Acylated Flavone O-Glucuronides from the Aerial Parts of Nepeta curviflora
Abstract
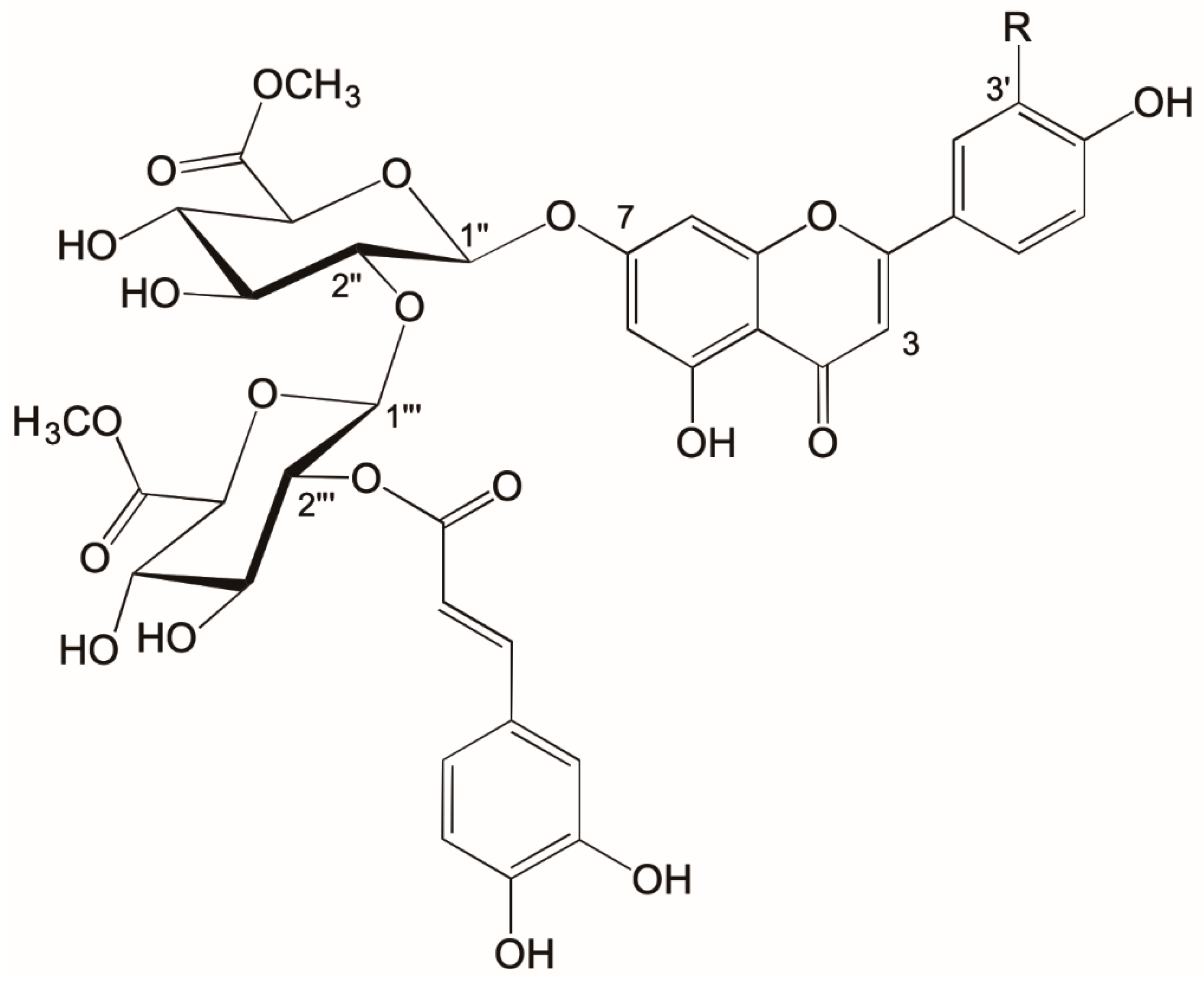
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Isolation of Flavones
3.3. Preparative HPLC
3.4. Analytical HPLC
3.5. Biological Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jamzad, Z.; Grayer, R.J.; Kite, G.C.; Simmonds, M.S.J.; Ingrouille, M.; Jalili, A. Leaf surface flavonoids in Iranian species of Nepeta (Lamiaceae) and some related genera. Biochem. Syst. Ecol. 2003, 31, 587–600. [Google Scholar] [CrossRef]
- Ali, L.; Ali, S.; Rizvi, T.S.; Khan, A.; Hassan, Z.; Al-Harrasi, A.; Hussain, J. Antioxidant Flavonoids from Nepeta floccosa Benth. Rec. Nat. Prod. 2015, 9, 567–571. [Google Scholar]
- Baser, K.H.C.; Kirimer, N.; Kurkcuoglu, M.; Demirci, B. Essential oils of Nepeta species growing in Turkey. Chem. Nat. Compd. 2000, 36, 356–359. [Google Scholar] [CrossRef]
- Dabiri, M.; Sefidkon, F. Composition of essential oil of Nepeta crassifolia Boiss and Buhse. Flav. Fragr. J. 2003, 18, 225–227. [Google Scholar] [CrossRef]
- Rapisarda, A.; Galati, E.M.; Tzakou, O.; Flores, M.; Miceli, N. Nepeta sibthorpii Bentham (Lamiaceae): Micromorphological analysis of leaves and flowers. Farmaco 2001, 56, 413–415. [Google Scholar] [CrossRef]
- Naghibi, F.; Mosaddegh, M.; Motamed, S.M.; Ghorbani, A. Labiatae family in folk medicine in Iran: From Ethnobotany to Pharmacology. Iran. J. Pharm. Res. 2005, 2, 63–79. [Google Scholar]
- Firoozabadi, A.; Kolouri, S.; Zarshenas, M.; Salehi, A.; Mosavat, S.H.; Dastgheib, S.A. Efficacy Nepeta menthoides Boiss and Buhse freeze-dried aqueous extract on anxiety of patients with depression: A double-blind randomized controlled clinical trial. Iran J. Med. Sci. 2016, 41, 164–170. [Google Scholar] [CrossRef]
- Dienaitėa, L.; Pukalskienėa, M.; Matiasb, A.A.; Pereirab, C.V.; Pukalskasa, A.; Venskutonisa, P.R. Valorization of six Nepeta species by assessing the antioxidant potential, phytochemical composition and bioactivity of their extracts in cell cultures. J. Funct. Foods 2018, 45, 512–522. [Google Scholar] [CrossRef]
- Barhoumi, L.M.; Al-Jaber, H.I.; Abu Zarga, M.H. Volatile Organic Compounds and Essential Oil Composition of Selected Organs of Nepeta curviflora Collected from two Region in Jordan. Jord. J. Chem. 2017, 12, 101–112. [Google Scholar]
