Development and Validation of a TLC-Densitometry Method for Histamine Monitoring in Fish and Fishery Products
Abstract
1. Introduction
2. Results and Discussion
2.1. Method Development
2.1.1. Derivatization Procedure
2.1.2. Optimization of Derivatization Procedure
2.1.3. Solvent System Effect
2.2. Method Validation
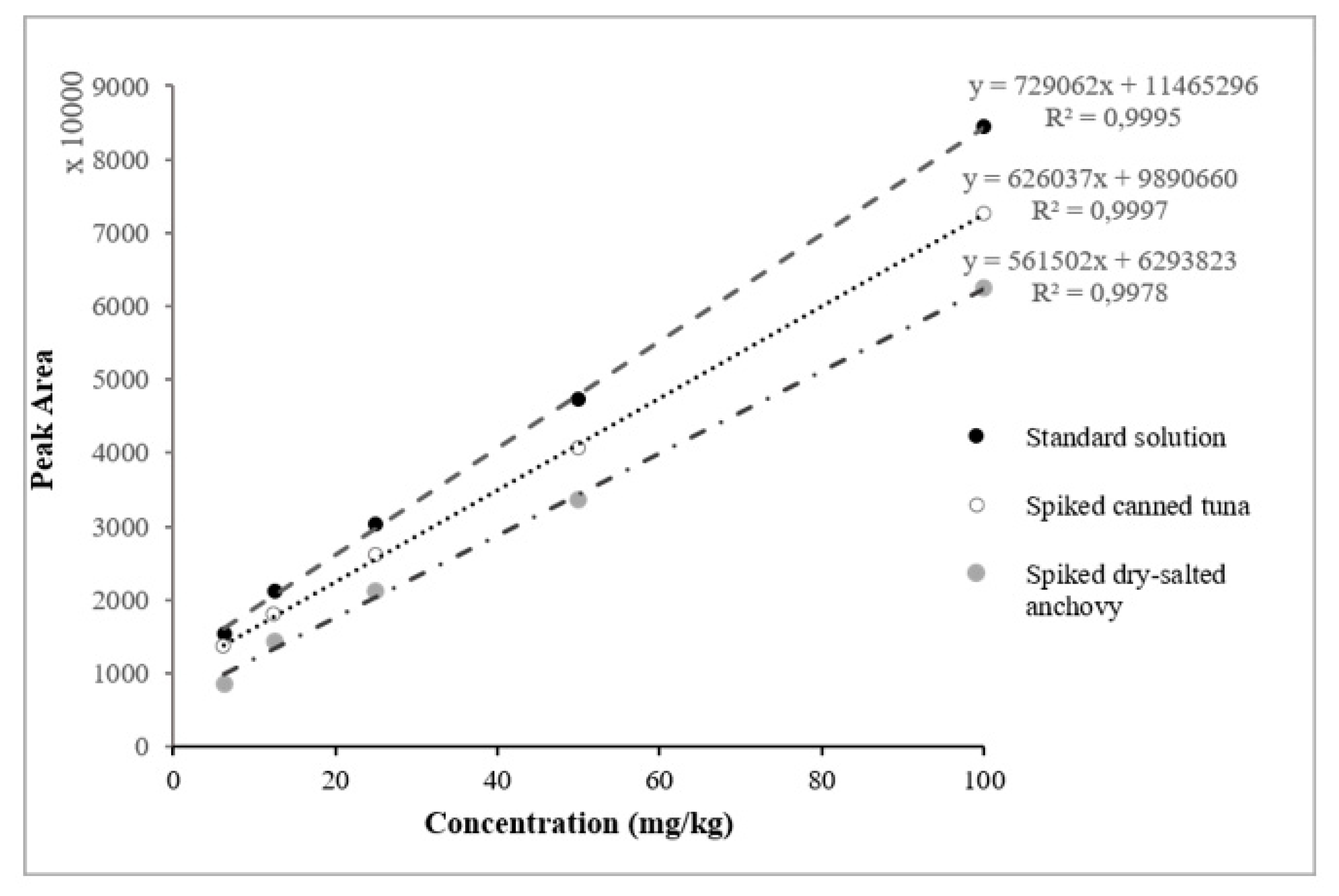
2.2.1. Linearity
2.2.2. Matrix Effect
2.2.3. Identity and Specificity
2.2.4. Limit of Detection (LOD) and Limit of Quantification (LOQ)
2.2.5. Precision, Accuracy, and Recovery
2.3. Determination of Histamine in Fish and Fishery Products
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Samples
3.3. Determination of Histamine by TLC-Densitometry
3.4. Preparation of Standard Solutions
3.5. Sample Preparation
3.5.1. Extraction
3.5.2. Derivatization
3.6. Method Validation and Statistical Analysis
3.7. HPLC-UV Method
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Burger, J.; Gochfeld, M. Perceptions of the risks and benefits of fish consumption: Individual choices to reduce risk and increase health benefits. Environ. Res. 2009, 109, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Prester, L. Biogenic amines in fish, fish products and shellfish: A review. Food Addit. Contam. Part A 2011, 28, 1547–1560. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.; Maeyama, K. Simultaneous electrochemical measurement method of histamine and Nτ-methylhistamine by high-performance liquid chromatography–amperometry with o-phthalaldehyde–sodium sulfite derivatization. Anal. Biochem. 2013, 432, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nei, D.; Nakamura, N.; Ishihara, K.; Kimura, M.; Satomi, M. A rapid screening of histamine concentration in fish fillet by direct analysis in real time mass spectrometry (DART-MS). Food Control 2017, 75, 181–186. [Google Scholar] [CrossRef]
- Tortorella, V.; Masciari, P.; Pezzi, M.; Mola, A.; Tiburzi, S.P.; Zinzi, M.C.; Scozzafava, A.; Verre, M. Histamine poisoning from ingestion of fish or scombroid syndrome. Case Rep. Emerg. Med. 2014, 2014, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.M.; Cattaneo, P.; Confalonieri, E.; Bernardi, C. Histamine food poisonings: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2018, 58, 1131–1151. [Google Scholar] [CrossRef]
