Ammonium Phosphate as Inhibitor to Mitigate the Corrosion of Steel Rebar in Chloride Contaminated Concrete Pore Solution
Abstract
1. Introduction
2. Experimental Details
2.1. Materials and Methods
2.2. Electrochemical Studies
2.3. Characterization of Passive Film
3. Results and Discussion
3.1. Potentiodynamic Polarization
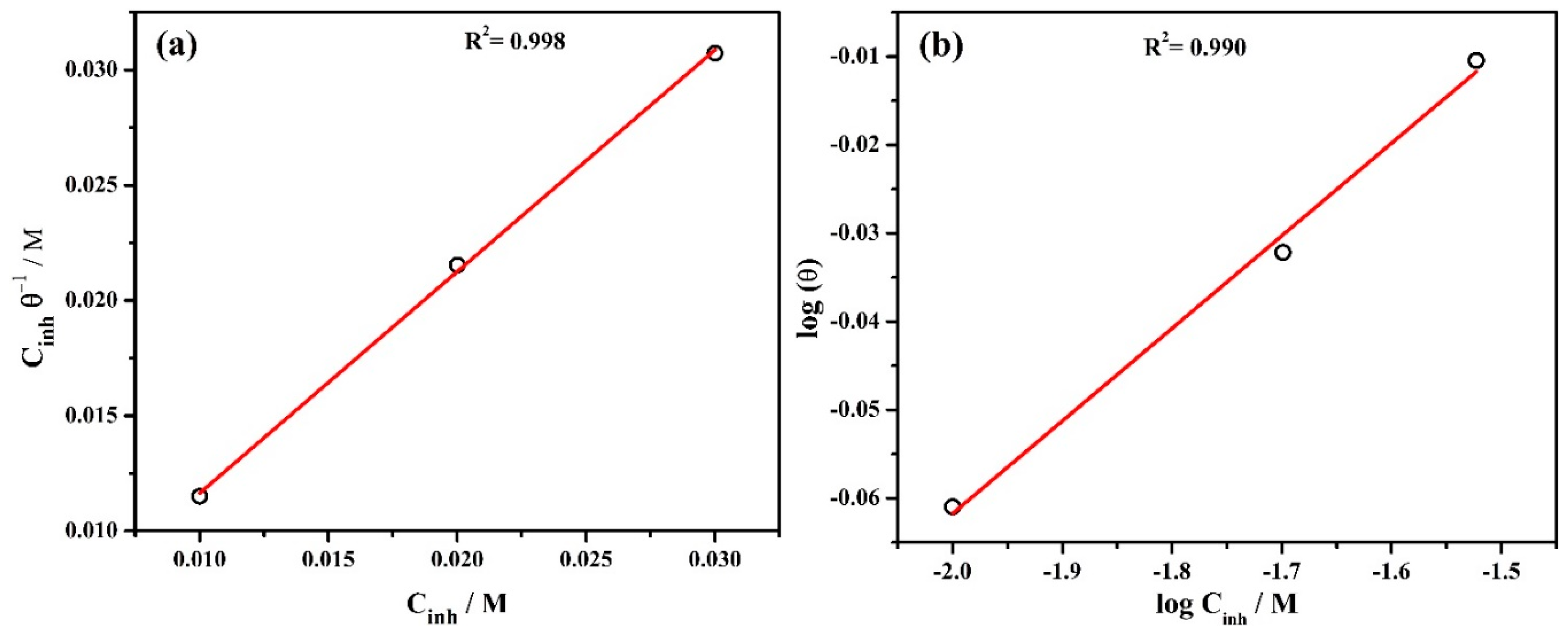
3.2. Adsorption Isotherm
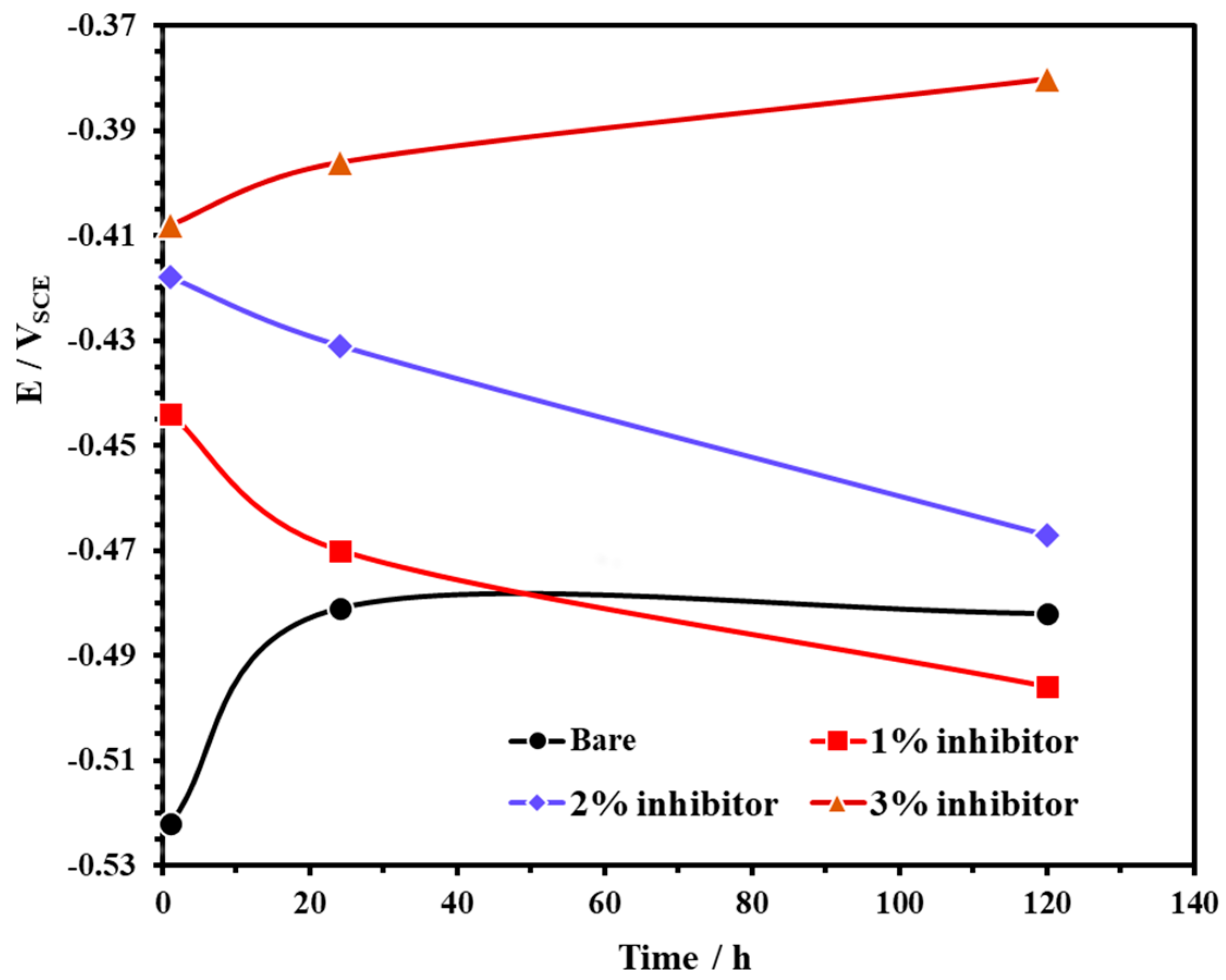
3.3. Open Circuit Potential (OCP) with Exposure Periods
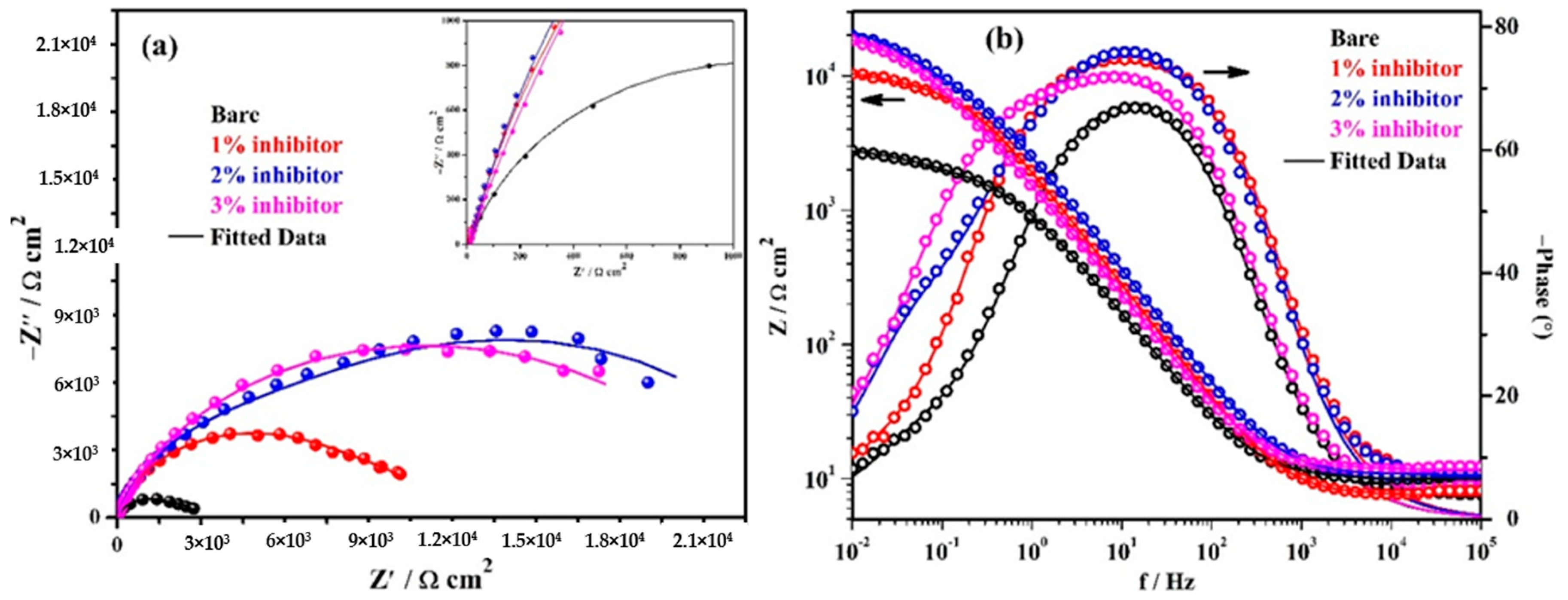
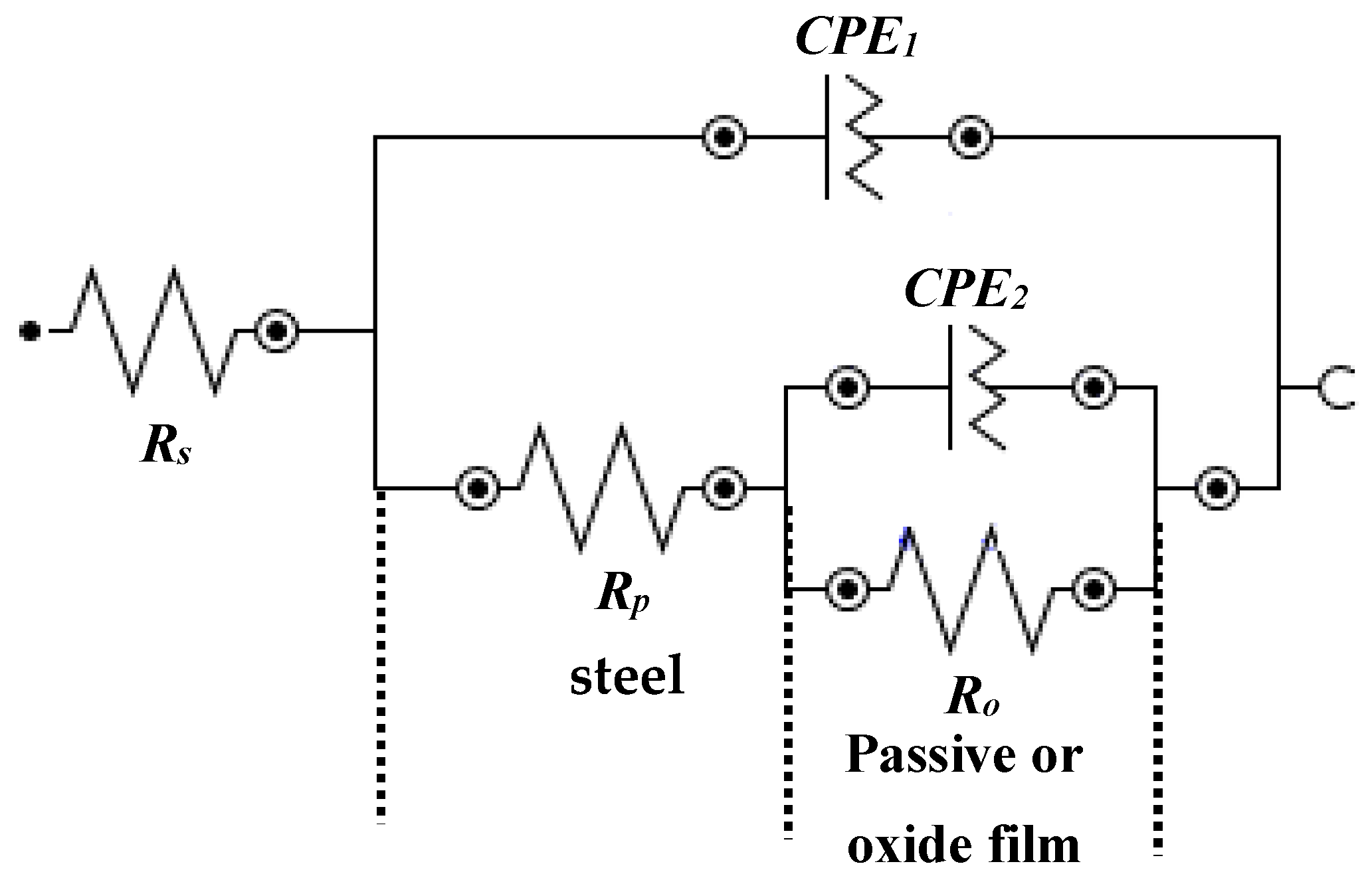
3.4. Electrochemical Impedance Spectroscopy (EIS) Studies with Exposure Periods
3.5. Nature of Corrosion Products/Passive Films Formed after 120 h of Exposure
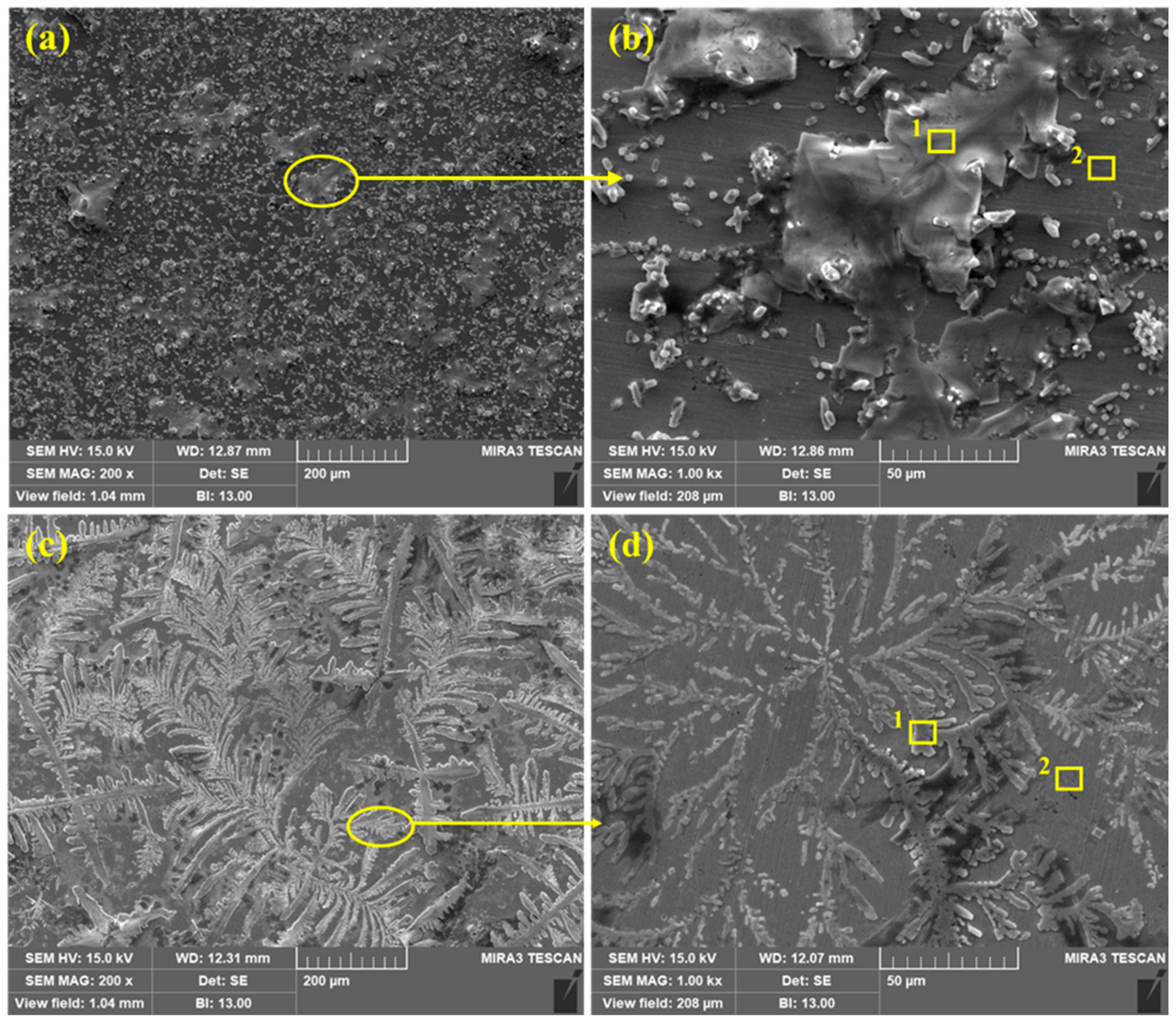
3.5.1. Scanning Electron Microscopy (SEM)
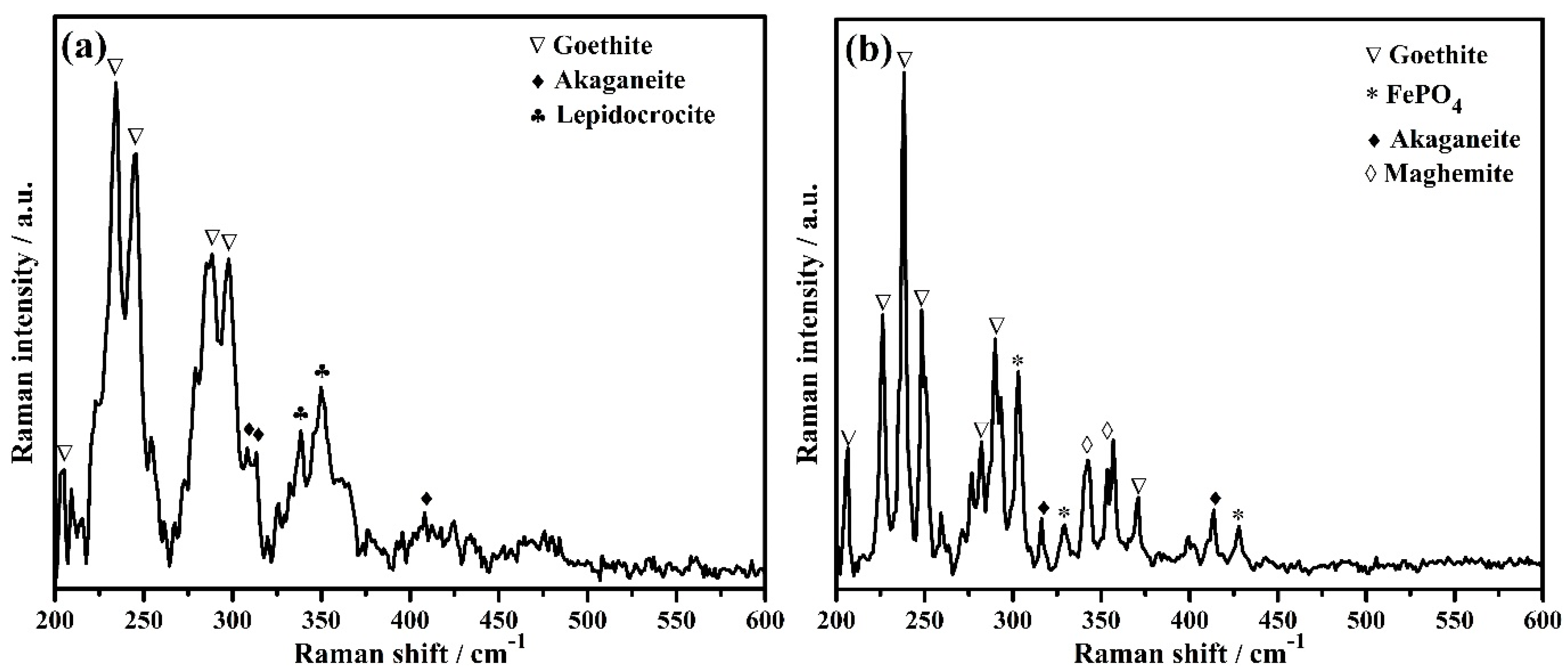
3.5.2. Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, H.; Liu, Y.; Chen, W.; Du, R.-G.; Lin, C. Corrosion behavior of reinforcing steel in simulated concrete pore solutions: A scanning micro-reference electrode study. Electrochim. Acta 2009, 54, 4067–4072. [Google Scholar] [CrossRef]
- Thangavel, K. The Threshold Limit for Chloride Corrosion of Reinforced Concrete. Corros. Rev. 2004, 22, 55–70. [Google Scholar] [CrossRef]
- Ahmad, S. Reinforcement corrosion in concrete structures, its monitoring and service life prediction––A review. Cem. Concr. Compos. 2003, 25, 459–471. [Google Scholar] [CrossRef]
- Biezma, M.V.; Cristóbal, J.R.S. Methodology to study cost of corrosion. Corros. Eng. Sci. Technol. 2005, 40, 344–352. [Google Scholar] [CrossRef]
- Page, C.L. Mechanism of corrosion protection in reinforced concrete marine structures. Nature 1975, 258, 514–515. [Google Scholar] [CrossRef]
- Singh, J.; Singh, D. The nature of rusts and corrosion characteristics of low alloy and plain carbon steels in three kinds of concrete pore solution with salinity and different pH. Corros. Sci. 2012, 56, 129–142. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Singh, J.; Yang, H.-M.; Lee, H.-S.; Ismail, M. Evaluation of corrosion resistance properties of N, N′-Dimethyl ethanolamine corrosion inhibitor in saturated Ca(OH)2 solution with different concentrations of chloride ions by electrochemical experiments. Constr. Build. Mater. 2016, 114, 223–231. [Google Scholar] [CrossRef]
- Huet, B.; L’Hostis, V.; Miserque, F.; Idrissi, H. Electrochemical behavior of mild steel in concrete: Influence of pH and carbonate content of concrete pore solution. Electrochim. Acta 2005, 51, 172–180. [Google Scholar] [CrossRef]
