Constituents of Huberantha jenkinsii and Their Biological Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Characterization
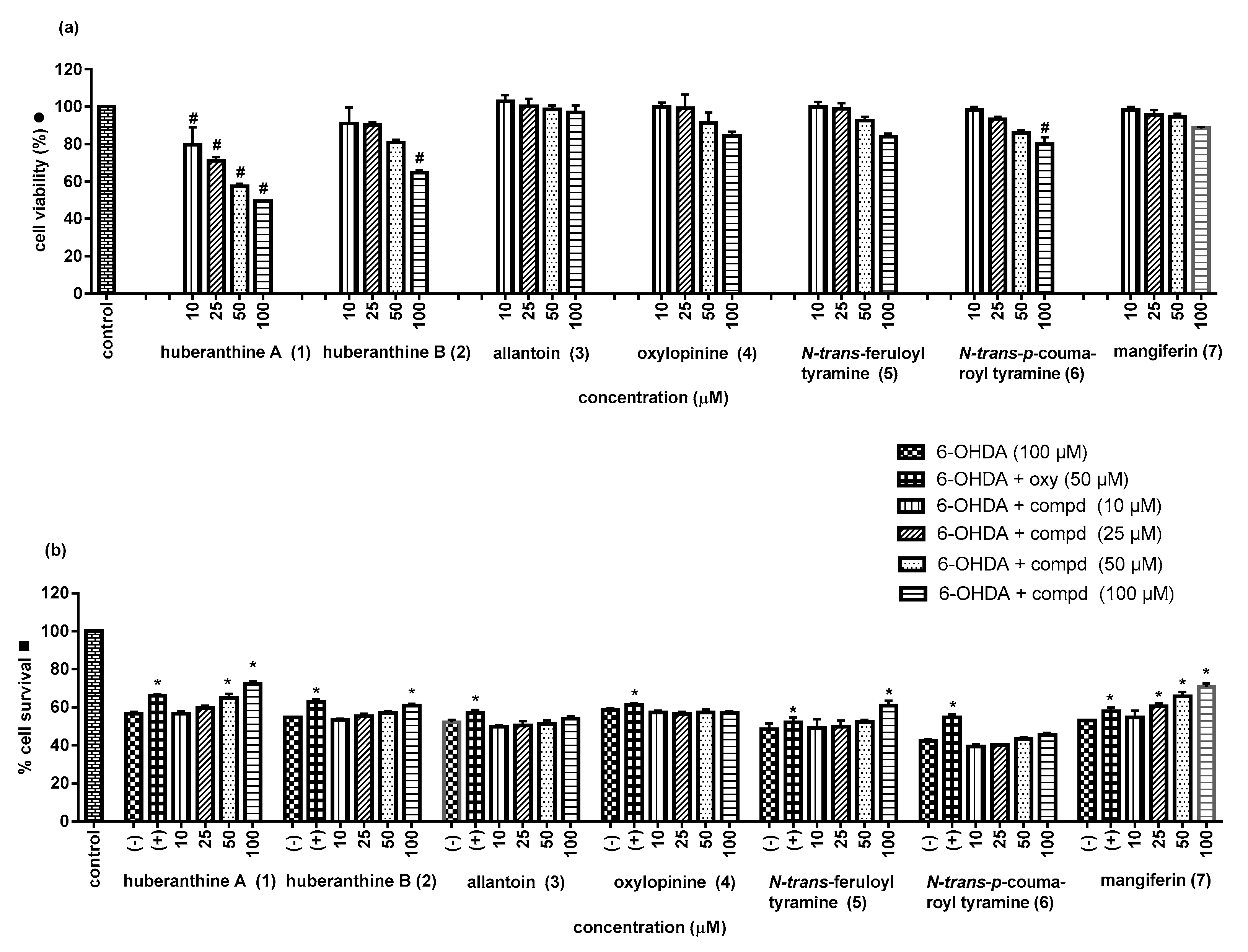
