Design, Synthesis, and Biological Investigation of Novel Classes of 3-Carene-Derived Potent Inhibitors of TDP1
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biology
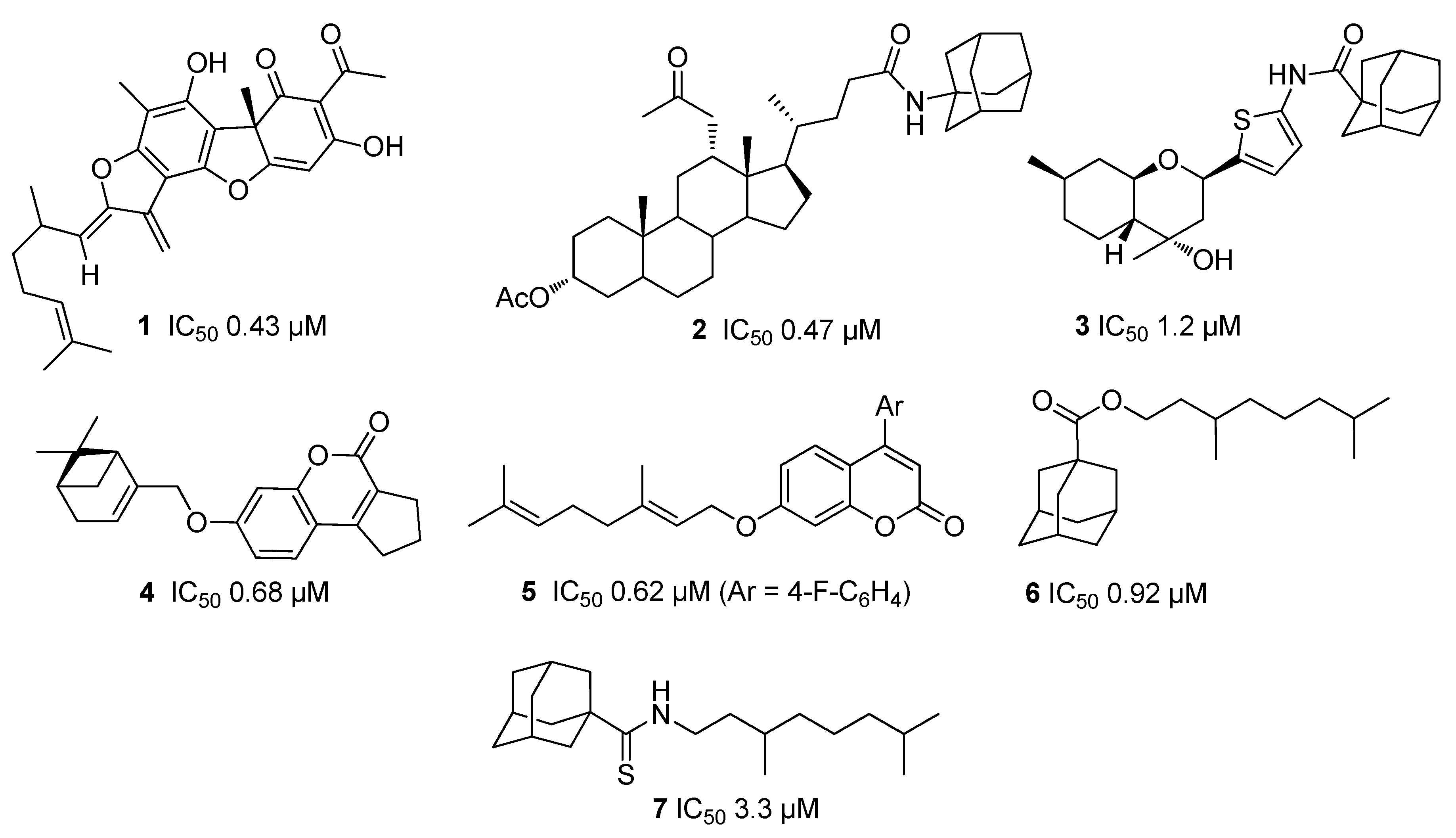
2.2.1. Structure–Activity Relationship Analysis
2.2.2. Cell Growth and Viability
Topotecan Cytotoxicity on HEK293FT Wild Type and TDP1−/− Cells
TDP1 Inhibitors’ Cytotoxicity on HEK293FT TDP1−/− Cells
Activity of 11h and 12k with Topotecan against Tumor Cells
2.2.3. Chemical Space
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure (GP)
3.1.2. Reaction of 2-Carene-Containing Mixture and 4-hydroxy-3-methoxybenzaldehyde 10a
3.1.3. Reaction of 2-Carene-Containing Mixture and Crotonaldehyde 10c
3.1.4. Reaction of 2-Carene-Containing Mixture and 2-Thiophenecarbaldehyde 10d
3.1.5. Reaction of 2-Carene-Containing Mixture and 3-methylthiophene-2-carbaldehyde 10e
3.1.6. Reaction of 2-Carene-Containing Mixture and 5-methylthiophene-2-carbaldehyde 10f
3.1.7. Reaction of 2-Carene-Containing Mixture and 4-bromothiophene-2-carbaldehyde 10g
3.1.8. Reaction of 2-Carene-Containing Mixture and 5-bromothiophene-2-carbaldehyde 10h
3.1.9. Reaction of 2-Carene-Containing Mixture and 5-nitrothiophene-2-carbaldehyde 10i
3.1.10. Reaction of 2-Carene-Containing Mixture and 5-nitrofuran-2-carbaldehyde 10j
3.1.11. Reaction of 2-Carene-Containing Mixture and thiophene-3-carbaldehyde 10k
3.1.12. Reaction of Limonene and 2-thiophenecarbaldehyde 10d
3.2. Real-Time Detection of TDP1 Activity
3.3. TDP1 Activity by Gel-Based Assay
3.4. Obtainment of TDP1 Knockout HEK293FT Clones
3.4.1. Plasmid Construction for Human TDP1 Gene Knockout
3.4.2. Knockout HEK293FT Clone Generation
3.4.3. Analysis of CRISPR/Cas9-Mediated Deletions in the TDP1 Gene
3.5. Cell Culture Assay
3.6. Calculation of Molecular Descriptors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, S.-Y.N.; Pommier, Y.; Marchand, C. Tyrosyl-DNA Phosphodiesterase 1 (Tdp1) inhibitors. Expert Opin. Ther. Patents 2011, 21, 1285–1292. [Google Scholar] [CrossRef]
- Laev, S.S.; Salakhutdinov, N.F.; Lavrik, O.I. Tyrosyl-DNA phosphodiesterase inhibitors: Progress and potential. Bioorganic Med. Chem. 2016, 24, 5017–5027. [Google Scholar] [CrossRef] [PubMed]
- Zakharenko, A.; Dyrkheeva, N.; Lavrik, O.I. Dual DNA topoisomerase 1 and tyrosyl-DNA phosphodiesterase 1 inhibition for improved anticancer activity. Med. Res. Rev. 2019, 39, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Kawale, A.S.; Povirk, L.F. Tyrosyl-DNA phosphodiesterases: Rescuing the genome from the risks of relaxation. Nucleic Acids Res. 2018, 46, 520–537. [Google Scholar] [CrossRef] [PubMed]
