Hydroxycinnamic Acids and Carotenoids of Dried Loquat Fruit cv. ‘Algar’ Affected by Freeze-, Convective-, Vacuum-Microwave- and Combined-Drying Methods
Abstract
1. Introduction
2. Results and Discussion
2.1. Drying Kinetics
2.2. Hydroxycinnamic Acid Derivatives
2.3. Carotenoids
2.4. Antioxidant Capacity
2.5. Color Coordinates
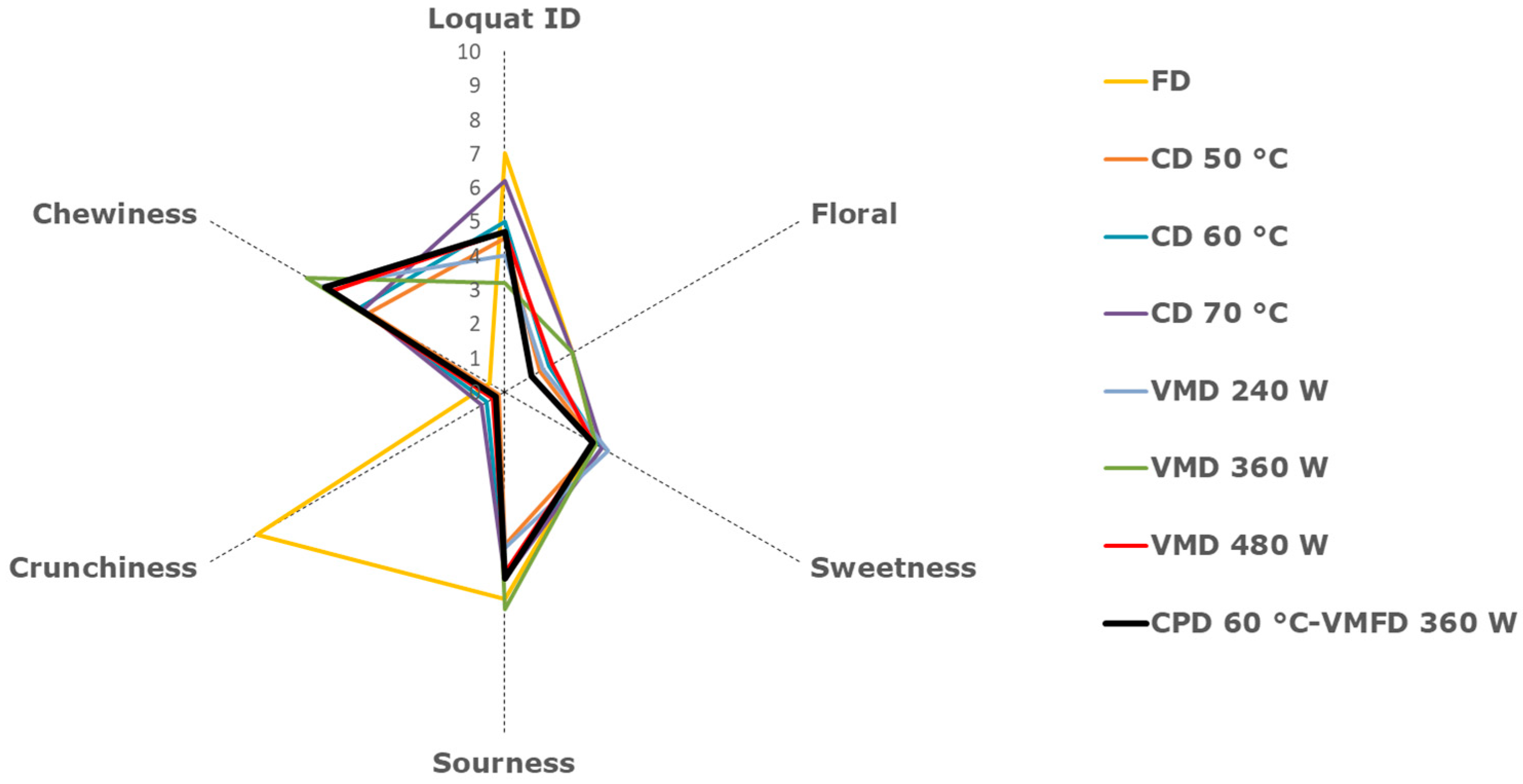
2.6. Descriptive Sensory Analysis
3. Materials and Methods
3.1. Plant Material and Sample Preparation
3.2. Drying Methodology
- Freeze drying (FD) was carried out in a freeze-dryer Christ Alpha 1-4 LSC (Martin Christ GmbH; Osterode am Harz, Germany) at a reduced pressure of 65 Pa for 24 h. The temperature within the drying chamber was −60 °C, while the heating plate was at ~30 °C. The freeze-dried loquats were considered as the control sample because it assures the expected quality, as a previous study indicated [39].
