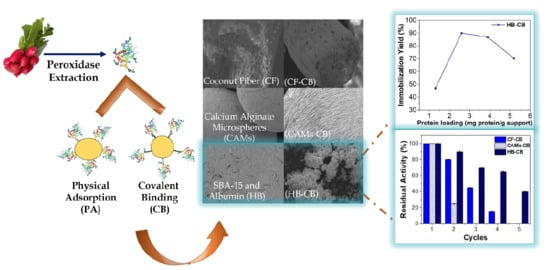
Immobilization of Low-Cost Alternative Vegetable Peroxidase (Raphanus sativus L. peroxidase): Choice of Support/Technique and Characterization
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Protein Loading
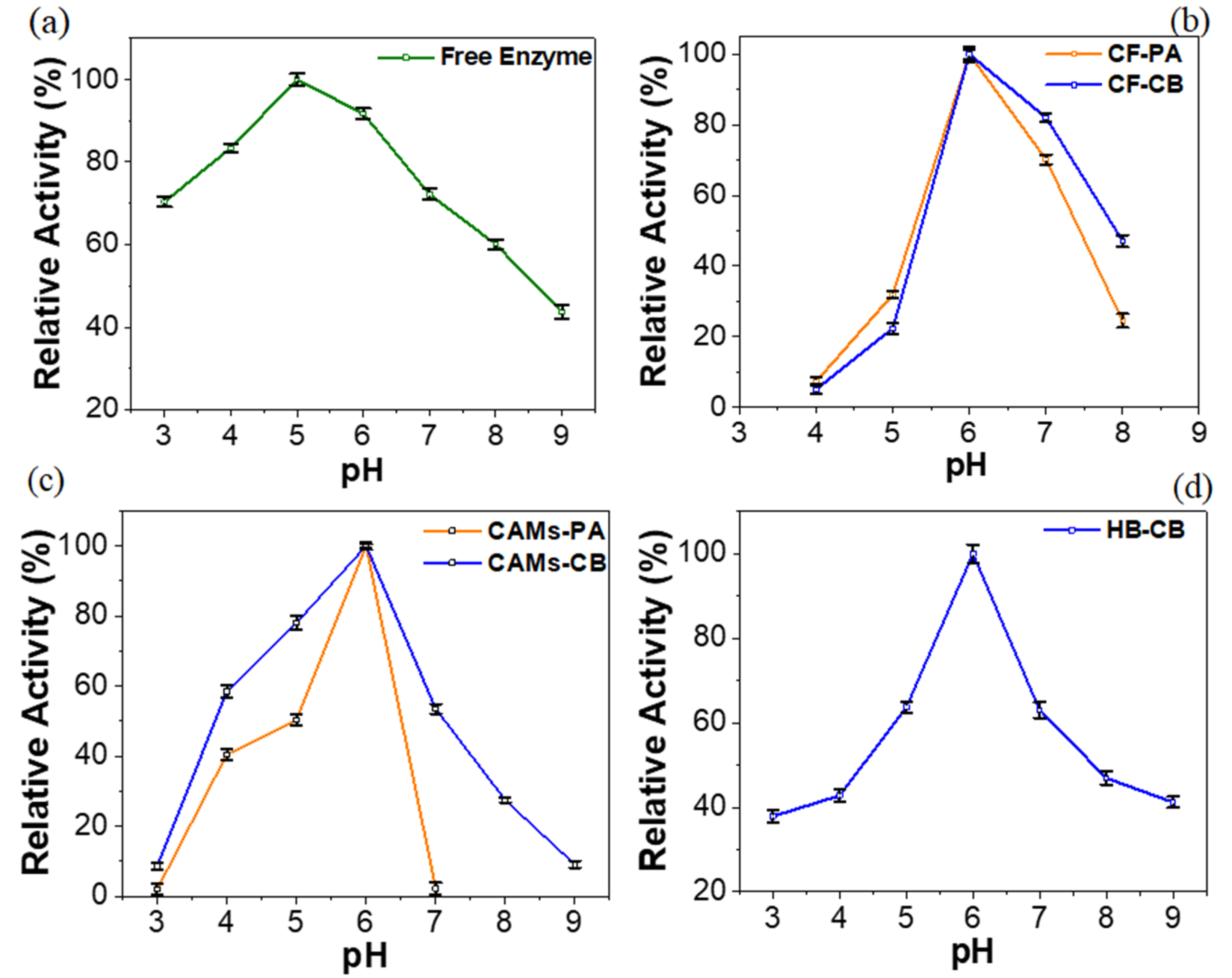
2.2. Effect of pH
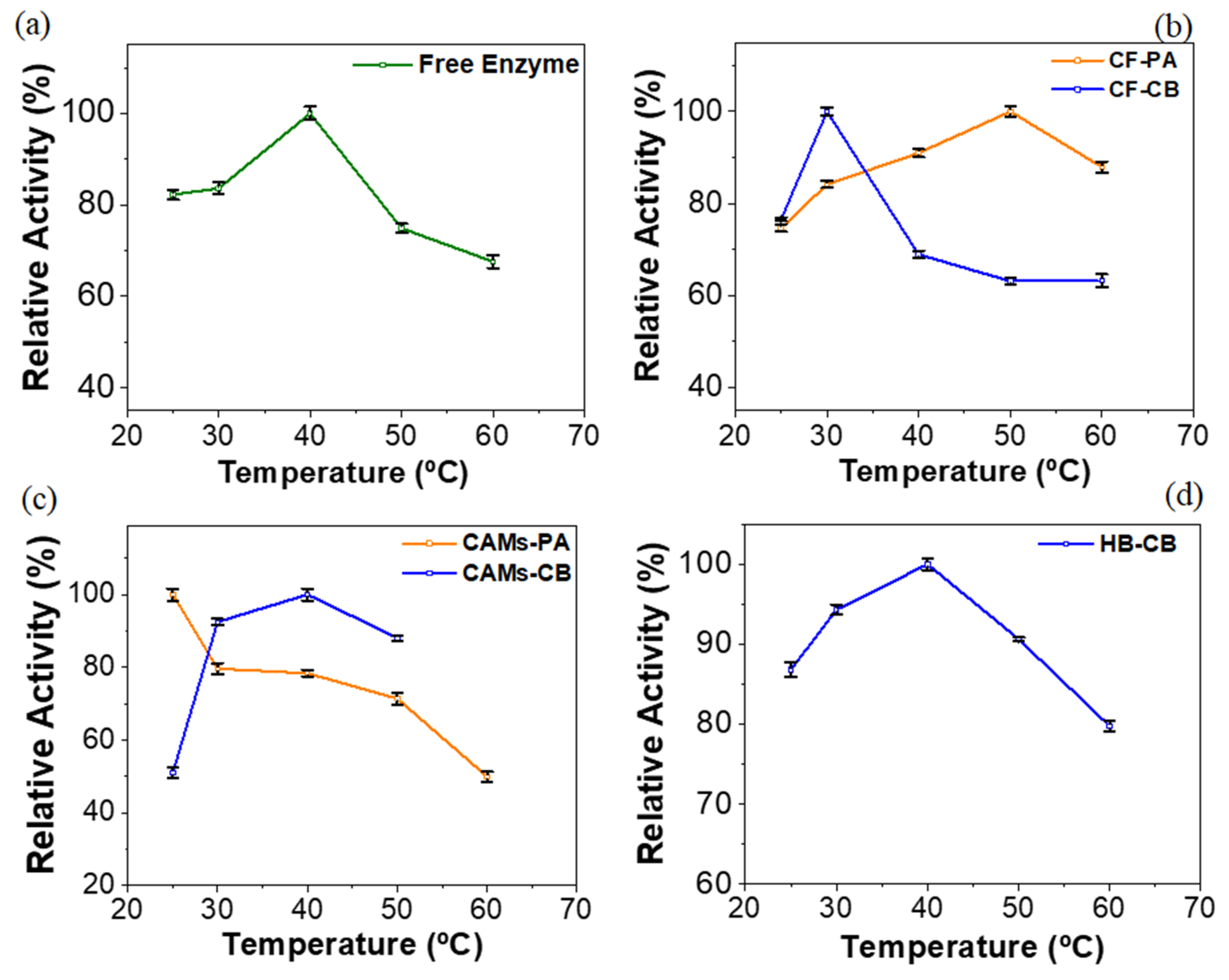
2.3. Effect of Temperature
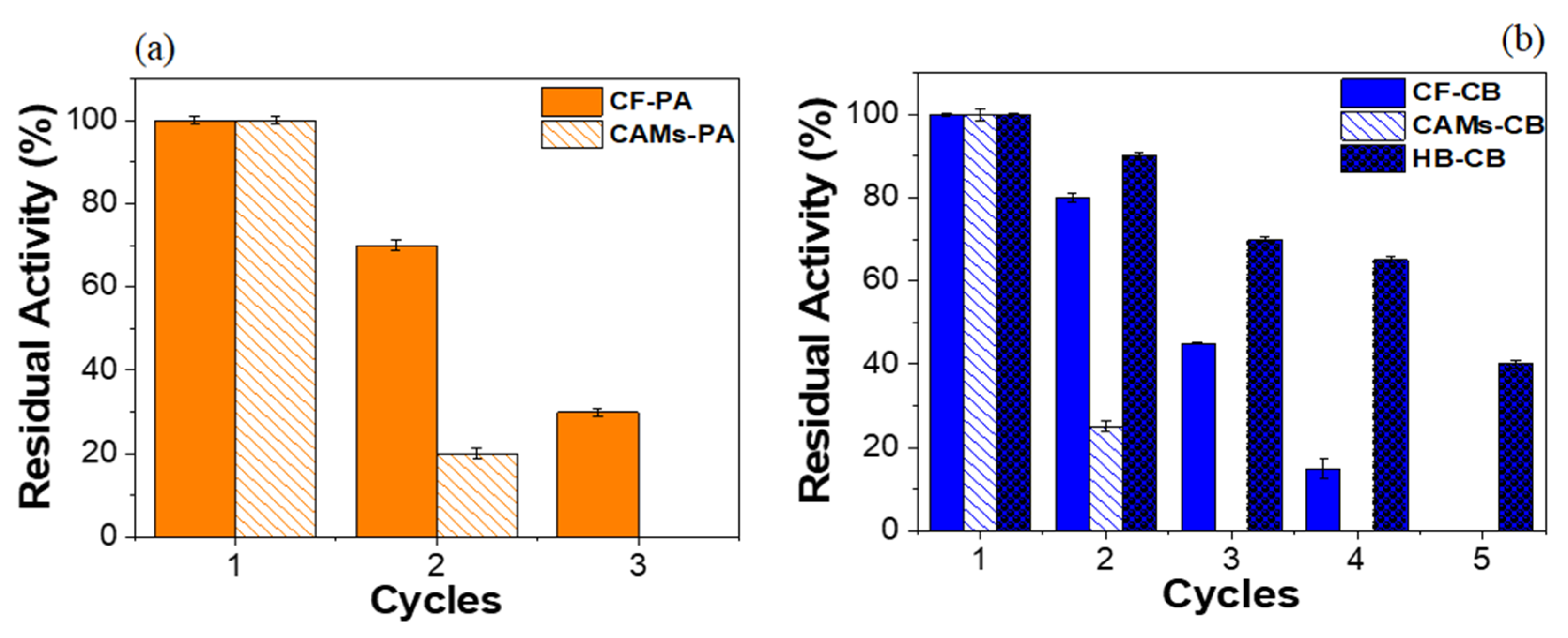
2.4. Operational Stability
2.5. Physicochemical and Morphological Characterization
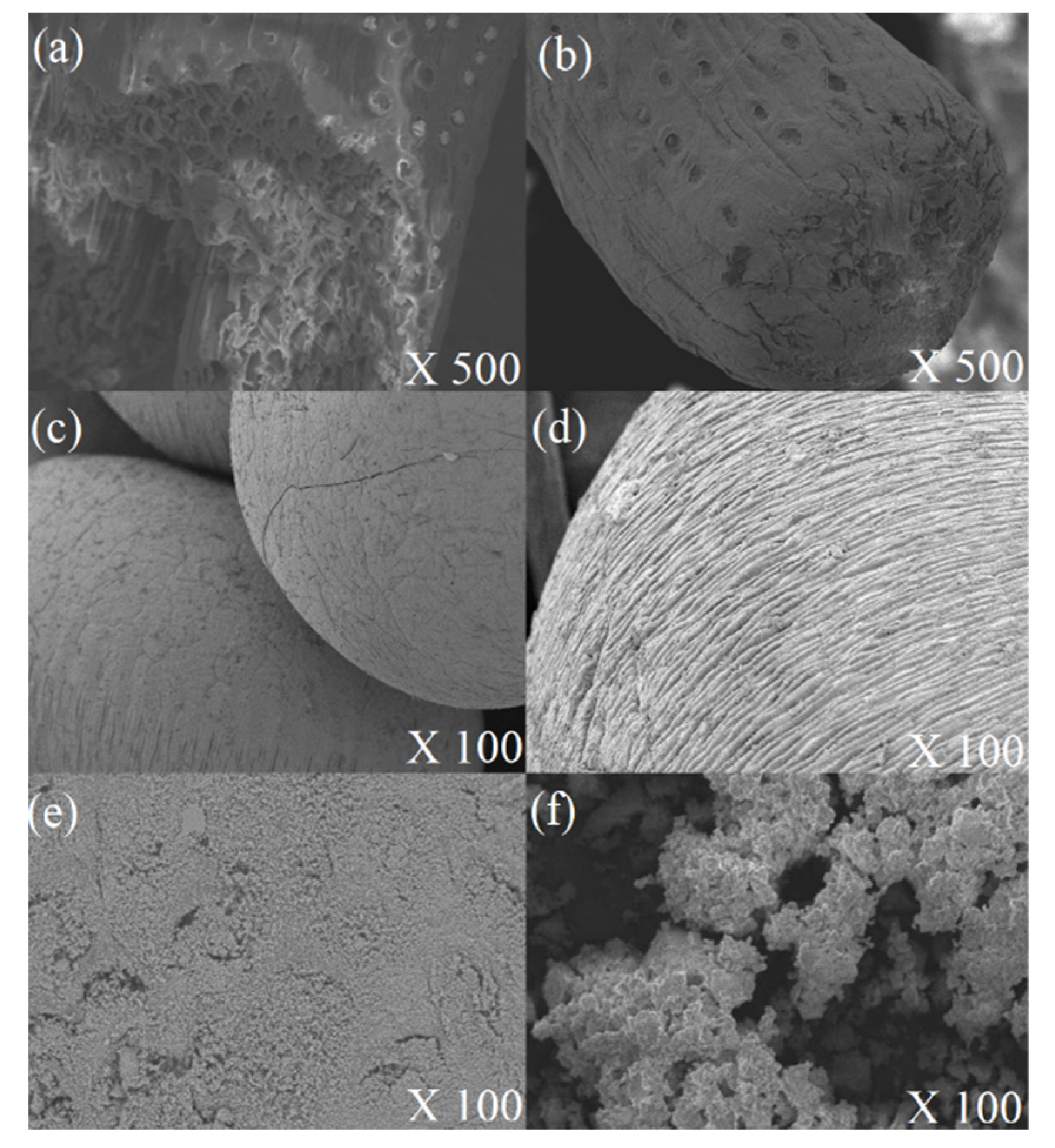
2.5.1. Scanning Electron Microscopy (SEM)
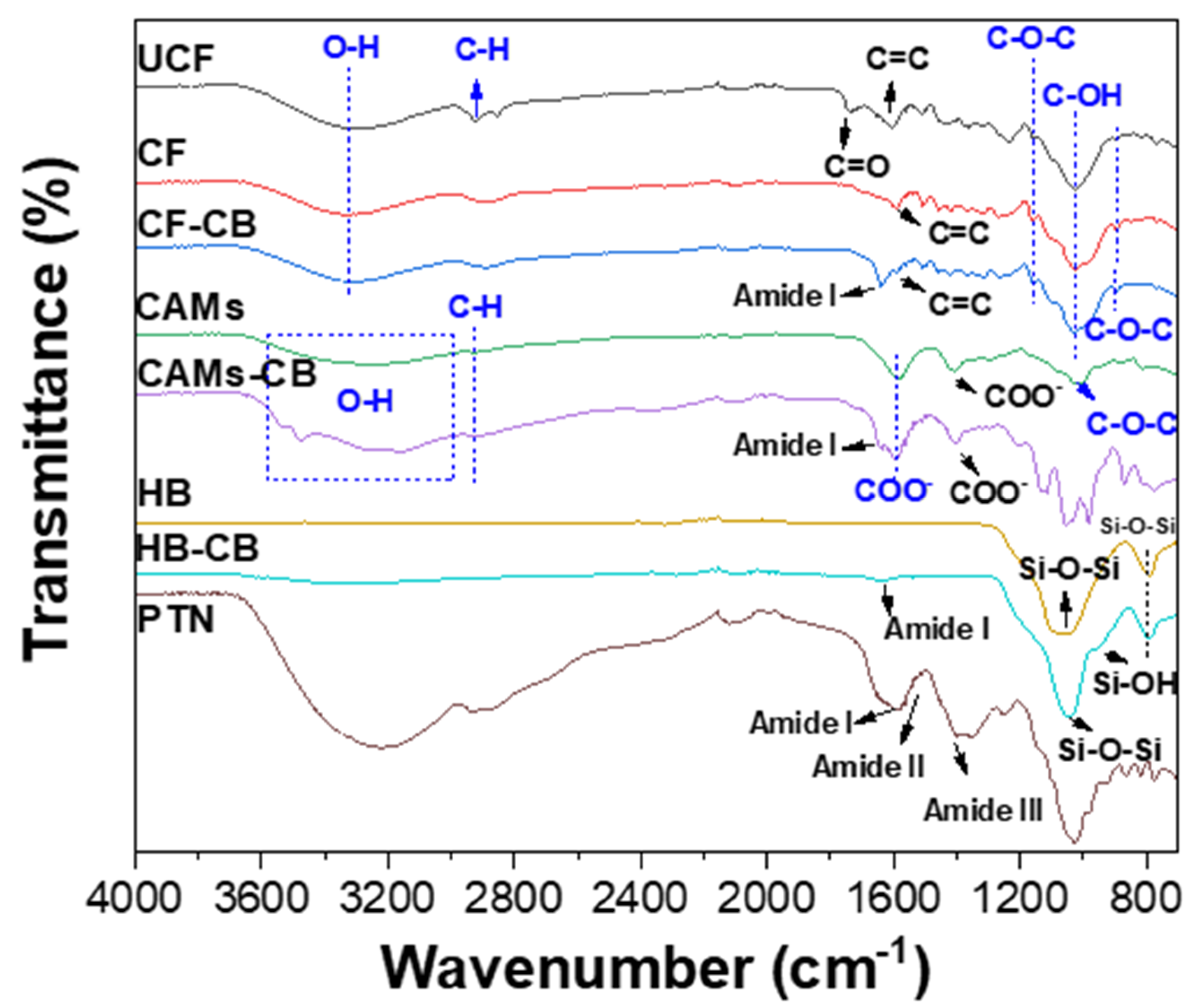
2.5.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5.3. Thermogravimetric Analysis (TGA)
3. Materials and Methods
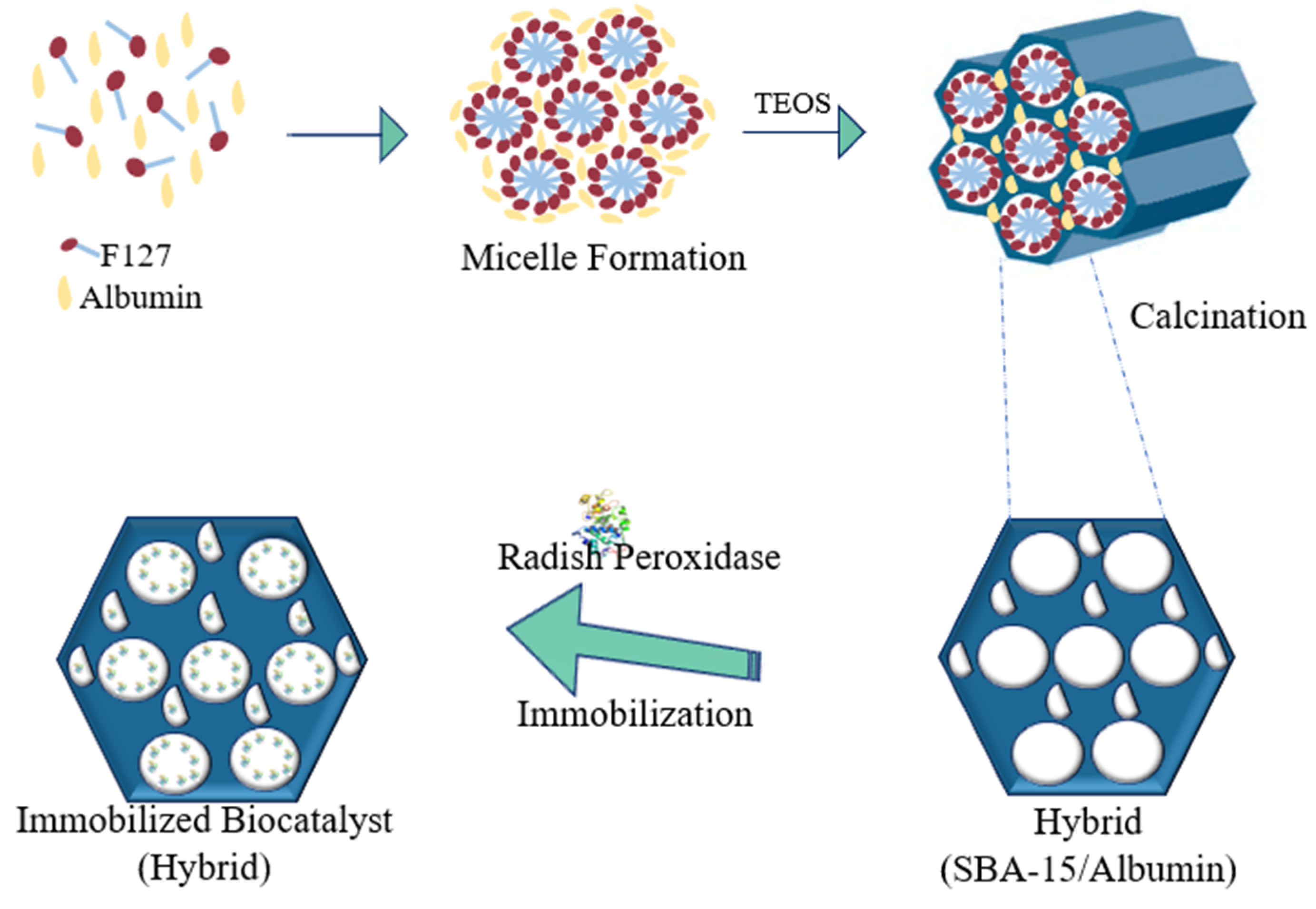
3.1. Preparation of the Supports
3.1.1. Coconut Fiber (CF)
3.1.2. Calcium Alginate Microspheres (CAMs)
3.1.3. SBA-15/Albumin Hybrid (HB)
3.2. Preparation of the Radish Crude Extract: Alternative Source of Peroxidase
3.3. Enzyme Immobilization
3.4. Peroxidase Activity Assay
3.5. Characterization of the Immobilized Biocatalysts (IBs)
3.5.1. Effect of pH, Temperature and Operational Stability
3.5.2. Scanning Electron Microscopy (SEM), Fourier-Transform Infrared Spectroscopy (FTIR), Thermogravimetric Analysis (TGA)
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Al-Bagmi, M.S.; Khan, M.S.; Ismael, M.A.; Al-Senaidy, A.M.; Bacha, A.B.; Husain, F.M.; Alamery, S.F. An efficient methodology for the purification of date palm peroxidase: Stability comparison with horseradish peroxidase (HRP). Saudi J. Biol. Sci. 2019, 26, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.; Kumari, V.; Kanwar, S.S. Comparative analysis of amino acid sequence diversity and physiochemical properties of peroxidase superfamily. J. Protein Res. Bioinform. 2020, 2, 003. [Google Scholar]
- Krainer, F.W.; Glieder, A. An updated view on horseradish peroxidases: Recombinant production and biotechnological applications. Appl. Microbiol. Biotechnol. 2015, 99, 1611–1625. [Google Scholar] [CrossRef]
- Lopes, L.C.; Brandão, I.V.; Sánchez, O.C.; Franceschi, E.; Borges, G.; Dariva, C.; Fricks, A.T. Horseradish peroxidase biocatalytic reaction monitoring using near-infrared (NIR) spectroscopy. Process. Biochem. 2018, 71, 127–133. [Google Scholar] [CrossRef]
- Almulaiky, Y.Q.; Al-Harbi, S.A. A novel peroxidase from Arabian balsam (Commiphora gileadensis) stems: Its purification, characterization and immobilization on a carboxymethylcellulose/Fe3O4 magnetic hybrid material. Int. J. Biol. Macromol. 2019, 133, 767–774. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Jang, S.; Lee, H.; Sung, D.; Chang, J.H. High efficient chromogenic catalysis of tetramethylbenzidine with horseradish peroxidase immobilized magnetic nanoparticles. Biochem. Eng. J. 2016, 105, 406–411. [Google Scholar] [CrossRef]
