Rapid Multi-Residue Detection Methods for Pesticides and Veterinary Drugs
Abstract
1. Introduction
2. Rapid Multi-Residue Detection Methods Based on Different Recognition Elements
2.1. Rapid Multi-Residue Detection Methods Based on Antibodies
2.1.1. Preparation and Application of Generic Antibodies
2.1.2. Preparation and Application of Broad-Spectrum Antibodies
2.1.3. Preparation and Application of Bispecific Antibodies
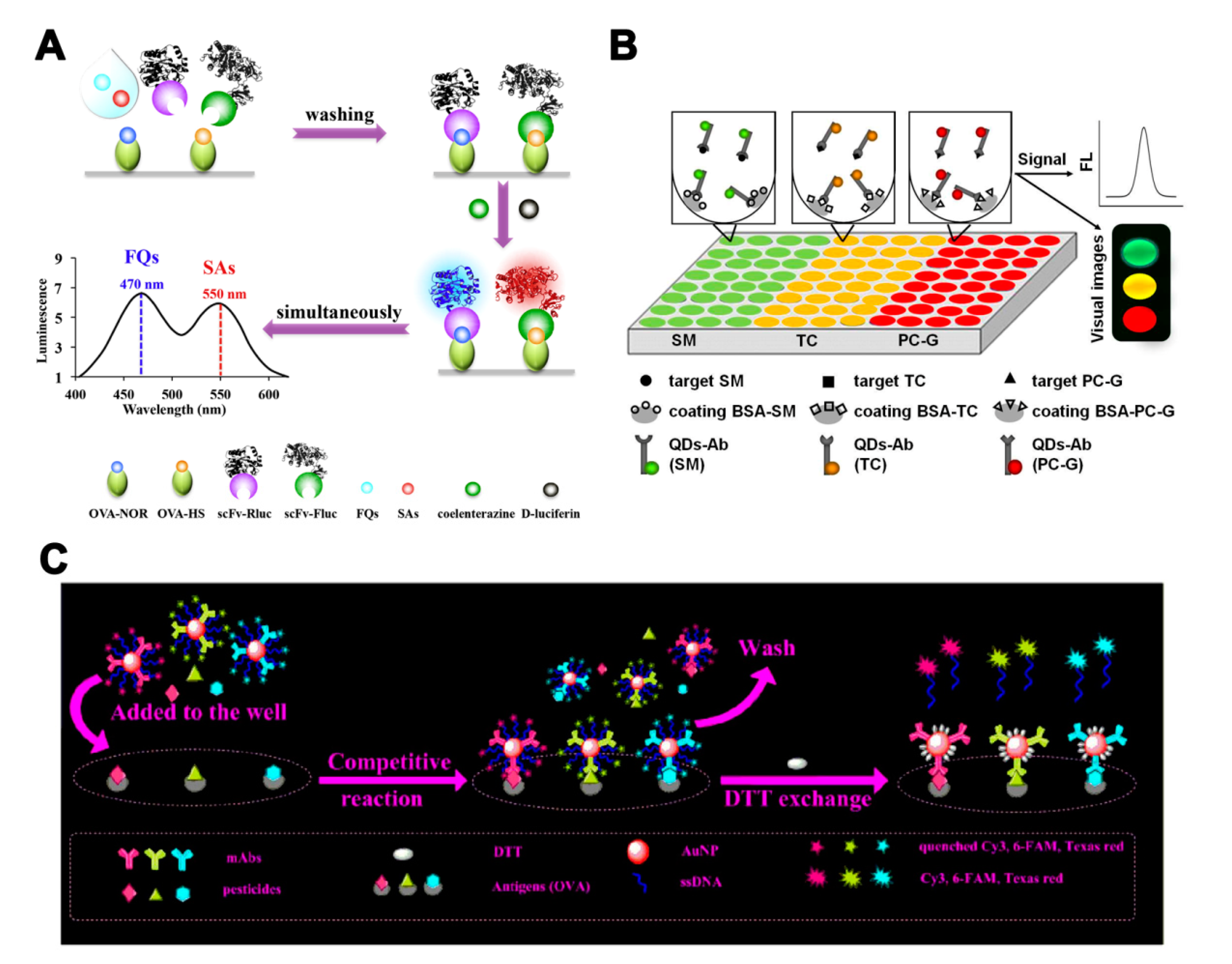
2.1.4. Preparation and Application of Multi-Antibodies
2.2. Rapid Multi-Residue Detection Methods Based on Aptamers
2.2.1. Preparation and Application of Group-Specific Aptamers
2.2.2. Preparation and Application of Broad-Spectrum Aptamers
2.2.3. Preparation and Application of Truncated Aptamers with Broad Specificity
2.2.4. Preparation and Application of Multi-Aptamers
2.3. Rapid Multi-Residue Detection Methods Based on Molecularly Imprinted Polymers
3. Rapid Multi-Residue Detection Methods Based on the Inherent Characteristics of Pesticides and Veterinary Drugs
3.1. Enzymatic Inhibition-Based Multi-Residue Detection
3.2. Near-Infrared Spectroscopy Based Multi-Residue Detections
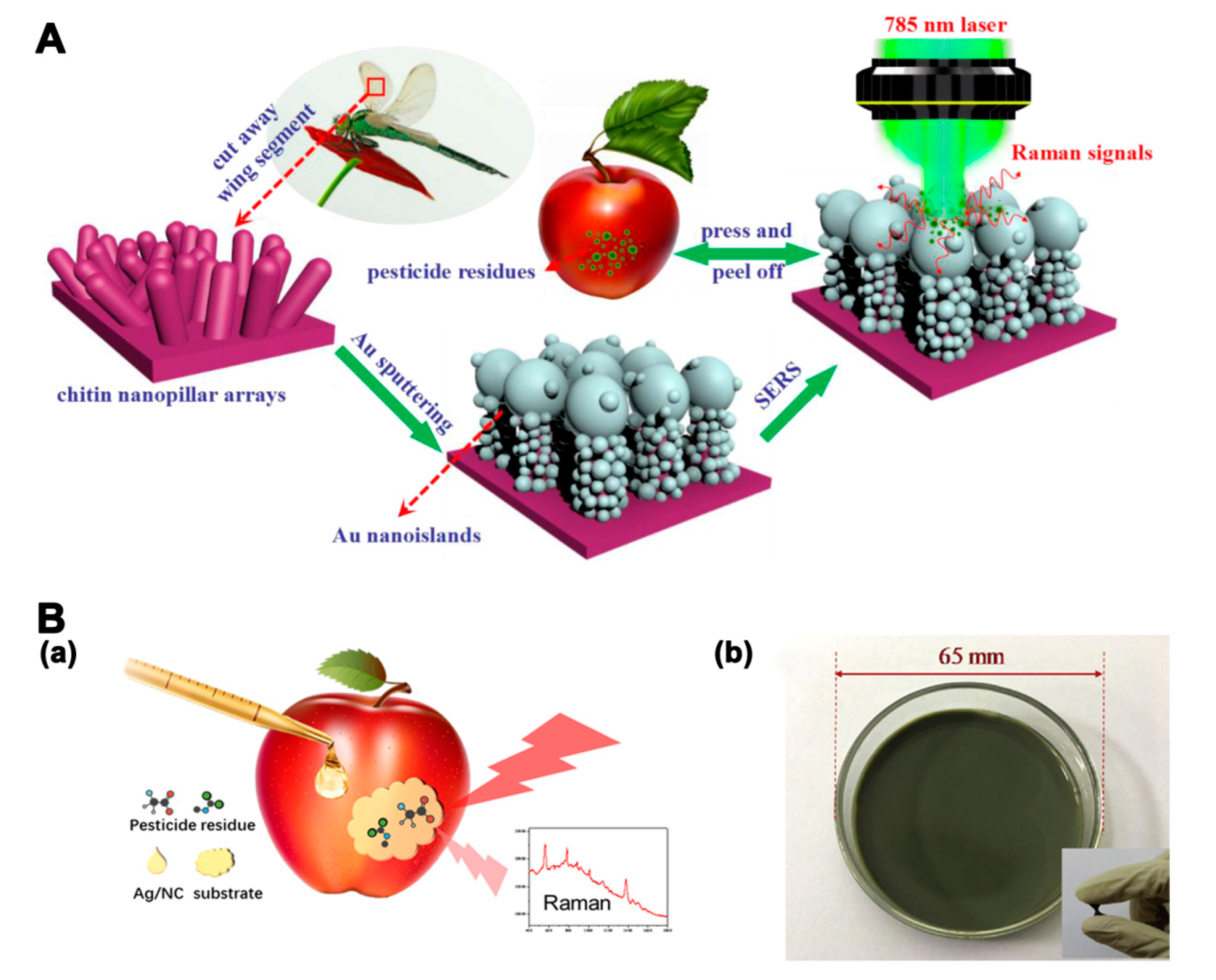
3.3. Surface-Enhanced Raman Scattering Based Multi-Residue Detections
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, J.-W.; Zou, X.-M.; Song, S.-H.; Chen, G.-H. Quantum dots applied to methodology on detection of pesticide and veterinary drug residues. J. Agric. Food Chem. 2018, 66, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Yin, Z.; Chai, T.; Mu, P.; Xu, N.; Song, Y.; Wang, X.; Jia, Q.; Qiu, J. Multi-residue determination of 210 drugs in pork by ultra-high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2016, 1463, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Mund, M.D.; Khan, U.H.; Tahir, U.; Mustafa, B.-E.; Fayyaz, A. Antimicrobial drug residues in poultry products and implications on public health: A review. Int. J. Food Prop. 2017, 20, 1433–1446. [Google Scholar] [CrossRef]
- Nsibande, S.A.; Forbes, P.B.C. Fluorescence detection of pesticides using quantum dot materials—A review. Anal. Chim. Acta 2016, 945, 9–22. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides: An update of human exposure and toxicity. Arch. Toxicol. 2017, 91, 549–599. [Google Scholar] [CrossRef]
- Baynes, R.E.; Dedonder, K.; Kissell, L.; Mzyk, D.; Marmulak, T.; Smith, G.; Tell, L.; Gehring, R.; Davis, J.; Riviere, J.E. Health concerns and management of select veterinary drug residues. Food Chem. Toxicol. 2016, 88, 112–122. [Google Scholar] [CrossRef]
- Alcantara-Duran, J.; Moreno-Gonzalez, D.; Gilbert-Lopez, B.; Molina-Diaz, A.; Garcia-Reyes, J.F. Matrix-effect free multi-residue analysis of veterinary drugs in food samples of animal origin by nanoflow liquid chromatography high resolution mass spectrometry. Food Chem. 2018, 245, 29–38. [Google Scholar] [CrossRef]
- Baghani, A.; Mesdaghinia, A.; Rafieiyan, M.; Soltan Dallal, M.M.; Douraghi, M. Tetracycline and ciprofloxacin multiresidues in beef and chicken meat samples using indirect competitive ELISA. J. Immunoass. Immunochem. 2019, 40, 328–342. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, D.-W.; Pu, H.; Wei, Q. Surface enhanced Raman spectroscopy (SERS): A novel reliable technique for rapid detection of common harmful chemical residues. Trends Food Sci. Technol. 2018, 75, 10–22. [Google Scholar] [CrossRef]
- Liu, M.; Khan, A.; Wang, Z.; Liu, Y.; Yang, G.; Deng, Y.; He, N. Aptasensors for pesticide detection. Biosens. Bioelectron. 2019, 130, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Perez-Fernandez, B.; Mercader, J.V.; Abad-Fuentes, A.; Checa-Orrego, B.I.; Costa-Garcia, A.; de la Escosura-Muniz, A. Direct competitive immunosensor for Imidacloprid pesticide detection on gold nanoparticle-modified electrodes. Talanta 2020, 209, 120465. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-F.; Sun, Y.-M.; Beier, R.C.; Lei, H.-T.; Gee, S.; Hammock, B.D.; Wang, H.; Wang, Z.; Sun, X.; Shen, Y.-D.; et al. Immunochemical techniques for multianalyte analysis of chemical residues in food and the environment: A review. Trac. Trends Anal. Chem. 2017, 88, 25–40. [Google Scholar] [CrossRef]
