Phenylpiperazine 5,5-Dimethylhydantoin Derivatives as First Synthetic Inhibitors of Msr(A) Efflux Pump in Staphylococcus epidermidis
Abstract
1. Introduction
2. Results
2.1. Chemistry
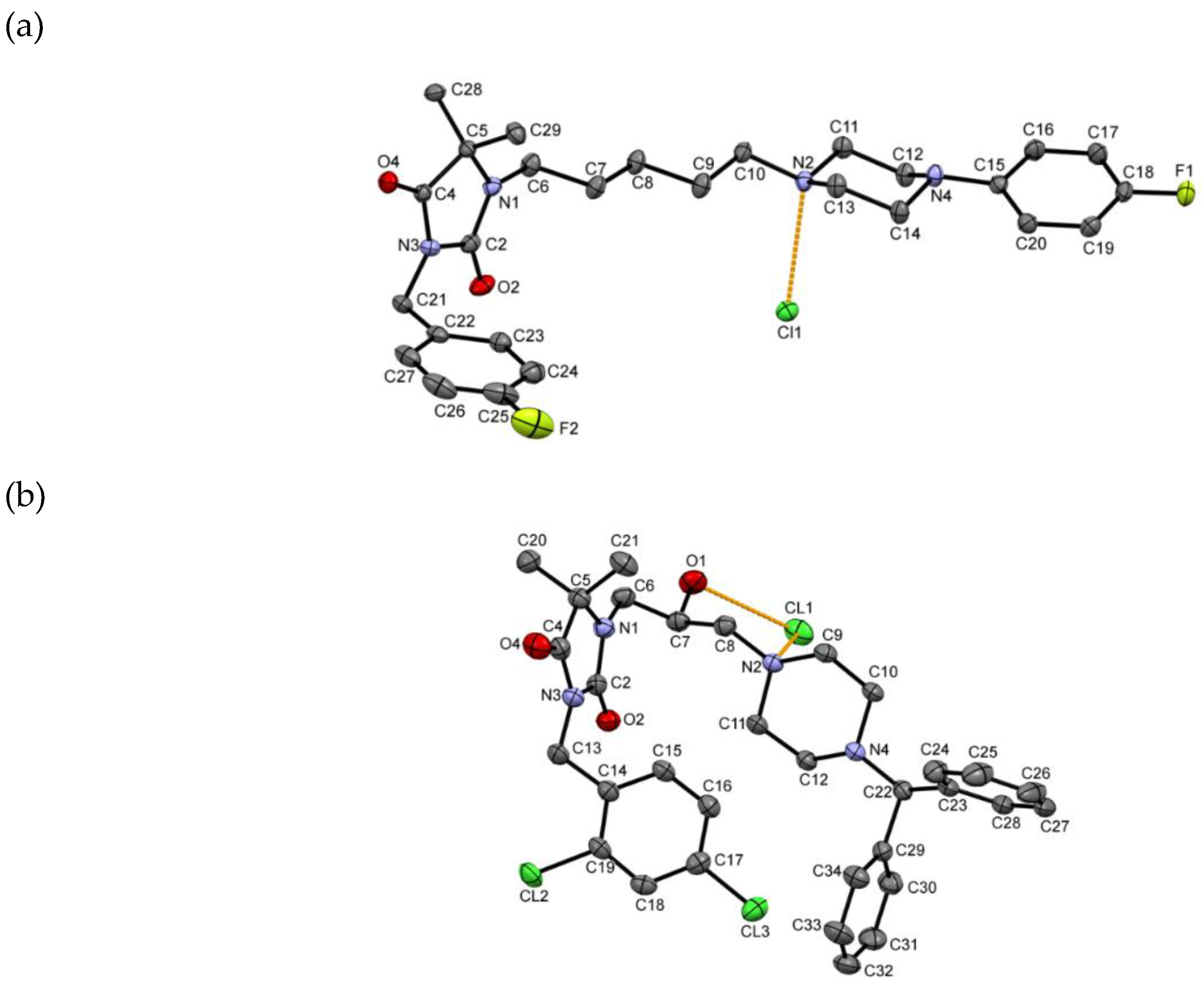
2.2. X-ray Crystallographic Studies
2.3. Biological Assays
2.3.1. Direct Antibacterial Activity
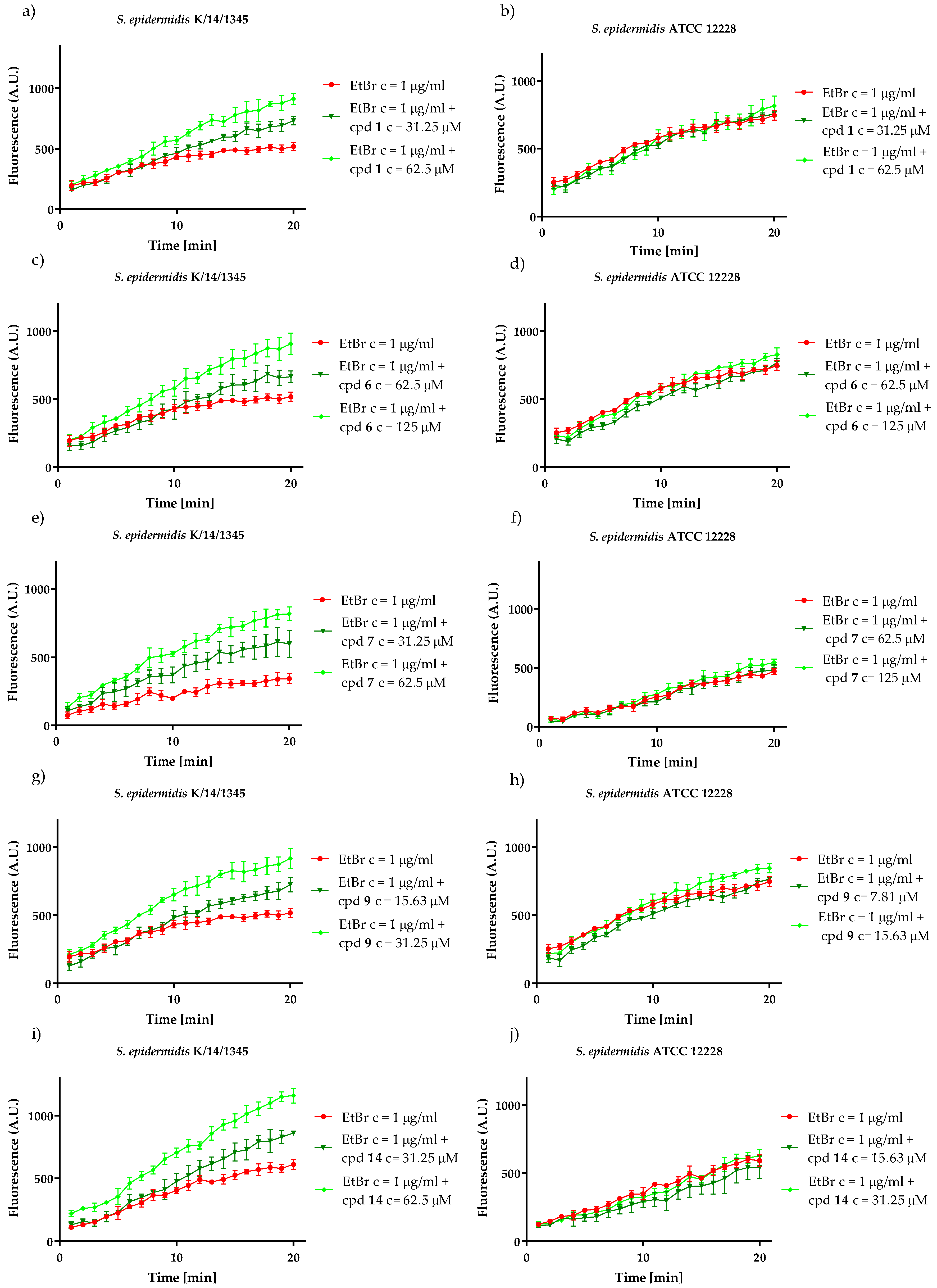
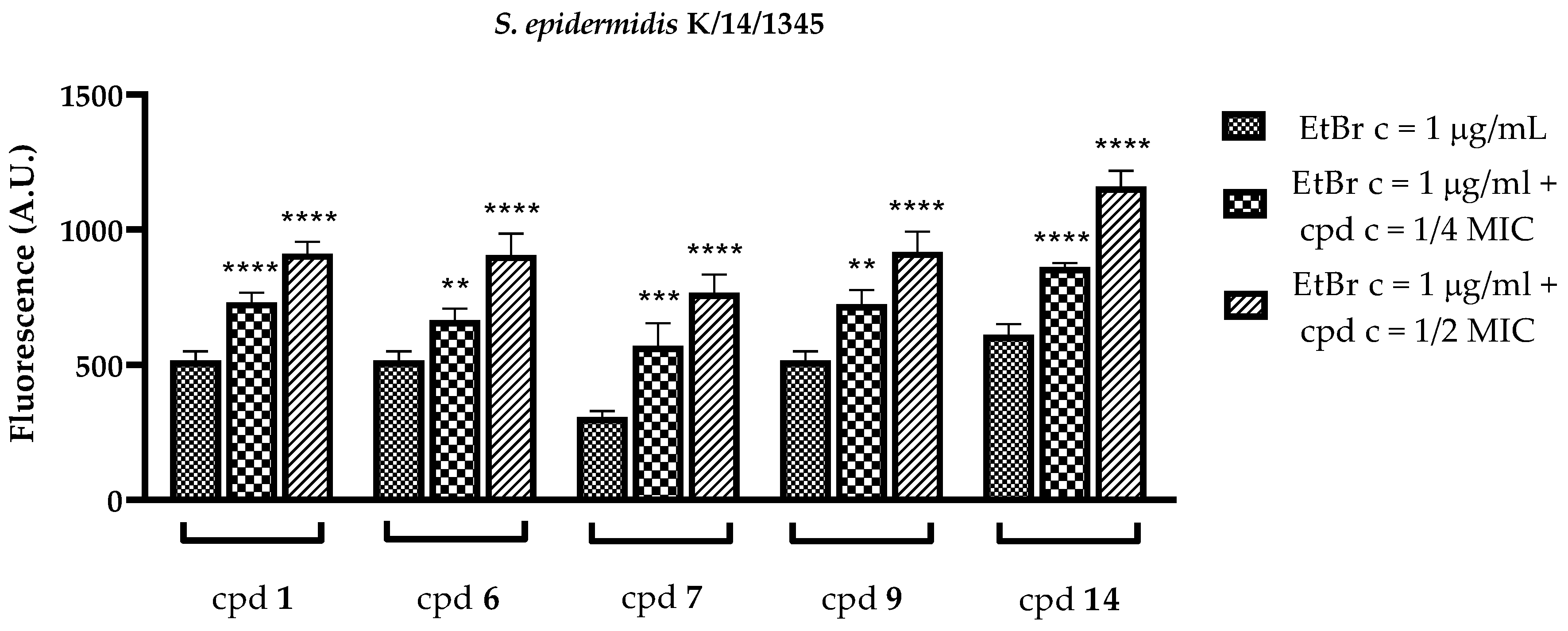
2.3.2. Inhibitory Action on Msr(A) Efflux Pump
2.3.3. Influence on the Activity of Erythromycin
3. Discussion
4. Experimental
4.1. Chemistry
4.1.1. 3-(4-Chlorobenzyl)-5,5-dimethylimidazolidine-2,4-dione (18)
4.1.2. 3-(4-Chlorobenzyl)-1-(6-bromohexyll)-5,5-dimethylimidazolidine-2,4-dione (22)
4.1.3. 3-(2,4-Dichlorobenzyl)-5,5-dimethyl-1-(oxiran-2-ylmethyl)imidazolidine-2,4-dione (24)
4.1.4. General Procedure to Obtain Final Products (8–11)
3-(4-Chlorobenzyl)-1-(6-(4-(2-fluorophenyl)piperazin-1-yl)hexyl)-5,5-dimethylimidazolidine-2,4-dione hydrochloride (8)
3-(2,4-Dichlorobenzyl)-1-(5-(4-(2-fluorophenyl)piperazin-1-yl)pentyl)-5,5-dimethylimidazolidine-2,4-dione hydrochloride (9)
3-(2,4-Dichlorobenzyl)-1-(5-(4-(4-fluorophenyl)piperazin-1-yl)pentyl)-5,5-dimethylimidazolidine-2,4-dione hydrochloride (10)
3-(2,4-Dichlorobenzyl)-1-(5-(4-(2,4-difluorophenyl)piperazin-1-yl)pentyl)-5,5-dimethylimidazolidine-2,4-dione hydrochloride (11)
4.1.5. General Procedure to Obtain Final Products (12–15)
3-(2,4-Dichlorobenzyl)-1-(3-(4-(2-fluorophenyl)piperazin-1-yl)-2-hydroxypropyl)-5,5-dimethylimidazolidine-2,4-dione hydrochloride (12)
3-(2,4-Dichlorobenzyl)-1-(3-(4-(4-fluorophenyl)piperazin-1-yl)-2-hydroxypropyl)-5,5-dimethylimidazolidine-2,4-dione hydrochloride (13)