- Lee, H.-S.; Singh, J.; Ismail, M. An effective and novel pore sealing agent to enhance the corrosion resistance performance of Al coating in artificial ocean water. Sci. Rep. 2017, 7, 41935. [Google Scholar] [CrossRef]
- Etteyeb, N.; Dhouibi, L.; Takenouti, H.; Alonso, M.; Triki, E. Corrosion inhibition of carbon steel in alkaline chloride media by Na3PO4. Electrochim. Acta 2007, 52, 7506–7512. [Google Scholar] [CrossRef]
- Ghods, P.; Isgor, B.; McRae, G.; Miller, T. The effect of concrete pore solution composition on the quality of passive oxide films on black steel reinforcement. Cem. Concr. Compos. 2009, 31, 2–11. [Google Scholar] [CrossRef]
- Söylev, T.; Richardson, M. Corrosion inhibitors for steel in concrete: State-of-the-art report. Constr. Build. Mater. 2008, 22, 609–622. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Singh, J.; Lee, H.-S.; Ismail, M.; Park, W.-J. Effect of LiNO2 inhibitor on corrosion characteristics of steel rebar in saturated Ca(OH)2 solution containing NaCl: An electrochemical study. Constr. Build. Mater. 2017, 133, 387–396. [Google Scholar] [CrossRef]
- Lee, H.-S.; Yang, H.-M.; Singh, J.K.; Prasad, S.K.; Yoo, B. Corrosion mitigation of steel rebars in chloride contaminated concrete pore solution using inhibitor: An electrochemical investigation. Constr. Build. Mater. 2018, 173, 443–451. [Google Scholar] [CrossRef]
- Gouda, V.K. Corrosion and corrosion inhibition of reinforcing steel. Br. Corros. J. 1970, 5, 198–203. [Google Scholar] [CrossRef]
- Ormellese, M.; Lazzari, L.; Goidanich, S.; Fumagalli, G.; Brenna, A. A study of organic substances as inhibitors for chloride-induced corrosion in concrete. Corros. Sci. 2009, 51, 2959–2968. [Google Scholar] [CrossRef]
- Ormellese, M.; Berra, M.; Bolzoni, F.M.; Pastore, T. Corrosion inhibitors for chlorides induced corrosion in reinforced concrete structures. Cem. Concr. Res. 2006, 36, 536–547. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Asghari, E. Influence of flow on the corrosion inhibition of St52-3 type steel by potassium hydrogen-phosphate. Corros. Sci. 2009, 51, 1828–1835. [Google Scholar] [CrossRef]
- Dhouibi, L.; Triki, E.; Raharinaivo, A.; Trabanelli, G.; Zucchi, F. Electrochemical methods for evaluating inhibitors of steel corrosion in concrete. Br. Corros. J. 2000, 35, 145–149. [Google Scholar] [CrossRef]
- Manna, M.; Chakrabarti, I.; Bandyopadhyay, N. Phosphate treatment of TMT rebar bundle to avoid early rusting: An option for single step process. Surf. Coat. Technol. 2006, 201, 1583–1588. [Google Scholar] [CrossRef]
- Ngala, V.; Page, C.; Page, M. Corrosion inhibitor systems for remedial treatment of reinforced concrete. Part 2: Sodium monofluorophosphate. Corros. Sci. 2003, 45, 1523–1537. [Google Scholar] [CrossRef]