- Jayan, H.; Pu, H.; Sun, D.-W. Recent development in rapid detection techniques for microorganism activities in food matrices using bio-recognition: A review. Trends Food Sci. Technol. 2020, 95, 233–246. [Google Scholar] [CrossRef]
- Acaroz, U.; Dietrich, R.; Knauer, M.; Maertlbauer, E. Development of a generic enzyme-immunoassay for the detection of fluoro(quinolone)-residues in foodstuffs based on a highly sensitive monoclonal antibody. Food Anal. Methods 2020, 13, 780–792. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Fang, G. Applications and recent developments of multi-analyte simultaneous analysis by enzyme-linked immunosorbent assays. J. Immunol. Methods 2011, 368, 1–23. [Google Scholar] [CrossRef]
- Piao, Y.Z.; Kim, Y.J.; Kim, Y.A.; Lee, H.-S.; Hammock, B.D.; Lee, Y.T. Development of ELISAs for the class-specific determination of organophosphorus pesticides. J. Agric. Food Chem. 2009, 57, 10004–10013. [Google Scholar] [CrossRef]
- Hua, X.; Liu, X.; Shi, H.; Wang, Y.; Kim, H.J.; Gee, S.J.; Wang, M.; Liu, F.; Hammock, B.D. Development of a heterologous enzyme-linked immunosorbent assay for organophosphorus pesticides with phage-borne peptide. Rsc Adv. 2014, 4, 42445–42453. [Google Scholar] [CrossRef]
- Johnson, J.C.; Van Emon, J.M.; Pullman, D.R.; Keeper, K.R. Development and evaluation of antisera for detection of the O,O-Diethyl phosphorothionate and phosphorothionothiolate organophosphorus pesticides by immunoassay. J. Agric. Food Chem. 1998, 46, 3116–3123. [Google Scholar] [CrossRef]
- Zhao, F.; Hu, C.; Wang, H.; Zhao, L.; Yang, Z. Development of a MAb-based immunoassay for the simultaneous determination of O,O-diethyl and O,O-dimethyl organophosphorus pesticides in vegetable and fruit samples pretreated with QuEChERS. Anal. Bioanal. Chem. 2015, 407, 8959–8970. [Google Scholar] [CrossRef]
- Li, C.; Luo, X.; Li, Y.; Yang, H.; Liang, X.; Wen, K.; Cao, Y.; Li, C.; Wang, W.; Shi, W.; et al. A class-selective immunoassay for sulfonamides residue detection in milk using a superior polyclonal antibody with broad specificity and highly uniform affinity. Molecules 2019, 24, 443. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, B.; Liu, G.; Zhang, Y.; Wang, J.; Wang, S. Substructure-activity relationship studies on antibody recognition for phenylurea compounds using competitive immunoassay and computational chemistry. Sci. Rep. 2018, 8, 3131. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, W.; Shen, X.; Li, X.; Huang, X.-A.; Xu, Z.; Sun, Y.; Chan, S.-W.; Zeng, L.; Eremin, S.A.; et al. Four hapten spacer sites modulating class specificity: Nondirectional multianalyte immunoassay for 31 β-agonists and analogues. Anal. Chem. 2018, 90, 2716–2724. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-L.; Shen, Y.-D.; Zheng, W.-X.; Beier, R.C.; Xie, G.-M.; Dong, J.-X.; Yang, J.-Y.; Wang, H.; Lei, H.-T.; She, Z.-G.; et al. Broad-specificity immunoassay for O,O-diethyl organophosphorus pesticides: Application of molecular modeling to improve assay sensitivity and study antibody recognition. Anal. Chem. 2010, 82, 9314–9321. [Google Scholar] [CrossRef] [PubMed]
- Narenderan, S.T.; Meyyanathan, S.N.; Babu, B. Review of pesticide residue analysis in fruits and vegetables. Pre-treatment, extraction and detection techniques. Food Res. Int. 2020, 133, 109141. [Google Scholar] [CrossRef] [PubMed]
- Aly, N.; Mosallam, E.; Ahmed, N.; El-Gendy, K. Development and validation of a novel enzyme-linked immunosorbent assay for monitoring ethylene thiourea in soil and vegetable samples. Food Chem. 2020, 325, 126931. [Google Scholar] [CrossRef] [PubMed]