- The process of convective drying (CD) was conducted using a drier designed and built at the Institute of Agriculture Engineering (Wrocław, Poland). Loquat samples of around 60 g were spread according to a methodology previously developed [39]. During CD, loquat samples represented by cylindrical particles were spread in a single layer over the mesh of a basket with 100 mm diameter. Drying in a single layer is required when determining the drying kinetics (references). A heavier sample would not form a single layer in the basket. The CD was operated at 3 temperatures: 50, 60 and 70 °C of the drying air flowing with the velocity 0.8 m s−1.
- The process of vacuum-microwave drying (VMD) of fresh samples was conducted according to a methodology previously developed [39]. The dryer was operated at 3 power levels: 240, 360 and 480 W, which corresponded to the specific microwave power stream values of 4, 6 and 8 W/g, respectively. These values were used in previous studies on drying other raw materials (references) and thus make possible relevant comparisons. Samples (~60 g) were placed in a cylindrical container of organic glass with a volume of 6.8 L, and with a pressure ranging from 4 to 6 kPa.
- The process of combined drying (CPD-VMFD) consisted of convective pre-drying (CPD) at 60 °C followed by vacuum-microwave finish drying (VMFD) at 360 W.
3.3. Identification and Quantification of Hydroxycinnamic acids and Carotenoids
3.4. Trolox Equivalent Antioxidant Capacity (TEAC ABTS+•) and Ferric-Reducing Antioxidant Potential (FRAP)
3.5. Color Measurement and Moisture Content
3.6. Descriptive Sensory Evaluation
3.7. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caballero, P.; Fernández, M.A. Loquat, production and market. In First International Symposium on Loquat; Options Méditerranéennes: Série A. Séminaires Méditerranéens; n. 58; CIHEAM: Zaragoza, Spain, 2003; pp. 11–20. [Google Scholar]
- Lin, S.Q. World loquat production and research with special reference to China. Acta Hortic. 2007, 750, 37–44. [Google Scholar] [CrossRef]
- Reig, C.; Mesejo, C.; Martínez-Fuentes, A.; Iglesias, D.J.; Agustí, M. Fruit load and root development in field-grown loquat trees (Eriobotrya japonica Lindl). J. Plant Growth Regul. 2013, 32, 281–290. [Google Scholar] [CrossRef]
- MAPA, Ministerio de Agricultura, Pesca y Alimentación. Anuario de Estadistica Agraria. 2018. Available online: https://www.mapa.gob.es/es/estadistica/temas/publicaciones/anuario-de-estadistica/default.aspx (accessed on 7 August 2020).