- Chaussadent, T.; Nobel-Pujol, V.; Farcas, F.; Mabille, I.; Fiaud, C. Effectiveness conditions of sodium monofluorophosphate as a corrosion inhibitor for concrete reinforcements. Cem. Concr. Res. 2006, 36, 556–561. [Google Scholar] [CrossRef]
- Alonso, C.; Andrade, C.; Argiz, C.; Malric, B. Na2PO3F as inhibitor of corroding reinforcement in carbonated concrete. Cem. Concr. Res. 1996, 26, 405–415. [Google Scholar] [CrossRef]
- Rajabalizadeh, Z.; Seifzadeh, D. Strontium phosphate conversion coating as an economical and environmentally-friendly pretreatment for electroless plating on AM60B magnesium alloy. Surf. Coat. Technol. 2016, 304, 450–458. [Google Scholar] [CrossRef]
- Zin’, I.M.; Lyon, S.; Pokhmurskii, V. Corrosion control of galvanized steel using a phosphate/calcium ion inhibitor mixture. Corros. Sci. 2003, 45, 777–788. [Google Scholar] [CrossRef]
- Simões, A.; Torres, J.P.N.; Picciochi, R.; Fernandes, J.C.S. Corrosion inhibition at galvanized steel cut edges by phosphate pigments. Electrochim. Acta 2009, 54, 3857–3865. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ghasemi, E.; Mahdavian, M.; Mahdavian, M. Corrosion inhibition by lithium zinc phosphate pigment. Corros. Sci. 2013, 77, 222–229. [Google Scholar] [CrossRef]
- Yohai, L.; Schreiner, W.; Vázquez, M.; Valcarce, M. Phosphate ions as effective inhibitors for carbon steel in carbonated solutions contaminated with chloride ions. Electrochim. Acta 2016, 202, 231–242. [Google Scholar] [CrossRef]
- Yohai, L.; Valcarce, M.; Vázquez, M. Testing phosphate ions as corrosion inhibitors for construction steel in mortars. Electrochim. Acta 2016, 202, 316–324. [Google Scholar] [CrossRef]
- Shi, J.; Sun, W. Effects of phosphate on the chloride-induced corrosion behavior of reinforcing steel in mortars. Cem. Concr. Compos. 2014, 45, 166–175. [Google Scholar] [CrossRef]
- González, J.; Ramírez, E.; Bautista, A. Protection of Steel Embedded in Chloride-Containing Concrete by means of Inhibitors. Cem. Concr. Res. 1998, 28, 577–589. [Google Scholar] [CrossRef]
- Song, H.-W.; Saraswathy, V.; Muralidharan, S.; Lee, C.-H.; Thangavel, K. Corrosion performance of steel in composite concrete system admixed with chloride and various alkaline nitrites. Corros. Eng. Sci. Technol. 2009, 44, 408–415. [Google Scholar] [CrossRef]
- Reou, J.; Ann, K. The electrochemical assessment of corrosion inhibition effect of calcium nitrite in blended concretes. Mater. Chem. Phys. 2008, 109, 526–533. [Google Scholar] [CrossRef]