- Lucena, I.V.; Brandão, I.V.; Mattedi, S.; Souza, R.L.; Soares, C.M.F.; Fricks, A.T.; Lima, A.S. Use of protic ionic liquids as adjuvants in PEG-based ATPS for the purification of radish peroxidase. Fluid Phase Equilib. 2017, 452, 1–8. [Google Scholar] [CrossRef]
- Centeno, D.A.; Solano, X.H.; Castillo, J.J. A new peroxidase from leaves of guinea grass (Panicum maximum): A potential biocatalyst to build amperometric biosensors. Bioelectrochemistry 2017, 116, 33–38. [Google Scholar] [CrossRef]
- Kandil, O.M.; El-Hakim, A.E.; Gad, A.A.M.; El-Ezz, N.M.T.A.; Mahmoud, M.S.; Hendawy, S.H.M.; Salama, D.B. Camel hydatidosis diagnostic kit: Optimization of turnip and horseradish peroxidase conjugates using glutaraldehyde method. J. Parasit Dis. 2020, 44, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.N.; Lopes, L.C.; Dariva, C.; Girardi, J.S.; Lucchese, A.M.; Alvarez, H.M.; Fricks, A.T. Bioepoxidation of isosafrol catalyzed by radish and turnip peroxidases. Afr. J. Biotechnol. 2015, 14, 1074–1080. [Google Scholar] [CrossRef][Green Version]
- Fritzke, W.; Salla, E.G.; Bagatini, M.D.; Bonadiman, B.S.R.; Skoronski, E.; Moroni, L.S.; Kempka, A.P. Peroxidase of Cedrela fissilis leaves: Biochemical characterization and toxicity of enzymatically decolored solution of textile dye Brilliant Sky-Blue G. Biocatal. Agric. Biotechnol. 2020, 24, 101553. [Google Scholar] [CrossRef]
- Kurnik, K.; Treder, K.; Skorupa-Kłaput, M.; Tretyn, A.; Tyburski, J. Removal of Phenol from Synthetic and Industrial Wastewater by Potato Pulp Peroxidases. Water Air Soil Pollut. 2015, 226, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Kumar, P.; Singh, S.; Yadav, A.; Dumée, L.F.; Sharma, R.S.; Mishra, V. Prosopis juliflora peroxidases for phenol remediation from industrial wastewater—An innovative practice for environmental sustainability. Environ. Technol. Innov. 2020, 19, 100865. [Google Scholar] [CrossRef]
- M’mbone, M.E.; Cheng, W.; Xu, L.; Wang, Y.; Karanja, B.K.; Zhu, X.; Cao, Y. Identification and transcript analysis of MATE genes involved in anthocyanin transport in radish (Raphanus sativus L.). Sci Hortic. 2018, 238, 195–203. [Google Scholar] [CrossRef]
- Correia, C.C.S.A.; Da Cunha, F.F.; Mantovani, E.C.; Da Silva, D.J.H.; Dias, S.H.B. Irrigation of radish cultivars in the region of Viçosa, Minas Gerais, Brazil. Rer. Cien. Agron. 2020, 51, e20175643. [Google Scholar] [CrossRef]
- Pei, Y.; Yao, N.; He, L.; Deng, D.; Li, W.; Zhang, W. Comparative study of the morphological, physiological and molecular characteristics between diploid and tetraploid radish (Raphanus sativus L.). Sci Hortic. 2019, 257, 108739. [Google Scholar] [CrossRef]
