New Series of Double-Modified Colchicine Derivatives: Synthesis, Cytotoxic Effect and Molecular Docking
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
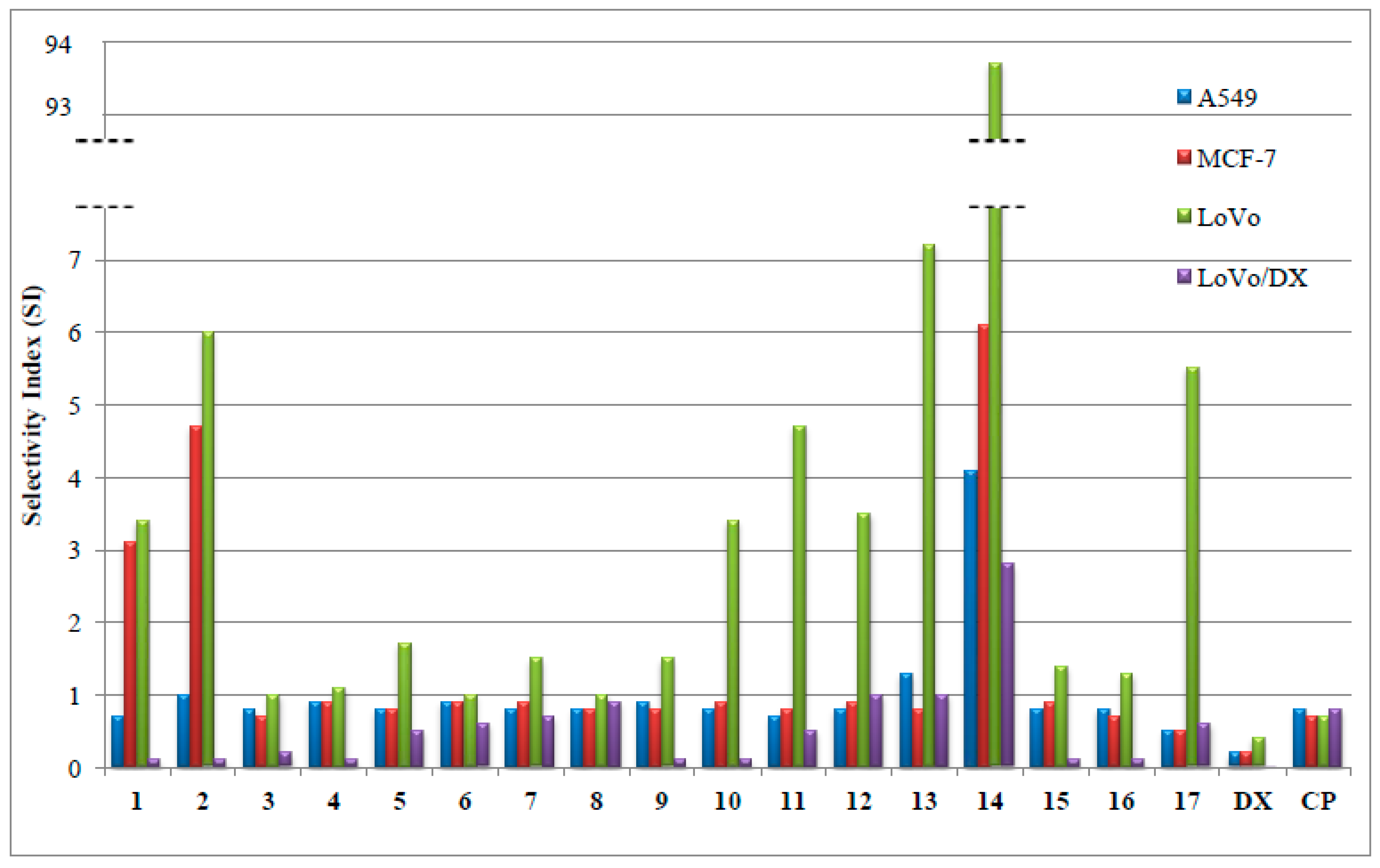
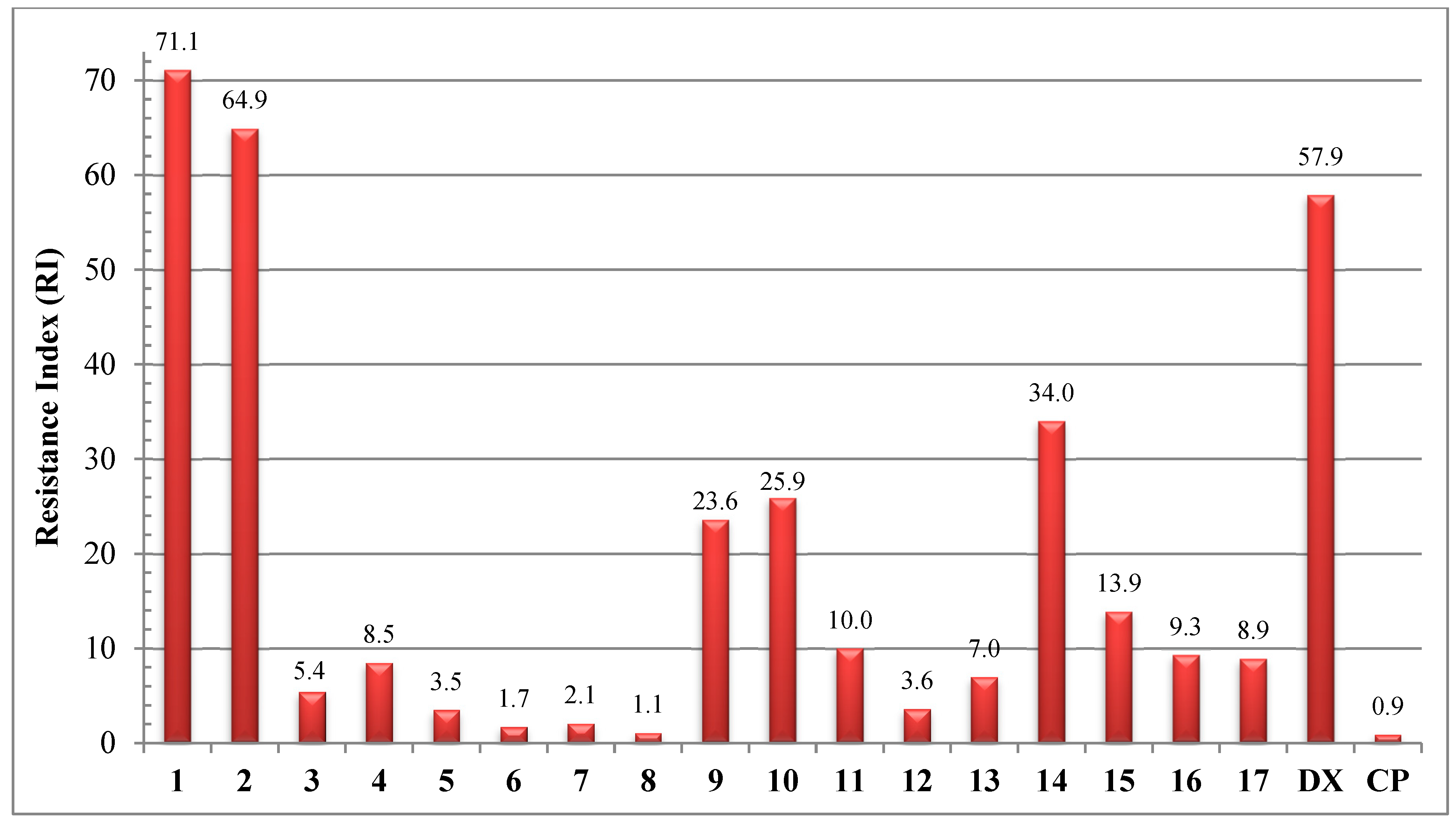
2.2. In Vitro Determination of Drug-Induced Inhibition of Human Cancer Cell Line Growth
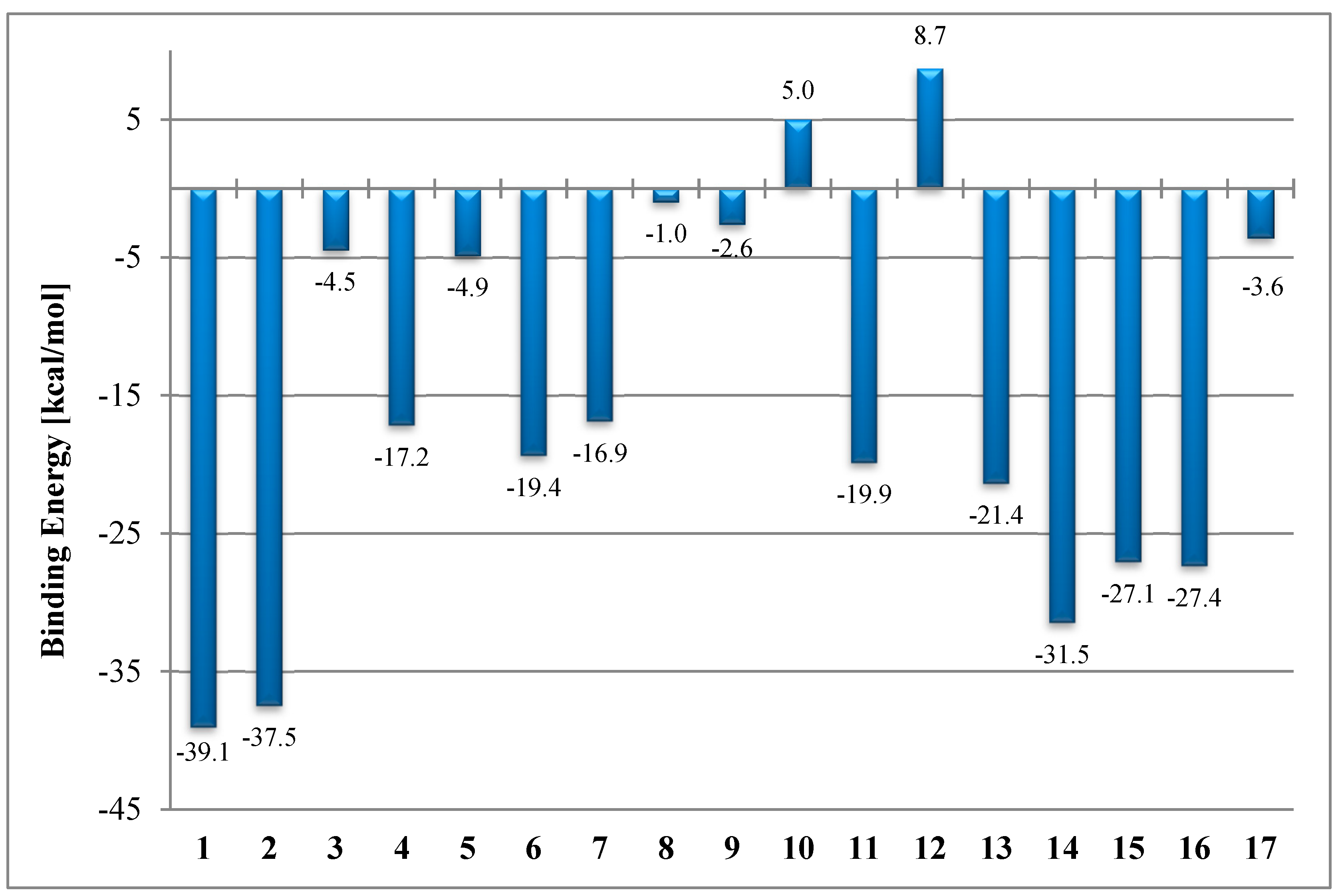
2.3. In Silico Determination of the Molecular Mode of Action
3. Materials and Methods
3.1. General
3.2. Spectroscopic Measurements
3.3. Synthesis
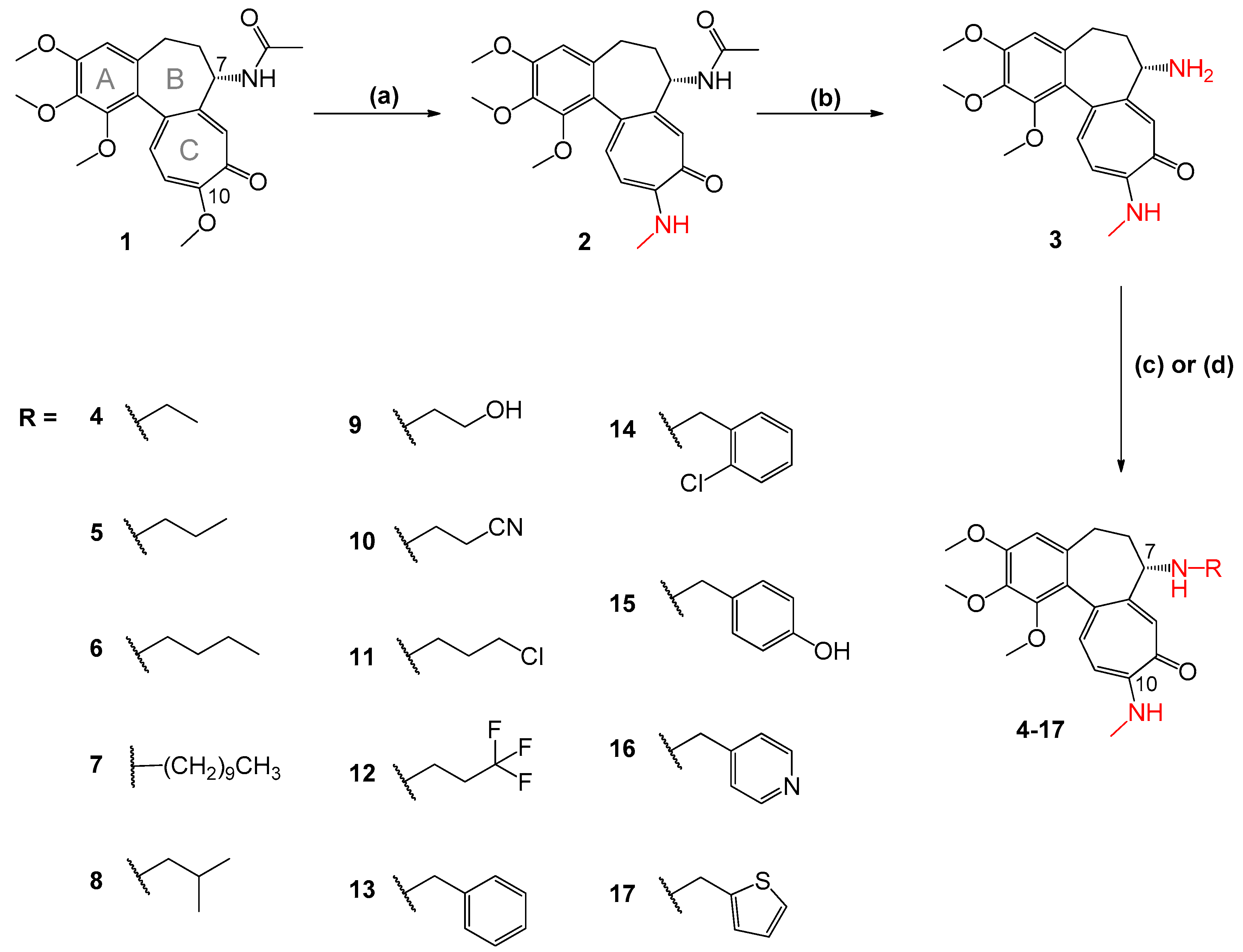
3.3.1. Synthesis of 2 and 3
3.3.2. General Procedure for the Synthesis of Colchicine Derivatives 5–9 and 12–17
Compound 5
Compound 6
Compound 7
Compound 8
Compound 9
Compound 12
Compound 13
Compound 14
Compound 15
Compound 16
Compound 17
3.3.3. General Procedure for the Synthesis of Colchicine Derivatives 4 and 11
Compound 4
Compound 11
3.3.4. Synthesis of 10
3.4. In Vitro Antiproliferative Activity
3.5. Molecular Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wood, K.W.; Cornwell, W.D.; Jackson, J.R. Past and future of the mitotic spindle as an oncology target. Curr. Opin. Pharmacol. 2001, 1, 370–377. [Google Scholar] [CrossRef]
- Vindya, N.G.; Sharma, N.; Yadav, M.; Ethiraj, K.R. Tubulins—The Target for Anticancer Therapy. Curr. Top. Med. Chem. 2015, 15, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Moscow, J.A.; Cowan, K.H. Multidrug Resistance. J. Natl. Cancer