Synthesis of 2-((2-(Benzo[d]oxazol-2-yl)-2H-imidazol-4-yl)amino)-phenols from 2-((5H-1,2,3-Dithiazol-5-ylidene)amino)phenols through Unprecedented Formation of Imidazole Ring from Two Methanimino Groups
Abstract
1. Introduction
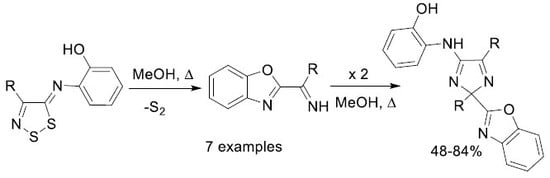
2. Results and Discussion
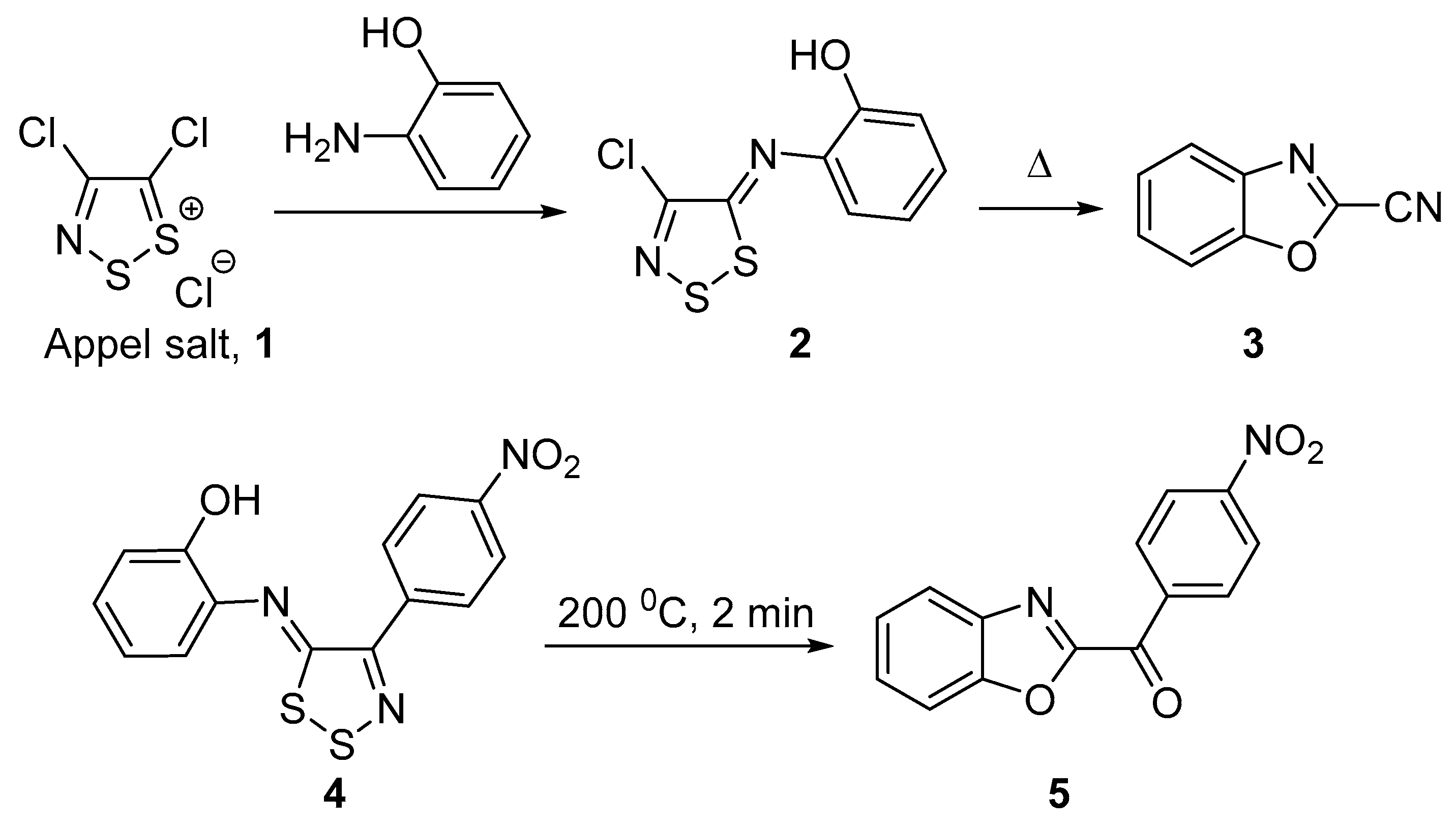
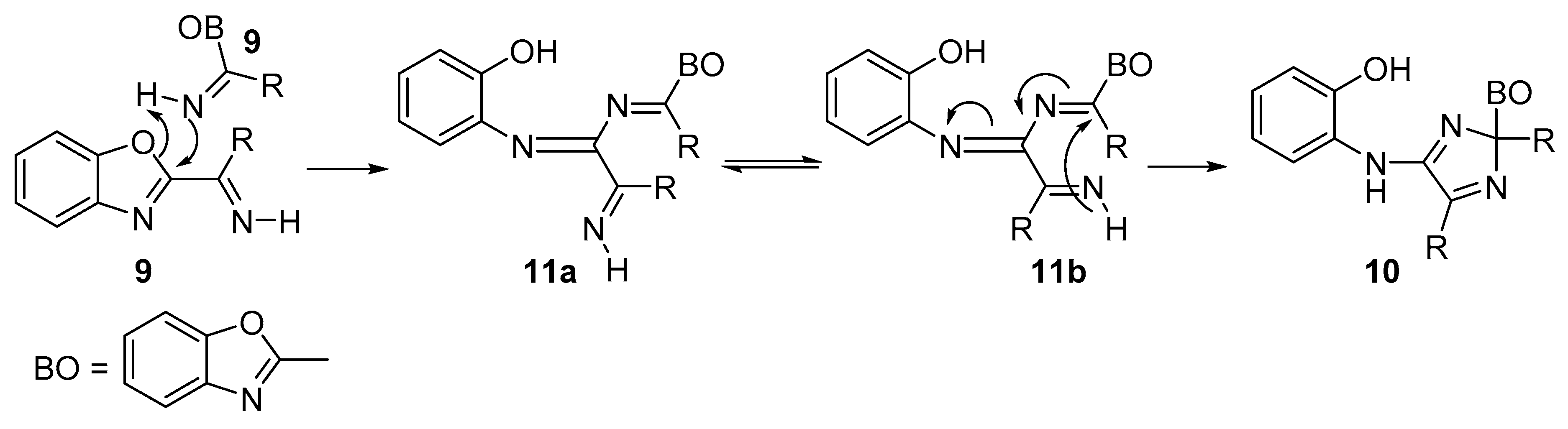
Mechanistic Rationale
3. Experimental Section
3.1. General Methods and Materials
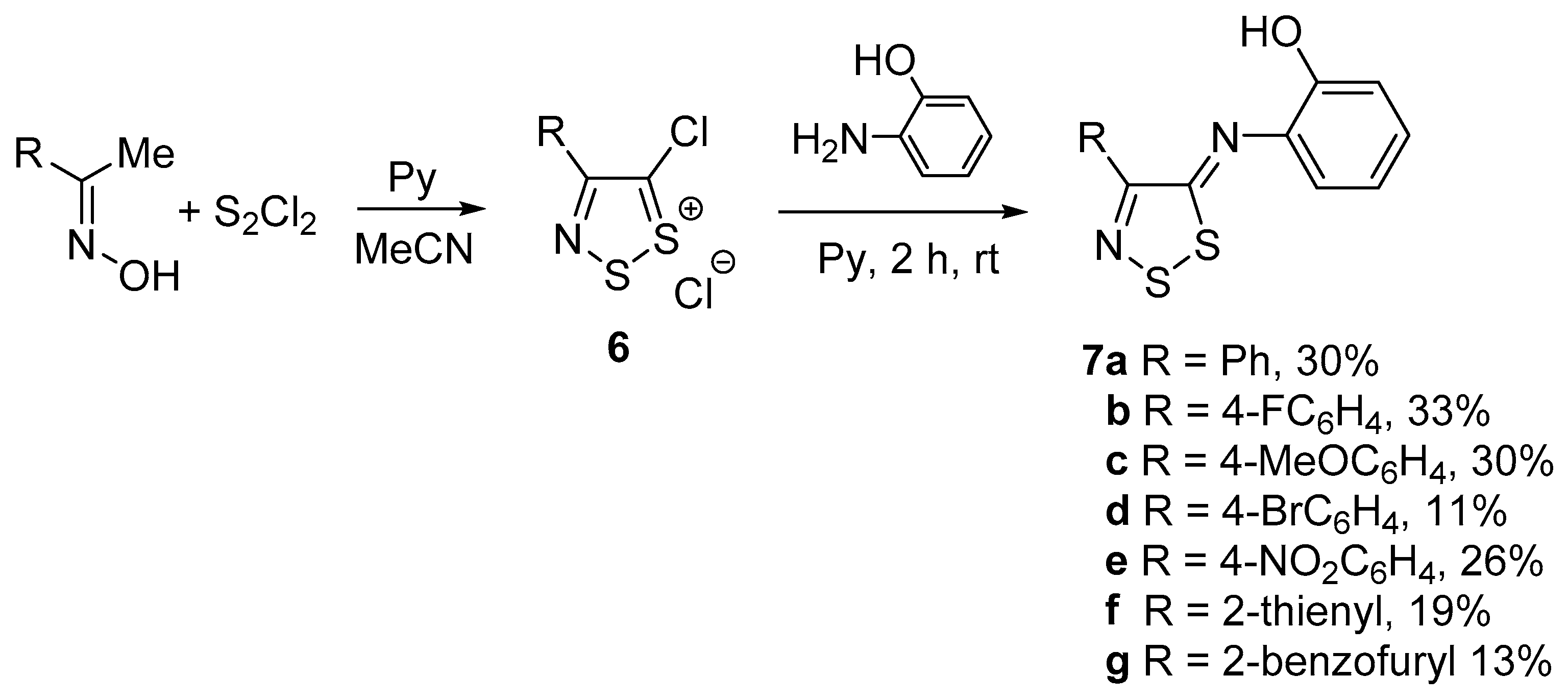
3.2. General Procedure for the Synthesis of 2-((4-aryl-5H-1,2,3-dithiazol-5-ylidene)amino)phenols (7)
3.3. General Procedure for the Thermolysis of 2-((4-aryl(hetaryl)-5H-1,2,3-dithiazol-5-ylidene)amino)phenols 7 in Various Solvents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Konstantinova, L.S.; Rakitin, O.A. Synthesis and properties of 1,2,3-dithiazoles. Russ. Chem. Rev. 2008, 77, 521–546. [Google Scholar] [CrossRef]
- Rakitin, O. 1,2-Oxa/thia-3-azoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier BV: Amsterdam, The Netherlands, 2008; Volume 6, pp. 1–36. [Google Scholar]
- Rakitin, O.A.; Zibarev, A.V. Synthesis and Applications of 5-Membered Chalcogen-Nitrogen π-Heterocycles with Three Heteroatoms. Asian J. Org. Chem. 2018, 7, 2397–2416. [Google Scholar] [CrossRef]
- Besson, T.; Rees, C.W.; Cottenceau, G.; Pons, A.-M. Antimicrobial evaluation of 3,1-benzoxazin-4-ones, 3,1-benzothiazin-4-ones, 4-alkoxyquinazolin-2-carbonitriles and N-arylimino-1,2,3-dithiazoles. Bioorg. Med. Chem. Lett. 1996, 6, 2343–2348. [Google Scholar] [CrossRef]
