Toxicity Studies on Graphene-Based Nanomaterials in Aquatic Organisms: Current Understanding
Abstract
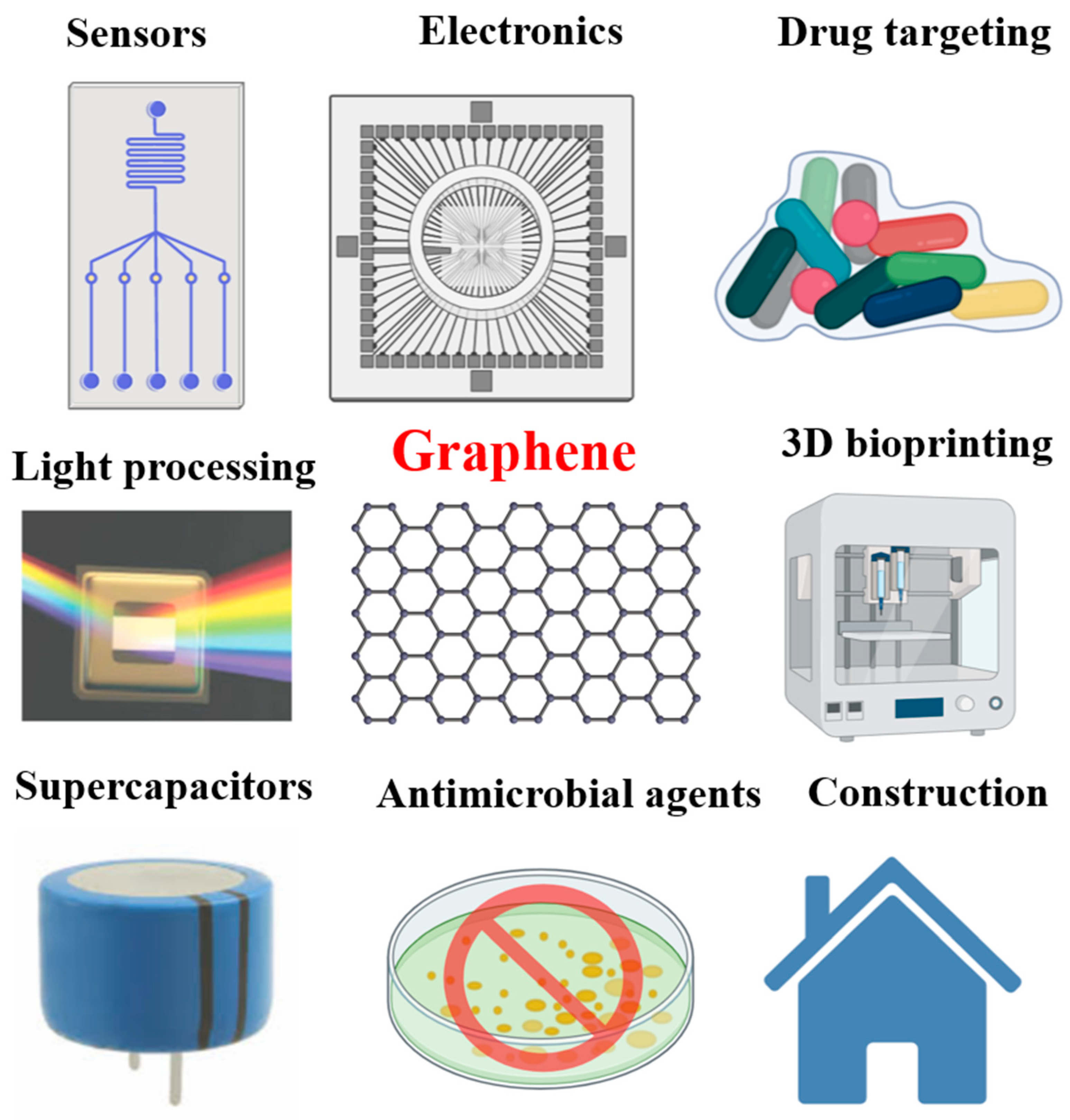
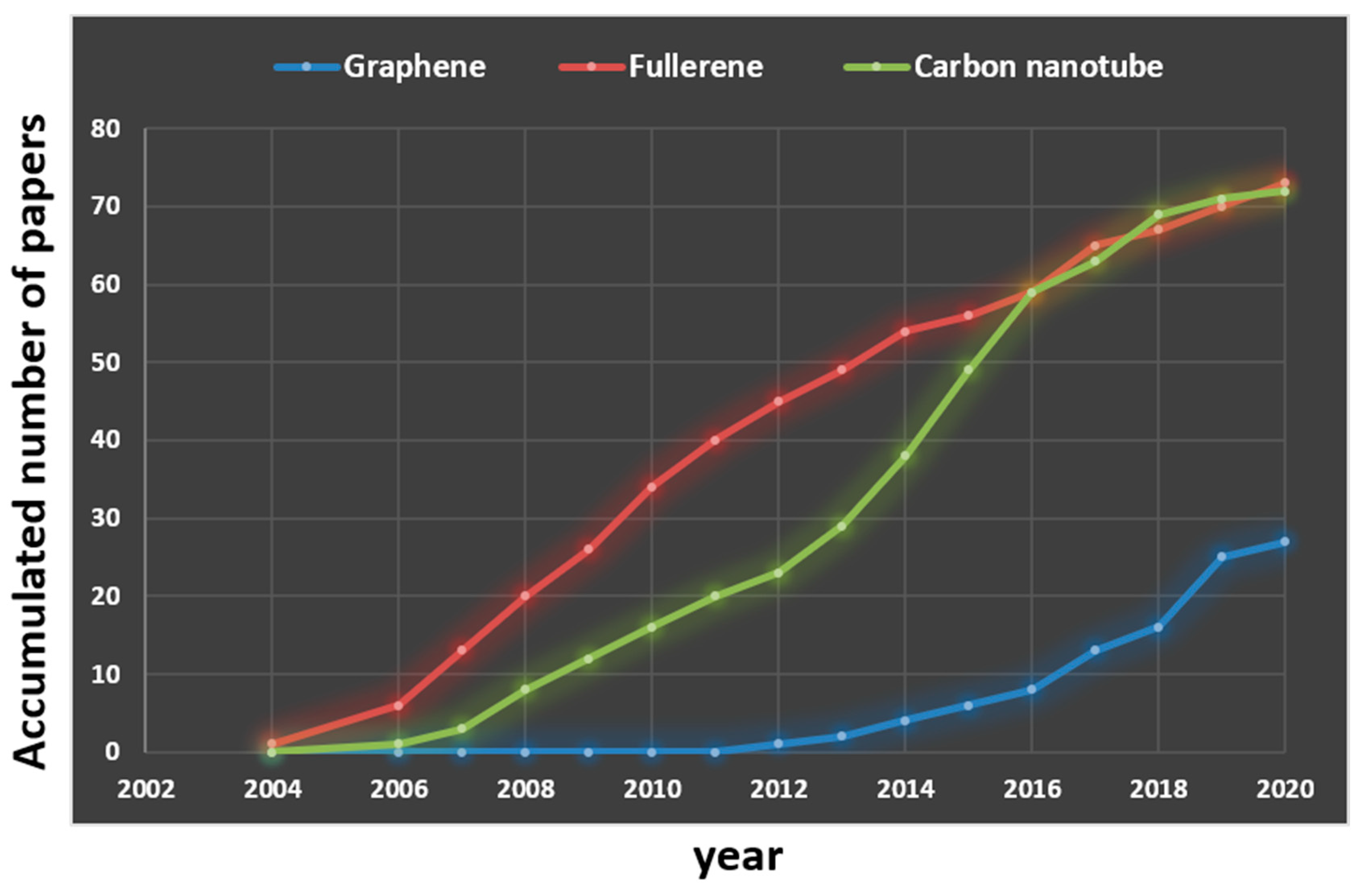
1. Introduction
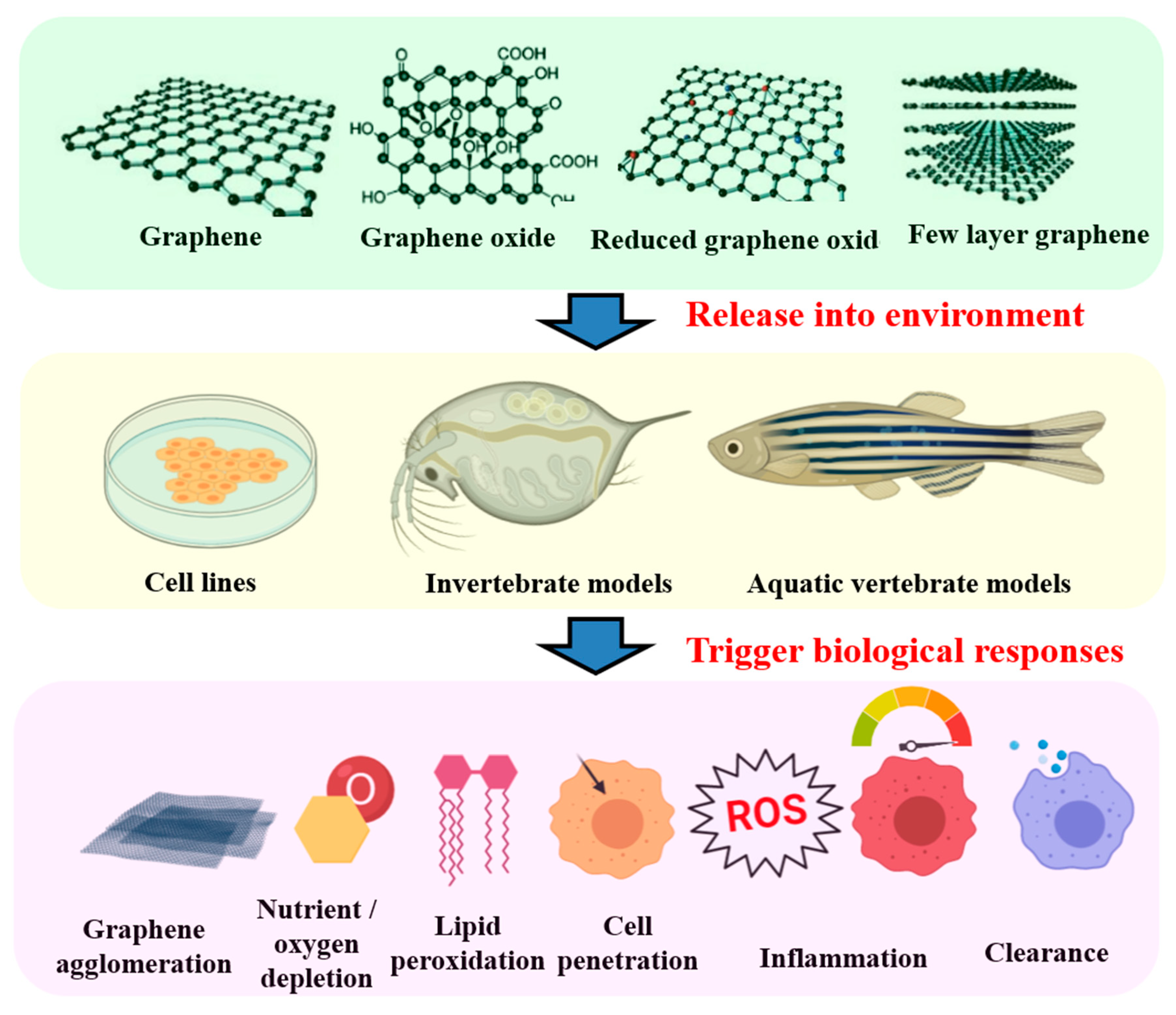
2. Graphene Chemistry
3. Exposure, Accumulation, and Bio-Distribution of Graphene in Invertebrates
4. Exposure of Graphene to Fish Cell, In Vitro
5. Exposure, Accumulation, and Bio-Distribution of Graphene in Fish Embryo and Larvae
6. Exposure, Accumulation, and Bio-Distribution of Graphene in Adult Fish
7. Current Understanding of Graphene and Graphene Oxide (GO) Toxicity and Knowledge Gaps along with Other Carbon Nanomaterials
8. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ema, M.; Gamo, M.; Honda, K. A review of toxicity studies on graphene-based nanomaterials in laboratory animals. Regul. Toxicol. Pharmacol. 2017, 85, 7–24. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, M.; Zhang, L.; Huang, J.; Yao, J.; Zhang, Z. Polyethylenimine-functionalized graphene oxide as an efficient gene delivery vector. J. Mater. Chem. 2011, 21, 7736–7741. [Google Scholar] [CrossRef]
- Munz, M.; Giusca, C.E.; Myers-Ward, R.L.; Gaskill, D.K.; Kazakova, O. Thickness-dependent hydrophobicity of epitaxial graphene. Acs Nano 2015, 9, 8401–8411. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M. All in the Graphene Family—A Recommended Nomenclature for Two-Dimensional Carbon Materials; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Zhao, J.; Wang, Z.; White, J.C.; Xing, B. Graphene in the aquatic environment: Adsorption, dispersion, toxicity and transformation. Environ. Sci. Technol. 2014, 48, 9995–10009. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and prospects. science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska, A.M.; Kurtycz, P.; Olszyna, A.R. Recent advances in graphene family materials toxicity investigations. J. Nanoparticle Res. 2012, 14, 1320. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Wang, J.; Wu, H.; Aksay, I.A.; Liu, J.; Lin, Y. Glucose oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009, 25, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Razmi, H.; Mohammad-Rezaei, R. Graphene quantum dots as a new substrate for immobilization and direct electrochemistry of glucose oxidase: Application to sensitive glucose determination. Biosens. Bioelectron. 2013, 41, 498–504. [Google Scholar] [CrossRef]
- Das, S.R.; Nian, Q.; Cargill, A.A.; Hondred, J.A.; Ding, S.; Saei, M.; Cheng, G.J.; Claussen, J.C. 3d nanostructured inkjet printed graphene via uv-pulsed laser irradiation enables paper-based electronics and electrochemical devices. Nanoscale 2016, 8, 15870–15879. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chen, L.; Chen, Y.; Bai, H.; Li, L. Three-dimensional porous graphene-based composite materials: Electrochemical synthesis and application. J. Mater. Chem. 2012, 22, 20968–20976. [Google Scholar] [CrossRef]
- Zhang, L.; Li, R.; Tang, B.; Wang, P. Solar-thermal conversion and thermal energy storage of graphene foam-based composites. Nanoscale 2016, 8, 14600–14607. [Google Scholar] [CrossRef]
- Pumera, M. Graphene-based nanomaterials for energy storage. Energy Environ. Sci. 2011, 4, 668–674. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.; Xu, Y.; Shang, G.; Wang, R.; Lin, Y. Review of and perspectives on the toxicology of graphene-based materials. Curr. Drug Metab. 2013, 14, 863–871. [Google Scholar] [CrossRef]
- Chang, H.; Tang, L.; Wang, Y.; Jiang, J.; Li, J. Graphene fluorescence resonance energy transfer aptasensor for the thrombin detection. Anal. Chem. 2010, 82, 2341–2346. [Google Scholar] [CrossRef]
- Lu, C.H.; Yang, H.H.; Zhu, C.L.; Chen, X.; Chen, G.N. A graphene platform for sensing biomolecules. Angew. Chem. Int. Ed. 2009, 48, 4785–4787. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, H.; Wang, K.; Cao, A.; Wei, J.; Li, C.; Jia, Y.; Li, Z.; Li, X.; Wu, D. Graphene-on-silicon schottky junction solar cells. Adv. Mater. 2010, 22, 2743–2748. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Sun, S.; Salim, T.; Wu, S.; Huang, X.; He, Q.; Lam, Y.M.; Zhang, H. Organic photovoltaic devices using highly flexible reduced graphene oxide films as transparent electrodes. Acs Nano 2011, 4, 5263–5268. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhu, J.; Sim, D.H.; Tay, Y.Y.; Lu, Z.; Zhang, X.; Sharma, Y.; Srinivasan, M.; Zhang, H.; Hng, H.H. Achieving high specific charge capacitances in fe 3 o 4/reduced graphene oxide nanocomposites. J. Mater. Chem. 2011, 21, 3422–3427. [Google Scholar] [CrossRef]