3-(2,4-Dichlorobenzyl)-1-(3-(4-(2,4-difluorophenyl)piperazin-1-yl)-2-hydroxypropyl)-5,5-dimethylimidazolidine-2,4-dione hydrochloride (14)
3-(2,4-Dichlorobenzyl)-1-(3-(4-benzhydrylpiperazin-1-yl)-2-hydroxypropyl)-5,5-dimethylimidazolidine-2,4-dione hydrochloride (15)
4.2. Crystallographic Studies
4.3. Microbiological Studies
4.3.1. Susceptibility Testing
4.3.2. Ethidium Bromide Accumulation Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Namvar, A.E.; Bastarahang, S.; Abbasi, N.; Ghehi, G.S.; Farhadbakhtiarian, S.; Arezi, P.; Hosseini, M.; Baravati, S.Z.; Jokar, Z.; Chermahin, S.G. Clinical characteristics of Staphylococcus epidermidis: A systematic review. GMS Hyg. Infect. Control 2014, 9, 1–10. [Google Scholar]
- Otto, M. Molecular basis of Staphylococcus epidermidis infections. Semin. Immunopathol. 2012, 34, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Chessa, D.; Ganau, G.; Mazzarello, V. An overview of Staphylococcus epidermidis and Staphylococcus aureus with a focus on developing countries. J. Infect. Dev. Ctries. 2015, 9, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Hischebeth, G.T.; Randau, T.M.; Ploeger, M.M.; Friedrich, M.J.; Kaup, E.; Jacobs, C.; Molitor, E.; Hoerauf, A.; Gravius, S.; Wimmer, M.D. Staphylococcus aureus versus Staphylococcus epidermidis in periprosthetic joint infection-Outcome analysis of methicillin-resistant versus methicillin-susceptible strains. Diagn. Microbiol. Infect. Dis. 2019, 93, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Trallero, E.; Montes, M.; Orden, B.; Tamayo, E.; García-Arenzana, J.M.; Marimón, J.M. Phenotypic