- Bernardi, M.M.; Kirsten, T.B.; Salzgeber, S.A.; Ricci, E.L.; Romoff, P.; Guilardi Lago, J.H.; Lourenc, L.M. Antidepressant-like effects of an apolar extract and chow enriched with Nepeta cataria (catnip) in mice. Psychol. Neurosci. 2010, 3, 251–258. [Google Scholar] [CrossRef]
- Hadi, N.; Sefidkon, F.; Shojaeiyan, A.; Šiler, B.; Jafari, A.A.; Mišić, D. Phenolic’s composition in four endemic Nepeta species from Iran cultivated under experimental field conditions: The possibility of the exploitation of Nepeta germplasm. Ind. Crop. Prod. 2017, 95, 475–484. [Google Scholar] [CrossRef]
- Joshi, N.; Sah, G.C. GC–MS analysis and antimicrobial activity of essential oil of Nepeta coerulescens. Int. J. Res. Pharm. Pharm. 2014, 3, 68–71. [Google Scholar]
- Kaviarasan, S.; Sundarapandiyan, R.; Anuradha, C.V. Protective action of fenugreek (Trigonella foenum graecum) seed polyphenols against alcohol-induced protein and lipid damage in rat liver. Cell. Biol. Toxicol. 2008, 24, 391–400. [Google Scholar] [CrossRef]
- Mahboubi, M.; Kazempour, N.; Ghazian, F.; Taghizadeh, M. Chemical composition: Antioxidant and antimicrobial activity of Nepeta persica Boiss essential oil. Herba Pol. 2011, 57, 62–71. [Google Scholar]
- Nestorović, J.; Mišić, D.; Šiler, B.; Soković, M.; Glamočlija, J.; ’Cirić, A.; Maksimović, V.; Grubišić, D. Nepeta lactone content in shoot cultures of three endemic Nepeta species and the evaluation of their antimicrobial activity. Fitoterapia 2010, 81, 621–626. [Google Scholar] [CrossRef]
- Süntar, I.; Nabavi, S.M.; Barreca, D.; Fischer, N.; Efferth, T. Pharmacological and chemical features of Nepeta L. genus: Its importance as a therapeutic agent. Phytother. Res. 2018, 32, 185–198. [Google Scholar]
- Mišić, D.; Šiler, B.; Gašić, U.; Avramov, S.; Živković, S.; Nestorović Živković, J.; Milutinović, M.; Tešičć, Ž. Simultaneous UHPLC/DAD/(+/-) HESI-MS/MS analysis of phenolic acids and Nepeta lactones in methanol extracts of Nepeta species: A possible application in chemotaxonomic studies. Phytochem. Anal. 2015, 26, 72–85. [Google Scholar]
- Formisano, C.; Rigano, D.; Senatore, F. Chemical constituents and biological activities of Nepeta species. Chem. Biodivers. 2011, 8, 1783–1818. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, C.Y.; Eom, S.H.; Kim, Y.K.; Park, N.I.; Park, S.U. Rosmarinic acid production from transformed root cultures of Nepeta Cataria L. Sci. Res. Essays 2010, 5, 1122–1126. [Google Scholar]