- Garcés, P.; Saura, P.; Mendez, A.; Zornoza, E.; Andrade, C. Effect of nitrite in corrosion of reinforcing steel in neutral and acid solutions simulating the electrolytic environments of micropores of concrete in the propagation period. Corros. Sci. 2008, 50, 498–509. [Google Scholar] [CrossRef]
- Medford, W.M. Testing Calcium Nitrite Corrosion Inhibitor in Concrete. Transp. Res. Rec. J. Transp. Res. Board 2002, 1795, 62–65. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C.; Acha, M.; Malric, B. Preliminary testing of Na2PO3F as a curative corrosion inhibitor for steel reinforcements in concrete. Cem. Concr. Res. 1992, 22, 869–881. [Google Scholar] [CrossRef]
- Nahali, H.; Ben Mansour, H.; Dhouibi, L.; Idrissi, H. Effect of Na3PO4 inhibitor on chloride diffusion in mortar. Constr. Build. Mater. 2017, 141, 589–597. [Google Scholar] [CrossRef]
- Nahali, H.; Dhouibi, L.; Idrissi, H. Effect of phosphate based inhibitor on the threshold chloride to initiate steel corrosion in saturated hydroxide solution. Constr. Build. Mater. 2014, 50, 87–94. [Google Scholar] [CrossRef]
- Jeong, H.-R.; Lee, H.-S.; Jalalzai, P.; Kwon, S.-J.; Singh, J.K.; Hussain, R.R.; Alyousef, R.; Alabduljabbar, H.; Aslam, F. Sodium Phosphate Post-treatment on Al Coating: Morphological and Corrosion Study. J. Therm. Spray Technol. 2019, 28, 1511–1531. [Google Scholar] [CrossRef]
- Lee, H.-S.; Kumar, A.; Mandal, S.; Singh, J.; Aslam, F.; Alyousef, R.; Alabduljabbar, H. Effect of Sodium Phosphate and Calcium Nitrate Sealing Treatment on Microstructure and Corrosion Resistance of Wire Arc Sprayed Aluminum Coatings. Coatings 2020, 10, 33. [Google Scholar] [CrossRef]
- Lee, H.-S.; Singh, J. Influence of calcium nitrate on morphology and corrosion characteristics of ammonium phosphate treated Aluminum coating deposited by arc thermal spraying process. Corros. Sci. 2019, 146, 254–268. [Google Scholar] [CrossRef]
- Singh, D.D.N.; Ghosh, R. Corrosion resistance performance of fusion bonded epoxy coated rebars used as reinforcement in concrete structures. J. Metal. Mater. Sci. 2003, 45, 73–83. [Google Scholar]
- Ghosh, R.; Singh, D. Kinetics, mechanism and characterisation of passive film formed on hot dip galvanized coating exposed in simulated concrete pore solution. Surf. Coat. Technol. 2007, 201, 7346–7359. [Google Scholar] [CrossRef]
- Imaz, N.; Ostra, M.; Vidal, M.; Díez, J.; Sarret, M.; García-Lecina, E. Corrosion behaviour of chromium coatings obtained by direct and reverse pulse plating electrodeposition in NaCl aqueous solution. Corros. Sci. 2014, 78, 251–259. [Google Scholar] [CrossRef]
- Cox, B.; Wong, Y.-M. Simulating porous oxide films on zirconium alloys. J. Nucl. Mater. 1995, 218, 324–334. [Google Scholar] [CrossRef]
