Abstract
(1) Background: voltage-gated sodium channels (Navs) are integral membrane proteins that allow the sodium ion flux into the excitable cells and initiate the action potential. They comprise an α (Navα) subunit that forms the channel pore and are coupled to one or more auxiliary β (Navβ) subunits that modulate the gating to a variable extent. (2) Methods: after performing homology in silico modeling for all nine isoforms (Nav1.1α to Nav1.9α), the Navα and Navβ protein-protein interaction (PPI) was analyzed chemometrically based on the primary and secondary structures as well as topological or spatial mapping. (3) Results: our findings reveal a unique isoform-specific correspondence between certain segments of the extracellular loops of the Navα subunits. Precisely, loop S5 in domain I forms part of the PPI and assists Navβ1 or Navβ3 on all nine mammalian isoforms. The implied molecular movements resemble macroscopic springs, all of which explains published voltage sensor effects on sodium channel fast inactivation in gating. (4) Conclusions: currently, the specific functions exerted by the Navβ1 or Navβ3 subunits on the modulation of Navα gating remain unknown. Our work determined functional interaction in the extracellular domains on theoretical grounds and we propose a schematic model of the gating mechanism of fast channel sodium current inactivation by educated guessing.
1. Introduction
1.1. The Sodium Channels
Concerning the voltage-gated sodium channels (Navs), a plethora of genes, their reading frames, expression patterns and functions have been reported for various organisms ranging from prokaryotic to eukaryotic cells. We treat the isoforms of ion channels in vertebrates with their greater gene complexities [1,2].
The Nav complex generally consists of a central α (Navα) subunit with the channel pore that is encoded by SCN1A to SCN5A (Nav1.1α to Nav1.5α, respectively) and SCN8A to SCN11A (Nav1.6α to Nav1.9α, respectively). The nine isoforms of the Navα subunit are expressed in specific tissue patterns and exhibit differences in gating behavior that adapts them to different physiological functions [3,4,5,6].
The pharmacological classification of these subtypes diverges according to their sensitivity and resistance to tetrodotoxin (TTX); Nav1.1α to Nav1.4α, Nav1.6α and Nav1.7α are sensitive to blocking by low nanomolar concentrations of TTX (TTX-S) and Nav1.5α, Nav1.8α and Nav1.9α are resistant to concentrations >1 μM TTX (TTX-R) [7].
More than a thousand point mutations have been identified in human Navs, while some of them have been associated with neurological, cardiovascular, muscular, and psychiatric disorders, such as epilepsy, arrhythmia, muscular paralysis, pain syndrome, and a broad spectrum in autism disorder [8,9,10,11,12].
Navs are targets for a wide variety of natural toxins and clinical therapeutic drugs [13,14,15,16].
1.2. The Navα Subunit
The Navα subunit of vertebrates consists of a single polypeptide chain with an approximate molecular mass of 260 kDa. It embraces the ion selective component that folds into four homologous but not identical domains (DI to DIV), each domain contains six transmembrane helical segments (S1 to S6), which are assembled around the ion selective pore [17,18].
The transmembrane helical segments S1, S2, S3, and S4 comprise the four voltage sensing domains (VSDs) in DI to DIV. They are located at the outer edge at each corner of the Navα subunit (Figure 1). The S4 helix constitutes the voltage sensor of each VSD. It has evolved into an amphipathic domain with a positively charged face. In response to the changes in the electric field produced by the depolarization of the membrane, the S4 moves towards the extracellular zone initiating conformational changes, which in turn open the pore [19,20,21,22]. The three S4 of the DI, DII, and DIII show faster kinetics in response to depolarization and allow sodium cations to enter the cells. The S4 of the DIV responds more slowly. Its movement releases an intracellular connector called the IFM inactivation gate. It contains three lipophilic residues (isoleucine, phenylalanine, methionine), and connects the S6 DIII to S1 DIV [23,24,25]. As a result, the inactivation gate moves to occlude the pore and leads the channel into an inactive state. Therefore, the activation and inactivation of the channel are linked in a structural, mechanical, and functional way [26,27].
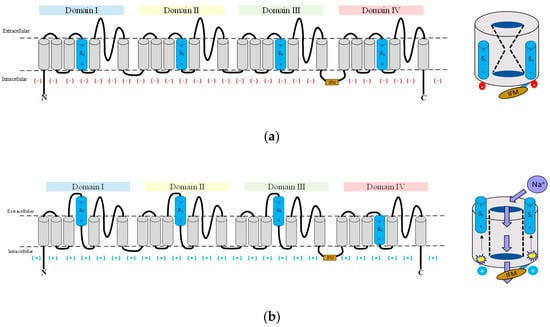
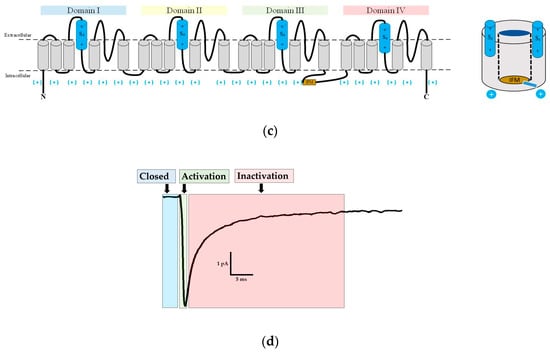
Figure 1.
Schematic representation of gating. The three schemes of eukaryotic Navs show the (a) closed; (b) open; and (c) inactivated gating states. (d) A typical membrane current of Rattus norvegicus of the Navα1.4 isoform responds to a depolarizing pulse reflecting the three main states of gating; IFM inactivation gate: brownish; S4 voltage sensors: sky blue.
1.3. The Navβ Subunits
Most Navs of vertebrate cells form biological units with associated β subunits (Navβs). There are four Navβ genes (SCN1B to SCN4B) that encode four proteins Navβ1 to Navβ4, respectively [28,29]. Like the Navα subunits, the Navβ subunits are individually expressed for tissue differentiation [30,31].
While the Navα subunit is sufficient for voltage detection and selective ion conductance, the Navβs subunits modulate peak values of sodium current and modify the kinetics of the activation and inactivation of the Navα subunit. They increase peak current density by augmentation of the channel density (number per area) on the cell surface. They effectively change the voltage range involved in activation and inactivation and improve inactivation and recovery rates of inactivation [5,28,32,33,34,35,36,37,38,39].
Prior to this chemometric study we carried out electrophysiological and site-directed mutagenesis experiments combined with molecular modelling to study modulation of Navα subunit by Navβ1 [40,41,42].
All Navβ subunits are type 1 membrane proteins. The extra-cellular amino-terminal region contains a single V-type amino-terminal immunoglobulin domain (IgD) and a short neck connected to a transmembrane helix (TMH) in addition to a carboxy-terminal intracellular region. The sequences similarities between Navβ1 and Navβ3 are higher than between Navβ2 and Navβ4 [43,44]. Navβ1 and Navβ3 are linked to Navα through non-covalent interactions, while Navβ2 and Navβ4 are linked by a disulfide bridge with Navα [45,46].
The cryo-electron microscope (cryo-EM) structures of Navs of insects, electric eel, rat, or human species reveal an identical three-dimensional (3D) architecture of their Navα subunits. Comparing the interface between Navα and Navβ (Navα/Navβ) of electric eel as well as Homo sapiens reveals that it is astonishingly well conserved [47,48,49,50,51,52].
The identification of the interaction sites for modulation of the Navα subunit with the Navβ subunits mainly stems from the mutagenic analysis and structural information of the individual Navβ subunits [36,40,41,42,46,49,53,54,55,56,57,58].
1.4. The Present Contributions
Currently, it remains an unanswered question how exactly the pore subunit (Navα) is modulated by the Navβ1 and Navβ3 subunits. Our present chemometric study aims at describing mechanistic behavior at the Navα/Navβ to shed light on the modulation by the nine isoforms (Nav1.1α to Nav1.9α) comparing three Mammalian species, namely Homo sapiens (hNav), Mus musculus (mNav) and Rattus norvegicus (rNav). To this end, well-established in silico methods were applied, like multiple sequence alignments, structural determinations, amino acid properties analysis, the generation of homology models as well as molecular electrostatic potentials, which can be color coded and projected on the molecular surfaces (MEPS).
To suit our chemometric analysis, the pore-bearing α subunit is dissected in the following topological parts. Herein after the entire pore subunit will be denominated as Navα or α for short. It is in turn composed of three topological segments: (i) the extracellular part or region (ECR) with its 16 extracellular loops (ECLs); (ii) the transmembrane helical part (TMH); and finally (iii) the intracellular region.
On theoretical ground, we determined (3D) structural features and sequence patterns as well as atom properties to describe the protein-protein interactions (PPI) between two pairs of proteins: not only Navα with Navβ1 subunits (Navα/Navβ1) but also Navα with Navβ3 subunits (Navα/Navβ3). The term PPI implies that both pairs were always treated in parallel for all three Mammalian species to provide a total and systematic view on chemometric patterns. While the (3D) structures of the former pair have been experimentally elucidated (by cryo-electron microscopy or crystallography), no (3D) structural information exists for the latter pair. This means, on the one hand we studied existing Navα/Navβ1 complexes, while on the other hand we directly applied our findings to create a hitherto unknown interface (symbol /) between Navα with Navβ3 (Navα/Navβ3). Herein after, the observed as well as the postulated interface will be denominated as IF, for short. The ectodomain of said IF contains the extracellular loops of the Navα subunit, which will be called ECLs in our study. All those (short) ECL segments in interaction with Navβ1 or Navβ3 subunits will be designated as IF-ECLs. All told, ECLs always belong to an α subunit, never to a β subunit because the latter does not possess loops, only antiparallel beta-strands which bend in turns and hair pins to form ordered beta-sheets (immunoglobulin domain, IgD, or all-beta fold). Finally, IF-ECLs embrace short amino acid segments, sometimes only a few individual residues, which we studied at either a sequential or even atomic scale.
2. Results
2.1. Determination of PPI in the Isoforms of the Navs
Our chemometric study produced detailed data, a fairly larger portion of which we present in the Supplementary Materials section. Precisely, the observed as well as the computed property patterns of interacting residues at the interface were described for eight PPI patches (thereupon called PPI-Id) by nine isoforms of three species for two pairs (Navα/Navβ1 and Navα/Navβ3), all of which yields 832 PPIs in 27 3D models (8 × 9 × 3 × 2) based on known 3D structures. When we take into account the four domains on each α subunit, the pore chain and the fact that each of those four domains (D1 to DIV) exposes four ECLs, then the sheer amount of 432 sets of calculations (16 loops × 9 isoforms × 3 species) were prepared, carried out, gathered, documented and interpreted. Table 1 provides a synopsis about the obtained results describing the interaction between Navα/Navβ1 or Navα/Navβ3. Of note, eight different computed polar interaction patterns were identified across all four domains (DI to DIV). They extend by far the extant literature for its systematic analysis and completeness (cf. 2.1.1.).

Table 1.
Synopsis of the studied PPI patterns. The interaction sites are labeled as PPI-Id with Arabic numerals from “1” to “8” to identify them. The resulting patterns are labeled with Roman numerals from “I” to “IX”. Certain PPI between Navα/Navβ1 and Navα/Navβ3 have been observed experimentally by structure elucidation. Their respective PDB entries and PPI-Id values are marked in bold face. They were used as templates for the reminder. The Y/N values in the table cells symbolize YES/NO referring to the presence / absence of contributions to PPI.