- Fricks, A.T.; Souza, D.P.B.; Oestreichera, E.G.; Antunes, O.A.C.; Girardi, J.S.; Oliveira, D.; Dariva, C. Evaluation of radish (Raphanus sativus L.) peroxidase activity after high-pressure treatment with carbon dioxide. J. Supercrit. Fluids. 2006, 38, 347–353. [Google Scholar] [CrossRef]
- Al-Sa’ady, A.J.R.; Al-Bahrani, M.H.A.; Aziz, G.M. Characterization and Immobilization of Peroxidase Extracted from Horse Radish and Decolorization of Some Dyes. J. Curr. Microbiol. App. Sci. 2018, 7, 328–339. [Google Scholar] [CrossRef]
- Fricks, A.T.; Dariva, C.; Alvarez, H.M.; Santosa, A.F.; Fortuny, M.; Queiroz, M.L.B.; Antunes, O.A.C. Compressed propane as a new and fast method of pre-purification of radish (Raphanus sativus L.) peroxidase. J. Supercrit. Fluids. 2010, 54, 153–158. [Google Scholar] [CrossRef]
- Pandey, V.P.; Awasthi, M.; Singh, S.; Tiwari, S.; Dwivedi, U.N. A comprehensive review on function and application of plant peroxidases. Biochem. Anal. Biochem. 2017, 6, 1000308. [Google Scholar] [CrossRef]
- Wasak, A.; Drozd, R.; Jankowiak, D.; Rakoczy, R. The influence of rotating magnetic field on bio-catalytic dye degradation using the horseradish peroxidase. Biochem. Eng. J. 2019, 147, 81–88. [Google Scholar] [CrossRef]
- Altinkaynak, C.; Tavlasoglu, S.; ÿzdemir, N.; Ocsoy, I. A new generation approach in enzyme immobilization: Organic-inorganic hybrid nanoflowers with enhanced catalytic activity and stability. Enzym. Microb. Tec. 2016, 93–94, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.R.; Pinto, D.C.G.A.; Silva, A.M.S. Horseradish peroxidase (HRP) as a tool in green chemistry. Rsc Adv. 2014, 4, 37244–37265. [Google Scholar] [CrossRef]
- Liu, D.M.; Chen, J.; Shi, Y.P. Advances on methods and easy separated support materials for enzymes immobilization. Trends. Analyt. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the Support Properties for Immobilization or Purification of Enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters necessary to define an immobilized enzyme preparation. Process. Biochem. 2020, 90, 66–80. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Wahab, R.A.; Elias, N.; Abdulla, F.; Ghosha, S.K. On the taught new tricks of enzymes immobilization: An all-inclusive Overview. React. Funct. Polym. 2020, 152, 104613. [Google Scholar] [CrossRef]
- Liu, D.M.; Dong, C. Recent advances in nano-carrier immobilized enzymes and their applications. Process. Biochem. 2020, 92, 464–475. [Google Scholar] [CrossRef]