Inst. 1988, 80, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Druley, T.E.; Stein, W.D.; Ruth, A.; Roninson, I.B. P-glycoprotein-mediated colchicine resistance in different cell lines correlates with the effects of colchicine on P-glycoprotein conformation. Biochemistry 2001, 40, 4323–4331. [Google Scholar] [CrossRef] [PubMed]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Capraro, H.G.; Brossi, A. Chapter 1 Tropolonic Colchicum Alkaloids. Alkaloids Chem. Pharmacol. 1984, 23, 1–70. [Google Scholar]
- Boyé, O.; Brossi, A. Tropolonic Colchicum Alkaloids and Allo Congeners. Alkaloids Chem. Pharmacol. 1992, 41, 125–176. [Google Scholar]
- Hastie, S.B. Interactions of colchicine with tubulin. Pharmacol. Ther. 1991, 51, 377–401. [Google Scholar] [CrossRef]
- Sapra, S.; Bhalla, Y.; Nandani; Sharma, S.; Singh, G.; Nepali, K.; Budhiraja, A.; Dhar, K.L. Colchicine and its various physicochemical and biological aspects. Med. Chem. Res. 2013, 22, 531–547. [Google Scholar] [CrossRef]
- Skoufias, D.A.; Wilson, L. Mechanism of Inhibition of Microtubule Polymerization by Colchicine: Inhibitory Potencies of Unliganded Colchicine and Tubulin-Colchicine Complexes. Biochemistry 1992, 31, 738–746. [Google Scholar] [CrossRef]