In Table 1 nine PPI patterns (Roman numerals) were detected as a result of the line-wise combination of Yes/No interaction features. Each line represents the eight identified potential contact zones in our study (PPI-Ids): (I) Id 6 has no PPI for hNav, mNav and rNav (1.1, 1.3 and 1.7); (II) Ids 1 to 8 have PPIs for hNav, mNav and rNav (1.2, 1.4 and 1.6); (III) Id 3 and 6 have no PPIs for hNav, mNav and rNav (1.5); (IV) Id 4 and 6 have no PPIs for hNav1.8α; (V) Id 3, 4 and 6 have no PPI for mNav1.8α; (VI) Id 2, 3, 4 and 6 have no PPI for rNav1.8α; (VII) Id 2, 4, 6, 7 and 8 have no PPI for hNav1.9α; (VIII) Id 3, 4, 6, 7 and 8 have no PPI for m, rNav1.9α; (IX) Id 1 has no PPI for eeNav1.4α.
2.1.1. PPI Analysis in the Structural Complex eeNav1.4α/eeNavβ1
Figure 2 presents the PPI for eeNav1.4α/eeNavβ1 (PDB: 5XSY [48]). Six interactions in three ECLs had already been published prior to our study: S1–S2 DIII, S5 DIV, and S6 DIV. However, our analysis of eel template unveiled a hitherto unpublished interaction site on ECL S5 DI (6 + 1 = 7 PPI-Ids).
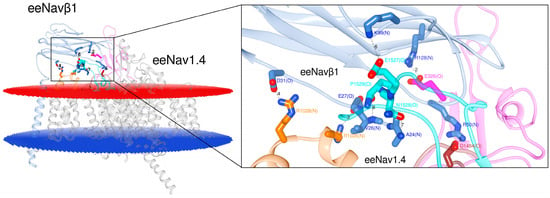
Figure 2.
Display of the 3D model for S6 DIV in eeNav1.4α/eeNavβ1 [48]. The box displays details about the interacting residues at the interface. Labels 2 to 8: PPI identification numbers (PPI-Ids) of computed polar interactions (Table S1, Supplementary Materials); the amino acids are labeled by one-letter-codes with their primary sequence residue numbers and interacting atoms, e.g. A24(N). Colors: extracellular membrane boundaries (dark red); intracellular membrane boundaries (navy blue); transmembrane and intracellular protein regions of Navα which do not participate in PPI (gray); Navβ1 subunit (cornflower blue); S5 DI: magenta; S1-S2 DIII: orange; S5 DIV: brown; S6 DIV: cyan; computed polar interactions: black dotted lines. Visualization achieved by Chimera Alpha 1.14.
2.1.2. PPI Analysis of the hNav1.4α/hNavβ1 and hNav1.4α/hNavβ3 Models
The 3D template complexes eeNav1.4α/eeNavβ1 [48] and hNav1.4α/hNavβ1 [49] possess a significant sequence identity (Navα ≈ 65% and Navβ ≈ 46%, resp.) in addition to a relatively high degree of conserved residues by homology. A measure of geometrical deformation is the so-called root-mean-square deviation (RMSD). The PDB entries 5XSY [48] versus 6AGF [49] were compared in terms of RMSD for both subunits: Navα ≈ 0.942, Navβ ≈ 0.955. A first inspection of both 3D templates by eyesight also revealed how well-conserved are all IF-ECLs with Navβ1 between both species.
After the detection of the seventh PPI site (cf. 2.1.1.) we inspected the 3D template hNav1.4α/hNavβ1 [49] and our homology model hNav1.4α/hNavβ3 [49,55] (Figure 3). At this stage we detected another PPI site on the ECL S5 DI. Hereupon we label the six published and the two detected PPI sites as follows: PPI-Ids 3 to 8 and PPI-Ids 1 and 2, respectively. Of note, PPI-Ids 3 to 8 were observed on 3D template eeNavα1.4/eeNavβ1 [48]. Albeit, side chain rotation by Chimera’s built-in rotamer library [59,60,61] had to be applied on the 3D template hNav1.4α/hNavβ1 [49]. Both detected PPI-Ids (1 and 2) lie on ECL S5 DI.
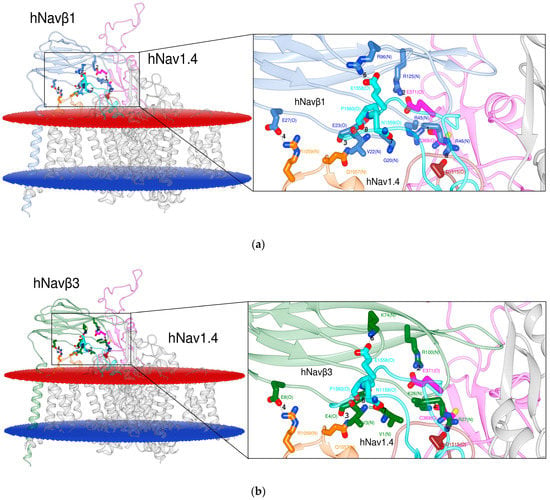
Figure 3.
Display of PPI models. Based on the 3D template in (a) hNav1.4α/hNavβ1 [49]; based on a homology model in (b) hNav1.4α/hNavβ3 [49,55] for S6 DIV. The box presents atomic details at the interface. Labels 1 to 8: Ids of computed polar interactions (Table S1, Supplementary Materials); the amino acids are labeled by one-letter-codes with their primary sequence residue numbers and interacting atoms in parentheses, e.g. bottommost D1515(O). Colors: extracellular membrane boundaries (dark red); intracellular membrane boundaries (navy blue); transmembrane and intracellular protein regions of Navα that do not participate in PPI (gray); Navβ1 subunit (cornflower blue); Navβ3 subunit (forest green); S5 DI: magenta; S1-S2 DIII: orange; S5 DIV: brown; S6 DIV: cyan); computed polar interactions: black dotted lines. Visualization achieved by Chimera Alpha 1.14 [61].
2.1.3. Identification of the Interacting Residues
Multiple sequence alignment (MSA) was performed by the Web-based Clustal Omega server [62] with the primary sequences of eeNav1.4α, hNav1.1α to hNav1.9α, mNav1.1α to mNav1.9α, rNav1.1α to rNav1.9α and all β subunits (eeNavβ1, hNavβ1 to hNavβ4, mNavβ1 to mNavβ4 and rNavβ1 to rNavβ4). We identified the conserved amino acids and replacements by homology since they constitute either structurally or functionally pivotal components for the channel (Table 2). As a most valuable asset, the hitherto known interacting residues of the 3D templates served as references to identify the interacting residues (Table 1 and Table S1). The 3D templates were as follows:eeNav1.4α/eeNavβ1, hNav1.4α/hNavβ1, and hNav1.4α/hNavβ3 (Figure 2 and Figure 3).

Table 2.
Multiple sequence alignments for either all nine α subunits or all four β subunits of three Mammalian organisms in addition to eel 3D template. MSA identified eight conserved or homologous residues on Navα and Navβ. Asterisks (*) in first column: 3D template structures from PDB. Capital letters in bold face: the eight residues. Lower case letters: amino acid neighbors of the eight residues for numberless identification. Colors: positively and negatively charged residues in blue and red, respectively; polar or non-polar residues in cyan or orange.
On Navα two unchanged residues were detected in sequence positions labeled as PPI-Ids 1 and 5 on ECLs S5 DI and S5 DIV, respectively. On the counter subunit β, both subunits contain partially conserved residues in positions PPI-Ids 2b, 3b, and 5b, but on PPI-Id 8b valine (V) remains unchanged. Intriguingly both βs also present two homologous residues by keeping their respective charges at positions 4 (negative) and 6 (positive) in all isoforms and species. In contrast, Table 2 also unravels that equivalent residues on Navβ2 or Navβ4 are not conserved when compared to locations on the three reference sequences of the eeNavβ1, hNavβ1 or hNavβ3 templates.
In the following we describe two PPI-Id cases to illustrate how Table 1, Table 2 and Table S1 are combined with Figure 2, Figure 3 and Figures S1–S9. Take the upper leftmost corner of Table 1. The value in the cell for PPI-Id 1 of Nav1.1α is “Y”, i.e. yes there is a PPI. This corresponds to cysteine (C) in Table S1 (first row entry of data crossed by 3rd col.: “agqCpeg(O)”. This corresponds to the string “agqCpEgym” which was generated by MSA in Table 2. Table S1 informs that C is in contact with arginine (R) of human Navβ1 (3rd col. under PPI-Id “1b” the value “R” in string “sckRrse(N)”). For this instance, the spatial configuration is depicted in atomic details (Figure S1). In this case the amide oxygen atom (>C=O) of cysteine forms a hydrogen bond with one nitrogen of β’s arginine. Table S1 informs about this PPI instance in a nongraphical way. The IF has two sides with PPI-Id 1 and PP-Id 1b. On the human pore subunit of isoform 1.1 (hNav1.1α) the string value “agqCpeg(O)” reports that the interacting residue is cysteine (C). It interacts through its backbone oxygen atom (O). On the other side the Table S1 holds the string value “sckRrse(N)” at position PPI-Id “1b” for Navβ1. This means that arginine is the counterpart. On an atomic scale nitrogen atom(s) of its monocationic guanidinium head group from its side chain can establish the hydrogen bonding with a strong polar attraction.
The Table 1 in its bottommost rightmost corner informs that no (value “N”) exists for PPI-Id 8 in case of rNav1.9α. Again, Table S1 lends insight not only on a molecular level but also at an atomic scale. The 1.9 isoform’s subunit α has a potential contribution by a cysteine at sequence position PPI-Id 8 (“kehCnss”), but the counter subunits (Navβ) present a nonresponding valine (V). This aliphatic residue is conserved on all four β subunits. As a direct result Table 1 summarizes this negative interaction with an “N” qualifier in its corresponding table cell along with all other “Yes” or “No” contributions for the systematic combinations of three Mammalian species, nine isoforms and eight potential PPI sites (labeled PPI-Ids 1 to 8). The two detailed illustrations underline the informative wealth of chemometric studies to complement limited experimental data. They do, however, also raise the molecular modeling practitioner’s challenges concerning management and presentation of data in huge volumes.
2.1.4. Structure Alignment of all Navβs Subunits
Figure 4 displays the results of the (3D) structure alignment (SA), i.e., the ectodomain (IgD) superpositions in space for the 3D templates, namely the cryo-EM structures of eeNavβ1, hNavβ1 in addition to the crystal structures of hNavβ2 to hNavβ4 (PDB: 5XSY [48], 6AGF [49], 5FEB [56], 4L1D [55], and 4MZ2 [54].
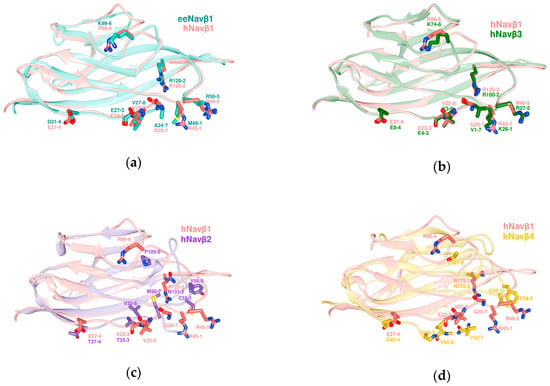
Figure 4.
Structure alignments of the ectodomains (IgD) of β subunit templates. (a) eeNavβ1 with hNavβ1; (b) hNavβ1 with hNavβ3; (c) hNavβ1 with hNavβ2; (d) hNavβ1 with hNavβ4; eeNavβ1: sea green; hNavβ1: salmon; hNavβ3: forest green; hNavβ2: purple; hNavβ4: golden; labels 1 to 8: positions of MSA residues according to Table 2. All superpositions were achieved by Chimera Alpha 1.14 with MatchMaker [61].