- Jayaperumal, D. Effect of alcohol based inhibitors on corrosion of mild steel in hydrochloric medium. Mater. Chem. Phys. 2010, 119, 478–484. [Google Scholar] [CrossRef]
- Ferreira, E.; Giacomelli, F.; Spinelli, A.; Giacomelli, C. Evaluation of the inhibitor effect of l-ascorbic acid on the corrosion of mild steel. Mater. Chem. Phys. 2004, 83, 129–134. [Google Scholar] [CrossRef]
- Dean, S.W. Electrochemical methods of corrosion testing. In Electrochemical Techniques for Corrosion; Baboian, R., Ed.; NACE: Houston, TX, USA, 1977; p. 52. [Google Scholar]
- Yohai, L.; Vázquez, M.; Valcarce, M. Phosphate ions as corrosion inhibitors for reinforcement steel in chloride-rich environments. Electrochim. Acta 2013, 102, 88–96. [Google Scholar] [CrossRef]
- Hassoune, M.; Bezzar, A.; Sail, L.; Ghomari, F. Corrosion inhibition of carbon steel by N,N-Dimethylaminoethanol in simulated concrete pore solution contaminated with NaCl. J. Adhes. Sci. Technol. 2017, 32, 68–90. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Tantawy, A.H. Synthesis and evaluation of novel series of Schiff base cationic surfactants as corrosion inhibitors for carbon steel in acidic/chloride media: Experimental and theoretical investigations. RSC Adv. 2016, 6, 8681–8700. [Google Scholar] [CrossRef]
- He, X.; Jiang, Y.; Li, C.; Wang, W.; Hou, B.; Wu, L. Inhibition properties and adsorption behavior of imidazole and 2-phenyl-2-imidazoline on AA5052 in 1.0 M HCl solution. Corros. Sci. 2014, 83, 124–136. [Google Scholar] [CrossRef]
- Deyab, M.; Essehli, R.; El Bali, B. Inhibition of copper corrosion in cooling seawater under flowing conditions by novel pyrophosphate. RSC Adv. 2015, 5, 64326–64334. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, F.; Liu, Y.; Wu, S.; Li, W.; Sun, W.; Guo, N.; Jiang, J. Molecule adsorption and corrosion mechanism of steel under protection of inhibitor in a simulated concrete solution with 3.5% NaCl. RSC Adv. 2018, 8, 20648–20654. [Google Scholar] [CrossRef]
- Murulana, L.C.; Kabanda, M.M.; Ebenso, E.E. Experimental and theoretical studies on the corrosion inhibition of mild steel by some sulphonamides in aqueous HCl. RSC Adv. 2015, 5, 28743–28761. [Google Scholar] [CrossRef]
- Yurt, A.; Ulutas, S.; Dal, H. Electrochemical and theoretical investigation on the corrosion of aluminium in acidic solution containing some Schiff bases. Appl. Surf. Sci. 2006, 253, 919–925. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Z.; Wu, S.; Jiang, J.; Chu, H. Effect of Inhibitor on Adsorption Behavior and Mechanism of Micro-Zone Corrosion on Carbon Steel. Materials 2019, 12, 1901. [Google Scholar] [CrossRef]