- Mihaylova, D.; Georgieva, L.; Pavlov, A. In vitro antioxidant activity and phenolic composition of Nepeta cataria L. extracts. Int. J. Agric. Sci. Tech. 2013, 1, 74–79. [Google Scholar]
- Modnicki, D.; Tokar, M.; Klimek, B. Flavonoids and phenolic acids of Nepeta cataria L: Var citriodora (Becker) balb (Lamiaceae). Acta. Pol. Pharm. 2007, 64, 247–252. [Google Scholar]
- Trivellini, A.; Lucchesini, M.; Maggini, R.; Mosadegh, H.; Villamarin, T.S.S.; Vernieri, P.; Mensuali-Sodi, A.; Pardossi, A. Lamiaceae phenols as multifaceted compounds: Bioactivity, industrial prospects and role of positive-stress. Ind. Crop. Prod. 2016, 83, 241–254. [Google Scholar] [CrossRef]
- Al-Qudah, M.A. Antioxidant activity and chemical composition of essential oils of fresh and dried Jordanian Nepeta curviflora Boiss. J. Biol. Act. Prod. Nat. 2016, 6, 101–111. [Google Scholar]
- Mancini, E.; Arnold, N.A.; Feo, V.D.; Formizano, C.; Rigano, D.; Piozzi, F.; Senatore, F. Phytotoxic effects of essential oils of Nepeta curviflora Boiss and Nepeta nuda L subsp albiflora growing wild in Lebanon. J. Plant. Inter. 2009, 4, 253–259. [Google Scholar]
- Musso, L.; Scaglia, B.; Al Haj, G.; Apostolides, N.A.; Adani, F.; Scarì, G.; Dallavalle, S.; Iriti, M. Chemical Characterization and Nematicidal Activity of the Essential Oil of Nepeta nuda L. ssp pubescens and Nepeta curviflora Boiss from Lebanon. J. Essent. Oil Bear. Pl. 2017, 20, 1424–1433. [Google Scholar] [CrossRef]
- Al-Bakri, A.G.; Afifi, F.U. Evaluation of antimicrobial activity of selected plant extracts by rapid XTT colorimetry and bacterial enumeration. J. Microbiol. Meth. 2007, 68, 19–25. [Google Scholar] [CrossRef]
- Barbour, E.K.; Al Sharif, M.; Sagherian, V.K.; Habre, A.N.; Talhouk, R.S.; Talhouk, S.N.J. Screening of selected indigenous plants of Lebanon for antimicrobial activity. J. Ethnopharmacol. 2004, 93, 1–7. [Google Scholar] [CrossRef]
- Cigremis, Y.; Ulukanli, Z.; Ilcim, A.; Akgoz, M. In vitro antioxidant and antimicrobial assays of acetone extracts from Nepeta meyeri Bentham. Eur. Rev. Med. Pharm. Sci. 2010, 14, 661–668. [Google Scholar]
- Proestos, C.; Lytoudi, K.; Mavromelanidou, O.; Zoumpoulakis, P.; Sinanoglou, V. Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils. Antioxidants 2013, 2, 11–22. [Google Scholar] [CrossRef]