- Soler, E.; Martinez-Calvo, J.; Llacer, G.; Badenes, M.L. Loquat In Spain: Production And Marketing. Acta Hortic. 2007, 45–48. [Google Scholar] [CrossRef]
- Commission of The European Communities: Commission Regulation (EC) No 1107/96 of 12 June 1996 on the Registration of Geographical Indications and Designations of Origin under the Procedure Laid Down in Article 17 of Council Regulation (EEC) No 2081/92; Commission of The European Communities: Brussels, Belgium, 12 June 1996.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Ferreres, F.; Gomes, D.; Valentão, P.; Gonçalves, R.; Pio, R.; Chagas, E.A.; Seabra, R.M.; Andrade, P.B. Improved loquat (Eriobotrya japonica Lind.) cultivars: Variation of phenolics and antioxidative potential. Food Chem. 2009, 114, 1019–1027. [Google Scholar] [CrossRef]
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care 2002, 5, 56–65. [Google Scholar] [CrossRef]
- Hadjipieri, M.; Georgiadou, E.C.; Marin, A.; Diaz-Mula, H.M.; Goulas, V.; Fotopoulos, V.; Tomás-Barberán, F.A.; Manganaris, G.A. Metabolic and transcriptional elucidation of the carotenoid biosynthesis pathway in peel and flesh tissue of loquat fruit during on-tree development. BMC Plant. Biol. 2017, 17, 102. [Google Scholar] [CrossRef]
- Cai, J.; Chen, T.; Zhang, Z.; Li, B.; Qin, G.; Tian, S. Metabolic dynamics during loquat fruit ripening and postharvest technologies. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Alos, E.; Martinez-Fuentes, A.; Reig, C.; Mesejo, C.; Zacarías, L.; Agustí, M.; Rodrigo, M.J. Involvement of ethylene in color changes and carotenoid biosynthesis in loquat fruit (Eriobotrya japonica Lindl. cv. Algerie). Postharvest Biol. Technol. 2019, 149, 129–138. [Google Scholar] [CrossRef]
- Lopes, M.M.D.A.; Sanches, A.G.; De Souza, K.O.; Silva, E.D.O. Loquat/Nispero—Eriobotrya japonica Lindl. In Exotic Fruits; Rodrigues, S., Silva, E., De Brito, E.S., Eds.; Academic Press: London, UK, 2018; pp. 285–292. [Google Scholar]
- Farina, V.; Cinquanta, L.; Vella, F.; Niro, S.; Panfili, G.; Metallo, A.; Cuccurullo, G.; Corona, O. Evolution of carotenoids, sensory profiles and volatile compounds in microwave-dried fruits of three different loquat cultivars (Eriobotrya japonica Lindl.). Plant Foods Hum. Nutr. 2020, 75, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Onwude, D.I.; Hashim, N.; Janius, R.B.; Nawi, N.M.; Abdan, K. modeling the thin-layer drying of fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 599–618. [Google Scholar] [CrossRef]