The SA of the subunits eeNavβ1 and hNavβ1 provides a visual means to identify the interacting residues for eeNav1.4α/eeNavβ1 [48] (Figure 2). As can be noticed, essential features remain in close spatial proximity while keeping their properties, except for one of the eight interaction sites: PPI-Id 1 (Figure 4a).
2.2. Pore Modulation by Navβ Concerning the Acceleration for Fast Gating Inactivation
Our hypothesis has been based on the displacement of S4 DIII towards the extracellular region during cell membrane depolarization, when it contacts Navβ1 or Navβ3 (Figure 5). It is not far-fetched to assume that if a voltage sensor gets close enough for noncovalent binding with IgD ectodomains, a spatial rearrangement will take place. Surface charges on Navα will come under the influence of positively charged lysines (K37 on Navβ1 or K18 on Navβ3, see our Table S2, cf. Figure 4c,d in [49,55]). Consequently, this displacement could trigger modulation of the pore subunit (Navα). Furthermore, we assume that this contact exert a domain rotation that affects the fast inactivation of Navα gating.
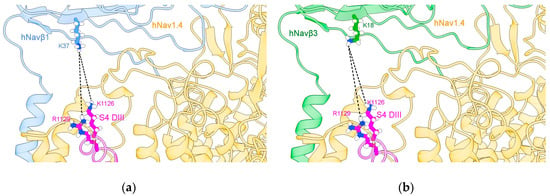
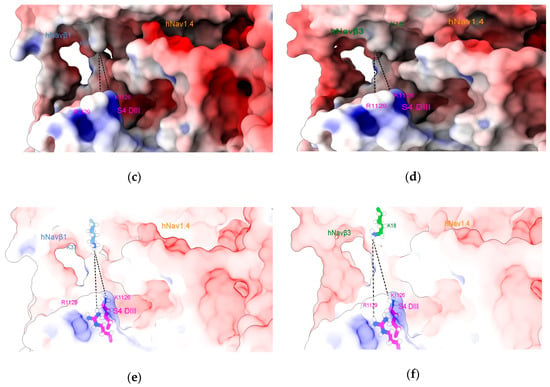
Figure 5.
S4 DIII voltage sensor of hNav1.4α [49] in close contact with hNavβ1 [49] or hNavβ3 [55]. (a) hNav1.4α interfaced with hNavβ1; (b) hNav1.4α interfaced with Navβ3; (c,e) the MEPS at the interface hNav1.4α/hNavβ1; (d,f) the MEPS at the interface between hNav1.4α and hNavβ3. The structures were prepared with Chimera add-on PDB2PQR [63] and MEPS calculated for PPI surfaces using the Adaptive Poisson-Boltzmann Solver (APBS) [64], a plug-in tool in Chimera Alpha 1.14 [61] and simulated under Chimera X [65]. S4 DIII voltage sensor: magenta; hNavβ1: cornflower blue; hNavβ3: green.
An electrostatic repulsion could be created with the Navβ1 or Navβ3 subunits. As a suggested mechanistic consequence, the IF-ECLs move and pull the ECR, which in turn transfers strain energy onto the Navα subunit. This happens precisely on S4 (DI and DIV) and could generate a conformational change in the channel that accelerates fast inactivation and finally closes the modulation cycle with S4 on DI and DIV returning to its starting positions.
Based on the present findings we propose a possible mechanism of α (pore) modulation by β subunits for the fast inactivation cycle (Figure 6). The acceleration of fast inactivation by the Navβ1 or Navβ3. In the PPI model, a rotamer library was applied to find favorable Van der Waals contacts [59,60,61] concerning Navα/Navβ1 and Navα/Navβ3 (Table S1).
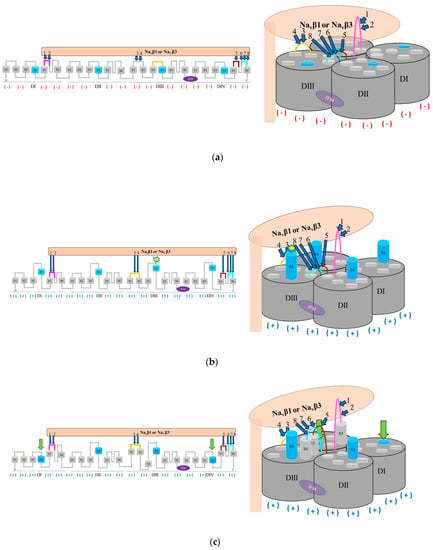
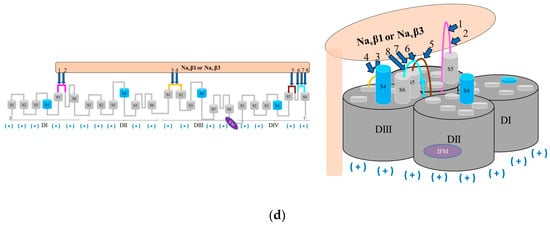
Figure 6.
Hypothetical modulation of fast inactivation of Navα gating by Navβ1 or Navβ3; (a) Navα in idle (closed) state in response to interacting Navβ1 or Navβ3; (b) Navα in open (activated) state in presence of Navβ1 or Navβ3 modulation; (c) fast inactivation modulated by Navβ1 or Navβ3; (d) fast inactivation triggered by the IFM inactivation gate; labels 1 to 8: Id of computed polar PPIs (Table S1); computed polar PPIs: navy blue arrows; return to its start position of S4 is forced by IF-ECLs by computed polar interactions with Navβ1 or Navβ3: green arrows; negative charges: red minus signs in parentheses; positive charges: navy blue plus signs in parentheses; Navα regions without PPI: dark and light gray; Navβ1 or Navβ3 subunit: light salmon; S5 DI: magenta; S1-S2 DIII: orange; S5 DIV: brown; S6 DIV: cyan; S4: sky blue, segments S1, S2, S3, S4, S5, and S6: light gray.
2.3. Determination of Relevant ECL Properties
We quantified all ECL sizes from the nine isoforms for the three species (Figure S10), the ECR properties and those for S5 and S6 ECLs (Figure S11), as well as the ECL properties on the α subunit (Figure S12) [66]. Each isoform and ECL presented inherent characteristics, which were discussed in more detail in Section 3.3.
Moreover, the volume and the solvent-accessible area (SAA)—surface area accessible to solvent—were calculated for each ECL of each isoform (Figures S13–S15) [61].
The polar surface area (PSA), nonpolar surface area (NPSA) and MEPS (negative potential: N-MEPS and positive potential: P-MEPS) were measured for the entire IF-ECL as a grand total and the individual values for each IF-ECL (Figures S16 and S17) [61,63,64].
The IF areas of the atoms were determined (Figure S18) besides the buried α and β IF area, i.e., the spatial intersection of IF-ECL with Navβ1 and Navβ3) (Table S3) [61]. To get rid of the loop length bias—longer loops tend to possess more chances of interacting residues than shorter loops—we decided to present normalized values, as a general rule: here we took the IF percentage of aforementioned grand total as the 100% basis. The following properties for IF-ECLs with both β subunits were computed: PSA, NPSA, P-MEPS and N-MEPS. In the next step, common treats (similarities) were detected and clustered into the following interaction patterns: S5 DI: (Nav1.1α and Nav1.3α), (Nav1.5α and Nav1.7α), (Nav1.2α, Nav1.4α and Nav1.6α), (Nav1.8α) and (Nav1.9α); S1-S2 DIII: (Nav1.1α and Nav1.4α), (Nav1.3α and Nav1.5α), (Nav1.2α and Nav1.6α), (Nav1.8α), and (Nav1.9α); S5 DIV: (Nav1.1α and Nav1.5α), (Nav1.2α, Nav1.3α and Nav1.6α), (Nav1.4α and Nav1.7α), (Nav1.8α), and (Nav1.9α). In the case of S6 DIV, however, no similarities have been found among the nine isoforms (Figures S19–S21). Supplementary Figure S23 illustrates the β1 subunit interface and its properties in all details.
2.4. PPI Patterns on Navs Isoforms
The underpinning of protein functions in most biological processes constitutes the plethora of atom-to-atom interactions between proteins and other biomolecules (cf. interactome). Predicting interactions on an atomic level remains one of the most challenging endeavors in structural biology [67,68,69].
During evolution protein structure is more conserved than its underlying primary sequence, i.e., sequences diverge from a common ancestor but maintain identical or similar functions with little changes due to homologous exchanges at their active sites [70,71,72]. Residues at the sensitive interfaces become significant for geometry or signaling and therefore tend to be conserved in the protein structure [73]. Another well-characterized property of interfaces refers to the existence of “hot spot” residues, which are the residues that make the largest contributions to complex formation [74]. In our study context, several reports have raised the question about what atomic components exactly protein interfaces are made of in order to improve prediction power for PPIs [75].
In vitro research to gain mechanistic insight into Navs at atomic scale has been a daunting task for decades due to its membrane-embedded location and multidomain complexity [6,76]. It is in such situations when chemometric approaches lend insight unraveling hitherto unnoticed atomic patterns.
Navβ subunits belong to the immunoglobulin (Ig) superfamily. Its overall structure is an all-beta (strands) fold, which is typical for cell adhesion molecules [77]. Variation in presence (or absence) of β subunits regulates α subunit expression to differentiate tissues. Furthermore, they modulate the pore unit kinetics [78,79] while the mechanism by which this phenomenon occurs has been extensively studied. However, understanding the gating mechanism of the channel and its modulation in details has just emerged in a bitwise manner [80]. Looking back into the channel’s history of research, in 1985 and 2000 respectively, Navβ1 and Navβ3 were first reported as cell (surface) adhesion proteins in interaction with the α pore unit through noncovalent bonds [81,82].
New aspects of subunit cooperativity came from A.P. Jackson’s laboratory with Namadurai et al. in 2014 [55] who have elucidated a trimeric crystal structure of Navβ3, i.e., three IgDs in a crystallographic unit cell. Notwithstanding, it has been an unsettled question if this trimer also reflects a biological unit? Recently, molecular dynamics studies indicated that spontaneous oligomerization of a full-length Navβ3 subunits to a trimer would probably be a very slow process if it occurred in cell membranes. The three TMH of Navβ3 would not interact strongly enough [83]. In addition, our team also analyzed whether the IgDs of the hNavβ1 subunits could form trimers [49]. We determined that in the three IgDs strong repulsion exists between the negative total charges of human Asp25 and Glu27 residues upon fitting them onto the spatial positions of each monomers of the Navβ3 trimeric structure (PDB: 4L1D [55]). So far, this finding has not been reported elsewhere. Both amino acids have not been exchanged during evaluation across Mammalian species, all of which hints at a pivotal PPI hot spot for Navα subunits (Figure 7, Table S1 and Figures S1–S9). Glass et al. (2020) [83] reasoned that if the hNavβ3 trimer were to interact with the VSDs of the pore-forming α protein in analogy to structurally known hNavβ1, a substantial rearrangement of the IgDs would be necessary.
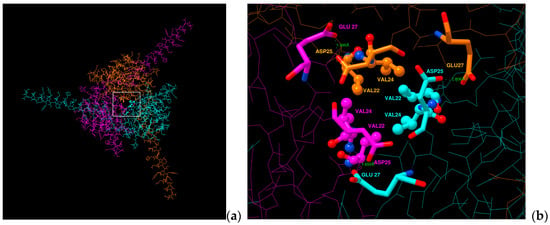

Figure 7.