- Oppedisano, F.; Catto, M.; Koutentis, P.A.; Nicolotti, O.; Pochini, L.; Koyioni, M.; Introcaso, A.; Michaelidou, S.S.; Carotti, A.; Indiveri, C. Inactivation of the glutamine/amino acid transporter ASCT2 by 1,2,3-dithiazoles: Proteoliposomes as a tool to gain insights in the molecular mechanism of action and of antitumor activity. Toxicol. Appl. Pharmacol. 2012, 265, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, A.; Koyioni, M.; Antoniades, I.; Pegeioti, D.; Eleftheriou, I.; Michaelidou, S.S.; Amelichev, S.A.; Konstantinova, L.S.; Rakitin, O.A.; Koutentis, P.A.; et al. 1,2,3-Dithiazoles–new reversible melanin synthesis inhibitors: A chemical genomics study. MedChemComm 2015, 6, 935–946. [Google Scholar] [CrossRef]
- Asquith, C.R.M.; Konstantinova, L.S.; Laitinen, T.; Meli, M.L.; Poso, A.; Rakitin, O.A.; Hofmann-Lehmann, R.; Hilton, S.T. Evaluation of Substituted 1,2,3-Dithiazoles as Inhibitors of the Feline Immunodeficiency Virus (FIV) Nucleocapsid Protein via a Proposed Zinc Ejection Mechanism. ChemMedChem 2016, 11, 2119–2126. [Google Scholar] [CrossRef]
- Asquith, C.R.M.; Meili, T.; Rakitin, O.A.; Baranovsky, I.V.; Konstantinova, L.S.; Poso, A.; Rakitin, O.A.; Hofmann-Lehmann, R. Synthesis and comparison of substituted 1,2,3-dithiazole and 1,2,3-thiaselenazole as inhibitors of the feline immunodeficiency virus (FIV) nucleocapsid protein as a model for HIV infection. Bioorganic Med. Chem. Lett. 2019, 29, 1765–1768. [Google Scholar] [CrossRef]
- Beer, L.; Cordes, A.W.; Haddon, R.C.; Itkis, M.E.; Oakley, R.T.; Reed, R.W.; Robertson, C.M. A π-stacked 1,2,3-dithiazolyl radical. Preparation and solid state characterization of (Cl2C3NS)(ClC2NS2). Chem. Commun. 2002, 1872–1873. [Google Scholar] [CrossRef]
- Rakitin, O.A. Stable heterocyclic radicals. Russ. Chem. Rev. 2011, 80, 647–659. [Google Scholar] [CrossRef]