- Kung, H.F.; Wang, T.Y.; Huang, Y.R.; Lin, C.S.; Wu, W.S.; Lin, C.M.; Tsai, Y.H. Isolation and identification of histamine-forming bacteria in tuna sandwiches. Food Control 2009, 20, 1013–1017. [Google Scholar] [CrossRef]
- Bajc, Z.; Gačnik, K. Densitometric TLC analysis of histamine in fish and fishery products. JPC J. Planar Chromatogr. 2009, 22, 15–17. [Google Scholar] [CrossRef]
- Kounnoun, A.; Maadoudi, M.E.; Cacciola, F.; Mondello, L.; Bougtaib, H.; Alahlah, N.; Amajoud, N.; EL Baaboua, A.; Louajri, A. Development and Validation of a High-Performance Liquid Chromatography Method for the Determination of Histamine in Fish Samples Using Fluorescence Detection with Pre-column Derivatization. Chromatographia 2020, 83, 893–901. [Google Scholar] [CrossRef]
- Tahmouzi, S.; Khaksar, R.; Ghasemlou, M. Development and validation of an HPLC-FLD method for rapid determination of histamine in skipjack tuna fish (Katsuwonus pelamis). Food Chem. 2011, 126, 756–761. [Google Scholar] [CrossRef]
- EC. Commission Regulation (EC) No 2073/2005 of 15 Novembre 2005 on the microbiological criteria for foodstuffs. Off. J. Eur. Comm. 2005, 338, 1–26. [Google Scholar]
- EU, European Union. Report EUR 26605 EN. In Equivalence Testing of Histamine Methods—Final Report; Administrative Arrangement NL SANCO/2011/G4/JRC32515/; Joint Research Centre: Brussels, Belgium, 2014. [Google Scholar]
- Peng, J.F.; Fang, K.T.; Xie, D.H.; Ding, B.; Yin, J.Y.; Cui, X.M.; Zhang, Y.; Liu, J.F. Development of an automated on-line pre-column derivatization procedure for sensitive determination of histamine in food with high-performance liquid chromatography–fluorescence detection. J. Chromatogr. A 2008, 1209, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.-S.; Wang, J.-T.; Choong, Y.-M. A rapid gas chromatographic method for the determination of histamine in fish and fish products. Food Chem. 2003, 82, 329–334. [Google Scholar] [CrossRef]
- Önal, A. A review: Current analytical methods for the determination of biogenic amines in foods. Food Chem. 2007, 103, 1475–1486. [Google Scholar] [CrossRef]
- Šimat, V.; Dalgaard, P. Use of small diameter column particles to enhance HPLC determination of histamine and other biogenic amines in seafood. LWT Food Sci. Technol. 2011, 44, 399–406. [Google Scholar] [CrossRef]
- Patange, S.B.; Mukundan, M.K.; Kumar, K.A. A simple and rapid method for colorimetric determination of histamine in fish flesh. Food Control 2005, 16, 465–472. [Google Scholar] [CrossRef]