- Fossen, T.; Slimestad, R.; Øvstedal, D.O.; Andersen, Ø.M. Covalent anthocyanin-flavonols complexes from flowers of chive, Allium schoenoprasum. Phytochemistry 2000, 54, 317–323. [Google Scholar] [CrossRef]
- Fossen, T.; Andersen, Ø.M. Spectroscopic techniques applied to flavonoids. In Flavonoids: Chemistry, Biochemistry and Applications; Andersen, Ø.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 37–142. [Google Scholar]
- Kowalska, I.; Stochmal, A.; Kapusta, I.; Janda, B.; Pizza, C.; Piacente, S.; Oleszek, W. Flavonoids from barrel medic (Medicago truncatula) aerial parts. J. Agric. Food Chem. 2007, 55, 2645–2652. [Google Scholar] [CrossRef]
- Stochmal, A.; Simonet, A.M.; Macias, F.A.; Oleszek, W. Alfalfa (Medicago sativa L.) flavonoids. 2. Tricin and chrysoeriol glycosides from aerial parts. J. Agric. Food Chem. 2001, 49, 5310–5314. [Google Scholar] [CrossRef]
- Parada, J.; Atria, A.N.; Wiese, G.; Rivas, E.; Corsini, G. Synthesis, characterization and antibacterial activity of cobalt (III) complex with phenanthroline and Maltose. J. Chil. Chem. Soc. 2014, 59, 2636–2639. [Google Scholar] [CrossRef][Green Version]
- Rahman, M.M.; Sheikh, M.M.I.; Sharmin, S.A.; Islam, M.S.; Rahman, M.A.; Rahman, M.M.; Alam, M.F. Antibacterial activity of leaf juice and extracts of Moringa oleifera Lam. Against some human patho-genic bacteria. Chiang Mai University. J. Nat. Sci. 2009, 8, 219–228. [Google Scholar]
- Güvenalp, Z.; Özbek, H.; Kuruüzüm-Uz, A.; Kazaz, C.; Demirezer, L.Ö. Secondary metabolites from Nepeta heliotropifolia. Turk. J. Chem. 2009, 33, 667–675. [Google Scholar]
- Olennikov, D.N.; Akobirshoeva, A.A. Flavonoids and phenylpropanoids of Nepeta glutinosa and Ziziphora Pamiroalaica. Chem. Nat. Compd. 2016, 52, 909–912. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Gil, M.I.; Ivanchev, S.; Tomas-Lorente, F. External and vacuolar flavonoids from Nepeta transcaucasica. Biochem. Syst. Ecol. 1992, 20, 589–590. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds can be obtained from the authors. |
| 1 (1H) | 1 (13C) | 2 (1H) | 2 (13C) | |
|---|---|---|---|---|
| Aglycone | ||||
| 2 | 164.39 | 164.66 | ||
| 3 | 6.85 s | 103.25 | 6.73 s | 103.31 |
| 4 | 182.10 | 182.03 | ||
| 5 | 161.28 | 161.34 | ||
| 6 | 6.39 d 2.2 | 99.25 | 6.39 d 2.1 | 99.29 |
| 7 | 162.19 | 162.22 | ||
| 8 | 6.76 d 2.2 | 94.44 | 6.73 d 2.1 | 94.34 |
| 9 | 157.01 | 157.06 | ||
| 10 | 105.52 | 105.57 | ||