- Interthal, H.; Pouliot, J.J.; Champoux, J.J. The tyrosyl-DNA phosphodiesterase Tdp1 is a member of the phospholipase D superfamily. Proc. Natl. Acad. Sci. USA 2001, 98, 12009–12014. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef]
- Dexheimer, T.S.; Antony, S.; Marchand, C.; Pommier, Y. Tyrosyl-DNA phosphodiesterase as a target for anticancer therapy. Anti-Cancer Agents Med. Chem. 2008, 8, 381–389. [Google Scholar] [CrossRef]
- Beretta, G.L.; Cossa, G.; Gatti, L.; Zunino, F.; Perego, P. Tyrosyl-DNA Phosphodiesterase 1 Targeting for Modulation of Camptothecin-Based Treatment. Curr. Med. Chem. 2010, 17, 1500–1508. [Google Scholar] [CrossRef]
- Ledesma, F.C.; El Khamisy, S.F.; Zuma, M.C.; Osborn, K.; Caldecott, K.W. A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature 2009, 461, 674–678. [Google Scholar] [CrossRef]
- Filimonov, A.S.; Chepanova, A.A.; Luzina, O.A.; Zakharenko, A.L.; Zakharova, O.D.; Ilina, E.S.; Dyrkheeva, N.S.; Kupryushkin, M.S.; Kolotaev, A.V.; Khachatryan, D.S.; et al. New Hydrazinothiazole Derivatives of Usnic Acid as Potent Tdp1 Inhibitors. Molecules 2019, 24, 3711. [Google Scholar] [CrossRef]
- Zakharenko, A.; Luzina, O.; Sokolov, D.N.; Kaledin, V.I.; Nikolin, V.; Popova, N.; Patel, J.; Zakharova, O.; Chepanova, A.; Zafar, A.; et al. Novel tyrosyl-DNA phosphodiesterase 1 inhibitors enhance the therapeutic impact of topotecan on in vivo tumor models. Eur. J. Med. Chem. 2019, 161, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Dyrkheeva, N.; Luzina, O.; Filimonov, A.; Zakharova, O.; Ilina, E.; Zakharenko, A.; Kuprushkin, M.; Nilov, D.; Gushchina, I.; Švedas, V.; et al. Inhibitory Effect of New Semisynthetic Usnic Acid Derivatives on Human Tyrosyl-DNA Phosphodiesterase 1. Planta Medica 2018, 85, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Salomatina, O.; Popadyuk, I.I.; Zakharenko, A.L.; Zakharova, O.D.; Fadeev, D.S.; Komarova, N.I.; Reynisson, J.; Arabshahi, H.J.; Chand, R.; Volcho, K.P.; et al. Novel Semisynthetic Derivatives of Bile Acids as Effective Tyrosyl-DNA Phosphodiesterase 1 Inhibitors. Molecules 2018, 23, 679. [Google Scholar] [CrossRef] [PubMed]
- Salakhutdinov, N.F.; Volcho, K.P.; Yarovaya, O.I. Monoterpenes as a renewable source of biologically active compounds. Pure Appl. Chem. 2017, 89, 1105–1117. [Google Scholar] [CrossRef]
- Patrusheva, O.; Volcho, K.; Salakhutdinov, N. Approaches to the synthesis of oxygen-containing heterocyclic compounds based on monoterpenoids. Russ. Chem. Rev. 2018, 87, 771–796. [Google Scholar] [CrossRef]
- Khomenko, T.; Zakharenko, A.; Odarchenko, T.; Arabshahi, H.J.; Sannikova, V.; Zakharova, O.; Korchagina, D.; Reynisson, J.; Volcho, K.; Salakhutdinov, N. New inhibitors of tyrosyl-DNA phosphodiesterase I (Tdp 1). Bioorg. Med. Chem. 2016, 24, 5573–5581. [Google Scholar] [CrossRef]