- Bhattacharyya, B.; Panda, D.; Gupta, S.; Banerjee, M. Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med. Res. Rev. 2008, 28, 155–183. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, R.B.G.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Wiesenfeld, P.L.; Garthoff, L.H.; Sobotka, T.J.; Suagee, J.K.; Barton, C.N. Acute oral toxicity of colchicine in rats: Effects of gender, vehicle matrix and pre-exposure to lipopolysaccharide. J. Appl. Toxicol. 2007, 27, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Spiller, H.A. Colchicine. In Encyclopedia of Toxicology: Third Edition; Wexler, P., Ed.; Elsevier Inc.: London, UK, 2014; pp. 1007–1008. ISBN 9780123864543. [Google Scholar]
- Roubille, F.; Kritikou, E.; Busseuil, D.; Barrere-Lemaire, S.; Tardif, J.-C. Colchicine: An Old Wine in a New Bottle? Antiinflamm. Antiallergy Agents Med. Chem. 2013, 12, 14–23. [Google Scholar] [CrossRef]
- Mendis, S. Colchicine cardiotoxicity following ingestion of Gloriosa superba tubers. Postgrad. Med. J. 1989, 65, 752–755. [Google Scholar] [CrossRef]
- Margolis, R.L.; Wilson, L. Addition of colchicine tubulin complex to microtubule ends: The mechanism of substoichiometric colchicine poisoning. Proc. Natl. Acad. Sci. USA 1977, 74, 3466–3470. [Google Scholar] [CrossRef]
- Kuncl, R.W.; Duncan, G.; Watson, D.; Alderson, K.; Rogawski, M.A.; Peper, M. Colchicine Myopathy and Neuropathy. N. Engl. J. Med. 1987, 316, 1562–1568. [Google Scholar] [CrossRef]
- Finkelstein, Y.; Aks, S.E.; Hutson, J.R.; Juurlink, D.N.; Nguyen, P.; Dubnov-Raz, G.; Pollak, U.; Koren, G.; Bentur, Y. Colchicine poisoning: The dark side of an ancient drug. Clin. Toxicol. 2010, 48, 407–414. [Google Scholar] [CrossRef]