- Shakila, R.J.; Vasundhara, T.S.; Kumudavally, K.V. A comparison of the TLC-densitometry and HPLC method for the determination of biogenic amines in fish and fishery products. Food Chem. 2001, 75, 255–259. [Google Scholar] [CrossRef]
- Sherma, J. Thin-layer chromatography in food and agricultural analysis. J. Chromatogr. A 2000, 880, 129–147. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists. Histamine in Seafood, Fluorimetric Method 977.13. In AOAC Off Method; AOAC: Urbana, IL, USA, 2005. [Google Scholar]
- Tao, Z.; Sato, M.; Han, Y.; Tan, Z.; Yamaguchi, T.; Nakano, T. A simple and rapid method for histamine analysis in fish and fishery products by TLC determination. Food Control 2011, 22, 1154–1157. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, Y.; Chen, Y.; Xu, X.; Jin, Z.; Ding, Y.; Yang, N.; Wu, F. Tuneable surface enhanced Raman spectroscopy hyphenated to chemically derivatized thin-layer chromatography plates for screening histamine in fish. Food Chem. 2017, 230, 547–552. [Google Scholar] [CrossRef]
- Yu, H.; Zhuang, D.; Hu, X.; Zhang, S.; He, Z.; Zeng, M.; Fang, X.; Chen, J.; Chen, X. Rapid determination of histamine in fish by thin-layer chromatography-image analysis method using diazotized visualization reagent prepared with p-nitroaniline. Anal. Meth. 2018, 10, 3386–3392. [Google Scholar] [CrossRef]
- Romano, A.; Klebanowski, H.; La Guerche, S.; Beneduce, L.; Spano, G.; Murat, M.L.; Lucas, P. Determination of biogenic amines in wine by thin-layer chromatography/densitometry. Food Chem. 2012, 135, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.; Pesek, J.J.; Matyska, M.T. The use of aqueous normal phase chromatography as an analytical tool for food analysis: Determination of histamine as a model system. Food Chem. 2013, 141, 4226–4230. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak, D.; Ciesielski, W.; Zakrzewski, R. Application of the Iodine-Azide Procedure for Detection of Biogenic Amines in TLC. J. Liq. Chrom. Relat. Technol. 2006, 29, 2425–2436. [Google Scholar] [CrossRef]
- Arulkumar, A.; Karthik, G.; Paramasivam, S.; Rabie, M.A. Histamine levels in Indian fish via enzymatic, TLC and HPLC methods during storage. J. Food Meas. Charact. 2017, 11, 281–289. [Google Scholar] [CrossRef]