- Szychowski, P.J.; Lech, K.; Sendra-Nadal, E.; Hernández, F.; Figiel, A.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Kinetics, biocompounds, antioxidant activity, and sensory attributes of quinces as affected by drying method. Food Chem. 2018, 255, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.Y.W.; Chua, B.L.; Figiel, A.; Chong, C.H.; Wojdyło, A.; Szumny, A.; Lech, K. Characterisation of the convective hot-air drying and vacuum microwave drying of Cassia alata: Antioxidant activity, essential oil volatile composition and quality studies. Molecules 2019, 24, 1625. [Google Scholar] [CrossRef]
- Zheng, M.; Xia, Q.; Lu, S. Study on drying methods and their influences on effective components of loquat flower tea. LWT Food Sci. Technol. 2015, 63, 14–20. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Figiel, A.; Lech, K.; Szumny, A.; Martínez-Tomé, J.; Carbonell-Barrachina, Á.A. Dying methods affect the aroma of Origanum majorana L. analyzed by GC–MS and descriptive sensory analysis. Ind. Crops Prod. 2015, 74, 218–227. [Google Scholar]
- Wojdyło, A.; Figiel, A.; Oszmiański, J. Effect of drying methods with the application of vacuum microwaves on the bioactive compounds, color, and antioxidant activity of strawberry fruits. J. Agric. Food Chem. 2009, 57, 1337–1343. [Google Scholar] [CrossRef]
- Figiel, A.; Michalska, A. Overall quality of fruits and vegetables products affected by the drying processes with the assistance of vacuum-microwaves. Int. J. Mol. Sci. 2016, 18, 71. [Google Scholar] [CrossRef]
- Figiel, A. Drying kinetics and quality of beetroots dehydrated by combination of convective and vacuum-microwave methods. J. Food Eng. 2010, 98, 461–470. [Google Scholar] [CrossRef]
- Boeckx, T.; Winters, A.; Webb, K.J.; Kingston-Smith, A.H. Detection of potential chloroplastic substrates for polyphenol oxidase suggests a role in undamaged leaves. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.P.; Figiel, A. Physicochemical properties of whole fruit plum powders obtained using different drying technologies. Food Chem. 2016, 207, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Samia El-Safy, F. Drying characteristics of loquat slices using different dehydration methods by comparative evaluation. World J. Dairy Food Sci. 2014, 9, 272–284. [Google Scholar]
- Fratianni, A.; Niro, S.; Alam, M.D.R.; Cinquanta, L.; Di Matteo, M.; Adiletta, G.; Panfili, G. Effect of a physical pre-treatment and drying on carotenoids of goji berries (Lycium barbarum L.). LWT Food Sci. Technol. 2018, 92, 318–323. [Google Scholar] [CrossRef]