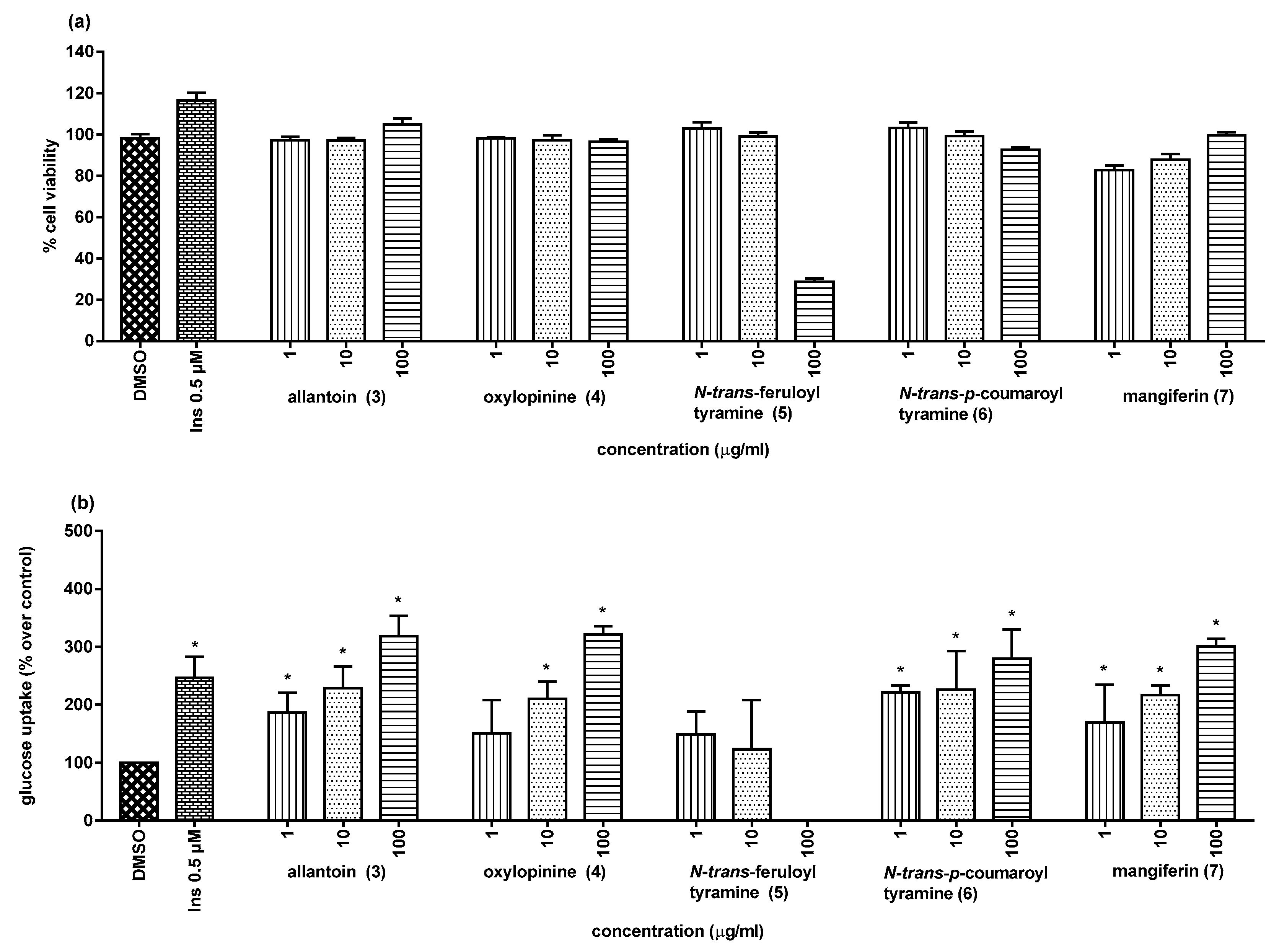
2.2. Biological Activities
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction, Isolation, and Purification