- Lapa-Guimaraes, J.; Pickova, J. New solvent systems for thin-layer chromatographic determination of nine biogenic amines in fish and squid. J. Chromatogr. A 2004, 1045, 223–232. [Google Scholar] [CrossRef]
- Shalaby, A.R. Simple, rapid and valid thin layer chromatographic method for determining biogenic amines in foods. Food Chem. 1999, 65, 117–121. [Google Scholar] [CrossRef]
- Mantoanelli, J.O.F.; Gonçalves, L.M.; Pereira, E.A. Dansyl Chloride as a Derivatizing Agent for the Analysis of Biogenic Amines by CZE-UV. Chromatographia 2020, 83, 767–778. [Google Scholar] [CrossRef]
- Duflos, G.; Dervin, C.; Malle, P.; Bouquelet, S. Relevance of matrix effect in determination of biogenic amines in plaice (Pleuronectes platessa) and whiting (Merlangus merlangus). J. AOAC Int. 1999, 82, 1097–1101. [Google Scholar] [CrossRef]
- Duflos, G.; Inglebert, G.; Himber, C.; Degremont, S.; Lombard, B.; Brisabois, A. Validation of standard method EN ISO 19343 for the detection and quantification of histamine in fish and fishery products using high-performance liquid chromatography. Int. J. Food Microbiol. 2019, 288, 97–101. [Google Scholar] [CrossRef]
- Altieri, I.; Semeraro, A.; Scalise, F.; Calderari, I.; Stacchini, P. European official control of food: Determination of histamine in fish products by a HPLC–UV-DAD method. Food Chem. 2016, 211, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Moratalla, M.L.; Bover-Cid, S.; Veciana-Nogués, T.; Vidal-Carou, M.C. Thin-layer chromatography for the identification and semi-quantification of biogenic amines produced by bacteria. J. Chromatogr. A 2009, 1216, 4128–4132. [Google Scholar] [CrossRef] [PubMed]
- Directive EC Council. 657/EC of 12 August, 1990 on implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. J. Eur. Communit. 2002, 221, 8–36. [Google Scholar]
- Afnor French Standardization Association. Analysis of Agri-Foodstuffs—Protocol of Characterization for the Validation of a Quantitative Method of Analysis by Construction of an Accuracy Profile; NF F03-110; Afnor: Paris, France, 2010. [Google Scholar]
- ISO International Organization for Standardization. Microbiology of the Food Chain-Detection and Quantification of Histamine in Fish and Fishery Products; ISO 19343; ISO: Geneva, Switzerland, 2017. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
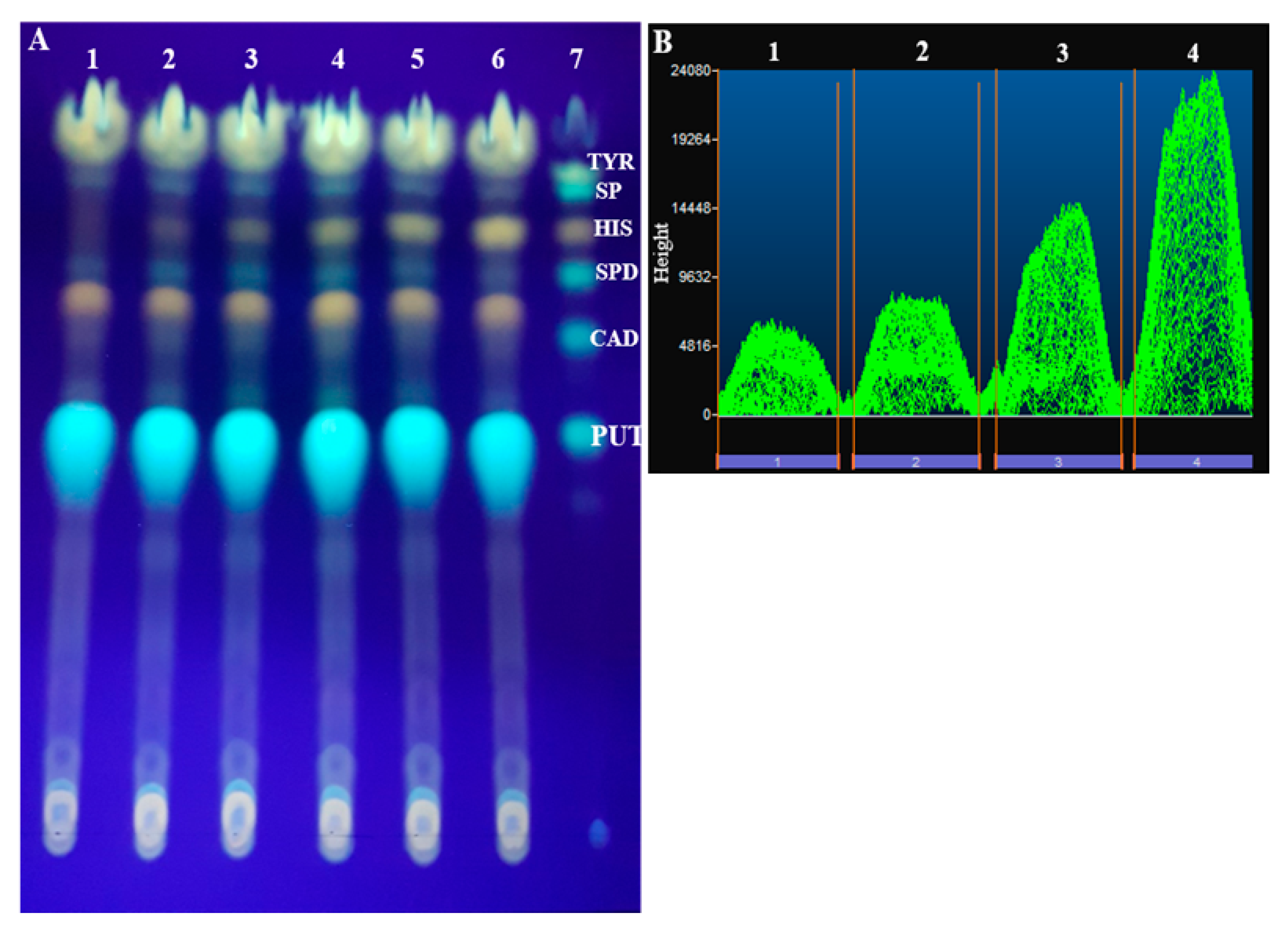
| Biogenic Amines | TLC Rf Values |
|---|---|
| Putrescine | 0.50 |
| Cadaverine | 0.61 |