- Azevedo-Meleiro, C.H.; Rodriguez-Amaya, D.B. Qualitative and Quantitative Differences in Carotenoid Composition among Cucurbita moschata, Cucurbita maxima, and Cucurbita pepo. J. Agric. Food Chem. 2007, 55, 4027–4033. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.-W.; Xu, S.-Y.; Sun, D.-W. Effect of microwave-vacuum drying on the carotenoids retention of carrot slices and chlorophyll retention of Chinese Chive leaves. Dry. Technol. 2015, 22, 563–575. [Google Scholar] [CrossRef]
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Escudero-Gilete, M.L.; Vicario, I.M.; Heredia, F.J. Study of the influence of carotenoid structure and individual carotenoids in the qualitative and quantitative attributes of orange juice colour. Food Res. Int. 2010, 43, 1289–1296. [Google Scholar] [CrossRef]
- Fratianni, A.; Cinquanta, L.; Panfili, G. Degradation of carotenoids in orange juice during microwave heating. LWT Food Sci. Technol. 2010, 43, 867–871. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Zhang, J.; Zhao, X.; Wu, Y.; Tan, S.; Zheng, Q.; Gao, X. Multivariate analysis illuminates the effects of vacuum drying on the extractable and nonextractable polyphenols profile of loquat fruit. J. Food Sci. 2019, 84, 726–737. [Google Scholar] [CrossRef]
- Rodrigues, S.; Silva, L.C.A.; Mulet, A.; Cárcel, J.A.; Fernandes, F.A. Development of dried probiotic apple cubes incorporated with Lactobacillus casei NRRL B-442. J. Funct. Foods. 2018, 41, 48–54. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Lech, K.; Calín-Sánchez, Á.; Rosas-Burgos, E.C.; Figiel, A.; Wojdyło, A.; Wasilewska, M.; Carbonell-Barrachina, Á.A. Quality of pomegranate pomace as affected by drying method. J. Food Sci. Technol. 2018, 55, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Figiel, A.; Legua, P.; Lech, K.; Carbonell-Barrachina, Á.A.; Hernández, F. Chemical composition, antioxidant capacity, and sensory quality of dried jujube fruits as affected by cultivar and drying method. Food Chem. 2016, 207, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Cano-Lamadrid, M.; Lech, K.; Michalska, A.; Wasilewska, M.; Figiel, A.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Influence of osmotic dehydration pre-treatment and combined drying method on physico-chemical and sensory properties of pomegranate arils, cultivar Mollar Elche. Food Chem. 2017, 232, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, P.; Wojdyło, A.; Lech, K.; Figiel, A. Influence of osmodehydration pretreatment and combined drying method on the bioactive potential of sour cherry fruits. Food Bioprocess. Technol. 2015, 8, 824–836. [Google Scholar] [CrossRef]