- Li, F.; Song, J.; Yang, H.; Gan, S.; Zhang, Q.; Han, D.; Ivaska, A.; Niu, L. One-step synthesis of graphene/sno2 nanocomposites and its application in electrochemical supercapacitors. Nanotechnology 2009, 20, 455602. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-graphene composite as a high performance photocatalyst. Acs Nano 2010, 4, 380–386. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Bai, H.; Wang, Y.; Sun, D.D. Gram-scale production of graphene oxide–tio2 nanorod composites: Towards high-activity photocatalytic materials. Appl. Catal. B Environ. 2011, 106, 76–82. [Google Scholar] [CrossRef]
- Dong, L.; Gari, R.R.S.; Li, Z.; Craig, M.M.; Hou, S. Graphene-supported platinum and platinum–ruthenium nanoparticles with high electrocatalytic activity for methanol and ethanol oxidation. Carbon 2010, 48, 781–787. [Google Scholar] [CrossRef]
- Jafri, R.I.; Rajalakshmi, N.; Ramaprabhu, S. Nitrogen doped graphene nanoplatelets as catalyst support for oxygen reduction reaction in proton exchange membrane fuel cell. J. Mater. Chem. 2010, 20, 7114–7117. [Google Scholar] [CrossRef]
- Wang, S.; Goh, B.M.; Manga, K.K.; Bao, Q.; Yang, P.; Loh, K.P. Graphene as atomic template and structural scaffold in the synthesis of graphene− organic hybrid wire with photovoltaic properties. Acs Nano 2010, 4, 6180–6186. [Google Scholar] [CrossRef]
- Han, T.H.; Lee, W.J.; Lee, D.H.; Kim, J.E.; Choi, E.Y.; Kim, S.O. Peptide/graphene hybrid assembly into core/shell nanowires. Adv. Mater. 2010, 22, 2060–2064. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yin, Z.; He, Q.; Huang, X.; Zhou, X.; Zhang, H. Electrochemical deposition of semiconductor oxides on reduced graphene oxide-based flexible, transparent, and conductive electrodes. J. Phys. Chem. C 2010, 114, 11816–11821. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, X.; Qi, X.; Wu, S.; Xue, C.; Boey, F.Y.; Yan, Q.; Chen, P.; Zhang, H. In situ synthesis of metal nanoparticles on single-layer graphene oxide and reduced graphene oxide surfaces. J. Phys. Chem. C 2009, 113, 10842–10846. [Google Scholar] [CrossRef]
- Muszynski, R.; Seger, B.; Kamat, P.V. Decorating graphene sheets with gold nanoparticles. J. Phys. Chem. C 2008, 112, 5263–5266. [Google Scholar] [CrossRef]
- Huang, X.; Li, S.; Huang, Y.; Wu, S.; Zhou, X.; Li, S.; Gan, C.L.; Boey, F.; Mirkin, C.A.; Zhang, H. Synthesis of hexagonal close-packed gold nanostructures. Nat. Commun. 2011, 2, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Hu, D.; Lin, C.-T.; Li, J.; Lin, Y. Aptamer/graphene oxide nanocomplex for in situ molecular probing in living cells. J. Am. Chem. Soc. 2010, 132, 9274–9276. [Google Scholar] [CrossRef]
- Qi, X.; Pu, K.Y.; Li, H.; Zhou, X.; Wu, S.; Fan, Q.L.; Liu, B.; Boey, F.; Huang, W.; Zhang, H. Amphiphilic graphene composites. Angew. Chem. Int. Ed. 2010, 49, 9426–9429. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Shan, C.; Li, F.; Han, D.; Niu, L. Stable, conductive supramolecular composite of graphene sheets with conjugated polyelectrolyte. Langmuir 2010, 26, 6708–6712. [Google Scholar] [CrossRef]
- Qi, X.; Pu, K.Y.; Zhou, X.; Li, H.; Liu, B.; Boey, F.; Huang, W.; Zhang, H. Conjugated-polyelectrolyte-functionalized reduced graphene oxide with excellent solubility and stability in polar solvents. Small 2010, 6, 663–669. [Google Scholar] [CrossRef]
- Dong, X.; Li, B.; Wei, A.; Cao, X.; Chan-Park, M.B.; Zhang, H.; Li, L.-J.; Huang, W.; Chen, P. One-step growth of graphene–carbon nanotube hybrid materials by chemical vapor deposition. Carbon 2011, 49, 2944–2949. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, J.; Zhi, L.; Zhang, Q.; Wei, T.; Feng, J.; Zhang, M.; Qian, W.; Wei, F. A three-dimensional carbon nanotube/graphene sandwich and its application as electrode in supercapacitors. Adv. Mater. 2010, 22, 3723–3728. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.; Kim, J.; Hosono, E.; Zhou, H.-s.; Kudo, T.; Honma, I. Large reversible li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217. [Google Scholar] [CrossRef]