- Khomenko, T.M.; Zakharenko, A.L.; Chepanova, A.A.; Ilina, E.S.; Zakharova, O.D.; I. Kaledin, V.; Nikolin, V.P.; A. Popova, N.; Korchagina, D.V.; Reynisson, J.; et al. Promising New Inhibitors of Tyrosyl-DNA Phosphodiesterase I (Tdp 1) Combining 4-Arylcoumarin and Monoterpenoid Moieties as Components of Complex Antitumor Therapy. Int. J. Mol. Sci. 2019, 21, 126. [Google Scholar] [CrossRef]
- Mozhaitsev, E.S.; Zakharenko, A.L.; Suslov, E.V.; Korchagina, D.V.; Zakharova, O.D.; Vasil’eva, I.A.; Chepanova, A.A.; Black, E.; Patel, J.; Chand, R.; et al. Novel Inhibitors of DNA Repair Enzyme TDP1 Combining Monoterpenoid and Adamantane Fragments. Anti-Cancer Agents Med. Chem. 2019, 19, 463–472. [Google Scholar] [CrossRef]
- Mozhaitsev, E.; Suslov, E.V.; Demidova, Y.; Korchagina, D.; Volcho, K.P.; Zakharenko, A.; Vasil’eva, I.; Kupryushkin, M.; Chepanova, A.; Ayine-Tora, D.M.; et al. The Development of Tyrosyl-DNA Phosphodyesterase 1 (TDP1) Inhibitors Based on the Amines Combining Aromatic/Heteroaromatic and Monoterpenoid Moieties. Lett. Drug Des. Discov. 2019, 16, 597–605. [Google Scholar] [CrossRef]
- Chepanova, A.A.; Mozhaitsev, E.S.; Munkuev, A.A.; Suslov, E.V.; Korchagina, D.V.; Zakharova, O.D.; Zakharenko, A.L.; Patel, J.; Ayine-Tora, D.M.; Reynisson, J.; et al. The Development of Tyrosyl-DNA Phosphodiesterase 1 Inhibitors. Combination of Monoterpene and Adamantine Moieties via Amide or Thioamide Bridges. Appl. Sci. 2019, 9, 2767. [Google Scholar] [CrossRef]
- Ponomarev, K.Y.; Suslov, E.V.; Zakharenko, A.L.; Zakharova, O.D.; Rogachev, A.D.; Korchagina, D.V.; Zafar, A.; Reynisson, J.; Nefedov, A.A.; Volcho, K.P.; et al. Aminoadamantanes containing monoterpene-derived fragments as potent tyrosyl-DNA phosphodiesterase 1 inhibitors. Bioorganic Chem. 2018, 76, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Li-Zhulanov, N.S.; Zakharenko, A.L.; Chepanova, A.A.; Patel, J.; Zafar, A.; Volcho, K.P.; Salakhutdinov, N.F.; Reynisson, J.; Leung, I.K.H.; Lavrik, O.I. A Novel Class of Tyrosyl-DNA Phosphodiesterase 1 Inhibitors That Contains the Octahydro-2H-chromen-4-ol Scaffold. Molecules 2018, 23, 2468. [Google Scholar] [CrossRef] [PubMed]
- Il’ina, I.V.; Volcho, K.P.; Korchagina, D.V.; Salnikov, G.E.; Genaev, A.M.; Karpova, E.V.; Salakhutdinov, N.F. Unusual reactions of (+)-2- and (+)-3-carene with aldehydes on K10 clay. Helv. Chim. Acta 2010, 93, 2135–2150. [Google Scholar] [CrossRef]
