Cannabidiol Determination on Peripheral Capillary Blood Using a Microsampling Method and Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry with On-Line Sample Preparation
Abstract
:1. Introduction
2. Results
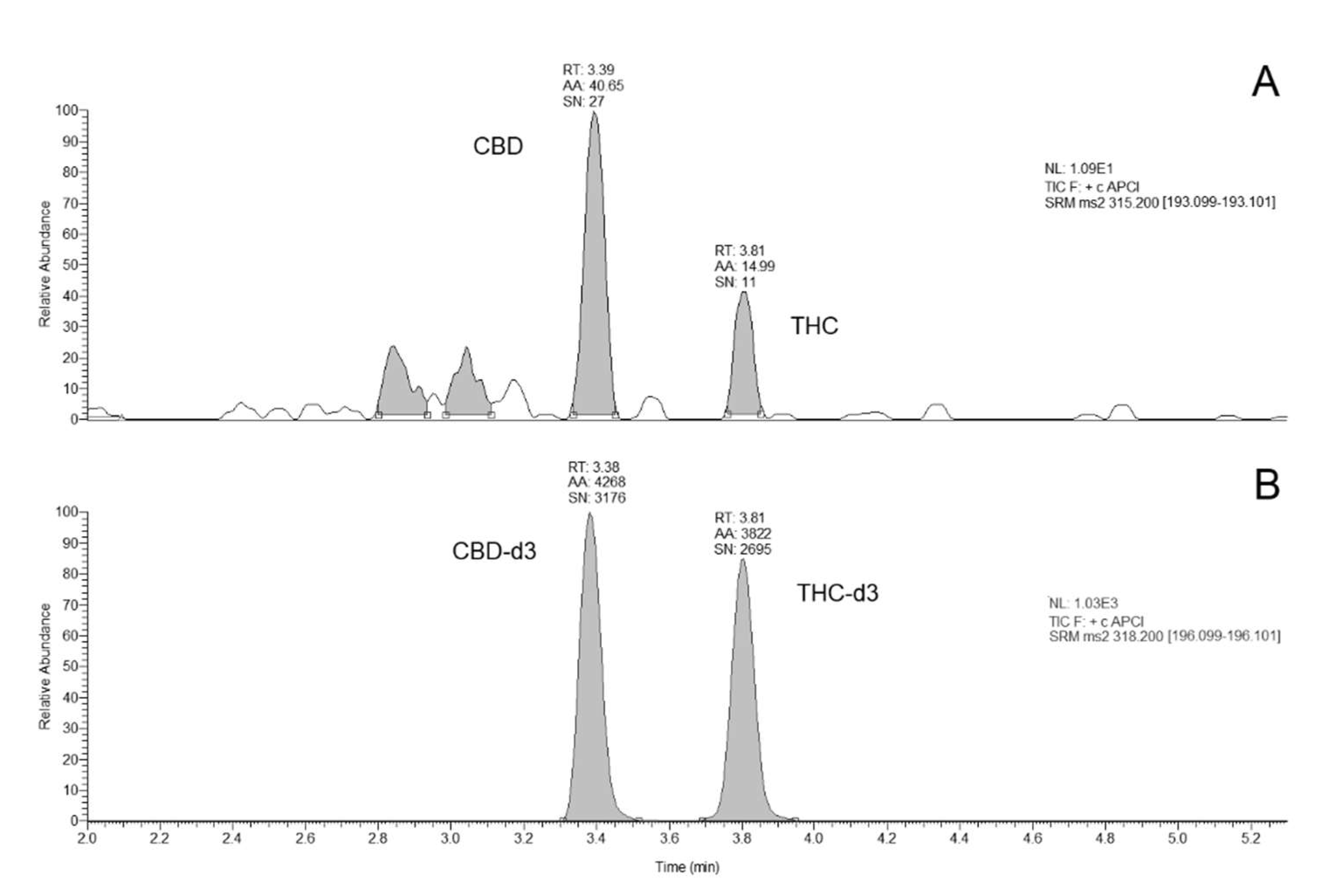
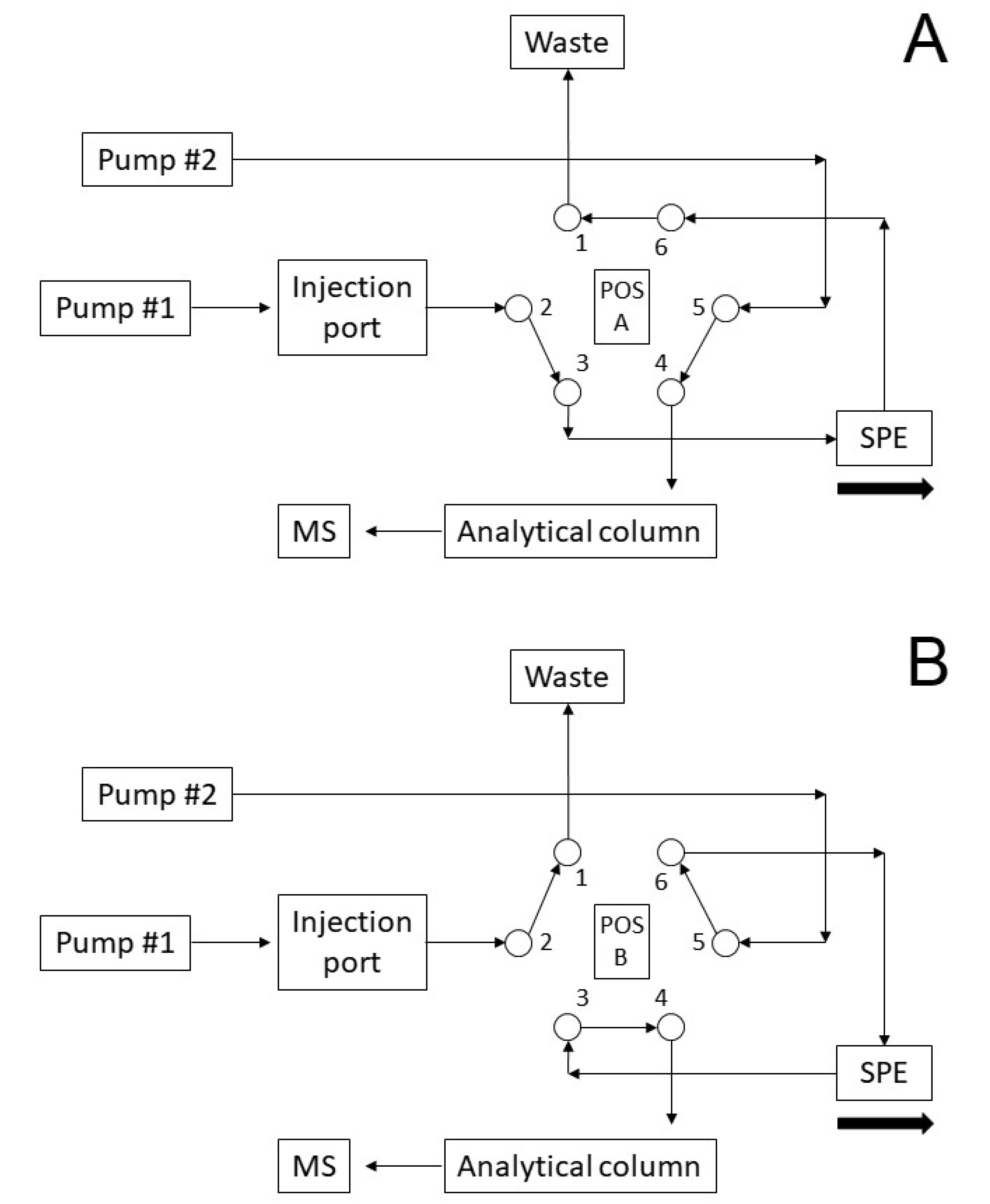
2.1. Method Development
2.2. Method Validation
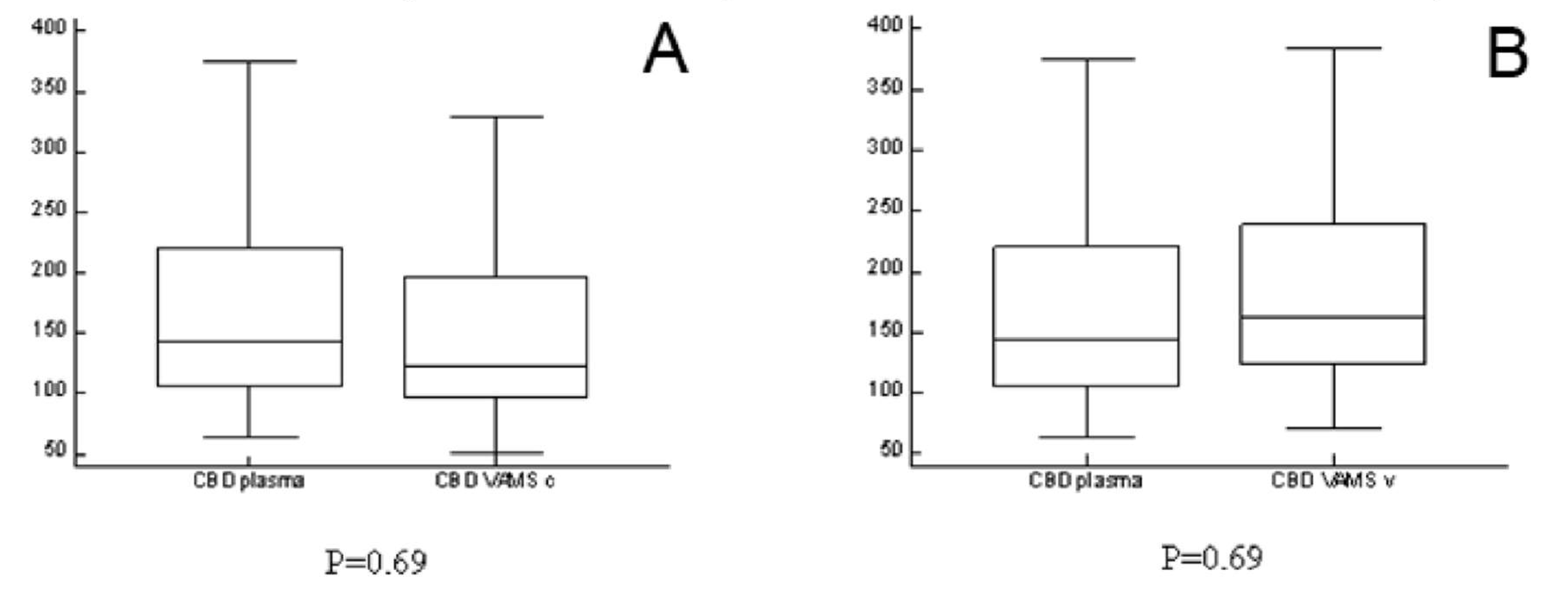
2.3. Analyses of Clinical Samples
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Collection
4.3. Preparation of VAMS Samples from Venous Blood
4.4. Preparation of Calibrators and Quality Control Samples
4.5. Analyte Extraction from VAMS
4.6. Chromatographic Separation
4.7. Mass Spectrometric Detection
4.8. Method Validation
4.8.1. Selectivity
4.8.2. Carry Over
4.8.3. Matrix Effects and Extraction Recovery
4.8.4. Linearity
4.8.5. Precision, Accuracy and LLOQ
4.8.6. Stability
4.9. Statistical Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brandt, C. Pharmacodynamic monitoring of antiepileptic drug therapy. Ther. Drug Monit. 2019, 41, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Striano, S.; Striano, P.; Capone, D.; Pisani, F. Limited place for plasma monitoring of new antiepileptic drugs in clinical practice. Med. Sci. Monit. 2008, 14, 173–178. [Google Scholar]
- Iapadre, G.; Balagura, G.; Zagaroli, L.; Striano, P.; Verrotti, A. Pharmacokinetics and drug interaction of antiepileptic drugs in children and adolescents. Pediatr. Drugs 2018, 20, 429–453. [Google Scholar] [CrossRef] [PubMed]