| 1′ | 121.12 | 121.50 | ||
| 2′ | 7.94 `d` 8.8 | 128.66 | 7.43 br | 119.25 |
| 3′ | 6.95 `d` 8.8 | 116.15 | 145.99 | |
| 4′ | 161.50 | 150.14 | ||
| 5′ | 6.95 `d` 8.8 | 116.15 | 6.92 d 8.5 | 116.18 |
| 6′ | 7.94 `d` 8.8 | 128.66 | 7.43 dd 2.2, 8.5 | 121.36 |
| 7-O-glucuronopyranosyl | ||||
| 1″ | 5.44 d 7.5 | 97.35 | 5.46 d 7.5 | 97.37 |
| 2″ | 3.52 m | 81.01 | 3.53 m | 81.07 |
| 3″ | 3.37 m | 74.73 | 3.37 m | 74.77 |
| 4″ | 3.37 m | 71.54 | 3.37 m | 71.58 |
| 5″ | 4.19 d 7.5 | 74.70 | 4.19 d 9.4 | 74.75 |
| 6″ | 169.10 | 169.15 | ||
| OCH3 | 3.63 s | 52.09 | 3.63 s | 52.14 |
| 2″-O-β-glucuronopyranosyl | ||||
| 1‴ | 4.88 d 8.2 | 101.42 | 4.89 d 8.3 | 101.46 |
| 2‴ | 4.62 d 8.2 | 73.52 | 4.63 dd 8.3, 9.6 | 73.58 |
| 3‴ | 3.47 m | 73.56 | 3.48 t 9.6 | 73.61 |
| 4‴ | 3.36 m | 72.02 | 3.36 t 9.6 | 72.08 |
| 5‴ | 3.85 d 8.2 | 75.37 | 3.85 d 9.6 | 75.42 |
| 6‴ | 169.17 | 169.22 | ||
| OCH3 | 3.52 s | 51.71 | 3.52 s | 51.75 |
| 2‴-O-caffeoyl | ||||
| C=O | 165.87 | 165.94 | ||
| α | 6.24 d 15.8 | 114.73 | 6.23 d 15.8 | 114.75 |
| β | 7.43 d 15.8 | 144.72 | 7.44 d 15.8 | 144.79 |
| 1⁗ | 125.86 | 125.89 | ||
| 2⁗ | 7.03 d 2.1 | 114.83 | 7.04 d 2.2 | 114.86 |
| 3⁗ | 145.65 | 145.72 | ||
| 4⁗ | 148.29 | 148.36 | ||
| 5⁗ | 6.76 m | 115.82 | 6.77 d 8.1 | 115.87 |
| 6⁗ | 6.97 dd 2.1, 8.5 | 121.30 | 6.97 dd 2.2, 8.1 | 121.36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabee, M.; Andersen, Ø.M.; Fossen, T.; Enerstvedt, K.H.; Abu Ali, H.; Rayyan, S. Acylated Flavone O-Glucuronides from the Aerial Parts of Nepeta curviflora. Molecules 2020, 25, 3782. https://doi.org/10.3390/molecules25173782
Rabee M, Andersen ØM, Fossen T, Enerstvedt KH, Abu Ali H, Rayyan S. Acylated Flavone O-Glucuronides from the Aerial Parts of Nepeta curviflora. Molecules. 2020; 25(17):3782. https://doi.org/10.3390/molecules25173782
Chicago/Turabian StyleRabee, Maysaa, Øyvind Moksheim Andersen, Torgils Fossen, Kjersti Hasle Enerstvedt, Hijazi Abu Ali, and Saleh Rayyan. 2020. "Acylated Flavone O-Glucuronides from the Aerial Parts of Nepeta curviflora" Molecules 25, no. 17: 3782. https://doi.org/10.3390/molecules25173782
APA StyleRabee, M., Andersen, Ø. M., Fossen, T., Enerstvedt, K. H., Abu Ali, H., & Rayyan, S. (2020). Acylated Flavone O-Glucuronides from the Aerial Parts of Nepeta curviflora. Molecules, 25(17), 3782. https://doi.org/10.3390/molecules25173782