- Pavlova, A.; Il’Ina, I.; Morozova, E.; Korchagina, D.; Kurbakova, S.; Sorokina, I.; Tolstikova, T.; Volcho, K.P.; Salakhutdinov, N. Potent Neuroprotective Activity of Monoterpene Derived 4-[(3aR,7aS)- 1,3,3a,4,5,7a-Hexahydro-3,3,6-trimethylisobenzofuran-1-yl]-2-methoxyphenol in MPTP Mice Model. Lett. Drug Des. Discov. 2013, 11, 611–617. [Google Scholar] [CrossRef]
- Acharya, S.P.; Brown, H.C. Hydroboration of Terpenes. III. Isomerization of (+)-3-Carene to (+)-2-Carene. Hydroboration of (+)-2-Carene ([UNK]4-Carene). Nuclear Magnetic Resonance Spectra with Absolute Configurational and Conformational Assignments for the 2-Caranols and 2-Caranones. J. Am. Chem. Soc. 1967, 89, 1925–1932. [Google Scholar] [CrossRef]
- Meyer, U.; Hoelderich, W. Application of basic zeolites in the decomposition reaction of 2-methyl-3-butyn-2-ol and the isomerization of 3-carene. J. Mol. Catal. A: Chem. 1999, 142, 213–222. [Google Scholar] [CrossRef]
- Julianto, T.S.; Jumina; Sastrohamidjojo, H.; Mustofa. Solvent-free isomerization of 3-carene to 2-carene using Na/o-chlorotoluene catalyst in trans-isolimonene production. Orient. J. Chem. 2017, 33, 3107–3111. [Google Scholar] [CrossRef]
- Eswaramoorthy, M.; Krishnasamy, V. Influence of coke on the aromatization of 3-carene in the vapour phase over zeolites. Indian J. Chem. 2001, 40, 264–269. [Google Scholar]
- Krishnasamy, V.; Yeddanapalli, L.M. Vapour phase catalytic transformations of terpene hydrocarbons in the C10H16 series. III. Dehydrogenation of Δ3-carene over modified chromia and chromia–alumina catalysts. Can. J. Chem. 1976, 54, 3458–3463. [Google Scholar] [CrossRef]
- Sidorenko, A.; Aho, A.; Ganbaatar, J.; Batsuren, D.; Utenkova, D.; Sen’Kov, G.; Wärnå, J.; Murzin, D.; Agabekov, V. Catalytic isomerization of α-pinene and 3-carene in the presence of modified layered aluminosilicates. Mol. Catal. 2017, 443, 193–202. [Google Scholar] [CrossRef]
- Sidorenko, A.; Il’Ina, I.; Kravtsova, A.; Aho, A.; Ardashov, O.V.; Li-Zhulanov, N.; Volcho, K.P.; Salakhutdinov, N.; Murzin, D.Y.; Agabekov, V. Preparation of chiral isobenzofurans from 3-carene in the presence of modified clays. Mol. Catal. 2018, 459, 38–45. [Google Scholar] [CrossRef]
- Pommier, Y.; Marchand, C.; Thibaut, L. Diamidine derivatives as inhibitors of human tyrosyl-DNA-phosphodiesterase (Tdp1). USA Patent 60/786,604, 27 March 2006. [Google Scholar]
- Zakharova, O.; Luzina, O.; Zakharenko, A.; Sokolov, D.; Filimonov, A.; Dyrkheeva, N.; Chepanova, A.; Ilina, E.; Ilyina, A.; Klabenkova, K.; et al. Synthesis and evaluation of aryliden- and hetarylidenfuranone derivatives of usnic acid as highly potent Tdp1 inhibitors. Bioorganic Med. Chem. 2018, 26, 4470–4480. [Google Scholar] [CrossRef] [PubMed]