- Liu, J.; Ma, R.T.; Shi, Y.P. “Recent advances on support materials for lipase immobilization and applicability as biocatalysts in inhibitors screening methods”—A review. Anal. Chim. Acta. 2020, 1101, 9–22. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M.A. General overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Manoel, E.A.; Lafuente, R.F.; Freire, D.M.G. Support engineering: Relation between development of new supports for immobilization of lipases and their applications. Biotechnol. Res. Innov. 2017, 1, 26–34. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N.; Cui, J. “Smart” chemistry and its application in peroxidase immobilization using different support materials. Int. J. Biol. Macromol. 2018, 119, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Salvi, H.M.; Yadav, G.D. Surface functionalization of SBA-15 for immobilization of lipase and its application in synthesis of alkyl levulinates: Optimization and kinetics. Biocatal. Agric. Biotechnol. 2019, 18, 101038. [Google Scholar] [CrossRef]
- Queiroz, M.L.B.; Conceição, K.C.; Melo, M.N.; Sánchez, O.C.; Alvarez, H.M.; Soares, C.M.F.; Fricks, A.T. Imobilização de peroxidase de raiz forte em bagaço de cana-de-açúcar. Quim. Nova 2018, 41, 1–6. [Google Scholar] [CrossRef]
- Bezerra, T.M.S.; Bassan, J.C.; Santos, V.T.O.; Ferraz, A.; Monti, R. Covalent immobilization of laccase in green coconut fiber and use in clarification of apple juice. Process. Biochem. 2015, 50, 417–423. [Google Scholar] [CrossRef]
- Brígida, A.I.S.; Pinheiro, A.D.T.; Ferreira, A.L.O.; Gonçalves, L.R.B. Immobilization of Candida antarctica Lipase B by adsorption to green coconut fiber. Appl. Biochem. Biotech. 2008, 146, 173–187. [Google Scholar] [CrossRef]
- Brígida, A.I.S.; Pinheiro, A.D.T.; Ferreira, A.L.O.; Pinto, G.A.S.; Gonçalves, L.R.B. Immobilization of Candida antarctica Lipase B by covalent attachment to green coconut fiber. Appl. Biochem. Biotech. 2007, 136, 67–80. [Google Scholar] [CrossRef]
- Cristóvão, R.O.; Tavares, A.P.M.; Brígida, A.I.; Loureiro, J.M.; Boaventura, R.A.R.; Macedo, E.A.; Coelho, M.A.Z. Immobilization of commercial laccase onto green coconut fiber by adsorption and its application for reactive textile dyes degradation. J. Mol. Catal. B Enzym. 2011, 72, 6–12. [Google Scholar] [CrossRef]
- Martins, A.P.; Sanches, R.A. Assessment of coconut fibers for textile applications. Matéria (Rio J.) 2019, 24, e-12428. [Google Scholar] [CrossRef]
- Rambo, M.K.D.; Alexandre, G.P.; Rambo, M.C.D.; Alves, A.R.; Garcia, W.T.; Baruque, E. Characterization of biomasses from the north and northeast regions of Brazil for processes in biorefineries. Food Sci. Technol. Campinas. 2015, 35, 605–611. [Google Scholar] [CrossRef]
- Martín, M.C.; López, O.V.; Ciolino, A.E.; Morata, V.I.; Villar, M.A.; Ninago, M.D. Immobilization of enological pectinase in calcium alginate hydrogels: A potential biocatalyst for winemaking. Biocatal. Agric. Biotechnol. 2019, 18, 101091. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Lignin peroxidase immobilization on Ca-alginate beads and its dye degradation performance in a packed bed reactor system. Biocatal Agric. Biotechnol. 2019, 20, 101205. [Google Scholar] [CrossRef]