- Min, K.L.; Ryu, J.Y.; Chang, M.J. Development and clinical applications of the dried blood spot method for therapeutic drug monitoring of anti-epileptic drugs. Basic Clin. Pharmacol. Toxicol. 2019, 125, 215–236. [Google Scholar] [CrossRef] [Green Version]
- D’urso, A.; Cangemi, G.; Barco, S.; Striano, P.; D’avolio, A.; De Grazia, U. LC-MS/MS-Based Quantification of 9 Antiepileptic Drugs From a Dried Sample Spot Device. Ther. Drug Monit. 2019, 41, 331–339. [Google Scholar] [CrossRef]
- Arzimanoglou, A.; Brandl, U.; Cross, J.H.; Gil-Nagel, A.; Lagae, L.; Landmark, C.J.; Specchio, N.; Nabbout, R.; Thiele, E.A.; Gubbay, O. Epilepsy and cannabidiol: A guide to treatment. Epileptic Disord. 2020, 22, 1–14. [Google Scholar] [CrossRef]
- Lattanzi, S.; Brigo, F.; Trinka, E.; Zaccara, G.; Striano, P.; Del Giovane, C.; Silvestrini, M. Adjunctive Cannabidiol in Patients with Dravet Syndrome: A Systematic Review and Meta-Analysis of Efficacy and Safety. CNS Drugs 2020, 34, 229–241. [Google Scholar] [CrossRef]
- Lattanzi, S.; Trinka, E.; Russo, E.; Striano, P.; Citraro, R.; Silvestrini, M.; Brigo, F. Cannabidiol as adjunctive treatment of seizures associated with Lennox–Gastaut syndrome and Dravet syndrome. Drugs Today 2019, 55, 177–196. [Google Scholar] [CrossRef]
- Ibeas Bih, C.; Chen, T.; Nunn, A.V.W.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef] [Green Version]
- De Caro, C.; Leo, A.; Citraro, R.; De Sarro, C.; Russo, R.; Calignano, A.; Russo, E. The potential role of cannabinoids in epilepsy treatment. Expert Rev. Neurother. 2017, 17, 1069–1079. [Google Scholar] [CrossRef]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geffrey, A.L.; Pollack, S.F.; Bruno, P.L.; Thiele, E.A. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015, 56, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Landmark, C.J.; Brandl, U. Pharmacology and drug interactions of cannabinoids. Epileptic Disord. 2020, 22, S16–S22. [Google Scholar] [CrossRef]
- Gherzi, M.; Milano, G.; Fucile, C.; Calevo, M.G.; Mancardi, M.M.; Nobili, L.; Astuni, P.; Marini, V.; Barco, S.; Cangemi, G.; et al. Safety and pharmacokinetics of medical cannabis preparation in a monocentric series of young patients with drug resistant epilepsy. Complement. Ther. Med. 2020, 51, 102402. [Google Scholar] [CrossRef]
- Ocque, A.J.; Hagler, C.E.; DiFrancesco, R.; Lombardo, J.; Morse, G.D. Development and validation of an assay to measure cannabidiol and Δ9-tetrahydrocannabinol in human EDTA plasma by UHPLC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1112, 56–60. [Google Scholar] [CrossRef]
- Biagini, D.; Antoni, S.; Lomonaco, T.; Ghimenti, S.; Salvo, P.; Bellagambi, F.G.; Scaramuzzo, R.T.; Ciantelli, M.; Cuttano, A.; Fuoco, R.; et al. Micro-extraction by packed sorbent combined with UHPLC-ESI-MS/MS for the determination of prostanoids and isoprostanoids in dried blood spots. Talanta 2020, 206, 120236. [Google Scholar] [CrossRef]
- Denniff, P.; Spooner, N. Volumetric absorptive microsampling: A dried sample collection technique for quantitative bioanalysis. Anal. Chem. 2014, 86, 8489–8495. [Google Scholar] [CrossRef]
- De Kesel, P.M.M.; Capiau, S.; Lambert, W.E.; Stove, C.P. Current strategies for coping with the hematocrit problem in dried blood spot analysis. Bioanalysis 2014, 6, 1871–1874. [Google Scholar] [CrossRef] [Green Version]