- Krzywik, J.; Mozga, W.; Aminpour, M.; Janczak, J.; Maj, E.; Wietrzyk, J.; Tuszyński, J.A.; Huczyński, A. Synthesis, antiproliferative activity and molecular docking studies of novel doubly modified colchicine amides and sulfonamides as anticancer agents. Molecules 2020, 25, 1789. [Google Scholar] [CrossRef]
- Kerekes, P.; Sharma, P.N.; Brossi, A.; Chignell, C.F.; Quinn, F.R. Synthesis and Biological Effects of Novel Thiocolchicines. 3. Evaluation of N-Acyldeacetylthiocolchicines, N-(Alkoxycarbonyl)deacetylthiocolchicines, and O-Ethyldemethylthiocolchicines. New Synthesis of Thiodemecolcine and Antileukemic Effects of 2-Demeth. J. Med. Chem. 1985, 28, 1204–1208. [Google Scholar] [CrossRef]
- Sun, L.; Hamel, E.; Lin, C.M.; Hastie, S.B.; Pyluck, A.; Lee, K.H. Antitumor Agents. 141. Synthesis and Biological Evaluation of Novel Thiocolchicine Analogs: N-Acyl-, N-Aroyl-, and N-(Substituted benzyl)deacetylthiocolchicines as Potent Cytotoxic and Antimitotic Compounds. J. Med. Chem. 1993, 36, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Gelmi, M.L.; Mottadelli, S.; Pocar, D.; Riva, A.; Bombardelli, E.; De Vincenzo, R.; Scambia, G. N-deacetyl-N-aminoacylthiocolchicine derivatives: Synthesis and biological evaluation on MDR-positive and MDR-negative human cancer cell lines. J. Med. Chem. 1999, 42, 5272–5276. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, S.K.; Kim, J.M.; Kim, M.H.; Kim, K.H.; Chun, K.W.; Cho, K.H.; Youn, J.Y.; Namgoong, S.K. New synthetic thiocolchicine derivatives as low-toxic anticancer agents. Arch. Pharm. (Weinheim) 2005, 338, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Marzo-Mas, A.; Barbier, P.; Breuzard, G.; Allegro, D.; Falomir, E.; Murga, J.; Carda, M.; Peyrot, V.; Marco, J.A. Interactions of long-chain homologues of colchicine with tubulin. Eur. J. Med. Chem. 2017, 126, 526–535. [Google Scholar] [CrossRef]
- Majcher, U.; Urbaniak, A.; Maj, E.; Moshari, M.; Delgado, M.; Wietrzyk, J.; Bartl, F.; Chambers, T.C.; Tuszynski, J.A.; Huczyński, A. Synthesis, antiproliferative activity and molecular docking of thiocolchicine urethanes. Bioorg. Chem. 2018, 81, 553–566. [Google Scholar] [CrossRef]
- Marzo-Mas, A.; Falomir, E.; Murga, J.; Carda, M.; Marco, J.A. Effects on tubulin polymerization and down-regulation of c-Myc, hTERT and VEGF genes by colchicine haloacetyl and haloaroyl derivatives. Eur. J. Med. Chem. 2018, 150, 591–600. [Google Scholar] [CrossRef]
- Testa, B.; Mayer, J.M. Hydrolysis in Drug and Prodrug Metabolism: Chemistry, Biochemistry, and Enzymology; Verlag Helvetica Chimica Acta: Zürich, Switzerland, 2006; ISBN 9783906390444. [Google Scholar]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T.; Savolainen, J. Prodrugs: Design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef]