3.4. Assay for Neuroprotective Activity
3.5. Assay for α-Glucosidase Activity
3.6. Assay for Glucose Uptake Stimulatory Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011; pp. 33–40.
- Furman, B.L.; Candasamy, M.; Bhattamisra, S.K.; Veettil, S.K. Reduction of blood glucose by plant extracts and their use in the treatment of diabetes mellitus; discrepancies in effectiveness between animal and human studies. J. Ethnopharmacol. 2019, 247, 112264. [Google Scholar] [CrossRef]
- Sairazi, N.S.M.; Sirajudeen, K.N.S. Natural products and their bioactive compounds: neuroprotective potentials against neurodegenerative diseases. Evid.-Based Complement. Altern. Med. 2020, 2020, 6565396–6565430. [Google Scholar] [CrossRef]
- Chatsumpun, N.; Sritularak, B.; Likhitwitayawuid, K. New biflavonoids with α-glucosidase and pancreatic lipase inhibitory activities from Boesenbergia rotunda. Molecules 2017, 22, 1862. [Google Scholar] [CrossRef]
- Tadtong, S.; Chatsumpun, N.; Sritularak, B.; Jongbunprasert, V.; Ploypradith, P.; Likhitwitayawuid, K. Effects of oxyresveratrol and its derivatives on cultured P19-derived neurons. Trop. J. Pharm. Res. 2017, 15, 2619. [Google Scholar] [CrossRef]
- Sangsen, Y.; Sooksawate, T.; Likhitwitayawuid, K.; Sritularak, B.; Wiwattanapatapee, R. A self-microemulsifying formulation of oxyresveratrol prevents amyloid beta protein-induced neurodegeneration in mice. Planta Med. 2018, 84, 820–828. [Google Scholar] [CrossRef]
- Chaowasku, T.; Johnson, D.M.; Van Der Ham, R.; Chatrou, L.W. Characterization of Hubera (Annonaceae), a new genus segregated from Polyalthia and allied to Miliusa. Phytotaxa 2012, 69, 33. [Google Scholar] [CrossRef]
- Chaowasku, T.; Johnson, D.M.; Van Der Ham, R.W.J.M.; Chatrou, L.W. Huberantha, a replacement name for Hubera (Annonaceae: Malmeoideae: Miliuseae). Kew Bull. 2015, 70. [Google Scholar] [CrossRef]