Display of a trimeric hNavβ1 model. (a) Navβ1 trimer seen top-down (b) close-up view from above of the alleged hotspot (c) Aligned sequences from Mammalian species. Negatively charged residues are identical to Asp25 and Glu27 on Navβ1 from Homo sapiens; white box: analysis area; green dotted lines: computed repulsion of charges; sticks: negative charged residues; sticks and balls: residues that form a possible hydrophobic patch. Data generated by Chimera Alpha 1.14 [61].
Since the crystal structures of α and β1 in complex exist in addition to the trimeric β3 and monomeric β1 (hNavβ3 [55], hNavβ1 [49]) it was possible to carry out structural biology studies by superpositioning them onto each other (Figure 8). Frequently other terms than superpositioning are used: 3D, spatial or structural alignment (SA), in addition to fitting or matching (cf. Magic fit under SPDBV or MatchMaker under Chimera). Here, we used hNav1.4α/hNavβ1 of the Navβ3 trimer [55] and hNav1.4α/hNavβ1 [49] (Figure 8 and Figure 9).

Figure 8.
Superposition of monomeric and trimeric 3D models of β proteins. (a) Experimentally determined homotrimeric hNavβ3 [55] (three colors: magenta, light blue, beige) in superposition with template hNav1.4/hNavβ1 (bluish/grey) [49] and (b) one IgD (out of three) subunit(s) of homotrimeric hNavβ3 (beige) in superposition with template hNav1.4α/hNavβ1. It can be seen—by eyesight—that in case a two out of the three subunits bump into the membrane. Extracellular membrane boundaries: dark red; intracellular membrane boundaries: navy blue; Navα subunit: gray; hNavβ1 subunit: cornflower blue; hNavβ3 chains A, B, and C: orange, cyan, and magenta, respectively.
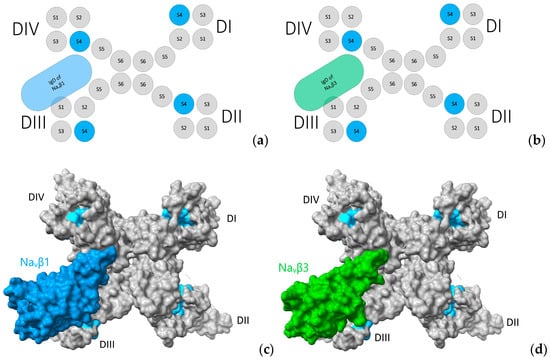
Figure 9.
Three-dimensional (3D) location of subunits Navβ1 and Navβ3. (a,b) Navα topology in complex with Navβ1 and Navβ3. Display of 3D models with solvent-excluded surface areas in panels (c,d). (c) Cryo-EM structure of hNavα1.4 in complex with Navβ1 [49] and (d) Cryo-EM structures of hNavα1.4 [49] in complex with crystal structure hNavβ3 [55] positioned according to structural analysis; S4: sky blue; in panels (c,d) the molecular surfaces are colored: Navβ1: cornflower blue; Navβ3: green forest; and grey color for Navα subunit surfaces. The same colors were applied to the panels (a,b) above. 3D models by Chimera X [65].
Compared to the sheer number of all-beta fold variations—AKA the Ig superfamily—the ectodomain IgD of hNavβ3 [55] is extremely similar to IgD of hNavβ1 [49]. Structural evidence concerning eeNav1.4α/eeNavβ1 [48], hNav1.4α/hNavβ1 [49], and hNav1.7α/hNavβ1 [51] was reported about a binding site in the TM region of Navβ1 between S1 and S2 helices on VSD DIII [55]. Noteworthy is the finding that the TM regions of the Navβ1 and Navβ3 subunits possess high sequence similarity. In vitro studies revealed that the TM region of Navβ3 non-covalently binds VSD DIII [80] in a way that Navβ1 does [48,49,51]. Albeit, there is no structural data to pinpoint the location of the Navβ3 binding site on α subunits.
Frequently, it has been assumed that the Navβ3 subunit interacts with the Navα subunit through the same mechanism as the Navβ1 subunit [84]. Nevertheless, in vitro studies demonstrated that both Navβ1 or Navβ3 attenuate lidocaine binding to Nav1.3α [85]. Said local anesthetic binds the S6 helix of domain IV by noncovalent bonds [86] and structural evidence affirms that Navβ1 forms IPP with Nav1.4α in the IF-ECL S6 DIV [48].
As working hypothesis, we proposed that the Navβ3 binding site on the α pore subunit is the same as that of Navβ1. The assumption was based on the structural data analyses of 3D aligned eeNav1.4α/eeNavβ1 with hNav1.4α/hNavβ1. The same conservation pattern is found again in template complex hNav1.4α/hNavβ3 in addition to all isoforms across the three species under scrutiny (Table S1 and Figures S1–S9).
In the analyzed (3D) template structures and our 3D models we found interaction patterns at the Navα/Navβ1 and Navα/Navβ3 interfaces. Upon inspection, it is safe to generalize our detailed findings that these interaction patterns significantly diverge between isoforms crossing species. In particular, we noted specific PPI patterns between Navα with either Navβ1 or Navβ3 subunits.
Applying Chimera’s combined two-dimensional (2D) with 3D alignment capacities for rational protein superposition (by MatchMaker), the templates of hNavβ1 [49] and hNavβ3 [55] were aligned as spatial references (Figure 4b). Thereupon, we identified all interacting residues between template hNav1.4α/hNavβ1 [49] and 3D model hNav1.4α/hNavβ3 [49,55] (Figure 3). The identification was assisted by 3D template eeNav1.4α/eeNavβ1 [48] as a most valuable reference to pinpoint conservation or homology for closely or far-distantly related organisms, here: three Mammalian species versus eel (Figure 2). As an asset for PPI validation, not only sequential but also structural similarities of hNavβ3 with hNavβ1 lie significantly above the twilight zone of homology with ≈ 50%, RMSD ≈ 1.2, respectively. Taken together all topology patterns, convincing evidence was unveiled by chemometrics, all of which indicate that hNavβ3 subunit binds and modulates Navα from the same position and via the same mechanism as hNavβ1 subunit, because the interacting residue pattern is almost the same (see our 3D model of hNav1.4α/Navβ3 and PDB entry 6AGF [49] with the hNav1.4α/Navβ1 in PDB format in SM). Sufficient(ly tiny) variations do exist, however, on both proteins. They could explain to some degree the differences in channel kinetics which has to be confirmed in future studies with more experimental research.
For the sake of inference power of our chemometric output data, we also studied the known structures of hNavβ2 [56] and hNavβ4 [54]. Again, 2D and 3D alignments were carried out with Chimera against reference structure hNavβ1 [49]. All critical data clearly lie in the boundaries of the twilight zone with ≈18.8 or 18.1%, RMSD: 4.52 or 7.12, respectively. With hNavβ1 [49], hNavβ2 [56] (Figure 4c) and hNavβ4 [54] (Figure 4d) aligned, the degree of positional mismatches of equivalent residues becomes obvious by eyesight, concerning β2 or β4 sequences vs known interacting residues of reference hNavβ1 [49]. The loss of conserved positions for the interacting residues of both subunits (β2, β4) strongly hints at the existence of a totally distinct PPI with the α pore subunit. This finding has not been reported in the literature.
At that stage we can characterize the more general topological behavior of all nine isoforms and lump them together in view of their distinct interaction patterns: (i) isoforms hNav, mNav and rNav (1.2, 1.4 and 1.6), present eight PPIs with Navβ1 or Navβ3 subunits; (ii) isoforms hNav, mNav and rNav (1.1, 1.3 and 1.7) present seven PPIs. Both share essential features, so we suggest they have a common effect on modulation. In (weak) contrast, isoforms hNav, mNav, and rNav (1.1, 1.3, 1.5, 1.7, 1.8, and 1.9) coincide in a non-interacting residue at position 6 in ECL S6 DIV. In stark contrast, isoforms hNav, mNav, and rNav (1.5, 1.8, and 1.9) always share two features: first, they all lack two or more PPIs and secondly, they all belong to binding type TTX-R, and they associate to an interaction reduction (PPI-Id in Table 1) on S1-S2 DIII ECL, while isoforms hNav, mNav, and rNav (1.9) do not at all interact through S6 DIV ECL. Wrapping up the findings into a mechanistic picture, gating of all those isoforms belonging to binding type TTX-R might also coincide in a common modulation mechanism. The absence of interaction in PPI-Id 6 in ECL S6 DIV concerns the following isoform: h, m, rNav (1.1, 1.3, 1.5, 1.7, 1.8, and 1.9). This interaction site (PPI-Id 6) exposes a strong salt bridge for isoforms h, m, rNav 1.2, 1.4, and 1.6. (Figures S1–S9). We infer that the presence or absence of that strong electrostatic signal at the IF could be a significant feature to trigger isoform-dependent variations in pore modulation.
3. Discussion
3.1. Hypothetical Acceleration of Fast Inactivation of Gating of the Navs
Cell membrane depolarization is associated with an upwards movement to expose the helical S4 voltage sensors into the cell membrane surface (Figure 6). S4 exposition to the surface implies a conformational change of the channel to enter an open state [19,20,21,22]. As a topological result, the three-amino acid inactivation gate (IFM) located between helices S6 DIII and S1 DIV swiftly connects to the pore and prevents sodium ions to enter (Na+ influx), all of which leads to an inactivated state. Thanks to the identification of interacting residues based on our systematic topological (sequential) and structural (spatial) models it is possible to link them to reported electrophysiological aspects about functional transition from activation to inactivation of all nine Nav isoforms (Figure 1) [26,27]. Of note, the variable N-linked glycosylation of the ectodomains (IgDs) does not affect our cheminformatic results because it does not belong to the interface between both subunits [84].
Zhu et al. (2017) [80] concluded from their in vitro studies that Navβ1 and Navβ3 accelerate the deactivation of S4 in Nav1.5, in addition to Navβ1 in S4 (DIII and DIV) or Navβ3 in S4 (DIII), respectively. In good keeping, Ferrera et al. (2006) [87] demonstrated that Navβ1 determines the electrical environment of the channel by changing the surface charges that electrostatically affected the activation of the channels.
In our modeled complexes Navα/Navβ1 and Navα/Navβ3 (Table S1 and Figures S1–S10), both β subunits are located in proximity to the S4 voltage sensor on DIII. In hypothetical terms, when the cell membrane is depolarized, S4 on DIII shifts in space and subsequently two conserved positively charged residues on S4 on DIII enter into repulsive contacts with a conserved lysine on both β subunits (Table S2). All three positive charges are clearly exposed on the solvent-accessible area on the protein surface (Figure 5). It is safe to conclude that a strong biochemical signal is triggered when this charge repulsion takes place at S4 DIII (Figure 8). As a direct consequence for the channel’s overall geometry, domain shift of IgDs (the ectodomains of Navβ1 and Navβ3) takes place to modulate fast inactivation mechanism. The segment S1–S2 on DIII serves as a flexible hinge to form a noncovalent association with β1 and β3 IgDs. In a slower response to depolarization, S5 on DI and S5 on DIV terminate the cycle by forcing S4 (DI and DIV) back into its initial position in a spring-like fashion. Finally, S6 on DIV arrests—like an anchor—both β subunits. On theoretical ground, the present findings explain the cooperativity between ECRs and certain Navβ subunits for channel pore modulation. From an evolutionary point-of-view, this makes sense, since calling-in external proteins (auxiliary βs) to assist the protein function (α pore) is achieved much faster than the adaption of loop segments by slow selection of random point mutations over time. Obviously, this happened at a time during cellular evolution when gene fusion already took place to transform the homotetrameric (bacterial) channel into a single chain pore protein, which is composed of four different domains (“monocadenar hetero-tetra-domain subunit”). In this context the existence of nine closely related mutants (isoforms) show the work of evolution from the near-past to present time when a common ancestral protein has been evolving along with differential gene expression and tissue specialization.