- El Alami El Hassani, N.; Baraket, A.; Boudjaoui, S.; Neto, E.T.T.; Bausells, J.; El Bari, N.; Bouchikhi, B.; Elaissari, A.; Errachid, A.; Zine, N. Development and application of a novel electrochemical immunosensor for tetracycline screening in honey using a fully integrated electrochemical BioMEMS. Biosens. Bioelectron. 2019, 130, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, S.; Zhang, X.; Li, C.; Dong, B.; Mujtaba, M.G.; Wei, Y.; Liang, X.; Yu, X.; Wen, K.; et al. Generic hapten synthesis, broad-specificity monoclonal antibodies preparation, and ultrasensitive ELISA for five antibacterial synergists in chicken and milk. J. Agric. Food Chem. 2018, 66, 11170–11179. [Google Scholar] [CrossRef]
- Liang, X.; Li, J.; Zhong, L.; Liu, B.; Wang, N.; Wang, Z.; Zou, M.; Zhang, Q. Highly broad-specific and sensitive direct competitive enzyme-linked immunosorbent assay for screening multi-antibacterial synergists: Assay optimization and application to animal-derived food. Food Agric. Immunol. 2020, 31, 150–164. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, J.; Zhang, C.; Huang, X.-a.; Sun, Y.; Xu, Z.; Lei, H. Broad-specificity chemiluminescence enzyme immunoassay for (fluoro)quinolones: Hapten design and molecular modeling study of antibody recognition. Anal. Chem. 2016, 88, 3909–3916. [Google Scholar] [CrossRef]
- Zhao, G.M.; Hou, P.L.; Huan, Y.J.; He, C.Q.; Wang, H.M.; He, H.B. Development of a recombinase polymerase amplification combined with a lateral flow dipstick assay for rapid detection of the Mycoplasma bovis. BMC Vet. Res. 2018, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.M.; Wang, H.M.; Hou, P.L.; He, C.Q.; He, H.B. Rapid visual detection of Mycobacterium avium subsp paratuberculosis by recombinase polymerase amplification combined with a lateral flow dipstick. J. Vet. Sci. 2018, 19, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, L.; Xu, L.; Song, S.; Kuang, H.; Cui, G.; Xu, C. Gold immunochromatographic sensor for the rapid detection of twenty-six sulfonamides in foods. Nano Res. 2017, 10, 2833–2844. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; Wang, Z.; Luo, P.; Xu, L.; Zheng, Q.; Kuang, H. A sensitive lateral flow immunoassay for the multiple residues of five adamantanes. Food Agric. Immunol. 2019, 30, 647–661. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, W.; Wang, P.; Su, X. A paper-based competitive lateral flow immunoassay for multi β-agonist residues by using a single monoclonal antibody labelled with red fluorescent nanoparticles. Microchim. Acta 2018, 185, 191. [Google Scholar] [CrossRef]
- Samdal, I.A.; Ballot, A.; Løvberg, K.E.; Miles, C.O. Multihapten approach leading to a sensitive ELISA with broad cross-reactivity to microcystins and nodularin. Environ. Sci. Technol. 2014, 48, 8035–8043. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, C.; Liu, Y.; Zhang, X.; Xie, Y.; Zhong, J.; Xu, C.; Liu, X. Simultaneous production of monoclonal antibodies against Bacillus thuringiensis (Bt) Cry1 toxins using a mixture immunization. Anal. Biochem. 2017, 531, 60–66. [Google Scholar] [CrossRef]
- Shi, H.; Li, H.; Hua, X.; Zheng, Z.; Zhu, G.; Wang, M. Characterization of multihapten antigens on antibody sensitivity and specificity for parathion. Anal. Lett. 2014, 47, 2699–2707. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, F.; Zhao, L.; Yang, Z. Development of a broad-specificity immunoassay for determination of organophosphorus pesticides using dual-generic hapten antigens. Food Anal. Methods 2015, 8, 420–427. [Google Scholar] [CrossRef]
- Zikos, C.; Evangelou, A.; Karachaliou, C.-E.; Gourma, G.; Blouchos, P.; Moschopoulou, G.; Yialouris, C.; Griffiths, J.; Johnson, G.; Petrou, P.; et al. Commercially available chemicals as immunizing haptens for the development of a polyclonal antibody recognizing carbendazim and other benzimidazole-type fungicides. Chemosphere 2015, 119, S16–S20. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, Z.; Tan, Y.; Lu, L.; Wang, L.; Liao, Y.; Beloglazova, N.; De Saeger, S.; Zheng, X.; Wu, A. Simultaneous raising of rabbit monoclonal antibodies to fluoroquinolones with diverse recognition functionalities via single mixture immunization. Anal. Chem. 2016, 88, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Yaneva, M.Y.; Ivanov, Y.L.; Godjevargova, T.I. Preparation of polyclonal antibodies with application for an organophosphorus pesticide immunoassay. Anal. Lett. 2017, 50, 1307–1324. [Google Scholar] [CrossRef]