- Cosentino, L.; Redondo-Horcajo, M.; Zhao, Y.; Santos, A.R.; Chowdury, K.F.; Vinader, V.; Abdallah, Q.M.A.; Abdel-Rahman, H.; Fournier-Dit-Chabert, J.; Shnyder, S.D.; et al. Synthesis and biological evaluation of colchicine B-ring analogues tethered with halogenated benzyl moieties. J. Med. Chem. 2012, 55, 11062–11066. [Google Scholar]
- Kiyoshi, A. N-Methyldeacetylcolchiceinamide Derivatives. Patent EP0607647(A1), 27 July 1994. [Google Scholar]
- Corey, E.J.; Suggs, J.W. Pyridinium chlorochromate. An efficient reagent for oxidation of primary and secondary alcohols to carbonyl compounds. Tetrahedron Lett. 1975, 16, 2647–2650. [Google Scholar] [CrossRef]
- Tateishi, T.; Soucek, P.; Caraco, Y.; Peter Gnengerich, F.; Wood, A.J.J. Colchicine biotransformation by human liver microsomes. Biochem. Pharmacol. 1997, 53, 111–116. [Google Scholar] [CrossRef]
- Niel, E.; Scherrmann, J.M. Colchicine today. Jt. Bone Spine 2006, 73, 672–678. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Compound | A549 | MCF-7 | LoVo | LoVo/DX | BALB/3T3 |
|---|---|---|---|---|---|
| IC50 [nM] | IC50 [nM] | IC50 [nM] | IC50 [nM] | IC50 [nM] | |
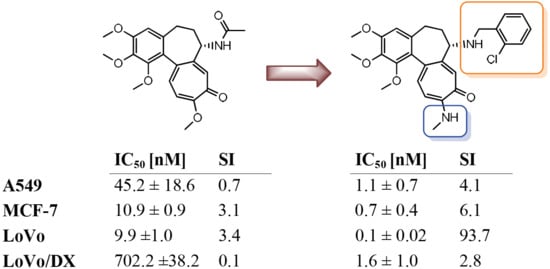
| 1 | 45.2 ± 18.6 | 10.9 ± 0.9 | 9.9 ± 1.0 | 702.2 ± 38.2 | 33.1 ± 16.0 |
| 2 | 7.2 ± 1.3 | 1.6 ± 0.5 | 1.2 ± 0.2 | 80.6 ± 18.7 | 7.5 ± 1.0 |
| 3 | 8.6 ± 3.9 | 9.8 ± 4.5 | 7.4 ± 2.7 | 39.5 ± 18.6 | 7.1 ± 3.6 |
| 4 | 9.4 ± 3.2 | 8.7 ± 3.3 | 7.3 ± 2.5 | 62.0 ± 7.5 | 8.0 ± 1.6 |