- Lekin, K.; Phan, H.; Winter, S.M.; Wong, J.W.L.; Leitch, A.A.; Laniel, D.; Yong, W.; Secco, R.A.; Tse, J.S.; Desgreniers, S.; et al. Heat, Pressure and Light-Induced Interconversion of Bisdithiazolyl Radicals and Dimers. J. Am. Chem. Soc. 2014, 136, 8050–8062. [Google Scholar] [CrossRef]
- Yu, X.; Mailman, A.; Lekin, K.; Assoud, A.; Robertson, C.M.; Noll, B.; Campana, C.F.; Howard, J.A.K.; Dube, P.A.; Oakley, R.T. Semiquinone-Bridged Bisdithiazolyl Radicals as Neutral Radical Conductors. J. Am. Chem. Soc. 2012, 134, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, L.S.; Baranovsky, I.V.; Irtegova, I.G.; Bagryanskaya, I.Y.; Shundrin, L.A.; Zibarev, A.V.; Rakitin, O.A. Fused 1,2,3-Dithiazoles: Convenient Synthesis, Structural Characterization, and Electrochemical Properties. Molecules 2016, 21, 596. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.M.; Beer, L.; Cordes, A.W.; Haddon, R.C.; Itkis, M.I.; Oakley, R.T.; Preuss, K.E.; Reed, R.W. Trans-4,4′-Dichloro-1,1′,2,2′,3,3′-tetrathiadiazafulvalene (DC-TAF) and Its 1:1 Radical Cation Salts [DC-TAF][X]: Preparation and Solid-State Properties of BF4-, ClO4-, and FSO3-Derivatives. J. Am. Chem. Soc. 1999, 121, 6657–6663. [Google Scholar] [CrossRef]
- Appel, R.; Janssen, H.; Siray, M.; Knoch, F. Synthese und Reaktionen des 4,5-Dichlor-1,2,3-dithiazolium-chlorids. Eur. J. Inorg. Chem. 1985, 118, 1632–1643. [Google Scholar] [CrossRef]
- Kim, K. Synthesis and Reactions of 1,2,3-Dithiazoles. Sulfur Rep. 1998, 21, 147–207. [Google Scholar] [CrossRef]