- Nnaji, N.J.; Nwaji, N.; Mack, J.; Nyokong, T. Corrosion Resistance of Aluminum against Acid Activation: Impact of Benzothiazole-Substituted Gallium Phthalocyanine. Molecules 2019, 24, 207. [Google Scholar] [CrossRef]
- Rivetti, M.L.S.; Neto, J.D.S.A.; Júnior, N.A.; Ribeiro, D.V. Corrosion Inhibitors for Reinforced Concrete. In Corrosion Inhibitors, Principles and Recent Applications; Aliofkhazraei, M., Ed.; BoD—Books on Demand: Norderstedt, Germany, 2018. [Google Scholar]
- Rausch, W. The Phosphating of Metals; Finishing Publications Ltd.: Hertfordshire, UK, 1990; p. 406. [Google Scholar]
- Krause, A. Über die Oxydation des Ferrohydroxyds an der Luft. Z. Anorg. Allg. Chem. 1928, 174, 145–160. [Google Scholar] [CrossRef]
- Shi, J.-J.; Sun, W. Electrochemical and analytical characterization of three corrosion inhibitors of steel in simulated concrete pore solutions. Int. J. Miner. Met. Mater. 2012, 19, 38–47. [Google Scholar] [CrossRef]
- Monticelli, C.; Frignani, A.; Balbo, A.; Zucchi, F. Influence of two specific inhibitors on steel corrosion in a synthetic solution simulating a carbonated concrete with chlorides. Mater. Corros. 2010, 62, 178–186. [Google Scholar] [CrossRef]
- Yohai, L.; Schreiner, W.; Valcarce, M.; Vázquez, M. Inhibiting Steel Corrosion in Simulated Concrete with Low Phosphate to Chloride Ratios. J. Electrochem. Soc. 2016, 163, C729–C737. [Google Scholar] [CrossRef]
- Rodrigues, J.F.Q.; Padilha, G.S.; Bortolozo, A.D.; Osório, W.R. Effect of sintering time on corrosion behavior of an Ag/Al/Nb/Ti/Zn alloy system. J. Alloys Compd. 2020, 834, 155039. [Google Scholar] [CrossRef]
- Satizabal, L.M.; Costa, D.; Moraes, P.B.; Bortolozo, A.D.; Osório, W.R. Microstructural array and solute content affecting electrochemical behavior of Sn Ag and Sn Bi alloys compared with a traditional Sn Pb alloy. Mater. Chem. Phys. 2019, 223, 410–425. [Google Scholar] [CrossRef]
- Verissimo, N.C.; Freitas, E.S.; Cheung, N.; Garcia, A.; Osório, W.R.; Cheung, N.; Osrio, W.R. The effects of Zn segregation and microstructure length scale on the corrosion behavior of a directionally solidified Mg-25 wt. %Zn alloy. J. Alloys Compd. 2017, 723, 649–660. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Constant-Phase-Element Behavior Caused by Resistivity Distributions in Films. J. Electrochem. Soc. 2010, 157, C458–C463. [Google Scholar] [CrossRef]
- Oh, S.J.; Cook, D.; Townsend, H. Characterization of Iron Oxides Commonly Formed as Corrosion Products on Steel. Hyperfine Interact. 1998, 112, 59–66. [Google Scholar] [CrossRef]