- Rahim, S.N.A.; Sulaiman, A.; Hamzah, F.; Hamid, K.H.K.; Rodhi, M.N.M.; Musa, M.; Edama, N.A. Enzymes encapsulation within calcium alginate-clay beads: Characterization and application for cassava slurry saccharification. Procedia Eng. 2013, 68, 411–417. [Google Scholar] [CrossRef]
- Tavares, T.S.; Rocha, E.P.; Nogueira, F.G.E.; Torres, J.A.; Silva, M.C.; Kuca, K.; Ramalho, T. D-FeOOH as support for immobilization peroxidase: Optimization via a chemometric approach. Molecules 2020, 25, 259. [Google Scholar] [CrossRef] [PubMed]
- Taratayko, A.; Larichev, Y.; Zaikovskii, V.; Mikheeva, N.; Mamontov, G. Ag-CeO2/SBA-15 composite prepared from Pluronic P123@SBA-15 hybrid as catalyst for room-temperature reduction of 4-nitrophenol. Catal. Today 2020, in press. [Google Scholar] [CrossRef]
- Chouyyok, W.; Panpranot, J.; Thanachayanant, C.; Prichanont, S. Effects of pH and pore characters of mesoporous silicas on horseradish peroxidase immobilization. J. Mol. Catal. Benzym. 2009, 56, 246–252. [Google Scholar] [CrossRef]
- Pitzalis, F.; Monduzzi, M.; Salis, A. A bienzymatic biocatalyst constituted by glucose oxidase and Horseradish peroxidase immobilized on ordered mesoporous silica. Micropor. Mesopor. Mat. 2017, 241, 145–154. [Google Scholar] [CrossRef]
- El-Nahass, M.N.; El-keiy, M.M.; Ali, E.M.M. Immobilization of horseradish peroxidase into cubic mesoporous silicate, SBA-16 with high activity and enhanced stability. Int. J. Biol. Macromol. 2018, 116, 1304–1309. [Google Scholar] [CrossRef]
- Zdarta, J.; Jedrzak, A.; Klapiszewski, L.; Jesionowski, T. Immobilization of cellulase on a functional inorganic-organic hybrid support: Stability and kinetic study. Catalysts 2017, 7, 374. [Google Scholar] [CrossRef]
- Mir, S.H.; Nagahara, L.A.; Thundat, T.; Tabari, P.M.; Furukawa, H.; Khosla, A. Review organic-inorganic hybrid functional materials: An integrated platform for applied technologies. J. Electromchem Soc. 2018, 165, 3137–3156. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial Applications of Enzymes: Recent Advances, Techniques and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Eisl, I.; Nidetzky, B. Advanced characterization of immobilized enzymes as heterogeneous biocatalysts. Catal. Today. 2016, 259, 66–80. [Google Scholar] [CrossRef]
