, R.; Kondo, T.; Hamada, Y.; Kuwata, H.; Kawata, M.; Yamazaki, M.; et al. Usefulness for the combination of G protein- and β-arrestin-biased ligands of μ-opioid receptors: Prevention of antinociceptive tolerance. Mol. Pain 2017, 13, 1744806917740030. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.Y.; Li, W.W.; Nwaneshiudu, C.; Irvine, K.A.; Clark, J.D. Pharmacological Characters of Oliceridine, a μ-Opioid Receptor G-Protein-Biased Ligand in Mice. Anesth. Analg. 2019, 129, 1414–1421. [Google Scholar] [CrossRef]
- Austin Zamarripa, C.; Edwards, S.R.; Qureshi, H.N.; Yi, J.N.; Blough, B.E.; Freeman, K.B. The G-protein biased mu-opioid agonist, TRV130, produces reinforcing and antinociceptive effects that are comparable to oxycodone in rats. Drug Alcohol Depend. 2018, 192, 158–162. [Google Scholar] [CrossRef]
- Schwienteck, K.L.; Faunce, K.E.; Rice, K.C.; Obeng, S.; Zhang, Y.; Blough, B.E.; Grim, T.W.; Negus, S.S.; Banks, M.L. Effectiveness comparisons of G-protein biased and unbiased mu opioid receptor ligands in warm water tail-withdrawal and drug discrimination in male and female rats. Neuropharmacology 2019, 150, 200–209. [Google Scholar] [CrossRef]
- Altarifi, A.A.; David, B.; Muchhala, K.H.; Blough, B.E.; Akbarali, H.; Negus, S.S. Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J. Psychopharmacol. 2017, 31, 730–739. [Google Scholar] [CrossRef]
- Granier, S.; Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Weis, W.I.; Kobilka, B.K. Structure of the δ-opioid receptor bound to naltrindole. Nature 2012, 485, 400–404. [Google Scholar] [CrossRef]
- Schneider, S.; Provasi, D.; Filizola, M. How oliceridine (TRV-130) binds and stabilizes a μ-opioid receptor conformational state that selectively triggers G protein signaling pathways. Biochemistry 2016, 55, 6456–6466. [Google Scholar] [CrossRef]
- Cheng, J.X.; Cheng, T.; Li, W.H.; Liu, G.X.; Zhu, W.L.; Tang, Y. Computational insights into the G-protein-biased activation and inactivation mechanisms of the μ opioid receptor. Acta Pharmacol. Sin. 2018, 39, 154–164. [Google Scholar] [CrossRef]
- Mafi, A.; Kim, S.-K.; Goddard, W.A. Mechanism of β-arrestin recruitment by the μ-opioid G protein-coupled receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 16346–16355. [Google Scholar] [CrossRef]
- Soergel, D.G.; Ann Subach, R.; Sadler, B.; Connell, J.; Marion, A.S.; Cowan, C.L.; Violin, J.D.; Lark, M.W. First clinical experience with TRV130: Pharmacokinetics and pharmacodynamics in healthy volunteers. J. Clin. Pharmacol. 2014, 54, 351–357. [Google Scholar] [CrossRef]
- Soergel, D.G.; Subach, R.A.; Burnham, N.; Lark, M.W.; James, I.E.; Sadler, B.M.; Skobieranda, F.; Violin, J.D.; Webster, L.R. Biased agonism of the l-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: A randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain 2014, 155, 1829–1835. [Google Scholar] [CrossRef]
- Viscusi, E.R.; Webster, L.; Kuss, M.; Daniels, S.; Bolognese, J.A.; Zuckerman, S.; Soergel, D.G.; Subach, R.A.; Cook, E.; Skobieranda, F. A randomized, phase 2 study investigating TRV130, a biased ligand of the -opioid receptor, for the intravenous treatment of acute pain. Pain 2016, 157, 264–272. [Google Scholar] [CrossRef]
- Singla, N.; Minkowitz, H.S.; Soergel, D.G.; Burt, D.A.; Subach, R.A.; Salamea, M.Y.; Fossler, M.J.; Skobieranda, F. A randomized, phase IIb study investigating oliceridine (TRV130), a novel µ-receptor G-protein pathway selective (µ-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty. J. Pain Res. 2017, 10, 2413–2424. [Google Scholar] [CrossRef]