and genotypic characterization of Streptococcus pyogenes isolates displaying the MLSB phenotype of macrolide resistance in Spain, 1999 to 2005. Antimicrob. Agents Chemother. 2007, 51, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Saderi, H.; Emadi, B.; Owlia, P. Phenotypic and genotypic study of macrolide, lincosamide and streptogramin B (MLSB) resistance in clinical isolates of Staphylococcus aureus in Tehran, Iran. Med. Sci. Monit. 2011, 17, BR48–BR53. [Google Scholar] [CrossRef]
- Dzierżanowska, D. Antybiotykoterapia Praktyczna, 6; Alfa Medica Press: Bielsko-Biała, Poland, 2018. [Google Scholar]
- Durmaz, S. Macrolide-lincosamide-streptogramin B resistance phenotypes in Staphylococcus aureus. Eur. J. Gen. Med. 2015, 11, 217–220. [Google Scholar] [CrossRef]
- Mišic, M. Prevalence of genotypes that determine resistance of staphylococci to macrolides and lincosamides in Serbia. Front. Public Health 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Ojo, K.K.; Striplin, M.J.; Ulep, C.C.; Close, N.S.; Zittle, J.; Luis, H.; Bernardo, M.; Leitao, J.; Roberts, M.C. Staphylococcus efflux msr(A) gene characterized in Streptococcus, Enterococcus, Corynebacterium, and Pseudomonas isolates. Antibact. Agents Chemother. 2006, 50, 1089–1091. [Google Scholar] [CrossRef]
- Juda, M.; Chudzik-Rzad, B.; Malm, A. The prevalence of genotypes that determine resistance to macrolides, lincosamides, and streptogramins B compared with spiramycin susceptibility among erythromycin-resistant Staphylococcus epidermidis. Mem. Inst. Oswaldo Cruz 2016, 111, 155–160. [Google Scholar] [CrossRef]