- Kustova, G.; Burgina, E.; Sadykov, V.A.; Poryvaev, S. Vibrational spectroscopic investigation of the goethite thermal decomposition products. Phys. Chem. Miner. 1992, 18, 379–382. [Google Scholar] [CrossRef]
- Li, S.; Hihara, L. A Micro-Raman Spectroscopic Study of Marine Atmospheric Corrosion of Carbon Steel: The Effect of Akaganeite. J. Electrochem. Soc. 2015, 162, C495–C502. [Google Scholar] [CrossRef]
- Zaghib, K.; Julien, C.M. Structure and electrochemistry of FePO4·2H2O hydrate. J. Power Sources 2005, 142, 279–284. [Google Scholar] [CrossRef]
- Zhang, L.; Brow, R.K. A Raman Study of Iron-Phosphate Crystalline Compounds and Glasses. J. Am. Ceram. Soc. 2011, 94, 3123–3130. [Google Scholar] [CrossRef]
Sample Availability: All samples are available from the authors. |
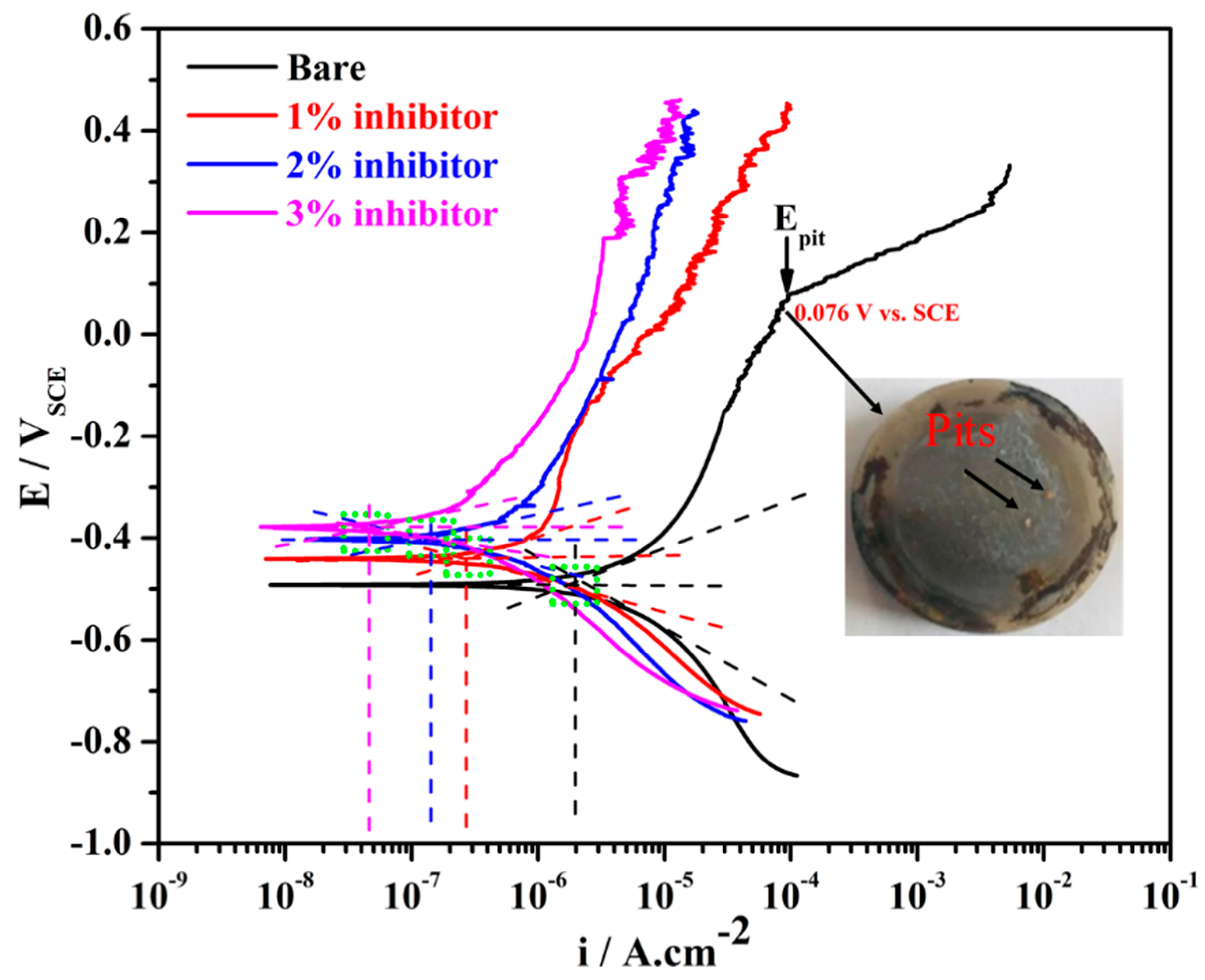


| Sample ID | Ecorr (mV) vs. SCE | icorr (µA·cm−2) | Corrosion Rate (µm·year−1) | Efficiency (%) |
|---|---|---|---|---|
| Bare | −486 (±5) | 2.52 (±0.20) | 29.28 (±1.46) | 0 |
| 1% | −439 (±2) | 0.33 (±0.01) | 3.83 (±0.33) | 86.90 (±6.52) |
| 2% | −401 (±2) | 0.18 (±0.03) | 2.09 (±0.06) | 92.86 (±4.18) |
| 3% | −379 (±1) | 0.06 (±0.002) | 0.70 (±0.02) | 97.62 (±5.97) |
| Surface Coverage (θ) | Isotherm Method | Kads (M−1) | ∆G0ads (kJ mol−1) | ||
|---|---|---|---|---|---|
| 1% Inhibitor | 2% Inhibitor | 3% Inhibitor | |||
| 0.869 | 0.9286 | 0.9762 | Langmuir | 500 | −24.92 |
| Freundlich | 1.40 | −10.61 | |||
| Time (h) | Sample ID | Electrochemical Parameters | Efficiency (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rs (Ω·cm2) | Rp (kΩ·cm2) | CPE1 | Ro (kΩ·cm2) | CPE2 | |||||
| Q1 (1 × 10−5) (Ω−1·cm−2·sn) | n1 | Q2 (1 × 10−5) (Ω−1·cm−2·sn) | n2 | ||||||
| 1 | Bare | 9.96 (±0.40) | 1.96 (±0.15) | 21.0 (±1.89) | 0.78 (±0.06) | 0.87 (±0.07) | 232.8 (±20.95) | 0.70 (±0.04) | 0 |
| 1% inhibitor | 10.55 (±0.84) | 8.04 (±0.65) | 10.1 (±0.56) | 0.82 (±0.03) | 2.61 (±0.10) | 34.1 (±2.05) | 0.74 (±0.03) | 66.67 (±4.00) | |
| 2% inhibitor | 10.72 (±0.70) | 10.87 (±0.67) | 8.6 (±0.48) | 0.87 (±0.06) | 9.10 (±0.82) | 12.8 (±0.38) | 0.80 (±0.05) | 90.44 (±6.91) | |
| 3% inhibitor | 11.75 (±0.82) | 10.41 (±0.86) | 8.8 (±1.58) | 0.86 (±0.07) | 8.21 (±0.29) | 14.89 (±0.91) | 0.78 (±0.02) | 89.40 (±3.58) | |
| 24 | Bare | 11.48 (±0.52) | 3.29 (±0.22) | 16.6 (±1.35) | 0.80 (±0.05) | 2.16 (±0.09) | 182.4 (±11.67) | 0.73 (±0.03) | 0 |
| 1% inhibitor | 18.21 (±0.75) | 3.87 (±0.08) | 15.5 (±1.24) | 0.81 (±0.02) | 2.53 (±0.05) | 95.0 (±2.00) | 0.74 (±0.04) | 14.62 (±1.09) | |
| 2% inhibitor | 13.62 (±0.66) | 6.63 (±0.23) | 12.3 (±1.16) | 0.85 (±0.02) | 4.23 (±0.33) | 21.5 (±0.43) | 0.75 (±0.04) | 48.94 (±2.20) | |