- Peng, J.; Liu, L.; Xu, L.; Song, S.; Kuang, H.; Cui, G.; Xu, C. Gold nanoparticle-based paper sensor for ultrasensitive and multiple detection of 32 (fluoro)quinolones by one monoclonal antibody. Nano Res. 2017, 10, 108–120. [Google Scholar] [CrossRef]
- Wang, S.T.; Gui, W.J.; Guo, Y.R.; Zhu, G.N. Preparation of a multi-hapten antigen and broad specificity polyclonal antibodies for a multiple pesticide immunoassay. Anal. Chim. Acta 2007, 587, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Manikwar, P.; Mulagapati, S.H.R.; Kasturirangan, S.; Moez, K.; Rainey, G.J.; Lobo, B. Characterization of a novel bispecific antibody with improved conformational and chemical stability. J. Pharm. Sci. 2020, 109, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.Y.; Guo, Y.R.; Wang, C.M.; Wu, J.X.; Zhu, G.N. Development of a bispecific monoclonal antibody to pesticide carbofuran and triazophos using hybrid hybridomas. J. Food Sci. 2009, 74, T1–T6. [Google Scholar] [CrossRef]
- Ouyang, H.; Wang, L.; Yang, S.; Wang, W.; Wang, L.; Liu, F.; Fu, Z. Chemiluminescence reaction kinetics-resolved multianalyte immunoassay strategy using a bispecific monoclonal antibody as the unique recognition reagent. Anal. Chem. 2015, 87, 2952–2958. [Google Scholar] [CrossRef]
- Hua, X.; Wang, L.; Li, G.; Fang, Q.; Wang, M.; Liu, F. Multi-analyte enzyme-linked immunosorbent assay for organophosphorus pesticides and neonicotinoid insecticides using a bispecific monoclonal antibody. Anal. Methods 2013, 5, 1556–1563. [Google Scholar] [CrossRef]
- Chen, M.; Wen, K.; Tao, X.; Xie, J.; Wang, L.; Li, Y.; Ding, S.; Jiang, H. Cloning, expression, purification and characterization of a bispecific single-chain diabody against fluoroquinolones and sulfonamides in Escherichia coli. Protein Expr. Purif. 2014, 100, 19–25. [Google Scholar] [CrossRef]
- Kim, H.-J.; González-Techera, A.; González-Sapienza, G.G.; Ahn, K.C.; Gee, S.J.; Hammock, B.D. Phage-borne peptidomimetics accelerate the development of polyclonal antibody-based heterologous immunoassays for the detection of pesticide metabolites. Environ. Sci. Technol. 2008, 42, 2047–2053. [Google Scholar] [CrossRef]
- Xu, Z.-L.; Dong, J.-X.; Wang, H.; Li, Z.-F.; Beier, R.C.; Jiang, Y.-M.; Lei, H.-T.; Shen, Y.-D.; Yang, J.-Y.; Sun, Y.-M. Production and characterization of a single-chain variable fragment linked alkaline phosphatase fusion protein for detection of O,O-diethyl organophosphorus pesticides in a one-step enzyme-linked immunosorbent assay. J. Agric. Food Chem. 2012, 60, 5076–5083. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, H.; Shen, Y.-D.; Li, Y.-J.; Dong, J.-X.; Xu, Z.-L.; Yang, J.-Y.; Sun, Y.-M.; Xiao, Z.-L. Bispecific monoclonal antibody-based multianalyte ELISA for furaltadone metabolite, malachite green, and leucomalachite green in aquatic products. J. Agric. Food Chem. 2016, 64, 8054–8061. [Google Scholar] [CrossRef]
- Moldenhauer, G. Bispecific antibodies from hybrid hybridoma. In Bispecific Antibodies; Kontermann, R.E., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 294–296. [Google Scholar]
- Han, S.; Zhou, T.; Yin, B.; He, P. A sensitive and semi-quantitative method for determination of multi-drug residues in animal body fluids using multiplex dipstick immunoassay. Anal. Chim. Acta 2016, 927, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Q.; Han, M.; Liu, J.; Zhao, P.; He, L.; Zhang, Y.; Niu, Y.; Yang, W.; Zhang, L. Near-infrared fluorescence-based multiplex lateral flow immunoassay for the simultaneous detection of four antibiotic residue families in milk. Biosens. Bioelectron. 2016, 79, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Huang, J.; Sun, Y.; Deng, R.; Teng, M.; Li, Q.; Yang, Y.; Hu, X.; Zhang, Z.; Zhang, G. A SERS-based multiple immuno-nanoprobe for ultrasensitive detection of neomycin and quinolone antibiotics via a lateral flow assay. Microchim. Acta 2018, 185, 84. [Google Scholar] [CrossRef]