| Spermidine | 0.69 |
| Histamine | 0.77 |
| Spermine | 0.82 |
| Tyramine | 0.85 |
| Nominal Concentration (mg/kg) | Observed Concentration a (Mean mg/kg ± SD) | Intra-Day Precision b (%) | Inter-Day Precision (%) | Trueness c (%) | Average Recovery d (Mean mg/kg ± SD) |
|---|---|---|---|---|---|
| 6.25 | 5.82 ± 0.28 | 4.82 | 11.16 | −6.88 | 93.18 ± 4,49 |
| 12.5 | 13.16 ± 0.53 | 4.09 | 5.37 | 5.28 | 105.28 ± 4.31 |
| 25 | 26.05 ± 0.98 | 3.78 | 6.35 | 4.20 | 104.21 ± 3.94 |
| 50 | 48.66 ± 1.89 | 3.90 | 7.29 | −2.68 | 97.33 ± 3.80 |
| 100 | 103.70 ± 2.27 | 2.19 | 5.80 | 3.70 | 103.70 ± 2.27 |
| Sample (n) | Histamine Levels (mg/kg) | |
|---|---|---|
| HPLC-UV Method | TLC Method | |
| Fresh mackerel (4) | nd 10.74 15.36 20.41 | nd nd 11.90 17.89 |
| Fresh Sardina pilchardus (2) | 9.82 16.32 | nd 14.53 |
| Fresh anchovy (2) | nd 35.21 | nd 39.23 |
| Fresh tuna (2) | 10.12 15.73 | nd 13.25 |
| Marinated anchovy (2) | nd 14.23 | nd 16.23 |
| Dry salted anchovy (2) | 17.53 nd | 22.36 nd |
| Canned mackerel (3) | 13.28 nd nd | 12.77 nd nd |
| Canned tuna (1) | nd | nd |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kounnoun, A.; Louajri, A.; Cacciola, F.; El Cadi, H.; Bougtaib, H.; Alahlah, N.; El Baaboua, A.; El Maadoudi, M. Development and Validation of a TLC-Densitometry Method for Histamine Monitoring in Fish and Fishery Products. Molecules 2020, 25, 3611. https://doi.org/10.3390/molecules25163611
Kounnoun A, Louajri A, Cacciola F, El Cadi H, Bougtaib H, Alahlah N, El Baaboua A, El Maadoudi M. Development and Validation of a TLC-Densitometry Method for Histamine Monitoring in Fish and Fishery Products. Molecules. 2020; 25(16):3611. https://doi.org/10.3390/molecules25163611
Chicago/Turabian StyleKounnoun, Ayoub, Adnane Louajri, Francesco Cacciola, Hafssa El Cadi, Hajar Bougtaib, Naoual Alahlah, Aicha El Baaboua, and Mohamed El Maadoudi. 2020. "Development and Validation of a TLC-Densitometry Method for Histamine Monitoring in Fish and Fishery Products" Molecules 25, no. 16: 3611. https://doi.org/10.3390/molecules25163611
APA StyleKounnoun, A., Louajri, A., Cacciola, F., El Cadi, H., Bougtaib, H., Alahlah, N., El Baaboua, A., & El Maadoudi, M. (2020). Development and Validation of a TLC-Densitometry Method for Histamine Monitoring in Fish and Fishery Products. Molecules, 25(16), 3611. https://doi.org/10.3390/molecules25163611