| 5 | 10.5 ± 0.7 | 10.4 ± 0.6 | 5.0 ± 0.1 | 17.2 ± 6.7 | 8.6 ± 0.4 |
| 6 | 8.1 ± 2.6 | 8.7 ± 3.1 | 7.8 ± 1.3 | 13.3 ± 5.8 | 7.5 ± 1.6 |
| 7 | 88.4 ± 3.2 | 75.2 ± 10.8 | 46.6 ± 15.5 | 99.5 ± 35.5 | 70.4 ± 1.2 |
| 8 | 9.6 ± 1.3 | 9.7 ± 1.5 | 7.8 ± 1.0 | 8.5 ± 1.1 | 7.5 ± 1.5 |
| 9 | 9.2 ± 2.2 | 10.1 ± 1.2 | 5.6 ± 1.5 | 132.0 ± 37.5 | 8.2 ± 0.9 |
| 10 | 9.0 ± 1.0 | 7.5 ± 0.7 | 2.0 ± 0.6 | 51.5 ± 8.6 | 6.7 ± 1.3 |
| 11 | 8.4 ± 2.2 | 7.6 ± 2.8 | 1.3 ± 0.3 | 12.8 ± 3.3 | 5.9 ± 2.5 |
| 12 | 9.5 ± 0.2 | 9.0 ± 1.7 | 2.3 ± 1.3 | 8.2 ± 0.6 | 7.9 ± 0.4 |
| 13 | 4.6 ± 1.8 | 7.3 ± 4.4 | 0.9 ± 0.4 | 6.0 ± 0.8 | 6.2 ± 0.7 |
| 14 | 1.1 ± 0.7 | 0.7 ± 0.4 | 0.1 ± 0.02 | 1.6 ± 1.0 | 4.5 ± 2.7 |
| 15 | 8.9 ± 1.4 | 8.5 ± 0.9 | 5.2 ± 0.5 | 72.2 ± 12.5 | 7.3 ± 0.7 |
| 16 | 8.9 ± 1.7 | 9.6 ± 1.8 | 5.2 ± 1.9 | 48.1 ± 14.5 | 6.9 ± 1.3 |
| 17 | 9.4 ± 0.5 | 9.3 ± 0.6 | 0.9 ± 0.3 | 7.9 ± 1.0 | 4.9 ± 2.8 |
| Doxorubicin | 172.0 ± 58.0 | 131.5 ± 63.7 | 83.2 ± 61.1 | 4814.7 ± 1996.9 | 32.3 ± 28.6 |
| Cisplatin | 4916.9 ± 1852.4 | 5812.2 ± 2610.6 | 5463.2 ± 1412.4 | 5013.9 ± 1562.2 | 3968.8 ± 995.1 |
| Compound | 3D Representation of the Interactions | 2D Representation of the Interactions | Binding Energy [kcal/mol] | Active Residues |
|---|---|---|---|---|
| 1 | 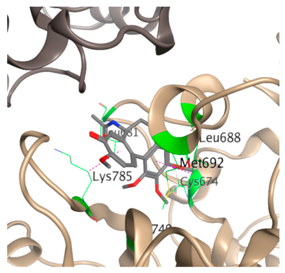 |  | −39.1 | Cys674 Leu681 Ala683 Leu688 Met692 Ala749 Lys785 |
| 2 | 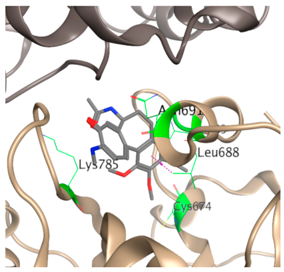 | 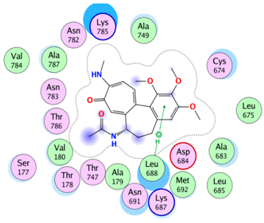 | −37.5 | Cys674 Ala683 Leu688 Asn691 Lys785 |