- Wu, S.-Y.; An, S.S.A.; Hulme, J. Current applications of graphene oxide in nanomedicine. Int. J. Nanomed. 2015, 10, 9. [Google Scholar]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Segal, M. Selling graphene by the ton. Nat. Nanotechnol. 2009, 4, 612. [Google Scholar] [CrossRef]
- Arvidsson, R.; Molander, S.; Sandén, B.A. Review of potential environmental and health risks of the nanomaterial graphene. Hum. Ecol. Risk Assess. Int. J. 2013, 19, 873–887. [Google Scholar] [CrossRef]
- Volkov, Y.; McIntyre, J.; Prina-Mello, A. Graphene toxicity as a double-edged sword of risks and exploitable opportunities: A critical analysis of the most recent trends and developments. 2d Mater. 2017, 4, 022001. [Google Scholar] [CrossRef]
- Kotchey, G.P.; Allen, B.L.; Vedala, H.; Yanamala, N.; Kapralov, A.A.; Tyurina, Y.Y.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. The enzymatic oxidation of graphene oxide. Acs Nano 2011, 5, 2098–2108. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.; Duch, M.C.; Mansukhani, N.D.; Hersam, M.C.; Bouchard, D. Colloidal properties and stability of graphene oxide nanomaterials in the aquatic environment. Environ. Sci. Technol. 2013, 47, 6288–6296. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhi, L.; Wu, Q.; Yu, Y.; Sun, Q.; Wang, D. P38 mapk-skn-1/nrf signaling cascade is required for intestinal barrier against graphene oxide toxicity in caenorhabditis elegans. Nanotoxicology 2016, 10, 1469–1479. [Google Scholar] [CrossRef]
- Ouyang, S.; Hu, X.; Zhou, Q. Envelopment–internalization synergistic effects and metabolic mechanisms of graphene oxide on single-cell chlorella vulgaris are dependent on the nanomaterial particle size. Acs Appl. Mater. Interfaces 2015, 7, 18104–18112. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, Q. Health and ecosystem risks of graphene. Chem. Rev. 2013, 113, 3815–3835. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, Y.; Fang, J.; Wang, D. Immune response is required for the control of in vivo translocation and chronic toxicity of graphene oxide. Nanoscale 2014, 6, 5894–5906. [Google Scholar] [CrossRef]
- Hazeem, L.J.; Bououdina, M.; Dewailly, E.; Slomianny, C.; Barras, A.; Coffinier, Y.; Szunerits, S.; Boukherroub, R. Toxicity effect of graphene oxide on growth and photosynthetic pigment of the marine alga picochlorum sp. During different growth stages. Environ. Sci. Pollut. Res. 2017, 24, 4144–4152. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Q.; Zou, W.; Hu, X. Molecular mechanisms of developmental toxicity induced by graphene oxide at predicted environmental concentrations. Environ. Sci. Technol. 2017, 51, 7861–7871. [Google Scholar] [CrossRef]
- Clemente, Z.; Castro, V.; Moura, M.; Jonsson, C.; Fraceto, L. Toxicity assessment of tio2 nanoparticles in zebrafish embryos under different exposure conditions. Aquat. Toxicol. 2014, 147, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, J.; Zhang, X.; Chang, Y.; Chen, Y. The impact of zno nanoparticle aggregates on the embryonic development of zebrafish (danio rerio). Nanotechnology 2009, 20, 195103. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, J.; Zhang, X.; Chang, Y.; Chen, Y. Trophic transfer of tio2 nanoparticles from daphnia to zebrafish in a simplified freshwater food chain. Chemosphere 2010, 79, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Ray, S. Levels of toxicity screening of environmental chemicals using aquatic invertebrates—A review. Invertebr. Exp. Models Toxic. Screen. IntechCroat. 2016, 1–11. [Google Scholar]
- Dasmahapatra, A.K.; Dasari, T.P.; Tchounwou, P.B. Graphene-based nanomaterials toxicity in fish. In Reviews of Environmental Contamination and Toxicology Volume 247; Springer: Berlin, Germany, 2018; pp. 1–58. [Google Scholar]
- Elmore, S.A.; Boorman, G.A. Environmental toxicologic pathology and human health. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1029–1049. [Google Scholar]
- Liao, L.; Peng, H.; Liu, Z. Chemistry makes graphene beyond graphene. J. Am. Chem. Soc. 2014, 136, 12194–12200. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascon, J. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.; Stankovich, S.; Dikin, D.; Herrera-Alonso, M.; Piner, R.; Adamson, D.; Schniepp, H.; Chen, X.; Ruoff, R. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef]
- Liu, Z.-B.; Xu, Y.-F.; Zhang, X.-Y.; Zhang, X.-L.; Chen, Y.-S.; Tian, J.-G. Porphyrin and fullerene covalently functionalized graphene hybrid materials with large nonlinear optical properties. J. Phys. Chem. B 2009, 113, 9681–9686. [Google Scholar] [CrossRef]