- Lan, M.; Guo, Y.; Zhao, Y.; Liu, Y.; Gui, W.; Zhu, G. Multi-residue detection of pesticides using a sensitive immunochip assay based on nanogold enhancement. Anal. Chim. Acta 2016, 938, 146–155. [Google Scholar] [CrossRef]
- Galvidis, I.A.; Wang, Z.; Nuriev, R.I.; Burkin, M.A. Broadening the detection spectrum of small analytes using a two-antibody-designed hybrid immunoassay. Anal. Chem. 2018, 90, 4901–4908. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, X.; Wang, Z.; Jiang, H.; Lv, Z.; Shen, J.; Xia, G.; Wen, K. Universal simultaneous multiplex ELISA of small molecules in milk based on dual luciferases. Anal. Chim. Acta 2018, 1001, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Yu, M.; Wang, Y.; Hu, W.; Cheng, D.; Swihart, M.T.; Song, Y. Multi-color quantum dot-based fluorescence immunoassay array for simultaneous visual detection of multiple antibiotic residues in milk. Biosens. Bioelectron. 2015, 72, 320–325. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Z.; Jin, M.; Du, P.; Chen, G.; Cui, X.; Zhang, Y.; Qin, G.; Yan, F.; Abd El-Aty, A.M.; et al. Fluorescence immunoassay for multiplex detection of organophosphate pesticides in agro-products based on signal amplification of gold nanoparticles and oligonucleotides. Food Chem. 2020, 326, 126813. [Google Scholar] [CrossRef]
- Capoferri, D.; Della Pelle, F.; Del Carlo, M.; Compagnone, D. Affinity sensing strategies for the detection of pesticides in food. Foods 2018, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Alkhamis, O.; Canoura, J.; Yu, H.; Liu, Y.; Xiao, Y. Innovative engineering and sensing strategies for aptamer-based small-molecule detection. Trac. Trends Anal. Chem. 2019, 121, 115699. [Google Scholar] [CrossRef]
- Jia, M.; Sha, J.; Li, Z.; Wang, W.; Zhang, H. High affinity truncated aptamers for ultra-sensitive colorimetric detection of bisphenol A with label-free aptasensor. Food Chem. 2020, 317, 126459. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, X.; Zhang, Q.; Zhang, C.; Liu, Y.; Tu, K.; Tu, J. Selection of DNA aptamers that bind to four organophosphorus pesticides. Biotechnol. Lett. 2012, 34, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Diao, D.; Lu, Z.; Li, X.; Guo, Q.; Huo, Y.; Xu, Q.; Li, Y.; Cao, S.; Wang, J.; et al. Selection of group-specific phthalic acid esters binding DNA aptamers via rationally designed target immobilization and applications for ultrasensitive and highly selective detection of phthalic acid esters. Anal. Chem 2017, 89, 5270–5277. [Google Scholar] [CrossRef]
- Nikolaus, N.; Strehlitz, B. DNA-aptamers binding aminoglycoside antibiotics. Sensors 2014, 14, 3737–3755. [Google Scholar] [CrossRef]
- Alam, K.K.; Chang, J.L.; Lange, M.J.; Nguyen, P.D.M.; Sawyer, A.W.; Burke, D.H. Poly-target selection identifies broad-spectrum RNA aptamers. Mol. Nucleic Acids 2018, 13, 605–619. [Google Scholar] [CrossRef]
- Niazi, J.H.; Lee, S.J.; Gu, M.B. Single-stranded DNA aptamers specific for antibiotics tetracyclines. Bioorg. Med. Chem. 2008, 16, 7245–7253. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Nguyen, V.-T.; Park, J.G.; Gu, M.B. Detection of iprobenfos and edifenphos using a new multi-aptasensor. Anal. Chim. Acta 2015, 868, 60–66. [Google Scholar] [CrossRef]
- Liu, D.L.; Li, Y.; Sun, R.; Xu, J.Y.; Chen, Y.; Sun, C.Y. Colorimetric detection of organophosphorus pesticides based on the broad-spectrum aptamer. J. Nanosci. Nanotechnol. 2020, 20, 2114–2121. [Google Scholar] [CrossRef]