3.2. Properties of the ECL Residues for Navα Subunits
The ECL sequence lengths are shown in Figure S10. Intriguingly, S5 on DI constitutes the longest ECL. Its aberrant size reflects that it encompasses a second PPI; PPI-Id 1 is conserved in every isoform and species; PPI-Id 2 is conserved in almost all isoforms with the exception of Nav1.8α and Nav1.9α. The ECL length of S5 on DIV contains a negatively charged residue (Id 5) which is conserved throughout all nine isoforms for all three species. Possibly, the ECLs of S5 on DI and DIV have evolved steadily in contact with Navβ1 and Navβ3 subunits, leading to speculations about their role as main binding sites for Navα modulation.
The chemometric properties describing S5 and S6 are documented in Figure S11. In all three species Nav1.4α (resp. Nav1.5α isoform) accounts for the highest (respectively, lowest) amount of polar and negatively charged residues on S5 and S6. Two isoforms contain the least number of nonpolar residues, namely Nav1.8α and Nav1.9α. Both show the highest percentage of polar ECR residues and in particular on ECL S5 and S6 for all three species. Our finding here reflects the extant literature speculating about the absence of experimental evidence for Nav1.9α cooperation with β subunits for gating. Figure S12 summaries the ECL properties. Nav1.4α and Nav1.6α possess the same number of PPIs. Despite this common treat, subtle differences may also explain why their kinetic behavior differs. The ECL of S5 on DI in Nav1.4α (resp. Nav1.6α) hosts the highest (resp. smallest) amount of polar and negatively charged amino acids. Intriguingly, the Nav1.6α isoform accumulates even more polar and negatively charged residues in its S5 loops on DIII. Domains DI and DIII are facing each other from diametrically opposing positions across the central pore part. Both have developed the longest S5 ECLs among all Navα. It can be speculated that their lengths could reflect the main electrostatic attraction of the Navα for the conduction of Na+ towards the channel pore. This balance of residues distributed in the S5 ECLs (DI and DIII) probably has evolved to provide the Nav1.4α and Nav1.6α isoforms a MEPS similar to a fingerprint with inherent characteristics to perform a specific function on the tissue. Aforementioned findings have not been reported in the extant literature that far.
3.3. Volume and Surface Properties of the ECL on Navα Subunits
Evolution leads to random point mutations or SNPs with variable consequences for survival of organisms. On molecular level it changes structures and functions [88,89]. Isoforms can be understood as transient states during divergent evolution to separate them from a common ancestral protein when cells evolve to more specialized tissues in organisms [90,91]. Biochemical signaling is not seldom located on exposed loop segments on cell surfaces with a remarkable conservation of signal-relevant residues amidst the variable loop segments. This observation is the rationale to combine 2D and 3D alignment techniques enabling us to reveal this hidden world of signaling or interacting amino acids at the α/β interface [92,93,94]. The type of protein structure—AKA fold unit—is more conserved than its underlying primary sequence. Moreover, unchanged structures keep the biochemical function, what sometimes can be observed even in extreme cases of sequence divergence [95,96,97]. Of note, each ECR has 16 ECLs on each isoform.
With respect to all nine isoforms, the Nav1.4α isoform has the largest volume and widest SAA concerning the ECR in general. Moreover, regarding the pore architecture, this holds true also for S5 and S6 of ECLs. In contrast, the Nav1.9α isoform it has the smallest volume and SAA in the ECR and ECLs S5 and S6 (Figure S13). This finding has not yet been reported by others.
Figures S14 and S15, respectively, display the molecular volume and SAA of the ECLs. The S5 DI ECLs on h, m, rNav1.4α have a fairly larger molecular volume than the other isoforms. In contrast, the molecular volume of the S6 DIV ECLs on Nav1.9α is significantly smaller than on all other isoforms. This finding nicely explains why Nav1.9α isoforms do not enter in contact both β subunits via ECL S6 DIV and has not yet been reported by others either.
Of all isoforms, the h, m, rNav1.4α (h, m, rNav1.8α and h, m, rNav1.9α) isoforms have the highest N-MEPS (P-MEPS) in the IF-ECLs (Figure S16). These patterns are identical for S5 DI and S6 DIV ECLs. On the other hand, Nav1.8α and Nav1.9α have higher P-MEPS for S1-S2 DIII ECLs. Intriguingly, Nav1.4 isoforms possess the smallest areas of N-MEPs in S1-S2 DIII ECLs (Figure S17). The isoform-dependent characteristic features of each isoform could be attributed to the affinity of electrostatic attraction to the Navβ1 and Navβ3 subunits.
3.4. Interface Properties Between Navα and Navβ1 or Navβ3
The observation that only a tiny portion of the total surface area belongs to structural or functional segments is reflected by high conservation at those segments [98,99,100,101]. Especially electrostatic forces often act as critical determinants for biochemical signaling or other protein functions like ligand recognition, affinities, or structural stability. PPI is said to take place at surface locations (patches) with geometric and chemical complementarity [101,102,103,104,105]. This way, ECLs on the sodium channel tend to keep physicochemical similarities on their surfaces all of which sum up into distinct interaction patterns.
The variable surface area between the IF-ECLs and both β subunits was documented in Figure S18. For most of the Navs isoforms, the following general interaction pattern holds in order of shrinking surface: S5 DI > S1–S2 DIII > S6 DIV > S5 DIV, with the exception of Nav1.9 isoforms, where the interface area of the IF-ECL S6 DIV and the Navβ1 and Navβ3 subunits was found to be much smaller compared to all other isoforms. The observation is in excellent keeping with the electrophysiological role of h, m, rNav1.9α, lacking PPI with both β subunits. Hence, it seems not far-fetched to infer that it does not interact with IF-ECL S6 DIV.
Figures S19–S22 inform about the percentage scores concerning IF-ECLs and both β subunits for the following properties: PSA, NPSA, P-MEPS, and N-MEPS, measured on an atomic scale, which only counts the PPI atoms (Figure S18). The isoforms Nav1.2 and Nav1.6 possess interaction patterns, which resemble those of the IF- ECLs S5 DI, S1-S2 DIII, and S5 DIV. Our finding here could explain why the interface surface properties in both subunits (Navα and Navβ) are conserved because the reflect similar modulation. Interestingly, Nav1.2α and Nav1.6α present the same PPI sites along with Nav1.4α. Yet, the size, residue properties, SAA, molecular volume, differ greatly between either Nav1.2α or Nav1.6α versus Nav1.4α, emphasizing the IF-ECLs on S5 DI. On the other hand, IF-ECLs on S6 DIV do not show any similarity between isoforms, all of which is in line with the pivotal role of IF-ECLs S6 DIV as a strong contributor to pore modulation because of its unique electrostatic forces on its surface.
4. Materials and Methods
4.1. In Silico Homology Modeling
Homology modeling techniques were carried out to generate (3D) structure models of the hitherto unknown β subunits of mice and rats (mNavβ1, mNavβ3, rNavβ1, and rNavβ3) as well as the isoforms of the following Navα/Navβ complexes: hNav1.1α, hNav1.3α, hNav1.6α, hNav1.8α, hNav1.9α, mNav1.1α to mNav1.9α, rNav1.1α to rNav1.4α, rNav1.6α to rNav1.9α. Two programs, MODELLER 9.22 [106] and Chimera alpha V.1.14 [61], were applied using the following cryo-EM as (3D) templates: hNav1.4α/hNavβ1 with chains A and B from PDB entry 6AGF [49], hNav1.2α with chain A from PDB entry 6J8E [50], hNav1.7α with chain A, from PDB entry 6J8G [51], rNav1.5α from PDB entry 6UZ0 [52]. The following crystal structures were also taken as templates: hNavβ2 from PDB entry 5FEB [56], hNavβ3 with chain A from PDB entry 4L1D [55], and finally hNavβ4 from PDB entry 4MZ2 [54].
4.2. Identification of Interacting Residues at the Interface Between Navα and Navβ
The advent of a complete sodium channel structure with pore part and auxiliary proteins from a higher (vertebrate) organism has ushered a new area of structural biology analysis to lend insight into the underpinnings of subunit modulation mechanisms. Th structure elucidation was a pivotal step because vertebrate Nav channels are composed of a monomeric (single-chain) α protein with four different domains (I to IV), in contrast to the hitherto known homo-tetrameric (4 chains) channels of bacterial species without auxiliary proteins. Precisely, our cheminformatic work exploits this first experimentally observed interface of Nav1.4α isoform complex from a vertebrate species: the eel (eeNavα/Navβ for short, PDB entry: 6AGF [48]). This template helped analyze the interacting amino acids which form the PPI in the ECR (here: eeNavα/Navβ) applying software tools to detect contacts at the interface and the databases of Dunbrack and Dynameomics rotamers [59,60]. MSA was always performed using web based Clustal Omega 1.2.4 under its default settings [62]. To get rid of the residue numbering problems in sight of variable sequence lengths, each identified residue as “interacting” (i.e., forming the PPI of eeNav1.4 α/eeNavβ1 [48]) was labelled as a small segment of seven adjacent amino acids. In its central position, the interacting residue is flanked by three amino acids on either side. The schematic pattern is “yyyXyyy”, where “y” symbolizes any amino acid, while “X” is the interacting amino acid.
4.3. The Input Structures as Templates for the PPI Models
To generate the interfaces the following templates were used: the cryo-EM structure of hNavβ1 from PDB entry 6AGF [49], chain A of hNavβ3 crystal structure from PDB entry 4L1D [55]. Moreover, the following homology models were generated for the mNavβ1 subunits, mNavβ3, rNavβ1, and rNavβ3, in addition to the computed isoform complexes hNav1.1α, hNav1.3α, hNav1.5α, hNav1.6α, hNav1.8α, hNav1.9α, mNav1.1α to mNav1.9α, rNav1.1α to rNav1.4α, rNav1.6α to rNav1.9α. We also used the cryo-EM structures of hNav1.4α isoform with chains A and B from PDB entry 6AGF [49]), hNav1.2α with chain A from PDB entry 6J8E [50]), hNav1.7α with chain A from PDB 6J8G [51]), rNav1.5α from PDB entry 6UZ0 [52]). Of note, no chimeric combinations were made crossing species.
4.4. Determination of the Extracellular Regions of all Navα Subunits
The structures and sequences of the ECLs of the isoforms hNav1.1α to hNav1.9α, mNav1.1α to mNav1.9α and rNav1.1α to rNav1.9α, were determined, based on the available template structures eeNav1.4α, hNav1.2α, hNav1.4α, rNav1.5α and hNav1.7α which had been downloaded from the Orientations of Proteins in Membranes (OPM) database [107]. OPM provides spatial information about the lipid bilayer packing of the transmembrane helical part for our channel models. OPM helped define the ECR, i.e., at which position the loop protrudes and re-enters TMH.