- Dadali, G.; Kılıç Apar, D.; Özbek, B. Microwave drying kinetics of Okra. Dry. Technol. 2007, 25, 917–924. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Bąbelewski, P. Phenolic and carotenoid profile of new goji cultivars and their anti-hyperglycemic, anti-aging and antioxidant properties. J. Funct. Foods 2018, 48, 632–642. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
Sample Availability: Samples of dried loquat are available from the authors. |
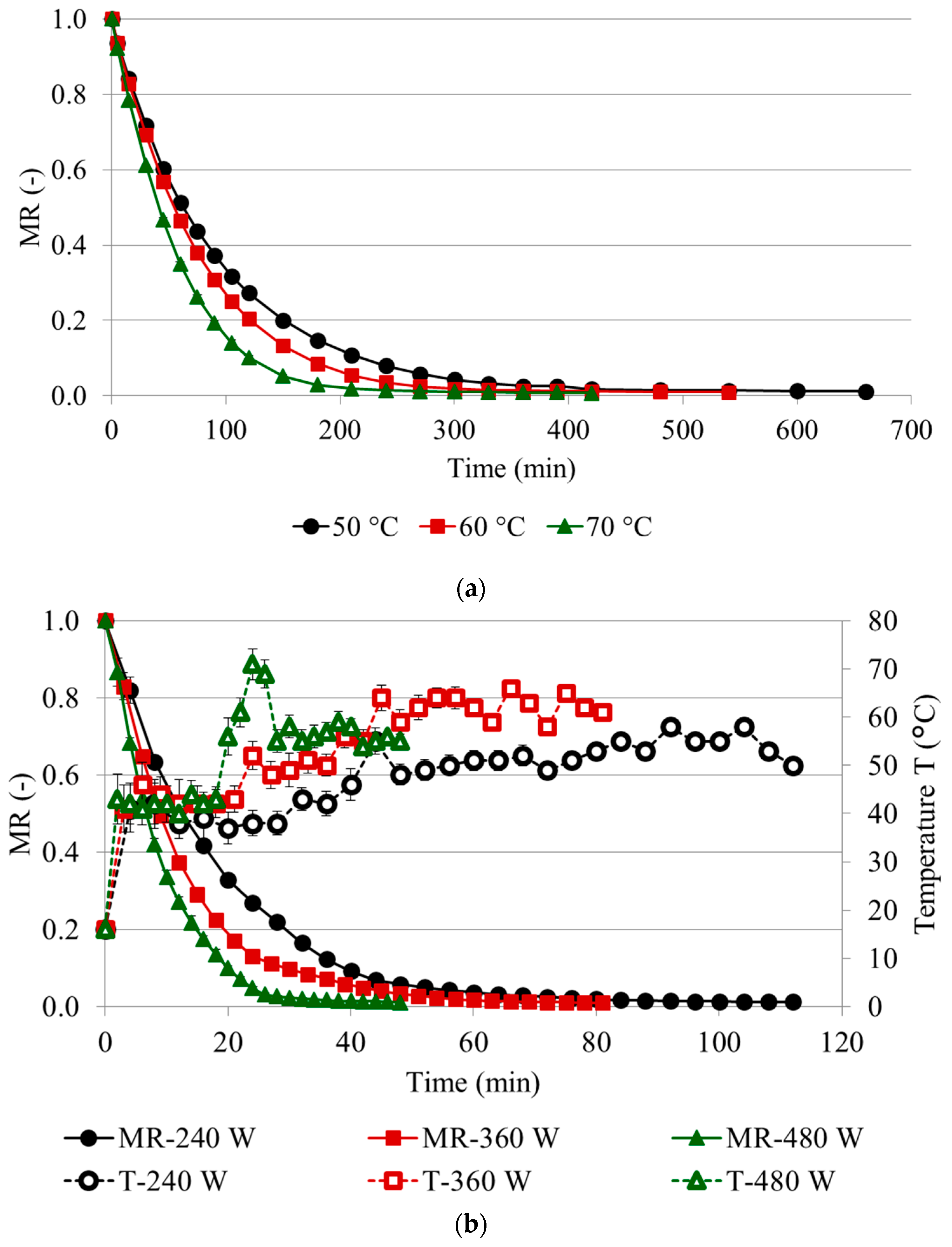
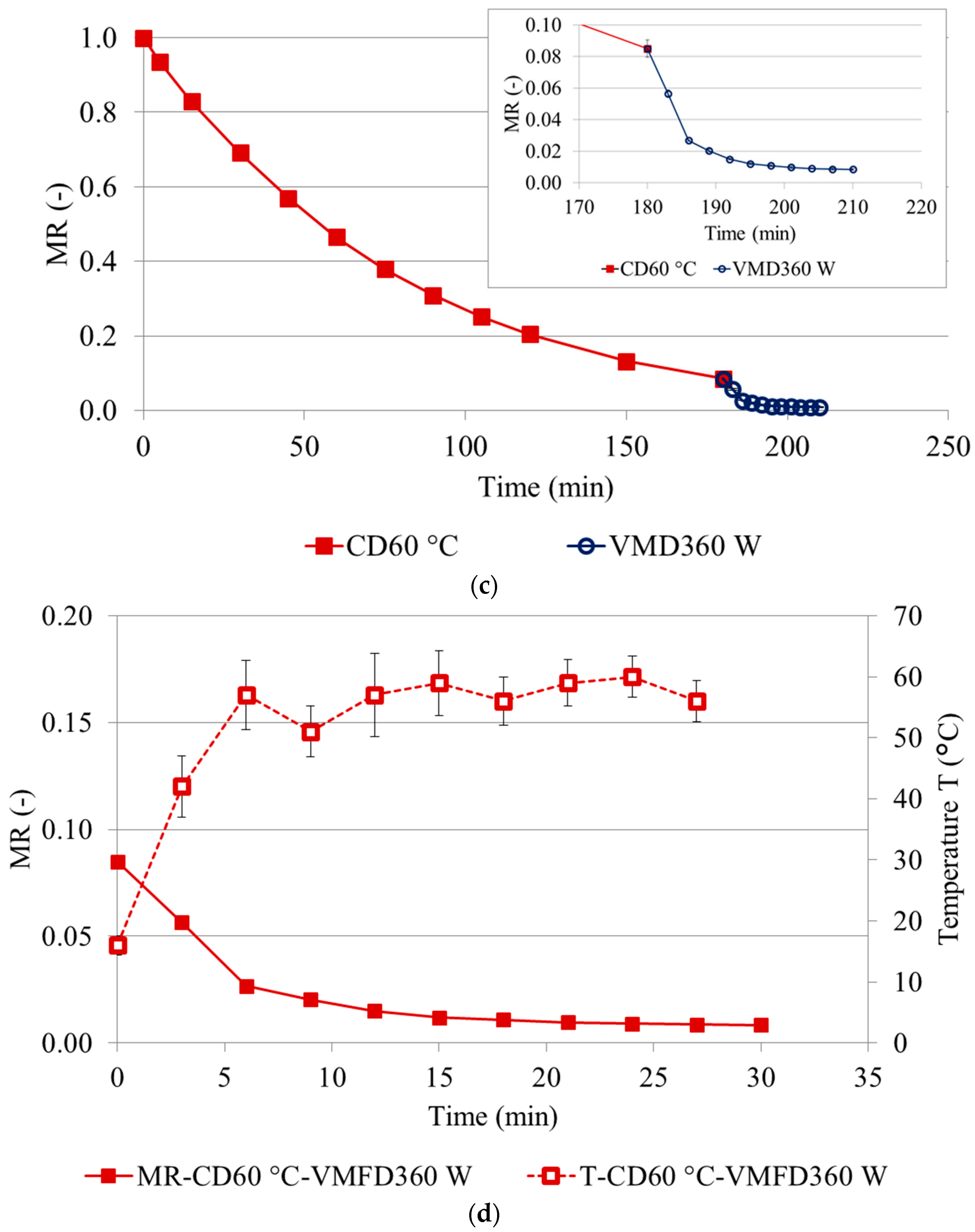

| Treatment ± | Drying Kinetics | RMSE ‡ | R2 | MC (g/100 g ww) | aw | Color Coordinates ¥ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | k | n | |||||||||||||
| CD | VMD | L* | a* | b* | C* | H°* | ΔE | ||||||||
| FD | - λ | - | - | - | - | - | - | 2.80 ± 0.09 c | 0.184 ± 0.001 c | 54.4 ± 0.3 a | 9.17 ± 0.12 ab | 20.9 ± 0.6 a | 22.8 ± 0.6 a | 66.3 ± 0.9 a | 7.1 ± 2.4 c |
| CD 50 | 1 | 0.013 | 0.96 | 660 | - | 0.0049 | 0.9998 | 8.38 ± 0.07 a | 0.383 ± 0.007 a | 38.2 ± 2.3 b | 10.20 ± 1.27 a | 15.7 ± 3.3 ab | 18.7 ± 3.5 b | 56.7 ± 2.5 bc | 11.2 ± 3.5 b |
| CD 60 | 1 | 0.011 | 1.04 | 540 | - | 0.0052 | 0.9997 | 6.76 ± 0.05 ab | 0.321 ± 0.001 ab | 41.0 ± 4.4 b | 12.10 ± 3.47 a | 18.5 ± 6.3 a | 22.3 ± 7.2 a | 56.5 ± 1.5 bc | 9.7 ± 3.4 c |
| CD 70 | 1 | 0.013 | 1.08 | 420 | - | 0.0059 | 0.9997 | 5.19 ± 0.43 b | 0.282 ± 0.009 b | 36.7 ± 1.8 c | 8.02 ± 2.21 b | 13.1 ± 2.0 ab | 15.4 ± 2.9 c | 58.7 ± 3.1 b | 13.8 ± 2.7 b |
| VMD 240 | 1 | 0.053 | 1.02 | - | 112 | 0.0089 | 0.9988 | 8.24 ± 0.29 a | 0.366 ± 0.030 a | 34.4 ± 2.5 d | 7.88 ± 2.03 b | 9.2 ± 3.1 b | 12.1 ± 3.7 d | 49.1 ± 2.3 d | 18.0 ± 4.0 a |
| VMD 360 | 1 | 0.072 | 1.03 | - | 81 | 0.0142 | 0.9970 | 7.06 ± 0.58 a | 0.352 ± 0.070 a | 33.4 ± 0.7 d | 7.55 ± 0.91 b | 7.6 ± 0.5 c | 10.7 ± 1 e | 45.4 ± 1.4 d | 19.8 ± 0.9 a |
| VMD 480 | 1 | 0.079 | 1.13 | - | 48 | 0.0098 | 0.9987 | 7.49 ± 0.09 a | 0.336 ± 0.012 a | 36.0 ± 1.3 c | 8.30 ± 0.39 b | 10.8 ± 2.1 b | 13.7 ± 1.9 cd | 52.2 ± 4.1 c | 15.6 ± 2.3 a |
| CPD-VMFD | 0.086 | 0.259 | 0.729 | 180 | 30 | 0.0046 | 0.9595 | 6.39 ± 0.23 ab | 0.303 ± 0.016 ab | 39.7 ± 0.1 b | 8.94 ± 0.95 ab | 15.7 ± 0.2 ab | 18.0 ± 0.7 b | 60.3 ± 2.3 b | 9.7 ± 0.9 c |
| Code | tR (min) | λmax (nm) | MS (m/z)//MS/MS (m/z) ¥ | Compounds |