- Koyioni, M.; Manoli, M.; Koutentis, P.A. The Reaction of DABCO with 4-Chloro-5H-1,2,3-dithiazoles: Synthesis and Chemistry of 4-[N-(2-Chloroethyl)piperazin-1-yl]-5H-1,2,3-dithiazoles. J. Org. Chem. 2015, 81, 615–631. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Michaelidou, S.S.; Koyioni, M.; Koutentis, P.A. Ring transformations of 2-hydroxy-(4-chloro-5H-1,2,3-dithiazol-5-ylideneamino)arenes. Tetrahedron 2015, 71, 7181–7190. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Michaelidou, S.S.; White, A.J.; Koutentis, P.A. Transformation of 2-(4-chloro-5H-1,2,3-dithiazol-5-ylideneamino)-6-ethoxy-4-phenylpyridine-3,5-dicarbonitrile into 4-aminopyrido[2,3-d]pyrimidines and 2-(pyrid-2-yl)guanidines. Tetrahedron 2015, 71, 1799–1807. [Google Scholar] [CrossRef]
- Koyioni, M.; Manoli, M.; Manolis, M.J.; Koutentis, P.A. Reinvestigating the Reaction of 1H-Pyrazol-5-amines with 4,5-Dichloro-1,2,3-dithiazolium Chloride: A Route to Pyrazolo[3,4-c]isothiazoles and Pyrazolo[3,4-d]thiazoles. J. Org. Chem. 2014, 79, 4025–4037. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Rakitin, O.A.; Rees, C.W.; Torroba, T.; White, A.J.P.; Williams, D.J. 1,2,4-Thiadiazole 4-oxides. J. Chem. Soc. Perkin Trans. 1999, 1, 2243–2248. [Google Scholar] [CrossRef]
- Clarke, D.; Emayan, K.; Rees, C.W. New synthesis of isothiazoles from primary enamines. J. Chem. Soc. Perkin Trans. 1 1998, 77–82. [Google Scholar] [CrossRef]
- Lee, H.; Kim, K. A facile synthesis of 2-cyano-4H-3,1-benzothiazines and 2-cyano-4H-3,1-benzoxazines. Heteroat. Chem. 1993, 4, 263–270. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Rakitin, O.A.; Rees, C.W.; Sivadasan, S.; Torroba, T. New route to 2-cyanobenzimidazoles. Tetrahedron 1998, 54, 9639–9650. [Google Scholar] [CrossRef]