4.5. Calculation of the Properties for all ECLs
The 432 amino acid segments, which define all ECLs under scrutiny were computed, i.e., D1 to DIV with 4 ectodomain loops, and each of them by nine isoforms for three species yield 16 × 9 × 3 = 432 topological models. They were “extracted” from the primary sequences of hNav1.1α to hNav1.9α, mNav1.1α to mNav1.9α and rNav1.1α to rNav1.9α. The ECL lengths and informative properties about the interacting amino acids were systematically computed and documented for subsequent PPI analyses. The plethora of data made scripting a most valuable asset under Chimera (see scripts at the end of SM). Chemometric properties—AKA descriptors or parameters—included polar and nonpolar area, cysteines, or aromatic amino acids. Of note, hydrogen bonding was observed and documented, but data presentation omitted, since constructing the intra- or intermolecular hydrogen networking is a standard option. The H-bonds at interfaces are readily on display, i.e., intermolecular hydrogen networks (in the contact zone, which is defined by an atom selection radius) between two proteins (see our 3D model of hNav1.4α/Navβ3 and PDB entry 6AGF [49] with the hNav1.4α/Navβ1 in PDB format in SM).
4.6. Calculation of the Chemical Surface Properties for All IF-ECLs
For all 3D models the potential energies of the structures were minimized and total and partial charges loaded with a water probe radius of 1.4 Å and a vertex density of 2.0 [108]. The molecular volume, total SAA as well as the polar or non-polar SAA of charged or uncharged atoms were estimated in Å2 for all ECLs [61]. As usual all data was computed for all nine isoforms of the three species.
4.7. Electrostatic Interactions of the IF-ECLs Surfaces (MEPS)
Structural input files (models and 3D templates) were prepared with Chimera add-on PDB2PQR, according to a protocol [63]. It computes Poisson-Boltzmann electrostatics, which constitutes a higher level of theory than electrostatic forces calculated based on Poisson–Boltzmann equation solver (APBS) [64]. The numerical output was converted for graphical display of MEPS. To this end, all those data points above and below a given threshold (±30) were excluded (empiric protocol). Electrostatic force values in the +30 to −30 range were considered for linear scaling the color code between +1 and −1 (unit one data normalization). The corresponding load at each vertex of the surface was assigned using the UCSF Chimera alpha V. 1.14 interface [61] in molecular selections for each ECL.
4.8. Calculation of the ECL Surface Properties at the Interface with Navβ1 and Navβ3 Subunits
At the interface the buried area, total SAA and polar and non-polar areas were calculated in Å2 applying scripts under Chimera [61]. For direct comparison some values were expressed as percentages to reflect the relative portion (%) of the total loop length (100 %) to account for the huge variation in length. In addition, residues with positive or negative charges were taken as basis to compare MEPS at the interface.
4.9. External Model Validation of the PPI Models
In a more general view, the advent of structural knowledge about the cell proteome has ushered a new area of PPI studies identifying hotspots of interaction between adjacent proteins [109]. After finishing our study, a fully automated interface generation was carried out. The web-based tool identified the same interacting residues (see final section of the Supplementary Materials [110]).
5. Conclusions
In this work, we analyzed observed protein-protein interfaces and postulated others in models derived from experimentally determined 3D templates. The models were generated for all nine existing isoforms of three species (human, rat, mouse). The interface concerned the residues of extracellular loops in close contact with the Navβ1 or Navβ3 subunits. Thanks to the chemometric analysis, we formulated a model for fast inactivation of the Navα pore gating modulated by the presence of either Navβ1 or Navβ3 auxiliary proteins. On theoretical ground, we gained mechanistic insight of the movements around the S4 DIII voltage sensor, which is modulated by β subunits.
We describe the modulation of the sodium channel activity in terms of a schematic PPI model between pore-containing transmembrane α protein and the auxiliary β proteins for all nine isoforms in three Mammalian species. Our structural models and topological analysis of sequences lead to the conclusion that their distinct interfaces reflect the observed differences in gating kinetics.
We computed chemometric patterns for criteria like non-covalent bonding, loop length, area or volume, solvent accessible area or buried surfaces and other electrostatic descriptors. Isoforms were grouped together according to common interaction patterns and opposed to others with different patterns, and all results were mechanistically related to reports on gating kinetics. The patterns included solvent accessible area or conserved positions for opposingly charged residues on either side of the interface. Our findings about subtle variations in the electrostatic patterns affect the individual modulation capacity of each isoform all of which is in keeping with electrophysiologic observations of gating kinetics and graphically resumed in our schematic drawings.
Our cheminformatic study was thoroughly based on observations taken from the extant literature, and our results are in line with their experimental findings. In addition, we report two hitherto unidentified interaction patterns (or patches) for the 3D templates as well as the proposed interface models. They fit into a larger mechanistic picture with the other interaction patches, which were first reported by Yan et al. with the advent of a complete sodium channel structure for vertebrate species (eel) [48].
This work could orient future research in molecular biology or help design site-directed mutagenesis studies at the subunit interface of voltage-gated sodium channels. In particular, molecular dynamics studies on supercomputers could simulate the gating trajectories over time and confirm that the isoform movements can be grouped together following the proposed cheminformatic patterns.
Supplementary Materials
The following are available online: Table S1. PPI of the residues of the Navα and Navβ subunits; Table S2. Residues of contact of Navs S4 DIII with the Navβ1 and Navβ3 subunits; Table S3. Interface area of the IF-ECLs of the Navs; Table S4. The output list of interacting residues between both subunits for the eel sodium channel; Figure S1. PPIs of the three h, m, rNav1.1 isoforms in complex with Navβ1 and Navβ3; Figure S2. PPIs of the three h, m, rNav1.2 isoforms in complex with Navβ1 and Navβ3; Figure S3. PPIs of the three Nav1.3 isoforms in complex with Navβ1 and Navβ3; Figure S4. PPIs of the three h, m, rNav1.4 isoforms in complex with Navβ1 and Navβ3; Figure S5. PPIs of the three h, m, rNav1.5 isoforms in complex with Navβ1 and Navβ3; Figure S6. PPIs of the three h, m, rNav1.6 isoforms in complex with Navβ1 and Navβ3; Figure S7. PPIs of the three h, m, rNav1.7 isoforms in complex with Navβ1 and Navβ3; Figure S8. PPIs of the three h, m, rNav1.8 isoforms in complex with Navβ1 and Navβ3; Figure S9. PPIs of the three h, m, rNav1.9 isoforms in complex with Navβ1 and Navβ3; Figure S10. Length of extracellular loops of the Navs; extracellular loops: S1-S2, S3-S4, S5 and S6; Figure S11. Properties of the residues of the extracellular region of the Nav; Figure S12. Properties of residues of Nav extracellular loops; Figure S13. Surface and volume properties of ECR and S5, and S6 extracellular loops; Figure S14. SAA of ECLs of the Navs; Figure S15. Molecular volume of ECLs of the Navs; Figure S16. Properties of total SAA of IF-ECLs of Navs; Figure S17. Properties of SAA of IF-ECLs of Nav; Figure S18. Total area of the atoms that form at the Navα/Navβ interface; Figure S19. Percentage scores of the surface properties for atoms at Navα/Navβ of ECL on S5 DI; Figure S20. Percentage of the surface properties of atoms that form at the Navα/Navβ interface of ECL S1-S2 DIII; Figure S21. Percentage of the surface properties of atoms that form at the Navα/Navβ interface of ECL S5 DIV; Figure S22. Percentage of the surface properties of atoms that form at the Navα/Navβ interface of ECL S6 DIV; Figure S23. Demonstration example of the surface of atoms that form the interface in hNavβ1 with the ECL S5 DI in the HMSR of hNavβ1/hNav1.4 complex.
Author Contributions
Authorship was limited to those who have contributed substantially to the work reported. F.V-D., S.L.-N., J.E.R.-C., and T.S. carried out the in silico research and graphical artwork. T.S. and F.V.-D. wrote the article. E.M.S.-S. provided bibliographic and in-house information from his ongoing electrophysiological experiments. Conceptualization by T.S.; methodology by T.S. and F.V.-D.; software by F.V.-D., S.L.-N., J.E.R.-C., and T.S.; in-house validation and formal analyses by E.M.S.-S., a research study by F.V.-D. and T.S.; Lab resources by T.S.; data curation by F.V.-D. and S.L.-N.; writing original Spanish version by F.V.-D.; writing, reviewing, and editing by T.S.; model visualization by F.V.-D., S.L.-N., and J.E.R.-C. and; supervision T.S.; project administration by T.S.; internal funding acquisitions by T.S. and E.M.S.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank the academic teachers Lourdes Milan, for supervising the PhD work of first author Fernando Villa. We also address our thanks to the Mexican CONACyT and VIEP of BUAP for scholarships and administrative support for Fernando Villa. We feel much beholden for the scientific discussions and advice of Anthony P. Jackson, Department of Biochemistry, University of Cambridge, UK, as well as for the internal funding for experimental work and publication fees by Ygnacio Martinez-Laguna, BUAP/VIEP (project ID: 00115) as well as BUAP-CA-120 (area name: Pharmaco-biology).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International Union of Pharmacology. XLVII. Nomenclature and Structure-Function Relationships of Voltage-Gated Sodium Channels. Pharmacol. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Fux, J.E.; Mehta, A.; Moffat, J.; Spafford, J.D. Eukaryotic Voltage-Gated Sodium Channels: On Their Origins, Asymmetries, Losses, Diversification and Adaptations. Front. Physiol. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G. Sodium channels, the electrogenisome and the electrogenistat: Lessons and questions from the clinic. J. Physiol. 2012, 590, 2601–2612. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.L.; Barchi, R.L.; Caldwell, J.H.; Hofmann, F.; Howe, J.R.; Hunter, J.C.; Kallen, R.G.; Mandel, G.; Meisler, M.H.; Netter, Y.B.; et al. Nomenclature of voltage-gated sodium channels. Neuron 2000, 28, 365–368. [Google Scholar] [CrossRef]
- O’Malley, H.A.; Isom, L.L. Sodium channel β subunits: Emerging targets in channelopathies. Annu. Rev. Physiol. 2015, 77, 481–504. [Google Scholar] [CrossRef]
- Catterall, W.A. From Ionic Currents to Molecular Mechanisms. Neuron 2000, 26, 13–25. [Google Scholar] [CrossRef]
- Ogata, N.; Ohishi, Y. Molecular diversity of structure and function of the voltage-gated Na+ channels. Jpn. J. Pharmacol. 2002, 88, 365–377. [Google Scholar] [CrossRef]
- Woolf, C.J.; Mannion, R.J. Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet 1999, 353, 1959–1964. [Google Scholar] [CrossRef]
- Catterall, W.A. Sodium Channels, Inherited Epilepsy, and Antiepileptic Drugs. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 317–338. [Google Scholar] [CrossRef]