- Bai, W.; Zhu, C.; Liu, J.; Yan, M.; Yang, S.; Chen, A. Gold nanoparticle-based colorimetric aptasensor for rapid detection of six organophosphorous pesticides. Environ. Toxicol. Chem. 2015, 34, 224–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fang, X.; Wu, J.; Hu, Z.; Jiang, Y.; Qi, H.; Zheng, L.; Xuan, X. An interdigitated microelectrode based aptasensor for real-time and ultratrace detection of four organophosphorus pesticides. Biosens Bioelectron 2020, 150, 111879. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Labuza, T.P.; He, L. Development of a single aptamer-based surface enhanced Raman scattering method for rapid detection of multiple pesticides. Analyst 2014, 139, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Ahmad Raston, N.H.; Gu, M.B. An ultra-sensitive colorimetric detection of tetracyclines using the shortest aptamer with highly enhanced affinity. Chem. Commun. 2014, 50, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zou, H.; Sun, C.; Ren, D.; Xiong, W.; Li, Y. Fluorescent aptasensor for detection of four tetracycline veterinary drugs in milk based on catalytic hairpin assembly reaction and displacement of G-quadruplex. Anal. Bioanal. Chem. 2018, 410, 2981–2989. [Google Scholar] [CrossRef]
- Ramezani, M.; Mohammad Danesh, N.; Lavaee, P.; Abnous, K.; Mohammad Taghdisi, S. A novel colorimetric triple-helix molecular switch aptasensor for ultrasensitive detection of tetracycline. Biosens. Bioelectron. 2015, 70, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lu, C.; Tang, Z.; Chen, X.; Wang, G.; Sun, F. Aptamer-functionalized magnetic nanoparticles for simultaneous fluorometric determination of oxytetracycline and kanamycin. Microchim. Acta 2015, 182, 2567–2575. [Google Scholar] [CrossRef]
- Youn, H.; Lee, K.; Her, J.; Jeon, J.; Mok, J.; So, J.; Shin, S.; Ban, C. Aptasensor for multiplex detection of antibiotics based on FRET strategy combined with aptamer/graphene oxide complex. Sci. Rep. 2019, 9, 7659. [Google Scholar] [CrossRef]
- Li, F.; Guo, Y.; Wang, X.; Sun, X. Multiplexed aptasensor based on metal ions labels for simultaneous detection of multiple antibiotic residues in milk. Biosens. Bioelectron. 2018, 115, 7–13. [Google Scholar] [CrossRef]
- He, L.; Shen, Z.; Wang, J.; Zeng, J.; Wang, W.; Wu, H.; Wang, Q.; Gan, N. Simultaneously responsive microfluidic chip aptasensor for determination of kanamycin, aflatoxin M1, and 17β-estradiol based on magnetic tripartite DNA assembly nanostructure probes. Microchim. Acta 2020, 187, 176. [Google Scholar] [CrossRef]
- Cheng, N.; Song, Y.; Fu, Q.; Du, D.; Luo, Y.; Wang, Y.; Xu, W.; Lin, Y. Aptasensor based on fluorophore-quencher nano-pair and smartphone spectrum reader for on-site quantification of multi-pesticides. Biosens. Bioelectron. 2018, 117, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, F.; Zhu, Y.; Xie, S.; Chen, M.; Xiong, Y.; Liu, Q.; Yang, H.; Chen, X. Intelligent platform for simultaneous detection of multiple aminoglycosides based on a ratiometric paper-based device with digital fluorescence detector readout. Acs Sens. 2019, 4, 3283–3290. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tang, W.; Wang, F.; Zhang, F.; Wang, Q.; He, P. Simultaneous detection of streptomycin and kanamycin based on an all-solid-state potentiometric aptasensor array with a dual-internal calibration system. Sens. Actuators B Chem. 2020, 311, 127857. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, W.; Guo, C.; Zhang, J.; Yu, L.; Zhang, G.; Wang, X.; Fang, G.; Sun, D. Magnetic molecularly imprinted electrochemical sensors: A review. Anal. Chim. Acta 2020, 1106, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A. Assessment of pesticides in environmental samples using voltammetric molecular imprinted based sensors: A review (2006–2015). Eur. Chem. Bull. 2016, 5, 69–76. [Google Scholar]
- Boulanouar, S.; Mezzache, S.; Combès, A.; Pichon, V. Molecularly imprinted polymers for the determination of organophosphorus pesticides in complex samples. Talanta 2018, 176, 465–478. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Electrochemical sensors based on magnetic molecularly imprinted polymers: A review. Anal. Chim. Acta 2017, 960, 1–17. [Google Scholar] [CrossRef]