|---|---|---|---|---|
| Hydroxycinnamic acid derivatives (H) | ||||
| 3-CQA | 3.04 | 321 | 359//191/179/135 | 3-caffeoylquinic acid |
| 3-p-CoQA | 3.75 | 310 | 337//191/173/163 | 3-p-coumaroyl quinic acid |
| 5-CAQ | 3.98 | 321 | 337//191/173/163 | 5-caffeoylquinic acid |
| SG | 4.20 | 299 | 385//162/223 | sinapoyl glucoside |
| 5-p-CoQA | 4.67 | 299 | 337//173 | 5-p-coumaroylquinic acid |
| 5-FQA | 5.53 | 324 | 367//193/191 | 5-feruloylquinic acid |
| Carotenoids (C) | ||||
| Zea | 7.25 | 450/480 | 569//551/533/476 | Zeaxanthin |
| β-C | 8.52 | 454/481 | 536//444 | β-Carotene |
| cis-β-C | 8.71 | 450/474 | 536//444 | 15,15-cis-β-Carotene |
| β-Cp | 10.13 | 446/479 | 791//698/535/443 | β-Cryptoxanthin–palmitate (C16:0) |
| Neox | 11.00 | 447.475 | 601//583/565/393 | Neoxanthin |
| Zeam | 11.97 | 455/478 | 807//551 | Zeaxanthin monopalmitate |
| β-Cpm | 12.13 | 451/482 | 791 | β-Cryptoxanthin monopalmitate |
| Sample ± | Hydroxycinnamic Acid Derivatives (H) †¥ | Carotenoids (C) ††¥ | Antioxidant Capacity ¥ | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-CQA | 3-p-CoQA | 5-CAQ | SG | 5-p-CoQA | 5-FQA | ∑H | Zea | β-C | cis-β-C | β-Cp | Neox | Zeam | β-Cpm | ∑C | ABTS+• | FRAP | |
| mg/kg ww | mg/kg ww | mmol Trolox/100 g ww | |||||||||||||||
| FD | 1282 ± 23 b | 274.2 ± 12.5 b | 2548 ± 23 b | 151.0 ± 10.3 a | 17.2 ± 1.2 b | 170.7 ± 1.2 a | 4394 c | 29.32 ± 2.55 b | 1182 ± 12 a | 83.55 ± 3.71 b | 445.5 ± 32.1 a | 189.0 ± 3.5 a | 225.6 ± 23.1 a | 446.4 ± 13.2 a | 2601 a | 3.08 ± 0.43 b | 2.21 ± 0.19 b |
| CD 50 °C | 1411 ± 12 a | 269.2 ± 13.4 b | 2913 ± 34 a | 114.9 ± 9.5 b | 8.41 ± 0.14 c | 165.2 ± 2.6 a | 4882 ab | 43.02 ± 3.66 a | 575.4 ± 21.3 b | 59.19 ± 2.43 c | 220.3 ± 12.5 b | 97.4 ± 5.3 b | 85.13 ± 10.31 b | 193.9 ± 8.3 b | 1274 b | 2.88 ± 0.27 bc | 1.54 ± 0.21 c |
| CD 60 °C | 1474 ± 21 a | 268.4 ± 10.8 b | 3055 ± 21 a | 163.7 ± 7.4 a | 9.21 ± 0.77 c | 168.5 ± 3.1 a | 5139 a | 41.55 ± 3.71 a | 520.3 ± 2.8 bc | 53.30 ± 3.65 c | 185.5 ± 10.4 c | 80.39 ± 4.7 b | 76.86 ± 9.43 b | 178.7 ± 2.54 bc | 1136 b | 3.14 ± 0.11 ab | 1.86 ± 0.10 c |
| CD 70 °C | 1455 ± 22 a | 289.4 ± 9.5 b | 3116 ± 18 a | 157.7 ± 10.7 a | 6.36 ± 8.51 c | 187.9 ± 4.8 a | 5211 a | 37.89 ± 3.21 a | 448.4 ± 8.6 c | 117.0 ± 11.8 a | 175.8 ± 10.6 c | 73.72 ± 5.32 b | 81.90 ± 9.11 b | 193.4 ± 2.7 b | 1128 b | 3.80 ± 0.54 a | 2.26 ± 0.09 b |