- Gourmelon, A.; Ahtiainen, J. Developing test guidelines on invertebrate development and reproduction for the assessment of chemicals, including potential endocrine active substances—the oecd perspective. Ecotoxicology 2007, 16, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Gornati, R.; Chiriva-Internati, M.; Bernardini, G. Ecotoxicology of nanomaterials: The role of invertebrate testing. Invertebr. Surviv. J. 2009, 6, 78–97. [Google Scholar]
- Emerich, D.F.; Thanos, C.G. The pinpoint promise of nanoparticle-based drug delivery and molecular diagnosis. Biomol. Eng. 2006, 23, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.S.; Edge, M.; Sandoval, G.; Verran, J.; Stratton, J.; Maltby, J. Photocatalytic coatings for environmental applications. Photochem. Photobiol. 2005, 81, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Adeleye, A.S.; Burgess, R.M.; Smolowitz, R.; Russo, S.M.; Ho, K.T. A 72-h exposure study with eastern oysters (crassostrea virginica) and the nanomaterial graphene oxide. Environ. Toxicol. Chem. 2019, 38, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Adeleye, A.S.; Burgess, R.M.; Russo, S.M.; Ho, K.T. Effects of graphene oxide nanomaterial exposures on the marine bivalve, crassostrea virginica. Aquat. Toxicol. 2019, 216, 105297. [Google Scholar] [CrossRef]
- De Marchi, L.; Neto, V.; Pretti, C.; Figueira, E.; Brambilla, L.; Rodriguez-Douton, M.J.; Rossella, F.; Tommasini, M.; Furtado, C.; Soares, A.M. Physiological and biochemical impacts of graphene oxide in polychaetes: The case of diopatra neapolitana. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 193, 50–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, T.; Shi, L.; Guo, X.; Si, X.; Yang, R.; Quan, X. The effects of humic acid on the toxicity of graphene oxide to scenedesmus obliquus and daphnia magna. Sci. Total Environ. 2019, 649, 163–171. [Google Scholar] [CrossRef]
- Guo, X.; Dong, S.; Petersen, E.J.; Gao, S.; Huang, Q.; Mao, L. Biological uptake and depuration of radio-labeled graphene by daphnia magna. Environ. Sci. Technol. 2013, 47, 12524–12531. [Google Scholar] [CrossRef]
- Souza, J.P.; Venturini, F.P.; Santos, F.; Zucolotto, V. Chronic toxicity in ceriodaphnia dubia induced by graphene oxide. Chemosphere 2018, 190, 218–224. [Google Scholar] [CrossRef]
- de Melo, C.B.; Côa, F.; Alves, O.L.; Martinez, D.S.T.; Barbieri, E. Co-exposure of graphene oxide with trace elements: Effects on acute ecotoxicity and routine metabolism in palaemon pandaliformis (shrimp). Chemosphere 2019, 223, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Lammel, T.; Boisseaux, P.; Navas, J.M. Potentiating effect of graphene nanomaterials on aromatic environmental pollutant-induced cytochrome p450 1a expression in the topminnow fish hepatoma cell line plhc-1. Environ. Toxicol. 2015, 30, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Lammel, T.; Navas, J.M. Graphene nanoplatelets spontaneously translocate into the cytosol and physically interact with cellular organelles in the fish cell line plhc-1. Aquat. Toxicol. 2014, 150, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, K.; Sundar, L.S.; Pereira, E.; Duarte, A.C. Graphene oxide induces cytotoxicity and oxidative stress in bluegill sunfish cells. J. Appl. Toxicol. 2018, 38, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Kalman, J.; Merino, C.; Fernández-Cruz, M.L.; Navas, J.M. Usefulness of fish cell lines for the initial characterization of toxicity and cellular fate of graphene-related materials (carbon nanofibers and graphene oxide). Chemosphere 2019, 218, 347–358. [Google Scholar] [CrossRef]
- Shah, B.R.; Mraz, J. Advances in nanotechnology for sustainable aquaculture and fisheries. Rev. Aquac. 2020, 12, 925–942. [Google Scholar] [CrossRef]
- Fako, V.E.; Furgeson, D.Y. Zebrafish as a correlative and predictive model for assessing biomaterial nanotoxicity. Adv. Drug Deliv. Rev. 2009, 61, 478–486. [Google Scholar] [CrossRef]
- d’Amora, M.; Camisasca, A.; Lettieri, S.; Giordani, S. Toxicity assessment of carbon nanomaterials in zebrafish during development. Nanomaterials 2017, 7, 414. [Google Scholar] [CrossRef]
- Wang, Z.G.; Rong, Z.; Jiang, D.; Jing, E.S.; Qian, X.; Jing, S.; Chen, Y.P.; Xin, Z.; Lu, G.; Li, J.Z. Toxicity of graphene quantum dots in zebrafish embryo. Biomed. Environ. Sci. 2015, 28, 341–351. [Google Scholar]