- Kok, M.G.M.; Fillet, M. Volumetric absorptive microsampling: Current advances and applications. J. Pharm. Biomed. Anal. 2018, 147, 288–296. [Google Scholar] [CrossRef]
- Mano, Y.; Kita, K.; Kusano, K. Hematocrit-independent recovery is a key for bioanalysis using volumetric absorptive microsampling devices, MitraTM. Bioanalysis 2015, 7, 1821–1829. [Google Scholar] [CrossRef]
- Spooner, N.; Denniff, P.; Michielsen, L.; De Vries, R.; Ji, Q.C.; Arnold, M.E.; Woods, K.; Woolf, E.J.; Xu, Y.; Boutet, V.; et al. A device for dried blood microsampling in quantitative bioanalysis: Overcoming the issues associated blood hematocrit. Bioanalysis 2015, 7, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Barco, S.; Castagnola, E.; Moscatelli, A.; Rudge, J.; Tripodi, G.; Cangemi, G. Volumetric adsorptive microsampling-liquid chromatography tandem mass spectrometry assay for the simultaneous quantification of four antibiotics in human blood: Method development, validation and comparison with dried blood spot. J. Pharm. Biomed. Anal. 2017, 145, 704–710. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, A.; Rudge, J.; Patsalos, P.N.; De Grazia, U. Volumetric Absorptive Microsampling: A New Sampling Tool for Therapeutic Drug Monitoring of Antiepileptic Drugs. Ther. Drug Monit. 2019, 41, 681–692. [Google Scholar] [CrossRef] [PubMed]
- EMA Committee for Medicinal Products for Human Use. Guideline on bioanalytical method validation. EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2**. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 7 August 2020).