- Claes, L.R.; Deprez, L.; Suls, A.; Baets, J.; Smets, K.; Van Dyck, T.; Deconinck, T.; Jordanova, A.; De Jonghe, P. The SCN1A variant database: A novel research and diagnostic tool. Hum. Mutat. 2009, 30, E904–E920. [Google Scholar] [CrossRef]
- Huang, W.; Liu, M.; Yan, S.; Yan, N. Structure-based assessment of disease-related mutations in human voltage-gated sodium channels. Protein Cell 2017, 8, 401–438. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.; Patowary, A.; Stanaway, I.B.; Mccord, E.; Nesbitt, R.R.; Archer, M.; Scheuer, T.; Nickerson, D.; Raskind, W.H.; Wijsman, E.M.; et al. Association of rare missense variants in the second intracellular loop of NaV1.7 sodium channels with familial autism. Mol. Psychiatry 2016, 23, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Cestele, S.; Yarov-Yarovoy, V.; Yu, F.H.; Konoki, K.; Scheuer, T. Voltage-gated ion channels and gating modifier toxins. Toxicon 2007, 49, 124–141. [Google Scholar] [CrossRef] [PubMed]
- Bagal, S.K.; E Marron, B.; Owen, R.M.; Storer, R.I.; A Swain, N. Voltage gated sodium channels as drug discovery targets. Channels 2015, 9, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.D.L.; Kraus, R.L. Voltage-Gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. J. Med. Chem. 2015, 58, 7093–7118. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.; Peigneur, S.; Tytgat, J. Neurotoxins and Their Binding Areas on Voltage-Gated Sodium Channels. Front. Pharmacol. 2011, 2, 71. [Google Scholar] [CrossRef] [PubMed]
- Mantegazza, M.; Catterall, W.A. Voltage-Gated Na+ Channels; Oxford University Press (OUP): Oxford, UK, 2012; Volume 80, pp. 41–54. [Google Scholar]
- Ahern, C.A.; Payandeh, J.; Bosmans, F.; Chanda, B. The hitchhiker’s guide to the voltage-gated sodium channel galaxy. J. Gen. Physiol. 2015, 147, 1–24. [Google Scholar] [CrossRef]
- Payandeh, J.; Scheuer, T.; Zheng, N.; Catterall, W.A. The crystal structure of a voltage-gated sodium channel. Nature 2011, 475, 353–358. [Google Scholar] [CrossRef]
- Long, S.B.; Tao, X.; Campbell, E.B.; MacKinnon, R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 2007, 450, 376–382. [Google Scholar] [CrossRef]
- Long, S.B. Crystal Structure of a Mammalian Voltage-Dependent Shaker Family K+ Channel. Science 2005, 309, 897–903. [Google Scholar] [CrossRef]
- Yu, F.H.; Yarov-Yarovoy, V.; Gutman, G.A.; Catterall, W.A. Overview of Molecular Relationships in the Voltage-Gated Ion Channel Superfamily. Pharmacol. Rev. 2005, 57, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Chanda, B.; Bezanilla, F. Tracking Voltage-dependent Conformational Changes in Skeletal Muscle Sodium Channel during Activation. J. Gen. Physiol. 2002, 120, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, F.; Martin-Eauclaire, M.-F.; Swartz, K.J. Deconstructing voltage sensor function and pharmacology in sodium channels. Nature 2008, 456, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Capes, D.L.; Goldschen-Ohm, M.P.; Arcisio-Miranda, M.; Bezanilla, F.; Chanda, B. Domain IV voltage-sensor movement is both sufficient and rate limiting for fast inactivation in sodium channels. J. Gen. Physiol. 2013, 142, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Voltage-gated sodium channels at 60: Structure, function and pathophysiology. J. Physiol. 2012, 590, 2577–2589. [Google Scholar] [CrossRef]
- Vargas, E.; Yarov-Yarovoy, V.; Khalili-Araghi, F.; Catterall, W.A.; Klein, M.L.; Tarek, M.; Lindahl, E.; Schulten, K.; Perozo, E.; Bezanilla, F.; et al. An emerging consensus on voltage-dependent gating from computational modeling and molecular dynamics simulations. J. Gen. Physiol. 2012, 140, 587–594. [Google Scholar] [CrossRef]
- Brackenbury, W.J.; Isom, L.L. Na+ Channel? Subunits: Overachievers of the Ion Channel Family. Front. Pharmacol. 2011, 2, 53. [Google Scholar] [CrossRef]
- Cusdin, F.S.; Clare, J.J.; Jackson, A.P. Trafficking and Cellular Distribution of Voltage-Gated Sodium Channels. Traffic 2008, 9, 17–26. [Google Scholar] [CrossRef]
- Patino, G.A.; Isom, L.L. Electrophysiology and beyond: Multiple roles of Na+ channel β subunits in development and disease. Neurosci. Lett. 2010, 486, 53–59. [Google Scholar] [CrossRef]
- Calhoun, J.D.; Isom, L.L. The Role of Non-pore-Forming β Subunits in Physiology and Pathophysiology of Voltage-Gated Sodium Channels. Handb. Exp. Pharmacol. 2014, 221, 51–89. [Google Scholar] [CrossRef]
- Catterall, W.A. Structure and function of voltage-gated ion channels. Annu. Rev. Biochem. 1995, 64, 493–531. [Google Scholar] [CrossRef] [PubMed]
- Isom, L.L.; De Jongh, K.S.; Patton, D.E.; Reber, B.F.; Offord, J.; Charbonneau, H.; Walsh, K.; Goldin, A.L.; Catterall, W.A. Primary structure and functional expression of the β1 subunit of the rat brain sodium channel. Science 1992, 256, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Isom, L.L.; Scheuer, T.; Brownstein, A.B.; Ragsdale, D.S.; Murphy, B.J.; Catterall, W.A. Functional Co-expression of the 1 and Type IIA Subunits of Sodium Channels in a Mammalian Cell Line. J. Boil. Chem. 1995, 270, 3306–3312. [Google Scholar] [CrossRef] [PubMed]
- McCormick, K.A.; Isom, L.L.; Ragsdale, D.; Smith, D.; Scheuer, T.; Catterall, W.A. Molecular Determinants of Na+Channel Function in the Extracellular Domain of the β1 Subunit. J. Boil. Chem. 1998, 273, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Makita, N.; Bennett, P.B.; George, A.L., Jr. Molecular determinants of β1 subunit-induced gating modulation in voltage-dependent Na+ channels. J Neurosci. 1996, 22, 7117–7127. [Google Scholar] [CrossRef]
- E Patton, D.; Isom, L.L.; A Catterall, W.; Goldin, A.L. The adult rat brain beta 1 subunit modifies activation and inactivation gating of multiple sodium channel alpha subunits. J. Boil. Chem. 1994, 269, 17649–17655. [Google Scholar]
- Yu, E.J.; Ko, S.-H.; Lenkowski, P.W.; Pance, A.; Patel, M.K.; Jackson, A.P. Distinct domains of the sodium channel β3-subunit modulate channel-gating kinetics and subcellular location. Biochem. J. 2005, 392, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cannon, S.C. Modulation of Na+channel inactivation by the ?1 subunit: A deletion analysis. Pflüg. Arch. Eur. J. Physiol. 1995, 431, 186–195. [Google Scholar] [CrossRef]
- Islas, A.A.; Sánchez-Solano, A.; Scior, T.; Millan-Perezpeña, L.; Salinas-Stefanon, E.M. Identification of Navβ1 Residues Involved in the Modulation of the Sodium Channel Nav1.4. PLoS ONE 2013, 8, e81995. [Google Scholar] [CrossRef]
- Scior, T.; Paiz-Candia, B.; Islas, Á.A.; Sánchez-Solano, A.; Peña,, L.M.-P.; Mancilla-Simbro, C.; Salinas-Stefanon, E.M. Predicting a double mutant in the twilight zone of low homology modeling for the skeletal muscle voltage-gated sodium channel subunit beta-1 (Na v 1.4 β1). Comput. Struct. Biotechnol. J. 2015, 13, 229–240. [Google Scholar] [CrossRef]
- Sánchez-Solano, A.; Islas, A.A.; Scior, T.; Paiz-Candia, B.; Millan-Perezpeña, L.; Salinas-Stefanon, E.M. Characterization of specific allosteric effects of the Na+ channel β1 subunit on the Nav1.4 isoform. Eur. Biophys. J. 2016, 46, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.S.; Watanabe, H.; Zhong, T.P.; Roden, D.M. Molecular cloning and analysis of zebrafish voltage-gated sodium channel beta subunit genes: Implications for the evolution of electrical signaling in vertebrates. BMC Evol. Boil. 2007, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Isom, L.L. Sodium channel beta subunits: Anything but auxiliary. Neuroscientist 2001, 7, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Buffington, S.A.; Rasband, M.N. Na+ channel-dependent recruitment of Navβ4 to axon initial segments and nodes of Ranvier. J. Neurosci. 2013, 33, 6191–6202. [Google Scholar] [CrossRef]
- Chen, C.; Calhoun, J.D.; Zhang, Y.; Lopez-Santiago, L.; Zhou, N.; Davis, T.H.; Salzer, J.L.; Isom, L.L. Identification of the Cysteine Residue Responsible for Disulfide Linkage of Na+Channel α and β2 Subunits. J. Boil. Chem. 2012, 287, 39061–39069. [Google Scholar] [CrossRef]
- Shen, H.; Zhou, Q.; Pan, X.; Li, Z.; Wu, J.; Yan, N. Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science 2017, 355, eaal4326. [Google Scholar] [CrossRef]
- Yan, Z.; Zhou, Q.; Wang, L.; Wu, J.; Zhao, Y.; Huang, G.; Peng, W.; Shen, H.; Lei, J.; Yan, N. Structure of the Na v 1.4-β1 Complex from Electric Eel. Cell 2017, 170, 470–482.e11. [Google Scholar] [CrossRef]
- Pan, X.; Li, Z.; Zhou, Q.; Shen, H.; Wu, K.; Huang, X.; Chen, J.; Zhang, J.; Zhu, X.; Lei, J.; et al. Structure of the human voltage-gated sodium channel Nav1.4 in complex with β1. Science 2018, 362, eaau2486. [Google Scholar] [CrossRef]
- Pan, X.; Li, Z.; Huang, X.; Huang, G.; Gao, S.; Shen, H.; Liu, L.; Lei, J.; Yan, N. Molecular basis for pore blockade of human Na+ channel Nav1.2 by the μ-conotoxin KIIIA. Science 2019, 363, 1309–1313. [Google Scholar] [CrossRef]
- Shen, H.; Liu, D.; Wu, K.; Lei, J.; Yan, N. Structures of human Nav1.7 channel in complex with auxiliary subunits and animal toxins. Science 2019, 363, 1303–1308. [Google Scholar] [CrossRef]
- Jiang, D.; Shi, H.; Tonggu, L.; El-Din, T.M.G.; Lenaeus, M.J.; Zhao, Y.; Yoshioka, C.; Zheng, N.; Catterall, W.A. Structure of the Cardiac Sodium Channel. Cell 2020, 180, 122–134.e10. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.H.; Westenbroek, R.E.; Silos-Santiago, I.; McCormick, K.A.; Lawson, D.; Ge, P.; Ferriera, H.; Lilly, J.; Distefano, P.S.; Catterall, W.A.; et al. Sodium Channel β4, a New Disulfide-Linked Auxiliary Subunit with Similarity to β2. J. Neurosci. 2003, 23, 7577–7585. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, J.M.; Das, S.; Van Petegem, F.; Bosmans, F. Crystallographic insights into sodium-channel modulation by the 4 subunit. Proc. Natl. Acad. Sci. USA 2013, 110, E5016–E5024. [Google Scholar] [CrossRef] [PubMed]
- Namadurai, S.; Balasuriya, D.; Rajappa, R.; Wiemhöfer, M.; Stott, K.; Klingauf, J.; Edwardson, J.M.; Chirgadze, D.Y.; Jackson, A.P. Crystal structure and molecular imaging of the Nav channel β3 subunit indicates a trimeric assembly. J. Boil. Chem. 2014, 289, 10797–10811. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Gilchrist, J.; Bosmans, F.; Van Petegem, F. Binary architecture of the Nav1.2- β2 signaling complex. Elife 2016, 5, e10960. [Google Scholar] [CrossRef]
- McCormick, K.A.; Srinivasan, J.; White, K.; Scheuer, T.; Catterall, W.A. The Extracellular Domain of the β1 Subunit Is Both Necessary and Sufficient for β1-like Modulation of Sodium Channel Gating. J. Boil. Chem. 1999, 274, 32638–32646. [Google Scholar] [CrossRef]
- Yereddi, N.R.; Cusdin, F.S.; Namadurai, S.; Packman, L.C.; Monie, T.P.; Slavny, P.; Clare, J.J.; Powell, A.J.; Jackson, A.P. The immunoglobulin domain of the sodium channel β3 subunit contains a surface-localized disulfide bond that is required for homophilic binding. FASEB J. 2012, 27, 568–580. [Google Scholar] [CrossRef]
- Shapovalov, M.V.; Dunbrack, R.L. A Smoothed Backbone-Dependent Rotamer Library for Proteins Derived from Adaptive Kernel Density Estimates and Regressions. Struct. 2011, 19, 844–858. [Google Scholar] [CrossRef]
- Scouras, A.D.; Daggett, V. The dynameomics rotamer library: Amino acid side chain conformations and dynamics from comprehensive molecular dynamics simulations in water. Protein Sci. 2011, 20, 341–352. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Available online. Met. Powder Rep. 2008, 63, 11. [CrossRef]
- Dolinsky, T.J.; Czodrowski, P.; Li, H.; Nielsen, J.E.; Jensen, J.H.; Klebe, G.; Baker, N. PDB2PQR: Expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 2007, 35, W522–W525. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef]
- Goddard, T.D.; Huang, C.C.; Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 2017, 27, 14–25. [Google Scholar] [CrossRef]
- Microsoft Corporation. Microsoft Excel. Available online: https://office.microsoft.com/excel (accessed on 20 May 2016).