- Fyfe, C.; Grossman, T.H.; Kerstein, K.; Sutcliffe, J. Resistance to macrolide antibiotics in public health pathogens. Cold Spring Harb. Perspect. Med. 2016, 6, 1–38. [Google Scholar] [CrossRef]
- Reynolds, E.; Ross, J.I.; Cove, J.H. Msr(A) and related macrolide/streptogramin resistance determinants: Incomplete transporters? Int. J. Antimicrob. Agents 2003, 22, 228–236. [Google Scholar] [CrossRef]
- Petinak, E.; Papagiannitsis, C. Resistance of staphylococci to macrolides-lincosamides-streptogramins B (MLSB): Epidemiology and mechanisms of resistance. In Staphylococcus Aureus; Hemeg, H., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Eady, E.A.; Ross, J.I.; Tipper, J.L.; Walters, C.E.; Cove, J.H.; Noble, W.C. Distribution of genes encoding erythromycin ribosomal methylases and an erythromycin efflux pump in epidemiologically distinct groups of staphylococci. J. Antimicrob. Chemother. 1993, 31, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Birtić, S.; Dussort, P.; Pierre, F.X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Dymek, A.; Armada, A.; Handzlik, J.; Viveiros, M.; Spengler, G.; Molnar, J.; Kieć-Kononowicz, K.; Amaral, L. The activity of 16 new hydantoin compounds on the intrinsic and overexpressed efflux pump system of Staphylococcus aureus. In Vivo 2012, 26, 223–229. [Google Scholar]