- Suthiphasilp, V.; Maneerat, W.; Rujanapun, N.; Duangyod, T.; Charoensup, R.; Deachathai, S.; Andersen, R.J.; Patrick, B.O.; Pyne, S.G.; Laphookhieo, S. α-Glucosidase inhibitory and nitric oxide production inhibitory activities of alkaloids isolated from a twig extract of Polyalthia cinnamomea. Bioorg. Med. Chem. 2020, 28, 115462. [Google Scholar] [CrossRef] [PubMed]
- Nantapap, S.; Sabgrueng, K.; Numtasaen, N.; Meepowpan, P.; Pompimon, W. Chemical constituents from aerial parts of Polyalthia evecta (Pierre) Finet & Gagnep. var. attopeuensis. Int. J. Chem. Sci. 2015, 13, 1705–1712. [Google Scholar]
- Zhang, J.; El-Shabrawy, A.-R.O.; El-Shanawany, M.A.; Schiff, P.L.; Slatkin, D.J. New azafluorene alkaloids from Oxandra xylopioides. J. Nat. Prod. 1987, 50, 800–806. [Google Scholar] [CrossRef]
- Al-Taweel, A.M.; Perveen, S.; El-Shafae, A.M.; Fawzy, G.A.; Malik, A.; Afza, N.; Iqbal, L.; Latif, M. Bioactive phenolic amides from Celtis africana. Molecules 2012, 17, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- Talamond, P.; Mondolot, L.; Gargadennec, A.; De Kochko, A.; Hamon, S.; Fruchier, A.; Campa, C. First report on mangiferin (C-glucosyl-xanthone) isolated from leaves of a wild coffee plant, Coffea pseudozanguebariae (Rubiaceae). Acta Bot. Gallica 2008, 155, 513–519. [Google Scholar] [CrossRef]
- González, M.C.; Zafra-Polo, C.; Blázquez, M.A.; Serrano, A.; Cortes, D. Cerasodine and cerasonine: New oxoprotoberberine alkaloids from Polyalthia cerasoides. J. Nat. Prod. 1997, 60, 108–110. [Google Scholar] [CrossRef]
- Shono, T.; Ishikawa, N.; Toume, K.; Arai, M.A.; Masu, H.; Koyano, T.; Kowithayakorn, T.; Ishibashi, M. Cerasoidine, a bis-aporphine alkaloid isolated from Polyalthia cerasoides during screening for Wnt signal inhibitors. J. Nat. Prod. 2016, 79, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Gadhiya, S.; Ponnala, S.; Harding, W.W.; Gadhiya, S. A divergent route to 9, 10-oxygenated tetrahydroprotoberberine and 8-oxoprotoberberine alkaloids: synthesis of (±)-isocorypalmine and oxypalmatine. Tetrahedron 2015, 71, 1227–1231. [Google Scholar] [CrossRef]