- Lee, H.-S.; Chang, Y.-G.; Kim, K. A facile synthesis of 3-substituted 2-cyanoquinazolin-4(3H)-ones and 3-alkyl-2-cyanothieno[3,2-d]pyrimidin-4(3H)-onesvia1,2,3-dithiazoles. J. Heterocycl. Chem. 1998, 35, 659–668. [Google Scholar] [CrossRef]
- English, R.F.; Rakitin, O.A.; Rees, C.W.; Vlasova, O.G. Conversion of imino-1,2,3-dithiazoles into 2-cyanobenzothiazoles, cyanoimidoyl chlorides and diatomic sulfur. J. Chem. Soc. Perkin Trans. 1997, 1, 201–206. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Reactions of tetracyanoethylene oxide with 1,2,3-dithiazoles. J. Chem. Soc. Perkin Trans. 1998, 1, 2505–2510. [Google Scholar] [CrossRef]
- Emayan, K.; Rees, C.W. The reaction of acetophenone oximes with disulfur dichloride; 4-aryl-5-arylimino-1,2,3-dithiazoles and pentathiepino[6,7-c]pyrrole. Bull. Soc. Chim. Belg. 1997, 106, 605–611. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Koyioni, M.; Michaelidou, S.S. The conversion of [(4-chloro-5H-1,2,3-dithiazol-5-ylidene)amino]azines into azine fused thiazole-2-carbonitriles. Org. Biomol. Chem. 2013, 11, 621–629. [Google Scholar] [CrossRef]
- Koyioni, M.; Manoli, M.; Koutentis, P.A. Synthesis of Fused 1,2,4-Dithiazines and 1,2,3,5-Trithiazepines. J. Org. Chem. 2014, 79, 9717–9727. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Bol’shakov, O.I.; Obruchnikova, N.V.; Laborie, H.; Tanga, A.; Sopena, V.; Lanneluc, I.; Picot, L.; Sable, S.; Thiéry, V.; et al. One-pot synthesis of 5-phenylimino, 5-thieno or 5-oxo-1,2,3-dithiazoles and evaluation of their antimicrobial and antitumor activity. Bioorg. Med. Chem. Lett. 2009, 19, 136–141. [Google Scholar] [CrossRef]