- Handzlik, J.; Bojarski, A.J.; Satała, G.; Kubacka, M.; Kucwaj, K.; Filipek, B.; Kieć-Kononowicz, K. SAR-studies on the importance of aromatic ring topologies in search for selective 5-HT7 receptor ligands among phenylpiperazine hydantoin derivatives. Eur. J. Med. Chem. 2014, 78, 324–339. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.; Piddock, L.J. How to measure export via bacterial multidrug resistance efflux pumps. MBio 2016, 7, e00840-16. [Google Scholar] [CrossRef]
- Kaczor, A.; Witek, K.; Podlewska, S.; Czekajewska, J.; Lubelska, A.; Zesławska, E.; Nitek, W.; Latacz, G.; Alibert, S.; Pagès, J.M.; et al. 5-arylideneimidazolones with amine at position 3 as potential antibiotic adjuvants against multidrug resistant bacteria. Molecules 2019, 24, 438. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Ciazovazzo, C.; Mallamo, M.; Mazzone, A.G.; Polidori, G. Crystal structure determination and refinement via SIR2014. J. Appl. Cryst. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard-Ninth Edition; CLSI Document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Otrębska-Machaj, E.; Chevalier, J.; Handzlik, J.; Szymańska, E.; Schabikowski, J.; Boyer, G.; Bolla, J.-M.; Kieć-Kononowicz, K.; Pagès, J.-M.; Alibert, S. Efflux pump blockers in Gram-negative bacteria: The new generation of hydantoin based-modulators to improve antibiotic activity. Front. Microbiol. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Couto, I.; Costa, S.S.; Viveiros, M.; Martins, M.; Amaral, L. Efflux-mediated response of Staphylococcus aureus exposed to ethidium bromide. J. Antimicrob. Chemother. 2008, 62, 504–513. [Google Scholar] [CrossRef]
- Viveiros, M.; Rodrigues, L.; Martins, M.; Couto, I.; Spengler, G.; Martins, A.; Amaral, L. Evaluation of efflux activity of bacteria by a semi-automated fluorometric system. Methods Mol. Biol. 2010, 642, 159–172. [Google Scholar]