| 3% inhibitor | 12.87 (±0.71) | 11.15 (±0.66) | 7.3 (±0.31) | 0.87 (±0.03) | 14.20 (±0.47) | 6.7 (±0.19) | 0.88 (±0.03) | 84.79 (±3.82) | |
| 120 | Bare | 11.12 (±0.39) | 1.88 (±0.04) | 23.8 (±2.09) | 0.76 (±0.02) | 2.66 (±0.14) | 155.7 (±12.76) | 0.73 (±0.04) | 0 |
| 1% inhibitor | 9.39 (±0.33) | 1.44 (±0.03) | 30.6 (±2.35) | 0.75 (±0.02) | 2.37 (±0.15) | 165.2 (±4.63) | 0.71 (±0.04) | −13.92 (±0.46) | |
| 2% inhibitor | 10.30 (±0.07) | 6.43 (±0.16) | 12.9 (±0.20) | 0.85 (±0.01) | 4.21 (±0.14) | 22.3 (±2.03) | 0.75 (±0.06) | 36.82 (±2.03) | |
| 3% inhibitor | 11.86 (±0.65) | 12.51 (±0.75) | 5.9 (±0.24) | 0.88 (±0.03) | 15.48 (±0.56) | 4.7 (±0.13) | 0.89 (±0.03) | 82.82 (±5.13) | |
| Sample ID | Point | O | Na | Cl | K | Ca | N | P | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Bare | 1 | 1.40 | 50.10 | 42.76 | 2.34 | 1.49 | 0 | 0 | Balance |
| 2 | 2.55 | 2.16 | 1.77 | 0.35 | 0.19 | 0 | 0 | Balance | |
| 3% | 1 | 16.91 | 34.81 | 30.63 | 0.61 | 0.08 | 0.71 | 1.21 | Balance |
| 2 | 6.15 | 0.72 | 0.33 | 0.27 | 0.14 | 1.12 | 0.35 | Balance |
| Sample ID | Raman Shift (cm−1) | Attribution | Reference |
|---|---|---|---|
| Bare | 205, 234, 245, 288, 297 | Goethite (α-FeOOH) | [71,72] |
| 308, 314, 410 | Akaganeite (β-FeOOH) | [71] | |
| 338, 349 | Lepidocrocite (γ-FeOOH) | [71,73] | |
| 3% inhibitor | 206, 226, 238, 248, 282, 290, 371 | Goethite (α-FeOOH) | [71,72] |
| 316, 413 | Akaganeite (β-FeOOH) | [71] | |
| 342, 353 | Maghemite (γ-Fe2O3) | [71] | |
| 303, 329, 428 | FePO4 (tertiary iron phosphate) | [74,75] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandal, S.; Singh, J.K.; Lee, D.-E.; Park, T. Ammonium Phosphate as Inhibitor to Mitigate the Corrosion of Steel Rebar in Chloride Contaminated Concrete Pore Solution. Molecules 2020, 25, 3785. https://doi.org/10.3390/molecules25173785
Mandal S, Singh JK, Lee D-E, Park T. Ammonium Phosphate as Inhibitor to Mitigate the Corrosion of Steel Rebar in Chloride Contaminated Concrete Pore Solution. Molecules. 2020; 25(17):3785. https://doi.org/10.3390/molecules25173785
Chicago/Turabian StyleMandal, Soumen, Jitendra Kumar Singh, Dong-Eun Lee, and Taejoon Park. 2020. "Ammonium Phosphate as Inhibitor to Mitigate the Corrosion of Steel Rebar in Chloride Contaminated Concrete Pore Solution" Molecules 25, no. 17: 3785. https://doi.org/10.3390/molecules25173785
APA StyleMandal, S., Singh, J. K., Lee, D.-E., & Park, T. (2020). Ammonium Phosphate as Inhibitor to Mitigate the Corrosion of Steel Rebar in Chloride Contaminated Concrete Pore Solution. Molecules, 25(17), 3785. https://doi.org/10.3390/molecules25173785