- Matta, C.F.; Boyd, R.J. The Quantum Theory of Atoms in Molecules: From Solid State to DNA and Drug Design; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2007. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; LeComte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Wang, X.; Xu, D.; Miao, C.; Zhang, Q.; Sun, W. N-Bromosuccinimide as an oxidant for the transition-metal-free synthesis of 2-aminobenzoxazoles from benzoxazoles and secondary amines. Org. Biomol. Chem. 2014, 12, 3108–3113. [Google Scholar] [CrossRef] [PubMed]
- Wertz, S.; Kodama, S.; Studer, A. Amination of Benzoxazoles and 1,3,4-Oxadiazoles Using 2,2,6,6-Tetramethylpiperidine-N-oxoammonium Tetrafluoroborate as an Organic Oxidant. Angew. Chem. Int. Ed. 2011, 50, 11511–11515. [Google Scholar] [CrossRef] [PubMed]
- Wagh, Y.S.; Tiwari, N.J.; Bhanage, B.M. Metal-free synthesis of 2-aminobenzoxazoles using hypervalent iodine reagent. Tetrahedron Lett. 2013, 54, 1290–1293. [Google Scholar] [CrossRef]
- Cook, S.; Jefferies, L.; Weber, S. Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement. Synlett 2014, 26, 331–334. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2007, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Medvedev, M.G.; Bushmarinov, I.S.; Sun, J.; Perdew, J.P.; Lyssenko, K.A. Density functional theory is straying from the path toward the exact functional. Science 2017, 355, 49–52. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Boominathan, S.S.K.; Hu, W.-P.; Senadi, G.C.; Vandavasi, J.K.; Wang, J.-J. A one-pot hypoiodite catalysed oxidative cycloetherification approach to benzoxazoles. Chem. Commun. 2014, 50, 6726–6728. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; He, Y.; Zhang, X.; Guo, S.; Wang, Y. Synthesis of heteroaryl ketones via tandem reaction of 1,1-dibromoethenes. Tetrahedron 2011, 67, 6369–6374. [Google Scholar] [CrossRef]