- Paixão, L.; Rodrigues, L.; Couto, I.; Martins, M.; Fernandes, P.; de Carvalho, C.C.C.R.; Monteiro, G.A.; Sansonetty, F.; Amaral, L.; Viveiros, M. Fluorometric determination of ethidium bromide efflux kinetics in Escherichia coli. J. Biol. Eng. 2009, 3, 1–13. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

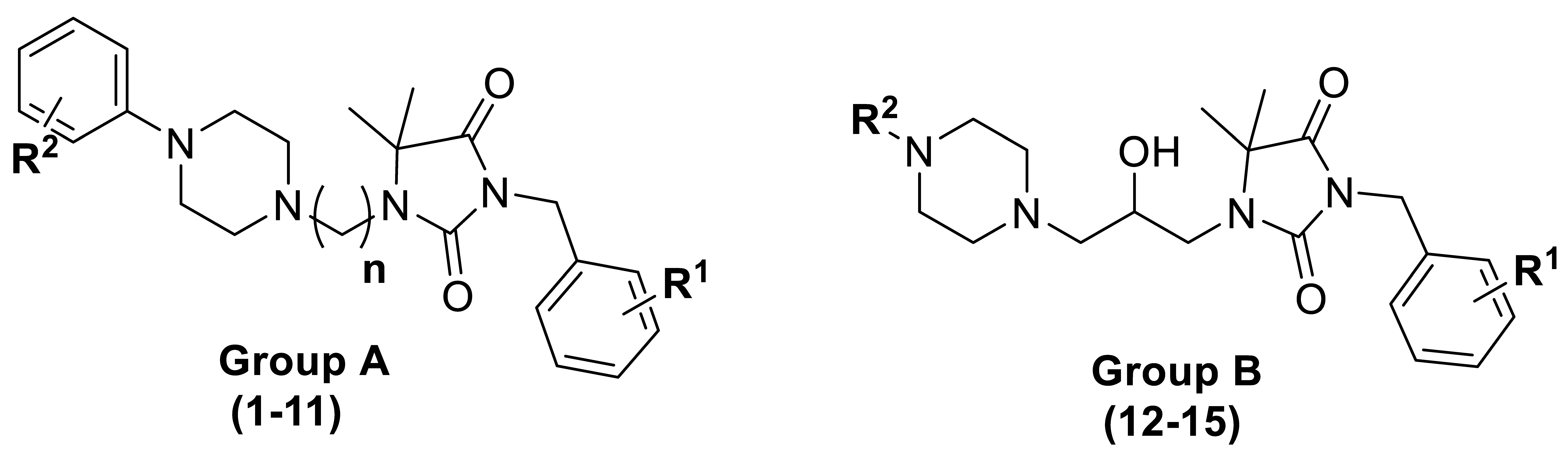
| Cpd | Group | R1 | n | R2 |
|---|---|---|---|---|
| 1 | A | H | 5 | 2,4-diF |
| 2 | A | H | 5 | H |
| 3 | A | H | 5 | 2-MeO |
| 4 | A | H | 5 | 3-MeO |
| 5 | A | H | 5 | 2-F |
| 6 | A | 4-F | 5 | 4-F |
| 7 | A | 4-F | 5 | 2,4-diF |
| 8 | A | 4-Cl | 6 | 2-F |
| 9 | A | 2,4-diCl | 5 | 2-F |
| 10 | A | 2,4-diCl | 5 | 4-F |
| 11 | A | 2,4-diCl | 5 | 2,4-diF |
| 12 | B | 2,4-diCl | - |  |
| 13 | B | 2,4-diCl | - | 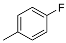 |
| 14 | B | 2,4-diCl | - |  |
| 15 | B | 2,4-diCl | - |  |
| Cmp. | Interaction | H···A (Å) | D···A (Å) | D-H···A (°) | Symmetry Code |
|---|---|---|---|---|---|
| 6 | N2-H2N···Cl1 | 2.14 | 3.023(1) | 176 | |
| C14-H14A···Cl1 | 2.72 | 3.558(1) | 142 | −x + 1, −y + 1, −z + 1 | |
| C20-H20···Cl1 | 2.85 | 3.753(1) | 159 | −x + 1, −y + 1, −z + 1 | |
| C28-H28C···Cl1 | 2.84 | 3.735(2) | 152 | −x, −y + 1, −z + 1 | |
| C10-H10A···O2 | 2.36 | 3.187(2) | 141 | −x, −y + 1, −z + 1 | |
| C12-H12B···O4 | 2.50 | 3.046(2) | 114 | x + 1, −y + 1/2, z + 1/2 | |
| C21-H21B···O4 | 2.66 | 3.385 (2) | 131 | −x − 1, y + 1/2, −z + 1/2 | |
| C13-H13B···N4 | 2.57 | 3.531(2) | 163 | −x + 1, −y, −z + 1 | |