- Zhu, Z.; Qian, J.; Zhao, X.; Qin, W.; Hu, R.; Zhang, H.; Li, D.; Xu, Z.; Tang, B.Z.; He, S. Stable and size-tunable aggregation-induced emission nanoparticles encapsulated with nanographene oxide and applications in three-photon fluorescence bioimaging. Acs Nano 2016, 10, 588–597. [Google Scholar] [CrossRef]
- Jeong, J.; Cho, H.-J.; Choi, M.; Lee, W.S.; Chung, B.H.; Lee, J.-S. In vivo toxicity assessment of angiogenesis and the live distribution of nano-graphene oxide and its pegylated derivatives using the developing zebrafish embryo. Carbon 2015, 93, 431–440. [Google Scholar] [CrossRef]
- Mold, D.E.; Dinitz, A.E.; Sambandan, D.R. Regulation of zebrafish zona pellucida gene activity in developing oocytes. Biol. Reprod. 2009, 81, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, P.; Zhang, L.; Huang, S.; Luo, L.; Huang, C. Toxicity of graphene oxide and multi-walled carbon nanotubes against human cells and zebrafish. Sci. China Chem. 2012, 55, 2209–2216. [Google Scholar] [CrossRef]
- Liu, X.T.; MU, X.Y.; WU, X.L.; Meng, L.X.; Guan, W.B.; Qiang, Y.; Hua, S.; Wang, C.J.; LI, X.F. Toxicity of multi-walled carbon nanotubes, graphene oxide, and reduced graphene oxide to zebrafish embryos. Biomed. Environ. Sci. 2014, 27, 676–683. [Google Scholar] [PubMed]
- Hu, X.; Wei, Z.; Mu, L. Graphene oxide nanosheets at trace concentrations elicit neurotoxicity in the offspring of zebrafish. Carbon 2017, 117, 182–191. [Google Scholar] [CrossRef]
- Xiong, G.; Deng, Y.; Liao, X.; Zhang, J.E.; Cheng, B.; Cao, Z.; Lu, H. Graphene oxide nanoparticles induce hepatic dysfunction through the regulation of innate immune signaling in zebrafish (danio rerio). Nanotoxicology 2020, 14, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Clemente, Z.; Silva, G.H.; de Souza Nunes, M.C.; Martinez, D.S.T.; Maurer-Morelli, C.V.; Thomaz, A.A.; Castro, V.L.S.S. Exploring the mechanisms of graphene oxide behavioral and morphological changes in zebrafish. Environ. Sci. Pollut. Res. 2019, 26, 30508–30523. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, C.; Ouyang, S.; Hu, X.; Zhou, Q. Mitigation in multiple effects of graphene oxide toxicity in zebrafish embryogenesis driven by humic acid. Environ. Sci. Technol. 2015, 49, 10147–10154. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, X.; Sun, J.; Zhou, Q. Specific nanotoxicity of graphene oxide during zebrafish embryogenesis. Nanotoxicology 2016, 10, 42–52. [Google Scholar] [CrossRef]
- Soares, J.; Pereira, T.; Costa, K.; Maraschin, T.; Basso, N.; Bogo, M. Developmental neurotoxic effects of graphene oxide exposure in zebrafish larvae (danio rerio). Colloids Surf. B: Biointerfaces 2017, 157, 335–346. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, C.; Khan, I.A.; Tang, Y.; Liu, S.; Yang, M. Toxic effects of different-sized graphene oxide particles on zebrafish embryonic development. Ecotoxicol. Environ. Saf. 2020, 197, 110608. [Google Scholar] [CrossRef]
- Chen, M.; Yin, J.; Liang, Y.; Yuan, S.; Wang, F.; Song, M.; Wang, H. Oxidative stress and immunotoxicity induced by graphene oxide in zebrafish. Aquat. Toxicol. 2016, 174, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Dong, S.; Petersen, E.J.; Niu, J.; Chang, X.; Wang, P.; Lin, S.; Gao, S.; Mao, L. Biological uptake, distribution, and depuration of radio-labeled graphene in adult zebrafish: Effects of graphene size and natural organic matter. Acs Nano 2017, 11, 2872–2885. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Lu, J.; Lin, G.; Su, H.; Sun, J.; Luan, T. Dysbiosis of gut microbiota by dietary exposure of three graphene-family materials in zebrafish (danio rerio). Environ. Pollut. 2019, 254, 112969. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.P.; Mansano, A.S.; Venturini, F.P.; Santos, F.; Zucolotto, V. Antioxidant metabolism of zebrafish after sub-lethal exposure to graphene oxide and recovery. Fish Physiol. Biochem. 2019, 45, 1289–1297. [Google Scholar] [CrossRef]
- Paital, B.; Guru, D.; Mohapatra, P.; Panda, B.; Parida, N.; Rath, S.; Kumar, V.; Saxena, P.S.; Srivastava, A. Ecotoxic impact assessment of graphene oxide on lipid peroxidation at mitochondrial level and redox modulation in fresh water fish anabas testudineus. Chemosphere 2019, 224, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Al-Rudainy, A.J.; Hawar, A. Hematological effects of graphene nanoparticles exposed to common carp cyprinus carpio l. Pak. J. Biotechnol. 2018, 15, 867–870. [Google Scholar]