- Gravenfors, Y.; Viklund, J.; Blid, J.; Ginman, T.; Karlström, S.; Kihlström, J.; Kolmodin, K.; Lindström, J.; Von Berg, S.; Von Kieseritzky, F.; et al. New Aminoimidazoles as β-Secretase (BACE-1) Inhibitors Showing Amyloid-β (Aβ) Lowering in Brain. J. Med. Chem. 2012, 55, 9297–9311. [Google Scholar] [CrossRef]
- Blid, J.; Ginman, T.; Gravenfors, Y.; Karlström, S.; Kolmodin, K.; Lindstroem, J.; Plobeck, N.; Rahm, F.; Swahn, B.-M.; Viklund, J.; et al. Preparation of phenylimidazolamine derivatives for use in treatment of neurodegenerative diseases. Patent: 2011, WO2011/2407. Chem. Abstr. 2011, 154, 109611. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

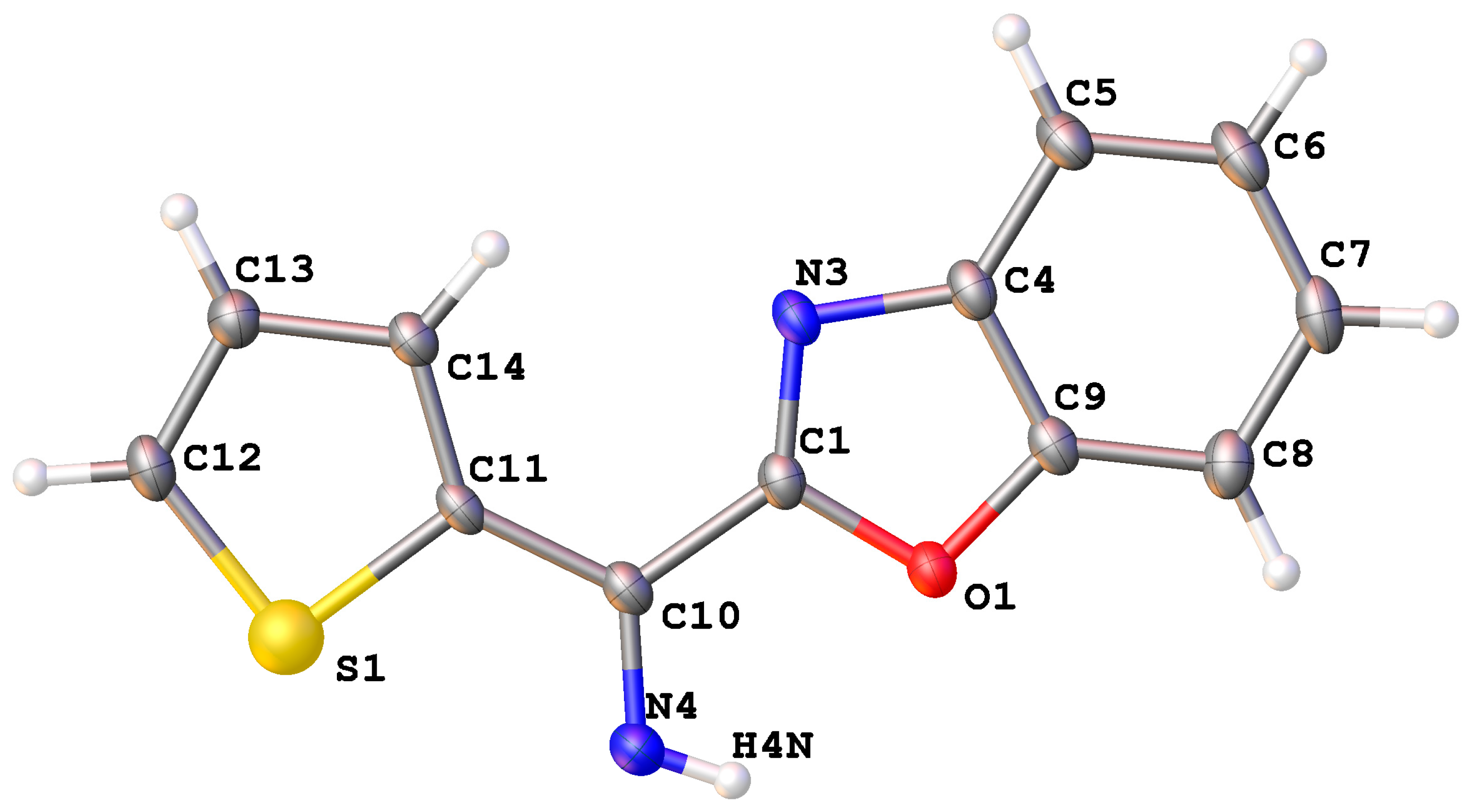


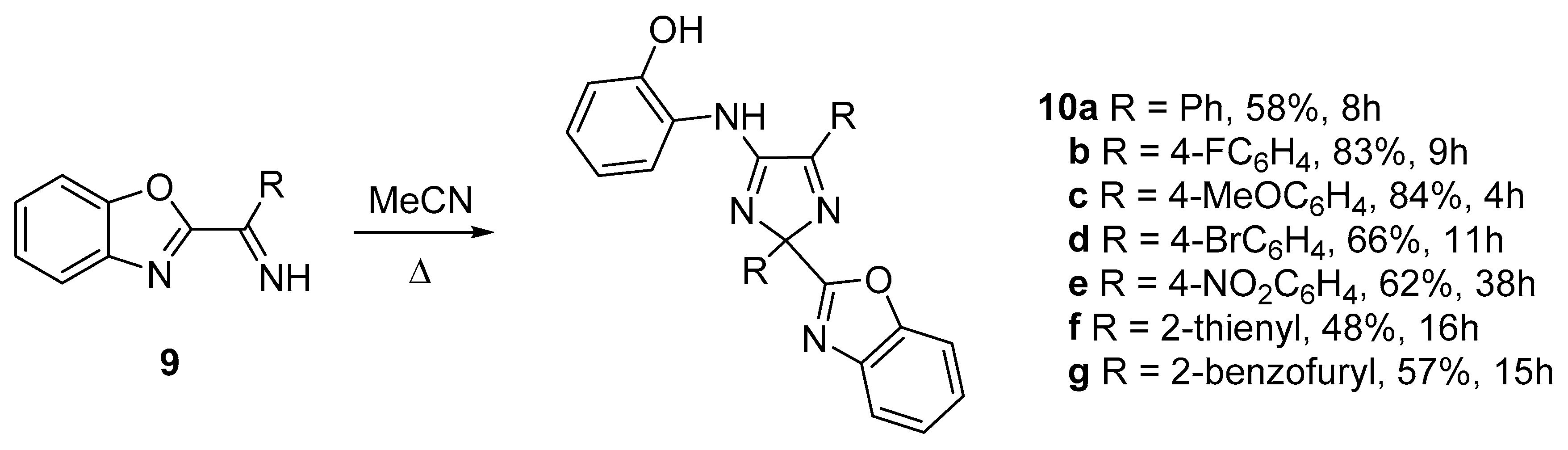
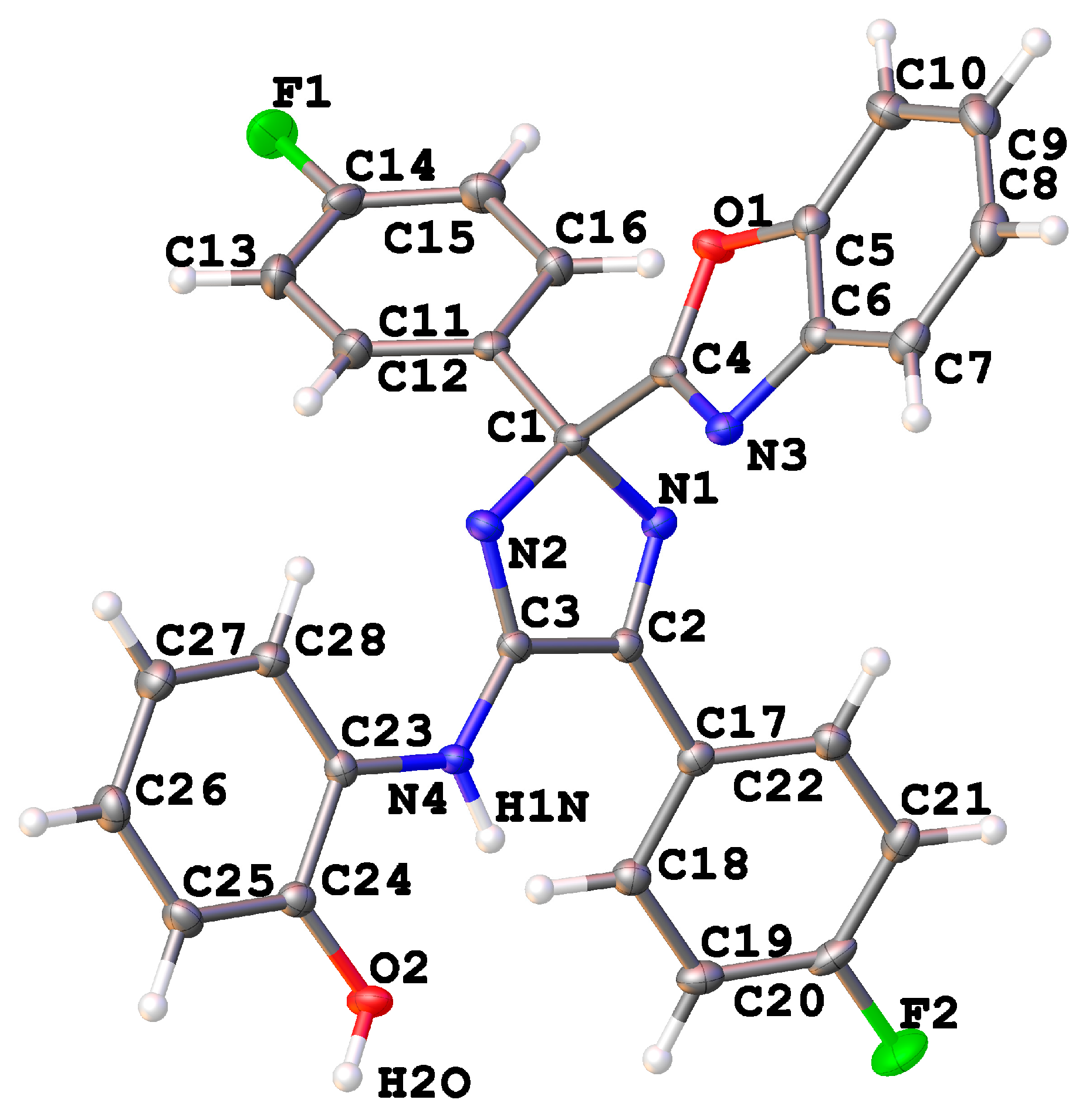
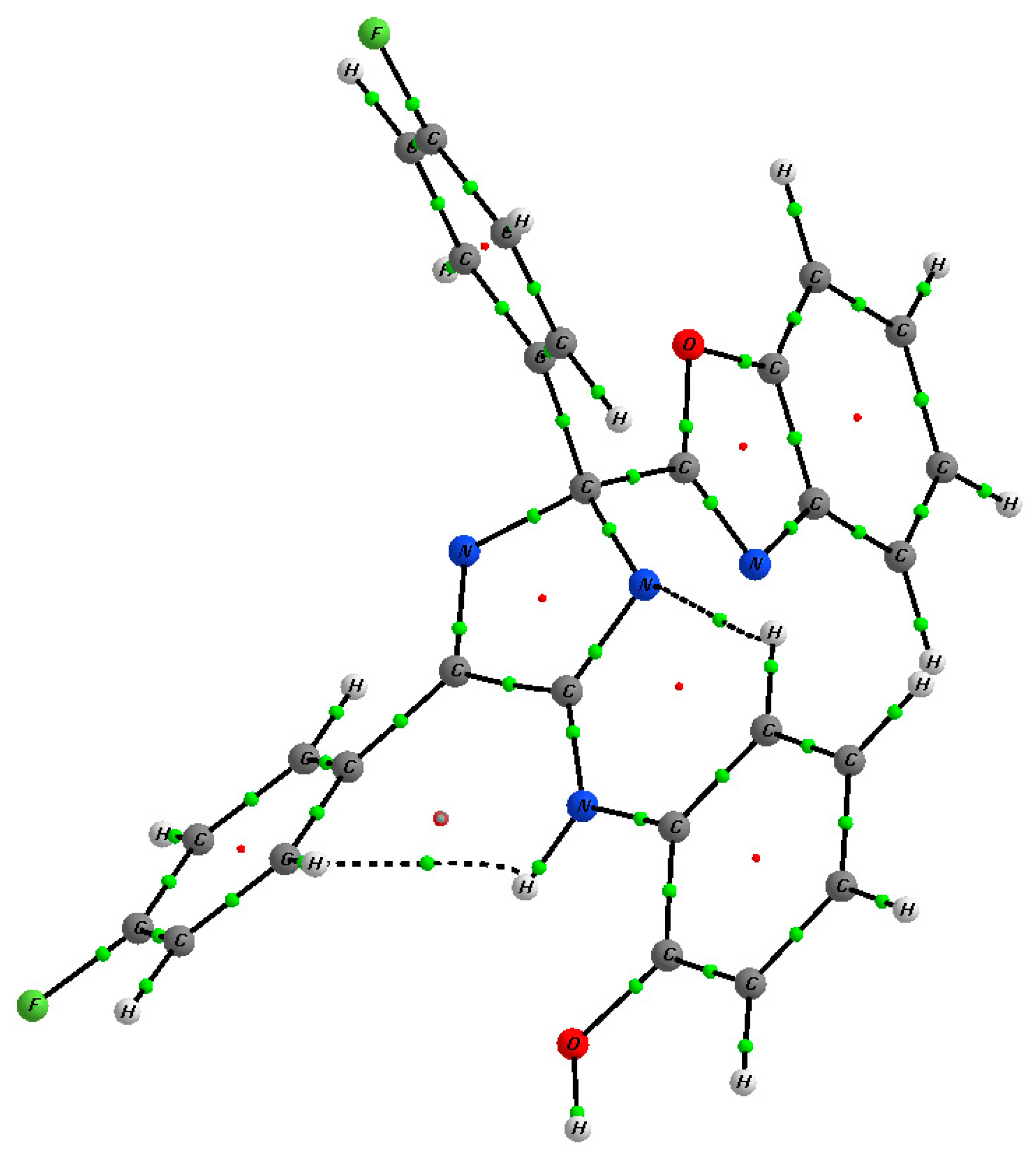
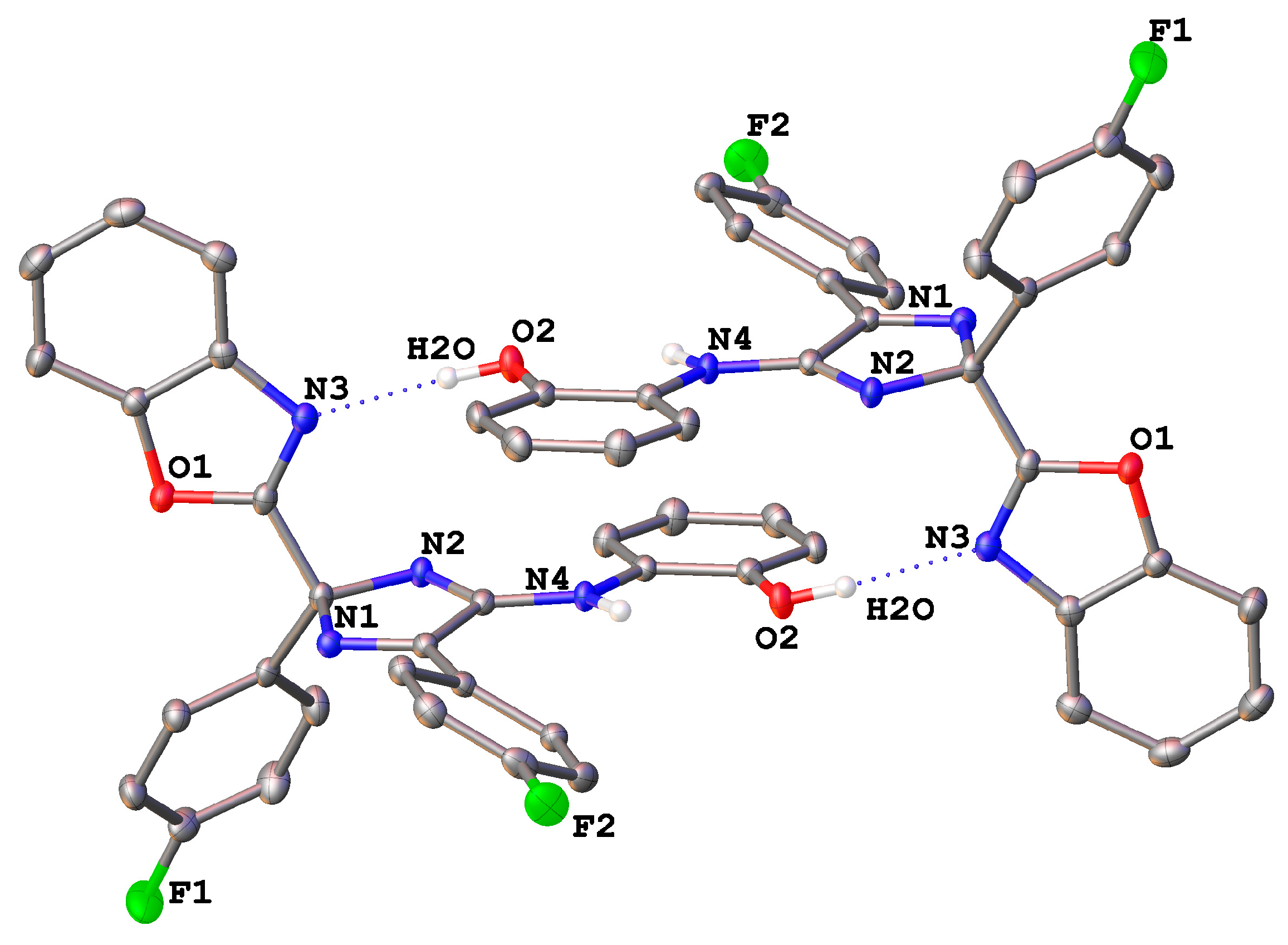
| 9f | 10b | |
|---|---|---|
| CCDC number | 1993040 | 1993041 |
| Empirical formula | C28H18F2N4O2 | C12H8N2OS |
| Formula weight | 480.46 | 228.26 |
| T, K | 120 | 120 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/n | P21/n |
| Z (Z’) | 4 (1) | 4(1) |
| a, Å | 12.3650(12) | 6.1355(5) |
| b, Å | 12.9901(13) | 7.5454(6) |
| c, Å | 14.3314(14) | 22.0813(19) |
| α, ° | 90 | 90 |
| β, ° | 107.871(2) | 94.779(2) |
| γ, ° | 90 | 90 |
| V, Å3 | 2190.9(4) | 1018.70(15) |
| Dcalc,gcm−3 | 1.457 | 1.488 |
| μ, cm−1 | 1.06 | 2.93 |
| F(000) | 992 | 472 |
| 2θmax, ° | 58 | 58 |
| Reflections collected | 25153 | 11900 |
| Reflections unique (Rint) | 5821 (0.0410) | 2710 (0.0357) |
| Reflections with I > 2σ(I) | 4558 | 2443 |
| Variables/restraints | 333 | 149 |
| R1 | 0.0425 | 0.0357 |
| wR2 | 0.1092 | 0.0962 |