| C29-H29C···F1 | 2.54 | 3.500(2) | 166 | −x + 1, −y, −z + 1 | |
| 15 | N2-H2N···Cl1 | 2.07 | 3.067(2) | 174 | |
| O1-H1···Cl1 | 2.12 | 3.049(2) | 168 | ||
| C9-H9A···Cl1 | 2.66 | 3.645(2) | 179 | X − 1, y, z | |
| C16-H16···Cl1 | 2.91 | 3.638(2) | 135 | X − 1, y, z | |
| C21-H21C···Cl1 | 2.95 | 3.755(2) | 140 | −x + 2, −y + 1, −z + 1 | |
| C6-H6B···O4 | 2.59 | 3.550(3) | 163 | x + 1, y, z | |
| C9-H9A···O1 | 2.36 | 3.325(3) | 166 | −x + 2, −y + 1, −z + 1 | |
| C18-H18···O2 | 2.58 | 3.463(3) | 156 | −x + 1, −y + 2, −z + 1 | |
| C21-H21A···O1 | 2.64 | 3.358(3) | 131 | X − 1, y, z |
| Compounds | MIC [µM] (µg/mL) | |
|---|---|---|
| S. epidermidis K/14/1345 | S. epidermidis ATCC 12228 | |
| 1 | >250 (130.26) 1 | 250 (130.26) |
| 2 | >250 (121.27) 1 | >250 (121.27) 1 |
| 3 | 1000 (515.09) | 250 (128.77) |
| 4 | >125 (64.39) 1 | 62.5 (32.19) |
| 5 | >500 (251.53) 1 | 250 (125.96) |
| 6 | >250 (130.26) 1 | 250 (130.26) |
| 7 | 125 (67.38) | 250 (134.76) |
| 8 | >62.5 (34.38) 1 | 62.5 (34.38) |
| 9 | >62.5 (35.74) 1 | 31.25 (17.88) |
| 10 | >31.25 (17.88) 1 | 31.25 (17.88) |
| 11 | >31.25 (18.44) 1 | 15.63 (9.22) |
| 12 | >125 (70) 1 | 62.5 (35) |
| 13 | >125 (69.99) 1 | 125 (69.99) |
| 14 | >125 (72.24) 1 | 62.5 (36.12) |
| 15 | >31.25 (19.76) 1 | 7.81 (4.94) |
| Erythromycin | 0.087 (64) | 0.00068 (0.5) |
| SA | SA | ||||
|---|---|---|---|---|---|
| S. epidermidis K/14/1345 | S. epidermidis ATCC 12228 | ||||
| RFI/µmol | RFI/µmol | ||||
| Compound | 1/2 MIC 1 | 1/4 MIC 1 | Compound | 1/2 MIC | 1/4 MIC |
| 1 | 12.22 | 13.31 | 1 | 1.48 | 0.094 |
| 6 | 6.02 | 4.59 | 6 | 0.87 | 0.49 |
| 7 | 22.16 | 23.77 | 7 | 0.65 | 0.23 |