- Dahan, A.; van Dam, C.J.; Niesters, M.; van Velzen, M.; Fossler, M.J.; Demitrack, M.A.; Olofsen, E. Benefit and Risk Evaluation of Biased μ-Receptor Agonist Oliceridine versus Morphine. Anesthesiology 2020, 133, 559–568. [Google Scholar] [CrossRef]
- Singla, N.K.; Skobieranda, F.; Soergel, D.G.; Salamea, M.; Burt, D.A.; Demitrack, M.A.; Viscusi, E.R. APOLLO-2: A Randomized, Placebo and Active-Controlled Phase III Study Investigating Oliceridine (TRV130), a G Protein–Biased Ligand at the μ-Opioid Receptor, for Management of Moderate to Severe Acute Pain Following Abdominoplasty. Pain Pract. 2019, 19, 715–731. [Google Scholar] [CrossRef]
- Viscusi, E.R.; Skobieranda, F.; Soergel, D.G.; Cook, E.; Burt, D.A.; Singla, N. APOLLO-1: A randomized placebo and activecontrolled phase iii study investigating oliceridine (TRV130), a G protein-biased ligand at the μ-opioid receptor, for management of moderateto-severe acute pain following bunionectomy. J. Pain Res. 2019, 12, 927–943. [Google Scholar] [CrossRef]
- Bergese, S.D.; Brzezinski, M.; Hammer, G.B.; Beard, T.L.; Pan, P.H.; Mace, S.E.; Berkowitz, R.D.; Cochrane, K.; Wase, L.; Minkowitz, H.S.; et al. ATHENA: A phase 3, open-label study of the safety and effectiveness of oliceridine (TRV130), a g-protein selective agonist at the µ-opioid receptor, in patients with moderate to severe acute pain requiring parenteral opioid therapy. J. Pain Res. 2019, 12, 3113–3126. [Google Scholar] [CrossRef]
- Manglik, A.; Lin, H.; Aryal, D.K.; McCorvy, J.D.; Dengler, D.; Corder, G.; Levit, A.; Kling, R.C.; Bernat, V.; Hübner, H.; et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature 2016, 537, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Mathiesen, J.M.; Sunahara, R.K.; Pardo, L.; Weis, W.I.; Kobilka, B.K.; Granier, S. Crystal structure of the μ-opioid receptor bound to a morphinan antagonist. Nature 2012, 485, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Disney, A.; Conibear, A.; Sutcliffe, K.; Dewey, W.; Husbands, S.; Bailey, C.; Kelly, E.; Henderson, G. The novel μ-opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. Br. J. Pharmacol. 2018, 175, 2653–2661. [Google Scholar] [CrossRef]
- Hill, R.; Lyndon, A.; Withey, S.; Roberts, J.; Kershaw, Y.; Maclachlan, J.; Lingford-Hughes, A.; Kelly, E.; Bailey, C.; Hickman, M.; et al. Ethanol reversal of tolerance to the respiratory depressant effects of morphine. Neuropsychopharmacology 2016, 41, 762–773. [Google Scholar] [CrossRef]
- Kudla, L.; Bugno, R.; Skupio, U.; Wiktorowska, L.; Solecki, W.; Wojtas, A.; Golembiowska, K.; Zádor, F.; Benyhe, S.; Buda, S.; et al. Functional characterization of a novel opioid, PZM21, and its effects on the behavioural responses to morphine. Br. J. Pharmacol. 2019, 176, 4434–4445. [Google Scholar] [CrossRef]
- Ding, H.; Kiguchi, N.; Perrey, D.; Nguyen, T.; Czoty, P.; Hsu, F.-C.; Zhang, Y.; Ko, M.C. Antinociceptive, Reinforcing, and Pruritic Effects of a G-Protein Signalling-Biased Mu Opioid Receptor Agonist, PZM21, in Nonhuman Primates. Br. J. Anaesth. 2020, in press. [Google Scholar] [CrossRef]
- Ma, M.; Sun, J.; Li, M.; Yu, Z.; Cheng, J.; Zhong, B.; Shi, W. Synthesis and evaluation of novel biased µ-opioid-receptor (µOR) agonists. Molecules 2019, 24, 259. [Google Scholar] [CrossRef]