- Mao, H.Y.; Laurent, S.; Chen, W.; Akhavan, O.; Imani, M.; Ashkarran, A.A.; Mahmoudi, M. Graphene: Promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev. 2013, 113, 3407–3424. [Google Scholar] [CrossRef]
- Tan, E.; Li, B.L.; Ariga, K.; Lim, C.-T.; Garaj, S.; Leong, D.T. Toxicity of two-dimensional layered materials and their heterostructures. Bioconjugate Chem. 2019, 30, 2287–2299. [Google Scholar] [CrossRef]
- Bidram, E.; Sulistio, A.; Cho, H.-J.; Amini, A.; Harris, T.; Zarrabi, A.; Qiao, G.; Stewart, A.; Dunstan, D.E. Targeted graphene oxide networks: Cytotoxicity and synergy with anticancer agents. Acs Appl. Mater. Interfaces 2018, 10, 43523–43532. [Google Scholar] [CrossRef]
- Sun, T.Y.; Bornhöft, N.A.; Hungerbühler, K.; Nowack, B. Dynamic probabilistic modeling of environmental emissions of engineered nanomaterials. Environ. Sci. Technol. 2016, 50, 4701–4711. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Y.; Li, N.-J.; Cheng, F.-Y.; Hsueh, J.-F.; Huang, C.-C.; Lu, F.-I.; Fu, T.-F.; Yan, S.-J.; Lee, Y.-H.; Wang, Y.-J. The effect of the chorion on size-dependent acute toxicity and underlying mechanisms of amine-modified silver nanoparticles in zebrafish embryos. Int. J. Mol. Sci. 2020, 21, 2864. [Google Scholar] [CrossRef] [PubMed]
- Dayal, N.; Thakur, M.; Soparkar, A.; Doctor, M.; Patil, P.; Joshi, D. Effective method to deliver test substance in adult zebrafish. Int. J. Adv. Res. 2016, 4, 543–551. [Google Scholar] [CrossRef]
| Animal | Route of Graphene Exposure | Adverse Outcome | Dosage Concentration and Time | Ref. |
|---|---|---|---|---|
| Tested in invertebrate species | ||||
| Crassostrea virginica | Waterborne exposure | Short-term GO exposures can induce oxidative stress, epithelial inflammation, and adversely affect overall Crassostrea virginica health. | 1 and 10 mg/L 72 h static renewal. | [77] |
| Crassostrea virginica | Waterborne exposure | Elevated lipid peroxidation and changes in glutathione-s-transferase (GST) activities were observed in gills and digestive gland tissues of the GO-exposed oysters. Oxidative damage, stress signaling leading to adverse effects on cellular health. | 2.5 and 5 mg/L 14 days | [78] |
| Diopatra neapolitana | Waterborne exposure | GO induced negative effects on the regenerative capacity, altered energy-related responses, especially glycogen content, and decrease in metabolism, cellular damage in Diopatra neapolitana. | 0.01, 0.10 and 1.00 mg/L 28 days | [79] |
| Daphnia magna | Waterborne exposure | GO induced significant toxicity to Daphnia magna. 21 days LC50 chronic toxicity 3.3 mg L−1. In the presence of HA, the decreased toxicity of GO was attributed to the alleviation of oxidative damage by HA. | 50.0, 65.0, 84.5, 110.0 and 143.0 mg/L 21 days | [80] |
| Daphnia magna | Waterborne exposure | 14C-labeled graphene accumulated 1% of the organism dry mass. Excretion of graphene at constant phase in depuration. Addition of algae and humic acid to water during the depuration period resulted in release of a significant fraction (~90%) of the accumulated graphene, some remained in the organism. Accumulated graphene in adult Daphnia was likely transferred to the neonates. | 250, 100, 50 and 25 µg/L 48 h Depuration 24 h | [81] |
| Ceriodaphnia dubia | Waterborne exposure | GO induced lethality, reproduction inhibition, ROS generation, reduction on feeding rates and accumulation on gut tract. There was a shift in the available energy for self-maintenance rather than feeding or reproduction activities. | Acute exposure: 0.1; 0.2; 0.4; 0.8; 1.6 and 3.2 mg/ L, 48 h Chronic exposure: 0.05; 0.1; 0.2; 0.4 and 0.8 mg/ L 7 days | [82] |
| Palaemon pandaliformis | Waterborne exposure | GO did not present acute ecotoxicity at concentrations up to 5.0 mg/L. The 96 h LC50 of Cd associated with GO was 1.7 times less than the 96 h LC50 of Cd alone and the 96 h LC50 of Zn associated with GO was 1.8 times less than the 96 h LC50 of Zn alone. The co-exposure of GO with trace elements impaired the routine metabolism of Palaemon pandaliformis. | GO - 0.1; 1.0; 2.5 and 5.0 mg/L 96 h Co-exposure of GO 1.0 mg/L with trace elements Cd 1.0 mg/L and Zn 1.0 mg/L | [83] |