| 9 | 24.82 | 25.66 | 9 | 4.29 | 1.52 |
| 14 | 14.32 | 13.04 | 14 | 1.9 | −5.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witek, K.; Latacz, G.; Kaczor, A.; Czekajewska, J.; Żesławska, E.; Chudzik, A.; Karczewska, E.; Nitek, W.; Kieć-Kononowicz, K.; Handzlik, J. Phenylpiperazine 5,5-Dimethylhydantoin Derivatives as First Synthetic Inhibitors of Msr(A) Efflux Pump in Staphylococcus epidermidis. Molecules 2020, 25, 3788. https://doi.org/10.3390/molecules25173788
Witek K, Latacz G, Kaczor A, Czekajewska J, Żesławska E, Chudzik A, Karczewska E, Nitek W, Kieć-Kononowicz K, Handzlik J. Phenylpiperazine 5,5-Dimethylhydantoin Derivatives as First Synthetic Inhibitors of Msr(A) Efflux Pump in Staphylococcus epidermidis. Molecules. 2020; 25(17):3788. https://doi.org/10.3390/molecules25173788
Chicago/Turabian StyleWitek, Karolina, Gniewomir Latacz, Aneta Kaczor, Joanna Czekajewska, Ewa Żesławska, Anna Chudzik, Elżbieta Karczewska, Wojciech Nitek, Katarzyna Kieć-Kononowicz, and Jadwiga Handzlik. 2020. "Phenylpiperazine 5,5-Dimethylhydantoin Derivatives as First Synthetic Inhibitors of Msr(A) Efflux Pump in Staphylococcus epidermidis" Molecules 25, no. 17: 3788. https://doi.org/10.3390/molecules25173788
APA StyleWitek, K., Latacz, G., Kaczor, A., Czekajewska, J., Żesławska, E., Chudzik, A., Karczewska, E., Nitek, W., Kieć-Kononowicz, K., & Handzlik, J. (2020). Phenylpiperazine 5,5-Dimethylhydantoin Derivatives as First Synthetic Inhibitors of Msr(A) Efflux Pump in Staphylococcus epidermidis. Molecules, 25(17), 3788. https://doi.org/10.3390/molecules25173788