- Antony, S.; Marchand, C.; Stephen, A.G.; Thibaut, L.; Agama, K.K.; Fisher, R.J.; Pommier, Y. Novel high-throughput electrochemiluminescent assay for identification of human tyrosyl-DNA phosphodiesterase (Tdp1) inhibitors and characterization of furamidine (NSC 305831) as an inhibitor of Tdp1. Nucleic Acids Res. 2007, 35, 4474–4484. [Google Scholar] [CrossRef]
- Majumdar, P.; Bathula, C.; Basu, S.M.; Das, S.K.; Agarwal, R.; Hati, S.; Singh, A.; Sen, S.; Das, B.B. Design, synthesis and evaluation of thiohydantoin derivatives as potent topoisomerase I (Top1) inhibitors with anticancer activity. Eur. J. Med. Chem. 2015, 102, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Volcho, K.P.; Tatarova, L.E.; Korchagina, D.V.; Salakhutdinov, N.F.; Aul’chenko, I.S.; Ione, K.G.; Barkhash, V.A. Cycloaddition of carbonyl compounds to olefins on aluminosilicate catalysts. Russ. J. Org. Chem. 1994, 30, 641–653. [Google Scholar]
- Sidorenko, A.; Kravtsova, A.; Mäki-Arvela, P.; Aho, A.; Sandberg, T.; Il’Ina, I.; Li-Zhulanov, N.; Korchagina, D.; Volcho, K.; Salakhutdinov, N.; et al. Synthesis of isobenzofuran derivatives from renewable 2-carene over halloysite nanotubes. Mol. Catal. 2020, 490, 110974. [Google Scholar] [CrossRef]
- Zakharenko, A.; Khomenko, T.; Zhukova, S.; Koval, O.A.; Zakharova, O.; Anarbaev, R.; Lebedeva, N.; Korchagina, D.; Komarova, N.; Vasiliev, V.; et al. Synthesis and biological evaluation of novel tyrosyl-DNA phosphodiesterase 1 inhibitors with a benzopentathiepine moiety. Bioorganic Med. Chem. 2015, 23, 2044–2052. [Google Scholar] [CrossRef]
- Li, J.; Summerlin, M.; Nitiss, K.C.; Nitiss, J.L.; Hanakahi, L. TDP1 is required for efficient non-homologous end joining in human cells. DNA Repair 2017, 60, 40–49. [Google Scholar] [CrossRef]
- Brettrager, E.J.; Segura, I.A.; Van Waardenburg, R.C.; Waardenburg, V. Tyrosyl-DNA Phosphodiesterase I N-Terminal Domain Modifications and Interactions Regulate Cellular Function. Genes 2019, 10, 897. [Google Scholar] [CrossRef]
- Zhu, F.; Logan, G.; Reynisson, J. Wine Compounds as a Source for HTS Screening Collections. A Feasibility Study. Mol. Informatics 2012, 31, 847–855. [Google Scholar] [CrossRef]
- Eurtivong, C.; Reynisson, J. The Development of a Weighted Index to Optimise Compound Libraries for High Throughput Screening. Mol. Informatics 2018, 38, 1800068. [Google Scholar] [CrossRef]
- Lebedeva, N.A.; Rechkunova, N.I.; Lavrik, O.I. AP-site cleavage activity of tyrosyl-DNA phosphodiesterase 1. FEBS Lett. 2011, 585, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; A. Scott, D.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- QikProp: Schrödinger, LLC, New York NY. 2020. Available online: https://www.schrodinger.com/citations (accessed on 11 May 2020).