- Chao, J.; Yu, M.-S.; Ho, Y.-S.; Wang, M.; Chang, R.C.C. Dietary oxyresveratrol prevents parkinsonian mimetic 6-hydroxydopamine neurotoxicity. Free Radic. Biol. Med. 2008, 45, 1019–1026. [Google Scholar] [CrossRef]
- Lee, H.J.; Noh, Y.H.; Lee, D.; Kim, Y.-S.; Kim, K.; Chung, Y.H.; Lee, W.; Kim, S. Baicalein attenuates 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. Eur. J. Cell Biol. 2005, 84, 897–905. [Google Scholar] [CrossRef]
- Shah, A.; Chao, J.; Legido-Quigley, C.; Chang, R.C.-C. Oxyresveratrol exerts ATF4- and Grp78-mediated neuroprotection against endoplasmic reticulum stress in experimental Parkinson’s disease. Nutr. Neurosci. 2019, 1–16. [Google Scholar] [CrossRef]
- Feng, S.-T.; Wang, Z.-Z.; Yuan, Y.-H.; Sun, H.-M.; Chen, N.-H.; Zhang, Y. Mangiferin: a multipotent natural product preventing neurodegeneration in Alzheimer’s and Parkinson’s disease models. Pharmacol. Res. 2019, 146, 104336. [Google Scholar] [CrossRef]
- San, H.T.; Boonsnongcheep, P.; Putalun, W.; Mekboonsonglarp, W.; Sritularak, B.; Likhitwitayawuid, K. α-Glucosidase inhibitory and glucose uptake stimulatory effects of phenolic compounds from Dendrobium christyanum. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- San, H.T.; Boonsnongchee, P.; Putalun, W.; Sritularak, B.; Likhitwitayawuid, K. Bergenin from Cissus javana DC. (Vitaceae) root extract enhances glucose uptake by rat L6 myotubes. Trop. J. Pharm. Res. 2020, 19, 1081–1086. [Google Scholar] [CrossRef]
- Panidthananon, W.; Chaowasku, T.; Sritularak, B.; Likhitwitayawuid, K. A new benzophenone C-glucoside and other constituents of Pseuduvaria fragrans and their α-glucosidase inhibitory activity. Molecules 2018, 23, 1600. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.M.; Rathod, V.K. Exploring the potential of Mangifera indica leaves extract versus mangiferin for therapeutic application. Agric. Nat. Resour. 2018, 52, 155–161. [Google Scholar] [CrossRef]