| 3 | 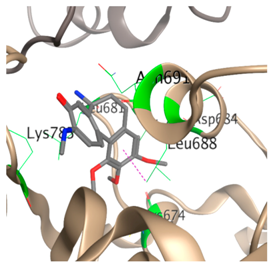 |  | −4.5 | Ser177 Cys674 Leu681 Ala683 Asp684 Leu688 Asn691 Lys785 |
| 14 | 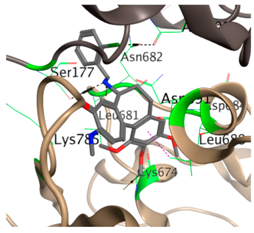 |  | −31.5 | Asn100 Ser177 Cys674 Leu681 Asn682 Asp684 Leu688 Lys785 |
 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzywik, J.; Aminpour, M.; Maj, E.; Mozga, W.; Wietrzyk, J.; Tuszyński, J.A.; Huczyński, A. New Series of Double-Modified Colchicine Derivatives: Synthesis, Cytotoxic Effect and Molecular Docking. Molecules 2020, 25, 3540. https://doi.org/10.3390/molecules25153540
Krzywik J, Aminpour M, Maj E, Mozga W, Wietrzyk J, Tuszyński JA, Huczyński A. New Series of Double-Modified Colchicine Derivatives: Synthesis, Cytotoxic Effect and Molecular Docking. Molecules. 2020; 25(15):3540. https://doi.org/10.3390/molecules25153540
Chicago/Turabian StyleKrzywik, Julia, Maral Aminpour, Ewa Maj, Witold Mozga, Joanna Wietrzyk, Jack A. Tuszyński, and Adam Huczyński. 2020. "New Series of Double-Modified Colchicine Derivatives: Synthesis, Cytotoxic Effect and Molecular Docking" Molecules 25, no. 15: 3540. https://doi.org/10.3390/molecules25153540
APA StyleKrzywik, J., Aminpour, M., Maj, E., Mozga, W., Wietrzyk, J., Tuszyński, J. A., & Huczyński, A. (2020). New Series of Double-Modified Colchicine Derivatives: Synthesis, Cytotoxic Effect and Molecular Docking. Molecules, 25(15), 3540. https://doi.org/10.3390/molecules25153540