| Cyprinus carpio L. | Waterborne exposure | Significant decrease in RBC count. No significant effect on WBC, PCV, and Hb. | 0, 10, 20 mg/L, 10 days | [109] |
| Tested in fish cell lines | ||||
| PLHC-1 | Co-exposure - increasing concentration of AhR agonist alone or in presence of GO and CXYG. Pre and post exposure – increasing concentration of BKF and α-MEM, to α-MEM + 4 mg/mL CXYG or to α-MEM + 16 mg/mL CXYG for another 24 h | GO and CXYG had potentiating effect on PAH- and PCB-induced Cyp1A expression at both the transcriptional and the enzymatic levels. It suggested surface chemistry of GO and CXYG did not had influence on the direct or indirect interaction with the selected AhR agonists. The obtained results suggest that a preceding and/or simultaneous exposure to GO or CXYG nanoplatelets may modify the toxicokinetics of aromatic environmental pollutants such as PAHs and PCBs. | GO and carboxyl graphene (CXYG) at 16 µg/mL, AhR agonist. | [84] |
| PLHC-1 | α-MEM medium | PLHC-1 cells demonstrated significantly reduced mitochondrial membrane potential (MMP) and increased ROS levels at 16 μg/mL GO and CXYG (72 h), but barely any decrease in cell viability. The observation of intracellular graphene accumulations not enclosed by membranes suggests that GO and CXYG internalization in fish hepatoma cells occurs through an endocytosis-independent mechanism. | GO: 0.125–16 µg/mL; CXYG: 0.25–32 µg/mL | [85] |
| BF2 | GO in milli Q water (stock solution) + Eagle’s medium | GO caused mitochondrial and lysosomal damage to BF-2 cells, oxidative stress, and morphological changes by GO through ROS, as indicated by the evaluated biomarkers LPO, GSH, SOD, CAT, and 8-OHdG. | 0, 10, 20, 40, 60, 80 and 100 μg/mL for 24 h | [86] |
| PLHC-1 and CLC | GRMs – Carbon nanofibers (CNFs) and graphene oxide (GO) | GO sheets were present within vesicles as well as free in the cytoplasm of both cell types. CNFs toxicity was inversely related to the graphitization degree. | 0–200 μg /mL of GRMs for 24 and 72 h | [87] |
| Tested at embryonic or larvae stages of fish | ||||
| Danio rerio | Waterborne exposure | Hatching delay of zebrafish embryos at a high dosage of 50 mg/L. Embryos exposed to GO exhibited significant cellular apoptosis only in the forehead and eye region, and no aggravation of cellular apoptosis was observed with increasing concentration of GO. | 0, 3.4, 7.6, 12.5, 25 and 50 mg/L 96 h post fertilization | [95] |
| Danio rerio | Waterborne exposure | GO impaired DNA modification, protein carbonylation, ROS generation (also superoxide radical) | 1–100 µg/L 2.5 hpf-7 dpf | [60] |
| Danio rerio (adult and embryo) | Waterborne exposure | GO translocated from the water to the brains of parental and offspring fish with a significant loss of claudin5a. GO did not trigger obvious neurotoxicity in parental zebrafish, whereas remarkable neurotoxicity occurred in the offspring, which exhibited a loss of dopaminergic neurons and reductions in acetylcholinesterase activity. | GO exposed to parental zebrafish 24 h prior mating 0.01–1 μg/L | [97] |
| Danio rerio | Waterborne exposure | Regardless of the presence of HA, larvae exposed to GO for 5 days showed an increase in locomotor activity, reduction in the yolk sac size, and total length and inhibition of AChE activity, but there was no difference in enzyme expression. Results indicated that HA is associated with the toxicity risk modulation by GO. | GO-100 mg/L & HA 20 mg/L alone or together for 5–7 days | [99] |
| Danio rerio | Waterborne exposure | GO adhered to and enveloped the chorion of zebrafish embryos mainly via hydroxyl group interactions, blocked the pore canals of the chorionic membrane, and caused marked hypoxia and hatching delay. GO induced excessive generation of reactive oxygen species and increased oxidative stress, DNA damage, and apoptosis | 0, 0.01, 0.1, 1, 100 mg/L for 24, 48 and 96 hpf | [101] |
| Danio rerio | Waterborne exposure of GO, Humic Acid (HA) and GO-HA | GO induced significant cardiac edema and hatching delay. HA decreased the interaction between GO and chorion, mitigated chorion damage by regulating morphology, structures, and surface negative charges of GO | GO 0–100 mg/L HA 0–100 mg/L 2.5 hpf-72 hpf | [100] |
| Danio rerio (larvae and adult) | Injections at ventral end of larvae | GO induced hepatic dysfunction through the ROS and PPAR-α mediated innate immune signaling in zebrafish | 0, 0.25, 0.5, and 1 mg/L for 72 h | [98] |