- Mohammadi, M.; Heshmati, M.K.; Sarabandi, K.; Fathi, M.; Lime, L.T.; Hamishehkar, H. Activated alginate-montmorillonite beads as an efficient carrier for pectinase immobilization. Int. J. Biol. Macromol. 2019, 137, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-deLuna, S.E.; Moreno-Cortez, I.E.; Garza-Navarro, M.A.; Lucio-Porto, R.; Pavón, L.L.; González-González, V.A. Thermal stability of the immobilization process of horseradish peroxidase in electrospun polymeric nanofibers. J. Appl. Polym. Sci. 2017, 134, 1–10. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Malkia, A.L.; Kumosania, T.A.; El-Shishtawyc, R.M. Horseradish peroxidase and chitosan: Activation, immobilization and comparative results. Int. J. Biol. Macromol. 2013, 60, 295–300. [Google Scholar] [CrossRef]
- Mazlan, S.Z.; Hanifah, S.A. Effects of temperature and pH on immobilized laccase activity in conjugated methacrylate-acrylate microspheres. Int. J. Polym. Sci. 2017, 1–8. [Google Scholar] [CrossRef]
- Amiour, S.D.; Hambaba, L. Effect of pH, temperature and some chemicals on polyphenoloxidase and peroxidase activities in harvested Deglet Nour and Ghars dates. Postharvest Biol. Technol. 2016, 111, 77–82. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Harbi, M.H.; Almulaiky, Y.Q.; Ibrahim, I.H.; El-Shishtawy, R.M. Immobilization of horseradish peroxidase on Fe3O4 magnetic nanoparticles. Electron. J. Biotechn. 2017, 27, 84–90. [Google Scholar] [CrossRef]
- Silva, J.C.; França, P.R.L.; Converti, A.; Porto, T.S. Pectin hydrolysis in cashew apple juice by Aspergillus aculeatus URM4953 polygalacturonase covalently-immobilized on calcium alginate beads: A kinetic and thermodynamic study. Int. J. Biol. Macromol. 2019, 126, 820–827. [Google Scholar] [CrossRef]
- Jamal, F.; Qidwai, T.; Singh, D.; Pandey, P.K. Biocatalytic activity of immobilized pointed gourd (Trichosanthes dioica) Peroxidase-concanavalin A complex on calcium alginate pectin gel. J. Mol. Catal. B Enzym. 2012, 74, 125–131. [Google Scholar] [CrossRef]
- Da Silva, R.M.; Gonçalves, L.R.B.; Rodrigues, S. Different strategies to co-immobilize dextransucrase and dextranase onto agarose based supports: Operational stability study. Int. J. Biol. Macromol. 2020, 156, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Pan, T.; Xie, Z.; Xu, X.; Jin, Z. Co-immobilization of β-fructofuranosidase and glucose oxidase improves the stability of Bi-enzymes and the production of lactosucrose. LWT Food Sci. Technol. 2020, 128, 109460. [Google Scholar] [CrossRef]
- Carvalho, T.; Pereira, A.d.S.; Bonomo, R.C.F.; Franco, M.; Finotelli, P.V.; Amaral, P.F.F. Simple physical adsorption technique to immobilize Yarrowia lipolytica lipase purified by different methods on magnetic nanoparticles: Adsorption isotherms and thermodynamic approach. Int. J. Biol. Macromol. 2020, 160, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Satar, R.; Jafri, M.A.; Rasool, M.; Ansari, S.A. Role of Glutaraldehyde in Imparting Stability to Immobilized β-Galactosidase Systems. Braz. Arch. Biol. Technol. 2017, 60, e17160311. [Google Scholar] [CrossRef]