- Fanali, G.; Cao, Y.; Ascenzi, P.; Trezza, V.; Rubino, T.; Parolaro, D.; Fasano, M. Binding of Δ9-tetrahydrocannabinol and diazepam to human serum albumin. IUBMB Life 2011, 63, 446–451. [Google Scholar] [CrossRef]
- Salomone, A.; Gerace, E.; D’Urso, F.; Di Corcia, D.; Vincenti, M. Simultaneous analysis of several synthetic cannabinoids, THC, CBD and CBN, in hair by ultra-high performance liquid chromatography tandem mass spectrometry. Method validation and application to real samples. J. Mass Spectrom. 2012, 47, 604–610. [Google Scholar] [CrossRef]
- Teixeira, H.; Verstraete, A.; Proença, P.; Corte-Real, F.; Monsanto, P.; Vieira, D.N. Validated method for the simultaneous determination of Δ9-THC and Δ9-THC-COOH in oral fluid, urine and whole blood using solid-phase extraction and liquid chromatography-mass spectrometry with electrospray ionization. Forensic Sci. Int. 2007, 170, 148–155. [Google Scholar] [CrossRef]
- Grauwiler, S.B.; Scholer, A.; Drewe, J. Development of a LC/MS/MS method for the analysis of cannabinoids in human EDTA-plasma and urine after small doses of Cannabis sativa extracts. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 850, 515–522. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Zarandona, I.; Ortiz, L.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Simultaneous quantification of major cannabinoids and metabolites in human urine and plasma by HPLC-MS/MS and enzyme-alkaline hydrolysis. Drug Test. Anal. 2017, 9, 626–633. [Google Scholar] [CrossRef]
- Concheiro, M.; de Castro, A.; Quintela, O.; Cruz, A.; López-Rivadulla, M. Development and validation of a method for the quantitation of Δ9tetrahydrocannabinol in oral fluid by liquid chromatography electrospray–mass-spectrometry. J. Chromatogr. B 2004, 810, 319–324. [Google Scholar] [CrossRef]
- Barco, S.; Fucile, C.; Manfredini, L.; De Grandis, E.; Gherzi, M.; Martelli, A.; Tripodi, G.; Mattioli, F.; Cangemi, G. A UHPLC-MS/MS method for the quantification of Δ9-tetrahydrocannabinol and cannabidiol in decoctions and in plasma samples for therapeutic monitoring of medical cannabis. Bioanalysis 2018, 10, 2003–2014. [Google Scholar] [CrossRef]
- Maralikova, B.; Weinmann, W. Simultaneous determination of Δ9-tetrahydrocannabinol, 11-hydroxy-Δ9-tetrahydrocannabinol and 11-nor-9-carboxy-Δ9-tetrahydrocannabinol in human plasma by high-performance liquid chromatography/tandem mass spectrometry. J. Mass Spectrom. 2004, 39, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Nadulski, T.; Sporkert, F.; Schnelle, M.; Stadelmann, A.M.; Roser, P.; Schefter, T.; Pragst, F. Simultaneous and sensitive analysis of THC, 11-OH-THC, THC-COOH, CBD, and CBN by GC-MS in plasma after oral application of small doses of THC and cannabis extract. J. Anal. Toxicol. 2005, 29, 782–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marasca, C.; Protti, M.; Mandrioli, R.; Atti, A.R.; Armirotti, A.; Cavalli, A.; De Ronchi, D.; Mercolini, L. Whole blood and oral fluid microsampling for the monitoring of patients under treatment with antidepressant drugs. J. Pharm. Biomed. Anal. 2020, 188, 113384. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-González, L.; Díaz-Louzao, C.; Lendoiro, E.; Otero-Antón, E.; Cadarso-Suárez, C.; López-Rivadulla, M.; Cruz, A.; de-Castro-Ríos, A. Volumetric Absorptive Microsampling (VAMS) for assaying immunosuppressants from venous whole blood by LC–MS/MS using a novel atmospheric pressure ionization probe (UniSprayTM). J. Pharm. Biomed. Anal. 2020, 189, 113422. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, G.S.; Vedar, C.; DiLiberto, M.A.; Zuppa, A.F. A patient-centric liquid chromatography-tandem mass spectrometry microsampling assay for analysis of cannabinoids in human whole blood: Application to pediatric pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1130, 121828. [Google Scholar] [CrossRef] [PubMed]
- Velghe, S.; Stove, C.P. Volumetric absorptive microsampling as an alternative tool for therapeutic drug monitoring of first-generation anti-epileptic drugs. Anal. Bioanal. Chem. 2018, 410, 2331–2341. [Google Scholar] [CrossRef] [Green Version]
- Protti, M.; Rudge, J.; Sberna, A.E.; Gerra, G.; Mercolini, L. Dried haematic microsamples and LC-MS/MS for the analysis of natural and synthetic cannabinoids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1044, 77–86. [Google Scholar] [CrossRef]
- Lomonaco, T.; Ghimenti, S.; Piga, I.; Biagini, D.; Onor, M.; Fuoco, R.; Paolicchi, A.; Ruocco, L.; Pellegrini, G.; Trivella, M.G.; et al. Monitoring of warfarin therapy: Preliminary results from a longitudinal pilot study. Microchem. J. 2018, 136, 170–176. [Google Scholar] [CrossRef]
- Qu, Y.; Brady, K.; Apilado, R.; O’Malley, T.; Reddy, S.; Chitkara, P.; Ibarra, C.; Alexander, R.V.; Dervieux, T. Capillary blood collected on volumetric absorptive microsampling (VAMS) device for monitoring hydroxychloroquine in rheumatoid arthritis patients. J. Pharm. Biomed. Anal. 2017, 140, 334–341. [Google Scholar] [CrossRef]
- Gu, H.; Liu, G.; Wang, J.; Aubry, A.F.; Arnold, M.E. Selecting the correct weighting factors for linear and quadratic calibration curves with least-squares regression algorithm in bioanalytical LC-MS/MS assays and impacts of using incorrect weighting factors on curve stability, data quality, and assay performance. Anal. Chem. 2014, 86, 8959–8966. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds THC, THC-d3, CBD, CBD-d3. are available from the authors. |
| INTER-DAY | ||||||
| CBD | THC | |||||
| Dev. Std. (σ) | CV % | Accuracy % | Dev. Std. (σ) | CV % | Accuracy % | |
| LLOQ | 0.01 | 6% | −13% | 0.01 | 5% | −12% |
| QC low | 0.22 | 10% | 14% | 0.32 | 9% | 12% |
| QC medium | 0.57 | 14% | −4% | 0.52 | 14% | −7% |
| QC high | 0.48 | 5% | −5% | 0.50 | 5% | −1% |
| INTRA-DAY | ||||||
| CBD | THC | |||||
| Dev. Std. (σ) | CV % | Accuracy % | Dev. Std. (σ) | CV % | Accuracy % | |
| LLOQ | 0.01 | 6% | 14% | 0.03 | 14% | 13% |
| QC low | 0.02 | 11% | 9% | 0.08 | 4% | 6% |
| QC medium | 0.07 | 6% | 12% | 0.11 | 10% | 11% |
| QC high | 1.48 | 13% | 6% | 0.40 | 4% | 5% |
| CBD | THC | ||||||
|---|---|---|---|---|---|---|---|
| T − 20 °C | T + 25 °C | T − 20 °C | T + 25 °C | ||||
| 7 days | 7 days | 7 days | 7 days | ||||
| LLOQ | 91% (6%) | LLOQ | 97% (8%) | LLOQ | 94% (3%) | LLOQ | 97% (8%) |
| QC high | 92% (7%) | QC high | 94% (1%) | QC high | 94% (1%) | QC high | 94% (1%) |
| 14 days | 14 days | 14 days | 14 days | ||||
| LLOQ | 99% (8%) | LLOQ | 94% (6%) | LLOQ | 91% (1%) | LLOQ | 94% (6%) |
| QC high | 97% (6%) | QC high | 91% (4%) | QC high | 92% (5%) | QC high | 91% (3%) |