| Danio rerio | Waterborne exposure of GO and reduced graphene (rGO) | GO had significant effects on the heart rate, while rGO affected the embryos hatching and the length of larvae in a dose-dependent manner | 1, 5, 10, 50, 100 mg/L for 96 h | [96] |
| Danio rerio | Waterborne exposure | GO induced cardiac and dopaminergic alterations, as well as neuronal gene expression and morphology modifications. Altered locomotion in terms of increase of turn angle suggesting parkinsonian-like motor symptoms (at low concentrations). | 5, 10, 50 or 100 mg/L for 6 days | [102] |
| Danio rerio | Waterborne exposure | GOs induced oxidative stress and apoptosis. In particular, the immune cell number, pro-inflammatory iNOS activity, and AChE activity (a neural development indicator) were found to be induced to some extent after GO exposure, suggesting the presence of both immunomodulatory and neurotoxic effects in zebrafish larvae. The waterborne-GO exposure on zebrafish during early development was not merely dependent on GO concentration but also the associated GO sizes. | GO particles (50–200 nm, <500 nm, and >500 nm) at 0.1, 1, 10, and 100 mg/L for 4–124 h post-fertilization | [103] |
| Danio rerio | Microinjection (4 nL/embryo) | Graphene induced no significant locomotion alterations, sleep behavior, and gene expression patterns. | Graphene at 1, 10, 50 µg/mL | [60] |
| Tested at adult stage of fish | ||||
| Danio rerio | Waterborne exposure | GO caused toxicity-Oxidative stress and tissue damage induced in fish by GO through ROS, indicated by the biomarkers of MDA, GSH, SOD, and CAT; GO caused immunotoxicity in fish indicated by increased expression of inflammatory cytokines, TNF-, IL-1, and IL-6. | 0, 1, 5, 10 or 50 mg/L GO for 14 days | [104] |
| Danio rerio | Carbon 14 labeled few-layered graphene (FLG) | At 48 h larger FLG (L-FLG) at 250 µg/L the amount of graphene was close to 48 mg/kg fish dry mass, 170-fold greater than body burden of the same concentration of smaller FLG (S-FLG). L-FLG accumulated in gut and S-FLG accumulated in gut and liver. L-FLG and S-FLG had significantly different impact on intestinal microbial community structure. | L-FLG- 300–700 nm S-FLG-30–70 nm 50 μg/L, 75 μg/L and 250 μg/L 4, 12, 24, 48, 72 h | [105] |
| Danio rerio | Graphene family materials (GFMs), Monolayer graphene powder (GR), graphene oxide nanosheet (GO), reduced graphene oxide powder (rGO) | GFMs led to different inflammatory responses and significantly altered the relative composition of the gut bacterial species. GFMs altered the intestinal morphology and antioxidant enzyme activities. | 1 µg in fish diet for 21 days | [106] |
| Danio rerio | Waterborne exposure | GO caused increase in oxidative stress, increase in lipid peroxidation, changes in SOD, CAT, GPx. After 168 h: GO toxic effects decreased, but homeostasis not fully recovered. | 2, 10, and 20 mg/L 48 h, recovery period 168 h | [107] |
| Anabas testudineus | Injection at base of caudal fin | GO induced oxidative stress in cell and mitochondria in fish | 200 µL from 1 g/L 100 mg/L, 10 mg/L of GO in aqueous solution 24 h | [108] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malhotra, N.; Villaflores, O.B.; Audira, G.; Siregar, P.; Lee, J.-S.; Ger, T.-R.; Hsiao, C.-D. Toxicity Studies on Graphene-Based Nanomaterials in Aquatic Organisms: Current Understanding. Molecules 2020, 25, 3618. https://doi.org/10.3390/molecules25163618
Malhotra N, Villaflores OB, Audira G, Siregar P, Lee J-S, Ger T-R, Hsiao C-D. Toxicity Studies on Graphene-Based Nanomaterials in Aquatic Organisms: Current Understanding. Molecules. 2020; 25(16):3618. https://doi.org/10.3390/molecules25163618
Chicago/Turabian StyleMalhotra, Nemi, Oliver B. Villaflores, Gilbert Audira, Petrus Siregar, Jiann-Shing Lee, Tzong-Rong Ger, and Chung-Der Hsiao. 2020. "Toxicity Studies on Graphene-Based Nanomaterials in Aquatic Organisms: Current Understanding" Molecules 25, no. 16: 3618. https://doi.org/10.3390/molecules25163618
APA StyleMalhotra, N., Villaflores, O. B., Audira, G., Siregar, P., Lee, J.-S., Ger, T.-R., & Hsiao, C.-D. (2020). Toxicity Studies on Graphene-Based Nanomaterials in Aquatic Organisms: Current Understanding. Molecules, 25(16), 3618. https://doi.org/10.3390/molecules25163618