- Xie, X.; Luo, P.; Han, J.; Chen, T.; Wang, Y.; Cai, Y.; Liu, Q. Horseradish peroxidase immobilized on the magnetic composite microspheres for high catalytic ability and operational stability. Enzym. Microb. Tech. 2019, 122, 26–35. [Google Scholar] [CrossRef]
- Brígida, A.I.S.; Calado, V.M.A.; Gonçalvez, L.R.B.; Coelho, M.A.Z. Effect of chemical treatments on properties of green coconut fiber. Carbohydr. Polym. 2010, 79, 832–838. [Google Scholar] [CrossRef]
- Praepanitchai, O.A.; Noomhorm, A.; Anal, A.K. Survival and behavior of encapsulated probiotics (Lactobacillus plantarum) in calcium-alginate-Soy protein isolate-based hydrogel beads in different processing conditions (pH and temperature) and in pasteurized mango juice. Biomed Res. Int. 2019, 1–8. [Google Scholar] [CrossRef]
- Cao, X.; Yu, J.; Zhang, Z.; Liu, S. Bioactivity of horseradish peroxidase entrapped in silica nanospheres. Biosens. Bioelestron. 2012, 35, 101–107. [Google Scholar] [CrossRef]
- Mothe, C.G.; de Miranda, I.C. Characterization of sugarcane and coconut fibers by thermal analysis and FTIR. J. Anal. Calorim. 2009, 97, 661–665. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, U.; Muzenda, E.; Shukla, M.; Rajulu, A.V. Extraction and characterization of cellulose from pretreated ficus (Peepal Tree) leaf fibers. J. Nat. Fibers. 2016, 13, 54–64. [Google Scholar] [CrossRef]
- Thite, V.S.; Nerurkar, A.S. Valorization of sugarcane bagasse by chemical pretreatment and enzyme mediated deconstruction. Sci. Rep. 2019, 9, 15904. [Google Scholar] [CrossRef] [PubMed]
- Razak, F.; Chyzna, V.; Morris, N.; Murphy, A.; Kennedy, J. Development of Amoxicillin loaded microspheres for anti-Helicobacter pylori infection using Ionic Gelation method. Int. J. Adv. Sci. Res. Manag. 2017, 2, 13–24. [Google Scholar]
- Jurić, S.; Đermić, E.; Topolovec-Pintarić, S.; Bedek, M.; Vinceković, M. Physicochemical properties and release characteristics of calcium alginate microspheres loaded with Trichoderma viride spores. J. Integr. Agric. 2019, 18, 2534–2548. [Google Scholar] [CrossRef]
- Badita, C.R.; Aranghel, D.; Burducea, C.; Mereuta, P. Characterization of sodium alginate based films. Rom. J. Phys. 2020, 65, 1–8. [Google Scholar]
- Zhao, H.; Zhang, T.; Qi, R.; Dai, J.; Liu, S.; Fei, T.; Lu, G. Organic-inorganic hybrid materials based on mesoporous silica derivatives for humidity sensing. Sens. Actuat. B-Chem. 2017, 248, 803–811. [Google Scholar] [CrossRef]
- Basu, G.; Mishra, L.; Jose, S.; Samanta, A.K. Accelerated retting cum softening of coconut fibre. Ind. Crops Prod. 2015, 77, 66–73. [Google Scholar] [CrossRef]