- Hermann, J.C.; Marti-Arbona, R.; Fedorov, A.A.; Fedorov, E.; Almo, S.C.; Shoichet, B.K.; Raushel, F.M. Structure-based activity prediction for an enzyme of unknown function. Nature 2007, 448, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Zahiri, J.; Bozorgmehr, J.H.; Masoudi-Nejad, A. Computational Prediction of Protein–Protein Interaction Networks: Algo-rithms and Resources. Curr. Genom. 2013, 14, 397–414. [Google Scholar] [CrossRef]
- Ezkurdia, I.; Bartoli, L.; Fariselli, P.; Casadio, R.; Valencia, A.; Tress, M.L. Progress and challenges in predicting protein-protein interaction sites. Briefings Bioinform. 2008, 10, 233–246. [Google Scholar] [CrossRef]
- Whisstock, J.; Lesk, A. Prediction of protein function from protein sequence and structure. Q. Rev. Biophys. 2003, 36, 307–340. [Google Scholar] [CrossRef]
- Izidoro, S.; De Melo-Minardi, R.C.; Pappa, G.L. GASS: Identifying enzyme active sites with genetic algorithms. Bioinformatics 2014, 31, 864–870. [Google Scholar] [CrossRef]
- Jacobson, M.P.; Kalyanaraman, C.; Zhao, S.; Tian, B. Leveraging structure for enzyme function prediction: Methods, opportunities, and challenges. Trends Biochem. Sci. 2014, 39, 363–371. [Google Scholar] [CrossRef]
- Ma, B.; Elkayam, T.; Wolfson, H.; Nussinov, R. Protein-protein interactions: Structurally conserved residues distinguish between binding sites and exposed protein surfaces. Proc. Natl. Acad. Sci. USA 2003, 100, 5772–5777. [Google Scholar] [CrossRef] [PubMed]
- Keskin, O.; Ma, B.; Nussinov, R. Hot Regions in Protein–Protein Interactions: The Organization and Contribution of Structurally Conserved Hot Spot Residues. J. Mol. Boil. 2005, 345, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Keskin, O.; Ma, B.; Nussinov, R.; Liang, J. Protein–Protein Interactions: Hot Spots and Structurally Conserved Residues often Locate in Complemented Pockets that Pre-organized in the Unbound States: Implications for Docking. J. Mol. Boil. 2004, 344, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Kruger, L.C.; Isom, L.L. Voltage-Gated Na+Channels: Not Just for Conduction. Cold Spring Harb. Perspect. Boil. 2016, 8, a029264. [Google Scholar] [CrossRef] [PubMed]
- Salvage, C.S.; Huang, C.L.H.; Jackson, A.P. Cell-Adhesion Properties of β-Subunits in the Regulation of Cardiomyocyte Sodium Channels. Biomolecules 2020, 10, 989. [Google Scholar] [CrossRef]
- Isom, L.L.; Ragsdale, D.S.; De Jongh, K.S.; E Westenbroek, R.; Reber, B.F.; Scheuer, T.; A Catterall, W. Structure and function of the beta 2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell 1995, 83, 90121–90123. [Google Scholar] [CrossRef]
- Molinarolo, S.; Granata, D.; Carnevale, V.; Ahern, C.A. Mining Protein Evolution for Insights into Mechanisms of Voltage-Dependent Sodium Channel Auxiliary Subunits. Handb. Exp. Pharmacol. 2018, 246, 33–49. [Google Scholar] [CrossRef]
- Zhu, W.; Voelker, T.L.; Varga, Z.; Schubert, A.R.; Nerbonne, J.M.; Silva, J. Mechanisms of noncovalent β subunit regulation of NaV channel gating. J. Gen. Physiol. 2017, 149, 813–831. [Google Scholar] [CrossRef]
- Messner, D.J.; A Catterall, W. The sodium channel from rat brain. Separation and characterization of subunits. J. Boil. Chem. 1985, 260, 10597–10604. [Google Scholar]
- Morgan, K.; Stevens, E.B.; Shah, B.; Cox, P.J.; Dixon, A.K.; Lee, K.; Pinnock, R.D.; Hughes, J.; Richardson, P.J.; Mizuguchi, K.; et al. beta 3: An additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc. Natl. Acad. Sci. USA 2000, 97, 2308–2313. [Google Scholar] [CrossRef]
- Glass, W.G.; Duncan, A.L.; Biggin, P.C. Computational Investigation of Voltage-Gated Sodium Channel β3 Subunit Dynamics. Front. Mol. Biosci. 2020, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Namadurai, S.; Yereddi, N.R.; Cusdin, F.S.; Huang, C.L.-H.; Chirgadze, D.Y.; Jackson, A.P. A new look at sodium channel β subunits. Open Boil. 2015, 5, 140192. [Google Scholar] [CrossRef] [PubMed]
- Lenkowski, P.W.; Shah, B.S.; E Dinn, A.; Lee, K.; Patel, M.K. Lidocaine block of neonatal Nav1.3 is differentially modulated by co-expression of β1 and β3 subunits. Eur. J. Pharmacol. 2003, 467, 23–30. [Google Scholar] [CrossRef]
- Ragsdale, D.; McPhee, J.; Scheuer, T.; Catterall, W. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science 1994, 265, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Ferrera, L.; Moran, O. β1-subunit modulates the Nav1.4 sodium channel by changing the surface charge. Exp. Brain Res. 2006, 172, 139–150. [Google Scholar] [CrossRef]
- Kinch, L.N.; Grishin, N.V. Evolution of protein structures and functions. Curr. Opin. Struct. Biol. 2002, 12, 400–408. [Google Scholar] [CrossRef]
- Starr, T.; Thornton, J.W. Epistasis in protein evolution. Protein Sci. 2016, 25, 1204–1218. [Google Scholar] [CrossRef]
- Kauvar, L.M.; O Villar, H. Deciphering cryptic similarities in protein binding sites. Curr. Opin. Biotechnol. 1998, 9, 390–394. [Google Scholar] [CrossRef]
- Russell, R.B.; Sasieni, P.D.; Sternberg, M.J. Supersites within superfolds. Binding site similarity in the absence of homology 1 1Edited by J. Thornton. J. Mol. Boil. 1998, 282, 903–918. [Google Scholar] [CrossRef]
- Spitzer, R.; Cleves, A.E.; Varela, R.; Jain, A.N. Protein function annotation by local binding site surface similarity. Proteins Struct. Funct. Bioinform. 2013, 82, 679–694. [Google Scholar] [CrossRef]
- Richards, F.M. Areas, volumes, packing and protein structure. Annu. Rev. Biophys. Bioeng. 1977, 6, 151–176. [Google Scholar] [CrossRef]
- Gainza, P.; Sverrisson, F.; Monti, F.; Rodolà, E.; Boscaini, D.; Bronstein, M.M.; Correia, B.E. Deciphering interaction fingerprints from protein molecular surfaces using geometric deep learning. Nat. Methods 2019, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Illergård, K.; Ardell, D.H.; Elofsson, A. Structure is three to ten times more conserved than sequence-A study of structural response in protein cores. Proteins: Struct. Funct. Bioinform. 2009, 77, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Sousounis, K.; E Haney, C.; Cao, J.; Sunchu, B.; Tsonis, P.A. Conservation of the three-dimensional structure in non-homologous or unrelated proteins. Hum. Genom. 2012, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Gibrat, J.-F.; Madej, T.; Bryant, S.H. Surprising similarities in structure comparison. Curr. Opin. Struct. Boil. 1996, 6, 377–385. [Google Scholar] [CrossRef]
- Caffrey, D.R.; Somaroo, S.; Hughes, J.D.; Mintseris, J.; Huang, E.S. Are protein–protein interfaces more conserved in sequence than the rest of the protein surface? Protein Sci. 2004, 13, 190–202. [Google Scholar] [CrossRef]
- Grishin, N.V.; Phillips, M.A. The subunit interfaces of oligomeric enzymes are conserved to a similar extent to the overall protein sequences. Protein Sci. 1994, 3, 2455–2458. [Google Scholar] [CrossRef] [PubMed]
- Binkowski, T.A.; Joachimiak, A. Protein Functional Surfaces: Global Shape Matching and Local Spatial Alignments of Ligand Binding Sites. BMC Struct. Boil. 2008, 8, 45. [Google Scholar] [CrossRef]
- A Marshall, S.; Morgan, C.S.; Mayo, S.L. Electrostatics significantly affect the stability of designed homeodomain variants. J. Mol. Boil. 2002, 316, 189–199. [Google Scholar] [CrossRef]
- Chothia, C.; Janin, J. Principles of protein–protein recognition. Nature 1975, 256, 705–708. [Google Scholar] [CrossRef]
- Sheinerman, F.B.; Norel, R.; Honig, B. Electrostatic aspects of protein–protein interactions. Curr. Opin. Struct. Boil. 2000, 10, 153–159. [Google Scholar] [CrossRef]
- Heifetz, A.; Katchalski-Katzir, E.; Eisenstein, M. Electrostatics in protein–protein docking. Protein Sci. 2002, 11, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Vizcarra, C.L.; Mayo, S.L. Electrostatics in computational protein design. Curr. Opin. Chem. Boil. 2005, 9, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Sali, A. Protein Structure Modeling with MODELLER. Adv. Struct. Saf. Stud. 2014, 1137, 1–15. [Google Scholar] [CrossRef]
- Lomize, M.A.; Lomize, A.L.; Pogozheva, I.D.; Mosberg, H.I. OPM: Orientations of Proteins in Membranes database. Bioinformatics 2006, 22, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, A.; Villa, L.; Vistoli, G. VEGA--an open platform to develop chemo-bio-informatics applications, using plug-in architecture and script programming. J. Comput. Mol. Des. 2004, 18, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Sarvagalla, S.; Cheung, C.H.A.; Tsai, J.Y.; Hsiehd, H.P.; Coumar, M.S. Disruption of protein-protein interactions: Hot spot detection, structure-based virtual screening and in vitro testing for the anti-cancer drug target-surviving. RSC. Adv. 2016, 6, 31947–31959. [Google Scholar] [CrossRef]
- Cukuroglu, E.; Gursoy, A.; Keskin, O. HotRegion: A database of predicted hot spot clusters. Nucleic Acids Res. 2011, 40, D829–D833. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).