- Farooq, S.; Nie, J.; Cheng, Y.; Yan, Z.; Li, J.; Bacha, S.A.S.; Mushtaq, A.; Zhang, H. Molecularly imprinted polymers’ application in pesticide residue detection. Analyst 2018, 143, 3971–3989. [Google Scholar] [CrossRef]
- Shi, C.; Liu, X.; Song, L.; Qiao, X.; Xu, Z. Biomimetic enzyme-linked immunosorbent assay using a hydrophilic molecularly imprinted membrane for recognition and fast determination of trichlorfon and acephate residues in vegetables. Food Anal. Methods 2015, 8, 2496–2503. [Google Scholar] [CrossRef]
- Tang, Q.; Shi, X.; Hou, X.; Zhou, J.; Xu, Z. Development of molecularly imprinted electrochemical sensors based on Fe3O4@MWNT-COOH/CS nanocomposite layers for detecting traces of acephate and trichlorfon. Analyst 2014, 139, 6403–6413. [Google Scholar] [CrossRef]
- Shi, X.; Lu, J.; Yin, H.; Qiao, X.; Xu, Z. A biomimetic sensor with signal enhancement of ferriferrous oxide-reduced graphene oxide nanocomposites for ultratrace levels quantification of methamidophos or omethoate in vegetables. Food Anal. Methods 2016, 10, 910–920. [Google Scholar] [CrossRef]
- Saylan, Y.; Akgönüllü, S.; Çimen, D.; Derazshamshir, A.; Bereli, N.; Yılmaz, F.; Denizli, A. Development of surface plasmon resonance sensors based on molecularly imprinted nanofilms for sensitive and selective detection of pesticides. Sens. Actuators B Chem. 2017, 241, 446–454. [Google Scholar] [CrossRef]
- Xu, Y.L.; Li, F.Y.; Ndikuryayo, F.; Yang, W.C.; Wang, H.M. Cholinesterases and engineered mutants for the detection of organophosphorus pesticide residues. Sensors 2018, 18, 4281. [Google Scholar] [CrossRef] [PubMed]
- Songa, E.A.; Okonkwo, J.O. Recent approaches to improving selectivity and sensitivity of enzyme-based biosensors for organophosphorus pesticides: A review. Talanta 2016, 155, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.; Gu, Y.; Wu, S.; Feng, L.; Xie, F.; He, Y. Research on a rapid detection method of pesticide residues in milk by enzyme inhibition. E3s Web Conf. 2019, 79, 03013. [Google Scholar]
- Tsagkaris, A.S.; Uttl, L.; Pulkrabova, J.; Hajslova, J. Screening of carbamate and organophosphate pesticides in food matrices using an affordable and simple spectrophotometric acetylcholinesterase Assay. Appl. Sci. 2020, 10, 565. [Google Scholar] [CrossRef]
- Sun, Z.; Tian, L.; Guo, M.; Xu, X.; Li, Q.; Weng, H. A double-film screening card for rapid detection of organophosphate and carbamate pesticide residues by one step in vegetables and fruits. Food Control. 2017, 81, 23–29. [Google Scholar] [CrossRef]
- Lang, Q.; Han, L.; Hou, C.; Wang, F.; Liu, A. A sensitive acetylcholinesterase biosensor based on gold nanorods modified electrode for detection of organophosphate pesticide. Talanta 2016, 156, 34–41. [Google Scholar] [CrossRef]
- Dzudzevic Cancar, H.; Soylemez, S.; Akpinar, Y.; Kesik, M.; Göker, S.; Gunbas, G.; Volkan, M.; Toppare, L. A novel acetylcholinesterase biosensor: Core–shell magnetic nanoparticles incorporating a conjugated polymer for the detection of organophosphorus pesticides. Acs Appl. Mater. Interfaces 2016, 8, 8058–8067. [Google Scholar] [CrossRef]
- Istamboulie, G.; Cortina-Puig, M.; Marty, J.-L.; Noguer, T. The use of Artificial Neural Networks for the selective detection of two organophosphate insecticides: Chlorpyrifos and chlorfenvinfos. Talanta 2009, 79, 507–511. [Google Scholar] [CrossRef]
- Alonso, G.A.; Istamboulie, G.; Noguer, T.; Marty, J.-L.; Muñoz, R. Rapid determination of pesticide mixtures using disposable biosensors based on genetically modified enzymes and artificial neural networks. Sens. Actuators B Chem. 2012, 164, 22–28. [Google Scholar] [CrossRef]
- El-Mesery, H.S.; Mao, H.; Abomohra, A.E. Applications of non-destructive technologies for agricultural and food products quality inspection. Sensors 2019, 19, 846. [Google Scholar] [CrossRef] [PubMed]
- Abasi, S.; Minaei, S.; Jamshidi, B.; Fathi, D. Dedicated non-destructive devices for food quality measurement: A review. Trends Food Sci. Technol. 2018, 78, 197–205. [Google Scholar] [CrossRef]