- Ma, J.; Kang, S.Y.; Meng, X.; Na Kang, A.; Park, J.H.; Park, Y.-K.; Jung, H.W. Effects of rhizome extract of Dioscorea batatas and its active compound, allantoin, on the regulation of myoblast differentiation and mitochondrial biogenesis in C2C12 myotubes. Molecuels 2018, 23, 2023. [Google Scholar] [CrossRef] [PubMed]
- Girón, M.D.; Sevillano, N.; Salto, R.; Haidour, A.; Manzano, M.; Jimenez, M.; Rueda, R.; López-Pedrosa, J.M. Salacia oblonga extract increases glucose transporter 4-mediated glucose uptake in L6 rat myotubes: role of mangiferin. Clin. Nutr. 2009, 28, 565–574. [Google Scholar] [CrossRef]
- Du, S.; Liu, H.; Lei, T.; Xie, X.; Wang, H.; He, X.; Tong, R.; Wang, Y. Mangiferin: An effective therapeutic agent against several disorders (review). Mol. Med. Rep. 2018, 18, 4775–4786. [Google Scholar] [CrossRef]
- Sekar, V.; Mani, S.; Malarvizhi, R.; Nithya, P.; Vasanthi, H.R. Antidiabetic effect of mangiferin in combination with oral hypoglycemic agents metformin and gliclazide. Phytomedicine 2019, 59, 152901. [Google Scholar] [CrossRef]
- Jyotshna; Khare, P.; Shanker, K.; Chi, Y. Mangiferin: A review of sources and interventions for biological activities. BioFactors 2016, 42, 504–514. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

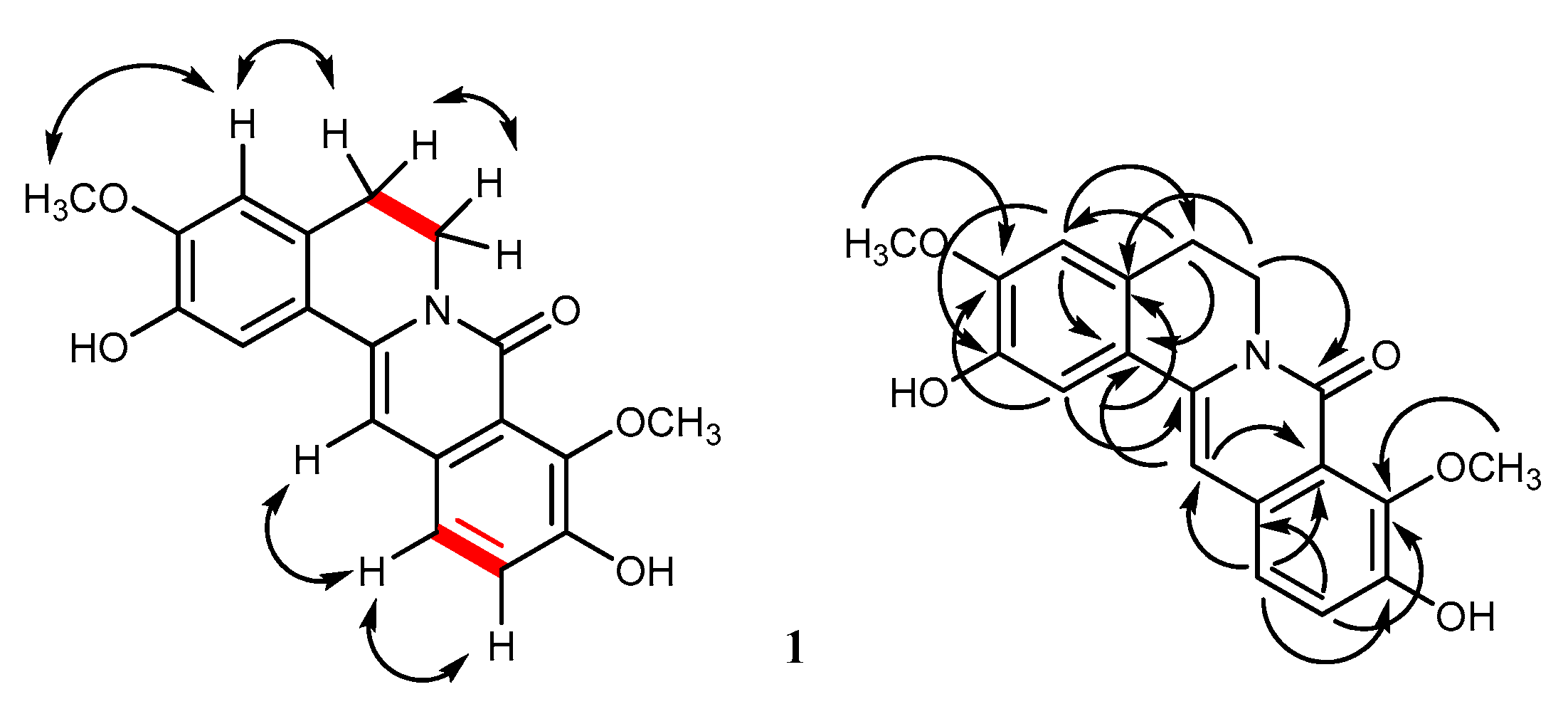
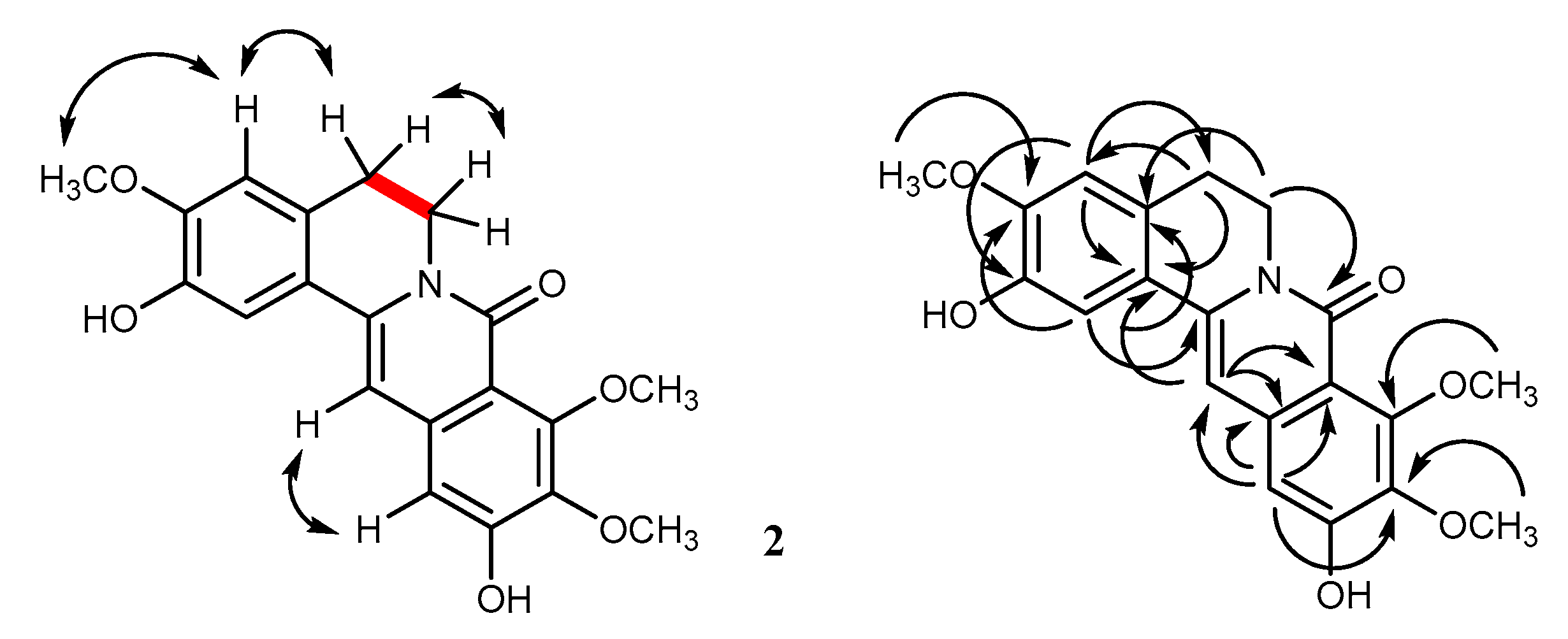
| Position | 1 * | 2 * | ||
|---|---|---|---|---|
| δH (Multiplicity, J in Hz) | δC | δH (Multiplicity, J in Hz) | δC | |
| 1 | 7.33 (1H, s) | 111.8 | 7.33 (1H, s) | 112.0 |
| 2 | 146.7 | 146.7 | ||
| 3 | 149.4 | 149.6 | ||
| 4 | 6.90 (1H, s) | 111.4 | 6.90 (1H, s) | 111.4 |
| 4a | 128.1 | 128.4 | ||
| 5 | 2.91 (2H, t, 6.0) | 28.6 | 2.89 (2H, dd, 6.5, 6.0) | 28.7 |
| 6 | 4.20 (2H, t, 6.0) | 40.1 | 4.16 (2H, dd, 6.5, 6.0) | 39.7 |
| 8 | 159.9 | 159.7 | ||
| 8a | 119.5 | 113.1 | ||
| 9 | 147.0 | 155.4 | ||
| 10 | 149.5 | 141.5 | ||
| 11 | 7.27 (1H, d, 8.4) | 122.6 | 155.2 | |