- Ma, M.; Li, X.; Tong, K.; Cheng, J.; Yu, Z.; Ren, F.; Zhong, B.; Shi, W. Discovery of Biased Mu-Opioid Receptor Agonists for the Treatment of Pain. ChemMedChem 2020, 15, 155–161. [Google Scholar] [CrossRef]
- Schmid, C.L.; Kennedy, N.M.; Ross, N.C.; Lovell, K.M.; Yue, Z.; Morgenweck, J.; Cameron, M.D.; Bannister, T.D.; Bohn, L.M. Bias Factor and Therapeutic Window Correlate to Predict Safer Opioid Analgesics. Cell 2017, 171, 1165.e13–1175.e13. [Google Scholar] [CrossRef]
- Black, J.W.; Leff, P. Operational models of pharmacological agonism. Proc. R. Soc. Biol. Sci. 1983, 220, 141–162. [Google Scholar] [CrossRef]
- Grim, T.W.; Schmid, C.L.; Stahl, E.L.; Pantouli, F.; Ho, J.H.; Acevedo-Canabal, A.; Kennedy, N.M.; Cameron, M.D.; Bannister, T.D.; Bohn, L.M. A G protein signaling-biased agonist at the μ-opioid receptor reverses morphine tolerance while preventing morphine withdrawal. Neuropsychopharmacology 2020, 45, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Gillis, A.; Gondin, A.B.; Kliewer, A.; Sanchez, J.; Lim, H.D.; Alamein, C.; Manandhar, P.; Santiago, M.; Fritzwanker, S.; Schmiedel, F.; et al. Low intrinsic efficacy for G protein activation can explain the improved side effect profiles of new opioid agonists. Sci. Signal. 2020, 13, eaaz3140. [Google Scholar] [CrossRef] [PubMed]
- Yudin, Y.; Rohacs, T. The G-protein-biased agents PZM21 and TRV130 are partial agonists of μ-opioid receptor-mediated signalling to ion channels. Br. J. Pharmacol. 2019, 176, 3110–3125. [Google Scholar] [CrossRef]
- Gillis, A.; Sreenivasan, V.; Christie, M.J. Intrinsic efficacy of opioid ligands and its importance for apparent bias, operational analysis and therapeutic window. Mol. Pharmacol. 2020, 14, mol.119.119214. [Google Scholar] [CrossRef]
- Dahan, A. Opioid-induced respiratory effects: New data on buprenorphine. Palliat. Med. 2006, 20, s3–s8. [Google Scholar]
- Wolff, R.F.; Aune, D.; Truyers, C.; Hernandez, A.V.; Misso, K.; Riemsma, R.; Kleijnen, J. Systematic review of efficacy and safety of buprenorphine versus fentanyl or morphine in patients with chronic moderate to severe pain. Curr. Med. Res. Opin. 2012, 28, 833–845. [Google Scholar] [CrossRef]
- Michel, M.C.; Charlton, S.J. Biased agonism in drug discovery-is it too soon to choose a path? Mol. Pharmacol. 2018, 93, 259–265. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo Neto, J.; Costanzini, A.; De Giorgio, R.; Lambert, D.G.; Ruzza, C.; Calò, G. Biased versus Partial Agonism in the Search for Safer Opioid Analgesics. Molecules 2020, 25, 3870. https://doi.org/10.3390/molecules25173870
Azevedo Neto J, Costanzini A, De Giorgio R, Lambert DG, Ruzza C, Calò G. Biased versus Partial Agonism in the Search for Safer Opioid Analgesics. Molecules. 2020; 25(17):3870. https://doi.org/10.3390/molecules25173870
Chicago/Turabian StyleAzevedo Neto, Joaquim, Anna Costanzini, Roberto De Giorgio, David G. Lambert, Chiara Ruzza, and Girolamo Calò. 2020. "Biased versus Partial Agonism in the Search for Safer Opioid Analgesics" Molecules 25, no. 17: 3870. https://doi.org/10.3390/molecules25173870
APA StyleAzevedo Neto, J., Costanzini, A., De Giorgio, R., Lambert, D. G., Ruzza, C., & Calò, G. (2020). Biased versus Partial Agonism in the Search for Safer Opioid Analgesics. Molecules, 25(17), 3870. https://doi.org/10.3390/molecules25173870