- Jamshidi, B.; Mohajerani, E.; Jamshidi, J. Developing a Vis/NIR spectroscopic system for fast and non-destructive pesticide residue monitoring in agricultural product. Measurement 2016, 89, 1–6. [Google Scholar] [CrossRef]
- Jamshidi, B.; Mohajerani, E.; Jamshidi, J.; Minaei, S.; Sharifi, A. Non-destructive detection of pesticide residues in cucumber using visible/near-infrared spectroscopy. Food Addit. Contam. Part. A 2015, 32, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Sun, J.; Xin, Z.; Mao, H.; Wu, X.; Li, Q. Visualizing distribution of pesticide residues in mulberry leaves using NIR hyperspectral imaging. J. Food Process. Eng. 2017, 40, e12510. [Google Scholar] [CrossRef]
- Su, W.-H.; Sun, D.-W.; He, J.-G.; Zhang, L.-B. Variation analysis in spectral indices of volatile chlorpyrifos and non-volatile imidacloprid in jujube (Ziziphus jujuba Mill.) using near-infrared hyperspectral imaging (NIR-HSI) and gas chromatograph-mass spectrometry (GC–MS). Comput. Electron. Agric. 2017, 139, 41–55. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, X.; Mao, H.; Wu, X.; Zhang, X.; Li, Q. Discrimination of pesticide residues in lettuce based on chemical molecular structure coupled with wavelet transform and near infrared hyperspectra. J. Food Process. Eng. 2017, 40, e12509. [Google Scholar] [CrossRef]
- Fan, M.; Andrade, G.F.S.; Brolo, A.G. A review on recent advances in the applications of surface-enhanced Raman scattering in analytical chemistry. Anal. Chim. Acta 2020, 1097, 1–29. [Google Scholar] [CrossRef]
- Yaseen, T.; Pu, H.; Sun, D.-W. Functionalization techniques for improving SERS substrates and their applications in food safety evaluation: A review of recent research trends. Trends Food Sci. Technol. 2018, 72, 162–174. [Google Scholar] [CrossRef]
- Luo, W.; Chen, M.; Hao, N.; Huang, X.; Zhao, X.; Zhu, Y.; Yang, H.; Chen, X. In situ synthesis of gold nanoparticles on pseudo-paper films as flexible SERS substrate for sensitive detection of surface organic residues. Talanta 2019, 197, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Pang, S.; He, L. In situ SERS detection of multi-class insecticides on plant surfaces. Anal. Methods 2015, 7, 6325–6330. [Google Scholar] [CrossRef]
- Yaseen, T.; Pu, H.; Sun, D.-W. Rapid detection of multiple organophosphorus pesticides (triazophos and parathion-methyl) residues in peach by SERS based on core-shell bimetallic Au@Ag NPs. Food Addit. Contam. Part A 2019, 36, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shi, G.; Zhu, Y.; Wang, Y.; Ma, W. Au-Decorated Dragonfly Wing Bioscaffold Arrays as Flexible Surface-Enhanced Raman Scattering (SERS) substrate for simultaneous determination of pesticide residues. Nanomaterials 2018, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.; Kim, J.; Kim, D.; Ko, Y.; Yamauchi, Y.; You, J. Nanoporous cellulose paper-based SERS platform for multiplex detection of hazardous pesticides. Cellulose 2019, 26, 4935–4944. [Google Scholar] [CrossRef]
- Chen, J.; Huang, M.; Kong, L.; Lin, M. Jellylike flexible nanocellulose SERS substrate for rapid in-situ non-invasive pesticide detection in fruits/vegetables. Carbohydr. Polym. 2019, 205, 596–600. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; E, Z.; Zhai, F.; Bing, X. Rapid Multi-Residue Detection Methods for Pesticides and Veterinary Drugs. Molecules 2020, 25, 3590. https://doi.org/10.3390/molecules25163590
Jia M, E Z, Zhai F, Bing X. Rapid Multi-Residue Detection Methods for Pesticides and Veterinary Drugs. Molecules. 2020; 25(16):3590. https://doi.org/10.3390/molecules25163590
Chicago/Turabian StyleJia, Min, Zhongbo E, Fei Zhai, and Xin Bing. 2020. "Rapid Multi-Residue Detection Methods for Pesticides and Veterinary Drugs" Molecules 25, no. 16: 3590. https://doi.org/10.3390/molecules25163590
APA StyleJia, M., E, Z., Zhai, F., & Bing, X. (2020). Rapid Multi-Residue Detection Methods for Pesticides and Veterinary Drugs. Molecules, 25(16), 3590. https://doi.org/10.3390/molecules25163590