| VMD 240 W | 982.0 ± 10.6 c | 258.0 ± 18.4 b | 1751 ± 20 d | 128.4 ± 11.7 ab | 24.3 ± 2.1 a | 152.5 ± 1.9 b | 3297 d | 16.02 ± 2.61 b | 364.1 ± 2.6 d | 10.91 ± 0.99 d | 179.4 ± 13.5 c | 78.25 ± 5.78 b | 68.93 ± 7.91 b | 151.4 ± 5.3 c | 869.0 d | 2.12 ± 0.88 c | 1.77 ± 0.12 c |
| VMD 360 W | 1273 ± 25 b | 352.0 ± 25.4 a | 2374 ± 16 b | 168.3 ± 12.6 a | 19.2 ± 1.9 b | 149.1 ± 5.6 b | 4336 c | 19.74 ± 1.34 c | 612.3 ± 7.5 b | 53.68 ± 1.23 c | 217.4 ± 23.5 b | 107.1 ± 2.5 b | 83.31 ± 2.55 b | 214.1 ± 7.2 b | 1308 b | 0.97 ± 0.07 d | 0.97 ± 0.04 d |
| VMD 480 W | 1059 ± 27 bc | 243.7 ± 25.3 b | 2499 ± 18 b | 158.1 ± 10.4 a | 22.0 ± 1.5 a | 126.7 ± 6.3 b | 4109 c | 16.25 ± 1.32 c | 408.8 ± 12.5 d | 69.08 ± 3.67 bc | 167.0 ± 12.4 c | 87.90 ± 7.31 b | 92.06 ± 4.65 b | 201.5 ± 9.1 b | 1043 c | 3.02 ± 0.51 b | 3.11 ± 0.11 a |
| CPD-VMFD | 1378 ± 15 b | 267.1 ± 11.0 b | 2621 ± 16 b | 199.7 ± 9.3 a | 23.0 ± 1.4 a | 162.7 ± 7.7 a | 4651 b | 30.29 ± 2.76 b | 455.2 ± 9.8 c | 55.27 ± 6.32 c | 181.8 ± 4.8 c | 78.18 ± 8.54 b | 92.16 ± 12.5 b | 197.5 ± 10.9 b | 1090 c | 2.23 ± 0.37 c | 2.29 ± 0.23 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Lluch, D.B.; Cano-Lamadrid, M.; Hernández, F.; Zimmer, A.; Lech, K.; Figiel, A.; Carbonell-Barrachina, Á.A.; Wojdyło, A. Hydroxycinnamic Acids and Carotenoids of Dried Loquat Fruit cv. ‘Algar’ Affected by Freeze-, Convective-, Vacuum-Microwave- and Combined-Drying Methods. Molecules 2020, 25, 3643. https://doi.org/10.3390/molecules25163643
López-Lluch DB, Cano-Lamadrid M, Hernández F, Zimmer A, Lech K, Figiel A, Carbonell-Barrachina ÁA, Wojdyło A. Hydroxycinnamic Acids and Carotenoids of Dried Loquat Fruit cv. ‘Algar’ Affected by Freeze-, Convective-, Vacuum-Microwave- and Combined-Drying Methods. Molecules. 2020; 25(16):3643. https://doi.org/10.3390/molecules25163643
Chicago/Turabian StyleLópez-Lluch, David Bernardo, Marina Cano-Lamadrid, Francisca Hernández, Aleksandra Zimmer, Krzysztof Lech, Adam Figiel, Ángel Antonio Carbonell-Barrachina, and Aneta Wojdyło. 2020. "Hydroxycinnamic Acids and Carotenoids of Dried Loquat Fruit cv. ‘Algar’ Affected by Freeze-, Convective-, Vacuum-Microwave- and Combined-Drying Methods" Molecules 25, no. 16: 3643. https://doi.org/10.3390/molecules25163643
APA StyleLópez-Lluch, D. B., Cano-Lamadrid, M., Hernández, F., Zimmer, A., Lech, K., Figiel, A., Carbonell-Barrachina, Á. A., & Wojdyło, A. (2020). Hydroxycinnamic Acids and Carotenoids of Dried Loquat Fruit cv. ‘Algar’ Affected by Freeze-, Convective-, Vacuum-Microwave- and Combined-Drying Methods. Molecules, 25(16), 3643. https://doi.org/10.3390/molecules25163643