| 12 | 7.33 (1H, d, 8.4) | 123.7 | 6.85 (1H, s) | 107.3 |
| 12a | 132.9 | 136.9 | ||
| 13 | 6.91 (1H, s) | 101.5 | 6.80 (1H, s) | 100.7 |
| 14 | 136.1 | 137.9 | ||
| 14a | 123.6 | 123.4 | ||
| MeO-2 | ||||
| MeO-3 | 3.89 (3H, s) | 56.3 | 3.90 (3H, s) | 61.5 |
| MeO-9 | 3.91 (3H, s) | 62.2 | 3.90 (3H, s) | 61.9 |
| MeO-10 | 3.88 (3H, s) | 56.3 | ||
| Sample | % Cell Survival | Sample | % Cell Survival |
|---|---|---|---|
| Control | 100 | ||
| Huberanthine A (1) | N-trans-Feruloyl tyramine (5) | ||
| 10 μM | 56.7 ± 1.2 | 10 μM | 48.9 ± 4.9 |
| 25 μM | 59.7 ± 1.1 | 25 μM | 49.8 ± 3.3 |
| 50 μM | 65.0 ± 2.1 * | 50 μM | 51.8 ± 1.1 |
| 100 μM | 72.4 ± 1.1 * | 100 μM | 60.9 ± 2.5 * |
| 6-OHDA (100 µM) | 56.8 ± 0.8 | 6-OHDA (100 µM) | 48.4 ± 3.3 |
| Oxyresveratrol (50 µM) | 66.0 ± 0.6 * | Oxyresveratrol (50 µM) | 56.1 ± 3.3 * |
| Huberanthine B (2) | N-trans-p-Coumaroyl tytyramine (6) | ||
| 10 μM | 53.6 ± 0.3 | 10 μM | 39.3 ± 1.4 |
| 25 μM | 55.3 ± 1.3 | 25 μM | 40.2 ± 0.3 |
| 50 μM | 57.1 ± 0.8 | 50 μM | 43.5 ± 0.8 |
| 100 μM | 60.9 ± 0.9 * | 100 μM | 45.5 ± 1.0 |
| 6-OHDA (100 µM) | 54.7 ± 0.4 | 6-OHDA (100 µM) | 42.5 ± 0.7 |
| Oxyresveratrol (50 µM) | 62.9 ± 1.2 * | Oxyresveratrol (50 µM) | 54.7 ± 1.5 * |
| Allantoin (3) | Mangiferin (7) | ||
| 10 μM | 49.7 ± 1.0 | 10 μM | 54.8 ± 3.5 |
| 25 μM | 50.5 ± 2.3 | 25 μM | 60.5 ± 1.7 * |
| 50 μM | 51.3 ± 1.9 | 50 μM | 65.7 ± 2.5 * |
| 100 μM | 54.2 ± 1.0 | 100 μM | 70.7 ± 1.8 * |
| 6-OHDA (100 µM) | 52.2 ± 1.2 | 6-OHDA (100 µM) | 53.1 ± 0.5 |
| Oxyresveratrol (50 µM) | 57.1 ± 1.6 * | Oxyresveratrol (50 µM) | 57.9 ± 1.8 * |
| Oxylopinine (4) | |||
| 10 μM | 45.5 ± 1.0 | ||
| 25 μM | 56.5 ± 1.0 | ||
| 50 μM | 57.2 ± 1.9 | ||
| 100 μM | 57.0 ± 0.8 | ||
| 6-OHDA (100 µM) | 58.4 ± 0.9 | ||
| Oxyresveratrol (50 µM) | 61.0 ± 1.2 * |
| Sample | % Glucose Uptake | % Enhancement |
|---|---|---|
| DMSO | 100 | 0 |
| Insulin (0.5 µM) | 246.6 ± 35.8 * | 146.6 |
| Allantoin (3) | ||
| 1 μg/mL (6.32 µM) | 186.5 ± 34.3 * | 86.5 |