| 21 days | 21 days | 21 days | 21 days | ||||
| LLOQ | 98% (4%) | LLOQ | 98% (4%) | LLOQ | 93% (5%) | LLOQ | 98% (6%) |
| QC high | 99% (3%) | QC high | 90% (3%) | QC high | 93% (6%) | QC high | 90% (9%) |
| 28 days | 28 days | 28 days | 28 days | ||||
| LLOQ | 99% (3%) | LLOQ | 97% (6%) | LLOQ | 94% (8%) | LLOQ | 97% (4%) |
| QC high | 98% (8%) | QC high | 91% (8%) | QC high | 98% (3%) | QC high | 91% (6%) |
| Dose (mg/kg/day) | Age (years) | Gender | CBD p (µg/L) | CBD VAMS v (µg/L) | CBD VAMS c (µg/L) | |
|---|---|---|---|---|---|---|
| Patient 1 | 17.5 | 6 | female | 374 | 383.45 | 330.14 |
| Patient 2 | 10.0 | 12 | male | 119 | 163.1 | 153.15 |
| Patient 3 | 17.5 | 17 | male | 143 | 141.27 | 112.22 |
| Patient 4 | 20.0 | 26 | male | 169 | 190.21 | 122.31 |
| Patient 5 | 10.0 | 8 | male | 64 | 72.15 | 52.19 |
| Time (min) | Divert Valve position | Pump #1 | Event SPE | Pump #2 | Event Analytical Column | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Flow rate (mL/min) | A% | B% | C% | Flow rate (mL/min) | A% | B% | ||||
| 0.00 | A | 1.0 | 100 | 0 | 0 | Loading | 0.4 | 50 | 50 | Equilibration |
| 0.10 | 1.0 | 100 | 0 | 0 | ||||||
| 0.10 | 2.5 | 0 | 100 | 0 | Washing | |||||
| 0.25 | 2.5 | 0 | 100 | 0 | ||||||
| 0.25 | 1.0 | 100 | 0 | 0 | ||||||
| 0.40 | B | Transfer | 0.4 | 50 | 50 | Loading | ||||
| 0.90 | A | |||||||||
| 1.00 | 0.4 | 0 | 100 | Separation | ||||||
| 1.50 | 1.0 | 100 | 0 | 0 | ||||||
| 1.50 | 2.0 | 0 | 0 | 100 | Washing | |||||
| 4.00 | 2.0 | 0 | 0 | 100 | ||||||
| 4.00 | 1.0 | 100 | 0 | 0 | Equilibration | |||||
| 5.00 | 0.4 | 0 | 100 | |||||||
| 5.00 | 0.4 | 50 | 50 | Equilibration | ||||||
| 7.50 | Stop Run | |||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigliasco, F.; Barco, S.; Dubois, S.; Marchese, F.; Striano, P.; Lomonaco, T.; Mattioli, F.; Tripodi, G.; Cangemi, G. Cannabidiol Determination on Peripheral Capillary Blood Using a Microsampling Method and Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry with On-Line Sample Preparation. Molecules 2020, 25, 3608. https://doi.org/10.3390/molecules25163608
Pigliasco F, Barco S, Dubois S, Marchese F, Striano P, Lomonaco T, Mattioli F, Tripodi G, Cangemi G. Cannabidiol Determination on Peripheral Capillary Blood Using a Microsampling Method and Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry with On-Line Sample Preparation. Molecules. 2020; 25(16):3608. https://doi.org/10.3390/molecules25163608
Chicago/Turabian StylePigliasco, Federica, Sebastiano Barco, Sara Dubois, Francesca Marchese, Pasquale Striano, Tommaso Lomonaco, Francesca Mattioli, Gino Tripodi, and Giuliana Cangemi. 2020. "Cannabidiol Determination on Peripheral Capillary Blood Using a Microsampling Method and Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry with On-Line Sample Preparation" Molecules 25, no. 16: 3608. https://doi.org/10.3390/molecules25163608
APA StylePigliasco, F., Barco, S., Dubois, S., Marchese, F., Striano, P., Lomonaco, T., Mattioli, F., Tripodi, G., & Cangemi, G. (2020). Cannabidiol Determination on Peripheral Capillary Blood Using a Microsampling Method and Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry with On-Line Sample Preparation. Molecules, 25(16), 3608. https://doi.org/10.3390/molecules25163608