| GOF | 1.024 | 1.036 |
| Largest difference in peak/hole (e/Å3) | 0.328/−0.242 | 0.415/−0.342 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranovsky, I.V.; Konstantinova, L.S.; Tolmachev, M.A.; Popov, V.V.; A. Lyssenko, K.; Rakitin, O.A. Synthesis of 2-((2-(Benzo[d]oxazol-2-yl)-2H-imidazol-4-yl)amino)-phenols from 2-((5H-1,2,3-Dithiazol-5-ylidene)amino)phenols through Unprecedented Formation of Imidazole Ring from Two Methanimino Groups. Molecules 2020, 25, 3768. https://doi.org/10.3390/molecules25173768
Baranovsky IV, Konstantinova LS, Tolmachev MA, Popov VV, A. Lyssenko K, Rakitin OA. Synthesis of 2-((2-(Benzo[d]oxazol-2-yl)-2H-imidazol-4-yl)amino)-phenols from 2-((5H-1,2,3-Dithiazol-5-ylidene)amino)phenols through Unprecedented Formation of Imidazole Ring from Two Methanimino Groups. Molecules. 2020; 25(17):3768. https://doi.org/10.3390/molecules25173768
Chicago/Turabian StyleBaranovsky, Ilia V., Lidia S. Konstantinova, Mikhail A. Tolmachev, Vadim V. Popov, Konstantin A. Lyssenko, and Oleg A. Rakitin. 2020. "Synthesis of 2-((2-(Benzo[d]oxazol-2-yl)-2H-imidazol-4-yl)amino)-phenols from 2-((5H-1,2,3-Dithiazol-5-ylidene)amino)phenols through Unprecedented Formation of Imidazole Ring from Two Methanimino Groups" Molecules 25, no. 17: 3768. https://doi.org/10.3390/molecules25173768
APA StyleBaranovsky, I. V., Konstantinova, L. S., Tolmachev, M. A., Popov, V. V., A. Lyssenko, K., & Rakitin, O. A. (2020). Synthesis of 2-((2-(Benzo[d]oxazol-2-yl)-2H-imidazol-4-yl)amino)-phenols from 2-((5H-1,2,3-Dithiazol-5-ylidene)amino)phenols through Unprecedented Formation of Imidazole Ring from Two Methanimino Groups. Molecules, 25(17), 3768. https://doi.org/10.3390/molecules25173768