| 10 μg/mL (63.24 µM) | 228.4 ± 37.7 * | 128.4 |
| 100 μg/mL (632.44 µM) | 318.2 ± 35.5 * | 218.2 |
| Oxylopinine (4) | ||
| 1 μg/mL (4.73 µM) | 150.7 ± 57.1 | NA |
| 10 μg/mL (47.34 µM) | 210.1 ± 29.6 * | 110.1 |
| 100 μg/mL (473.44 µM) | 321.0 ± 14.6 * | 221.0 |
| N-trans-Feruloyl tyramine (5) | ||
| 1 μg/mL (3.19 µM) | 148.7 ± 39.7 | NA |
| 10 μg/mL (31.91 µM) | 123.7 ± 84.6 | NA |
| 100 μg/mL (319.13 µM) | NC | NA |
| N-trans-p-Coumaroyl tyramine (6) | ||
| 1 μg/mL (3.53 µM) | 221.0 ± 12.5 * | 121.0 |
| 10 μg/mL (35.29 µM) | 225.7 ± 67.3 * | 125.7 |
| 100 μg/mL (352.95 µM) | 279.7 ± 49.9 * | 179.7 |
| Mangiferin (7) | ||
| 1 μg/mL (2.32 µM) | 169.6 ± 14.9 * | 69.6 |
| 10 μg/mL (23.68 µM) | 216.9 ± 16.4 * | 116.9 |
| 100 μg/mL (236.77 µM) | 300.7 ± 12.9 * | 200.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
San, H.T.; Chaowasku, T.; Mekboonsonglarp, W.; Rodsiri, R.; Sritularak, B.; Buraphaka, H.; Putalun, W.; Likhitwitayawuid, K. Constituents of Huberantha jenkinsii and Their Biological Activities. Molecules 2020, 25, 3533. https://doi.org/10.3390/molecules25153533
San HT, Chaowasku T, Mekboonsonglarp W, Rodsiri R, Sritularak B, Buraphaka H, Putalun W, Likhitwitayawuid K. Constituents of Huberantha jenkinsii and Their Biological Activities. Molecules. 2020; 25(15):3533. https://doi.org/10.3390/molecules25153533
Chicago/Turabian StyleSan, Htoo Tint, Tanawat Chaowasku, Wanwimon Mekboonsonglarp, Ratchanee Rodsiri, Boonchoo Sritularak, Hathairat Buraphaka, Waraporn Putalun, and Kittisak Likhitwitayawuid. 2020. "Constituents of Huberantha jenkinsii and Their Biological Activities" Molecules 25, no. 15: 3533. https://doi.org/10.3390/molecules25153533
APA StyleSan, H. T., Chaowasku, T., Mekboonsonglarp, W., Rodsiri, R., Sritularak, B., Buraphaka, H., Putalun, W., & Likhitwitayawuid, K. (2020). Constituents of Huberantha jenkinsii and Their Biological Activities. Molecules, 25(15), 3533. https://doi.org/10.3390/molecules25153533