- Widnyana, A.; Rian, I.G.; Surata, I.W.; Nindhia, T.G.T. Tensile Properties of coconut Coir single fiber with alkali treatment and reinforcement effect on unsaturated polyester polymer. Mater. Today Proc. 2020, 22, 300–305. [Google Scholar] [CrossRef]
- Mandal, S.; Kumar, S.S.; Krishnamoorthy, B.; Basu, S.K. Development and evaluation of calcium alginate beads prepared by sequential and simultaneous methods. Braz. J. Pharm. Sci. 2010, 46, 785–793. [Google Scholar] [CrossRef]
- Méndez, J.C.; Arellano, U.; Solís, S.; Asomoza, M.; Lara, V.H.; Padilha, A.J.; Wang, J.A. Synthesis of hybrid materials, immobilization of lipase in SBA-15 modified with CaO. J. Appl. Res. Technol. 2018, 16, 498–510. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Sample Availability: Samples of the immobilized biocatalysts and supports are available from the authors. |

| Mass Loss (%) | Moisture Content 1 (%) | |||
|---|---|---|---|---|
| Sample | Region I 25–200 °C | Region II 200–600 °C | Region III Above 600 °C | |
| UCF | 7.18 | 65.36 | 12.37 | ---- |
| CF | 8.71 | 76.03 | 11.39 | 3.80 ± 0.37 |
| CF-PA | 10.28 | 72.17 | 13.31 | 5.40 ± 0.05 |
| CF-CB | 11.40 | 73.97 | 11.73 | 3.78 ±0.22 |
| CAM | 13.99 | 43.86 | 14.77 | 1.46 ± 0.14 |
| CAM-PA | 22.24 | 44.64 | 20.40 | 1.56 ± 0.25 |
| CAM-CB | 35.80 | 43.20 | 15.12 | 1.47 ± 0.03 |
| HB | 9.31 | 3.91 | 1.21 | 10.99 ± 0.64 |
| HB-CB | 17.77 | 4.00 | 0.03 | 16.07 ± 0.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, G.S.d.S.; Oliveira, M.E.P.S.; dos Santos, A.B.S.; Sánchez, O.C.; Soares, C.M.F.; Fricks, A.T. Immobilization of Low-Cost Alternative Vegetable Peroxidase (Raphanus sativus L. peroxidase): Choice of Support/Technique and Characterization. Molecules 2020, 25, 3668. https://doi.org/10.3390/molecules25163668
Barbosa GSdS, Oliveira MEPS, dos Santos ABS, Sánchez OC, Soares CMF, Fricks AT. Immobilization of Low-Cost Alternative Vegetable Peroxidase (Raphanus sativus L. peroxidase): Choice of Support/Technique and Characterization. Molecules. 2020; 25(16):3668. https://doi.org/10.3390/molecules25163668
Chicago/Turabian StyleBarbosa, Gabrielle Souza da Silva, Maria Emanuela P. S. Oliveira, Ana Beatriz S. dos Santos, Osmar Calderón Sánchez, Cleide Mara Faria Soares, and Alini Tinoco Fricks. 2020. "Immobilization of Low-Cost Alternative Vegetable Peroxidase (Raphanus sativus L. peroxidase): Choice of Support/Technique and Characterization" Molecules 25, no. 16: 3668. https://doi.org/10.3390/molecules25163668
APA StyleBarbosa, G. S. d. S., Oliveira, M. E. P. S., dos Santos, A. B. S., Sánchez, O. C., Soares, C. M. F., & Fricks, A. T. (2020). Immobilization of Low-Cost Alternative Vegetable Peroxidase (Raphanus sativus L. peroxidase): Choice of Support/Technique and Characterization. Molecules, 25(16), 3668. https://doi.org/10.3390/molecules25163668