- Ioakimidis, L.; Thoukydidis, L.; Mirza, A.; Naeem, S.; Reynisson, J. Benchmarking the Reliability of QikProp. Correlation between Experimental and Predicted Values. QSAR Comb. Sci. 2008, 27, 445–456. [Google Scholar] [CrossRef]
- Liu, S.; Kurzrock, R. Toxicity of targeted therapy: Implications for response and impact of genetic polymorphisms. Cancer Treat. Rev. 2014, 40, 883–891. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds of structural types 11 and 12 are available from the authors. |


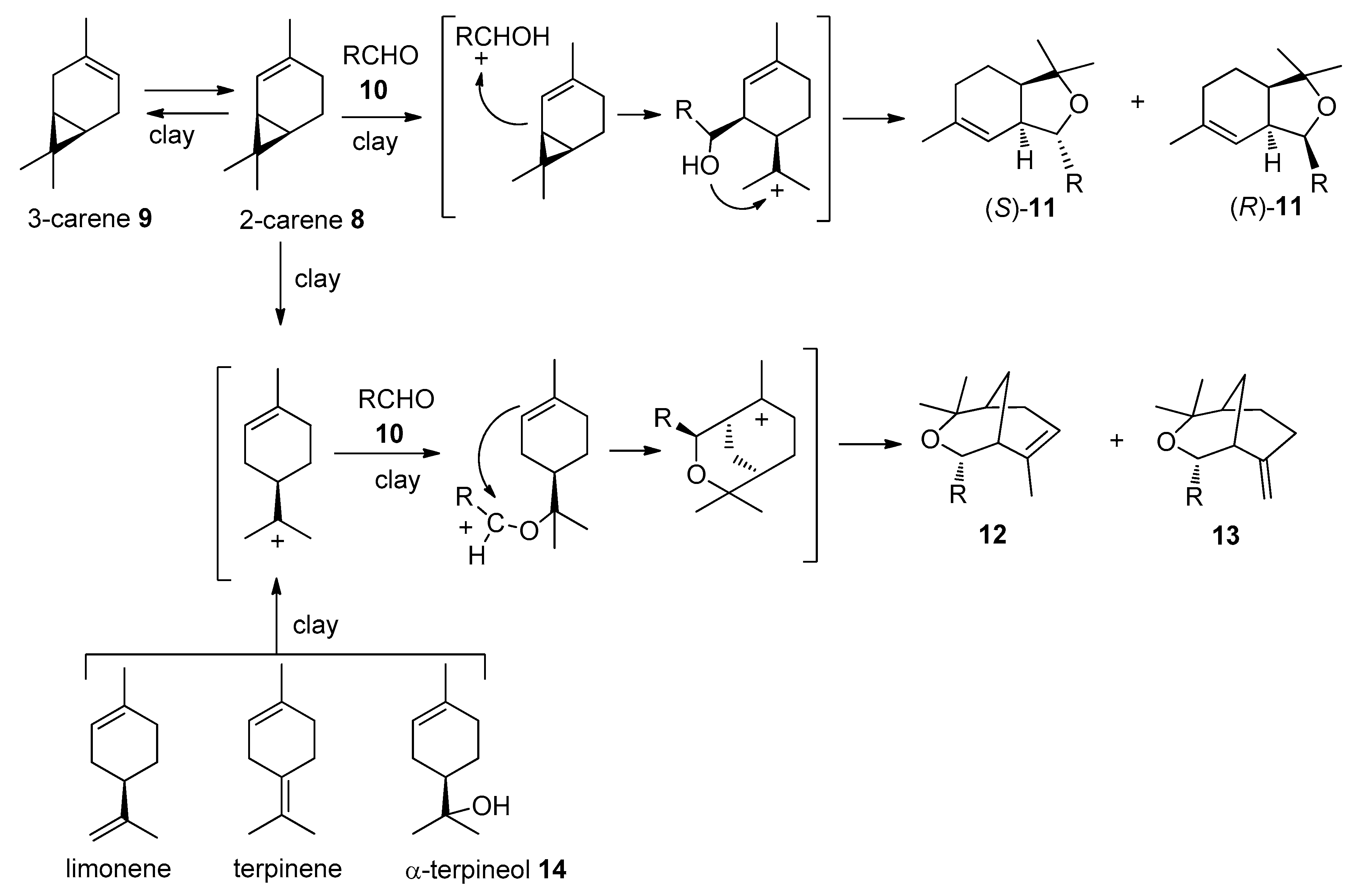




| R | RCHO | Product 11 (Yield, (S)-/(R) Ratio) | Products 12 and 13 (Total Yield, %, 12/13) |
|---|---|---|---|
| 4-Hydroxy-3-methoxyphenyl | 10a | 11a (81%, (S)-/(R) = 1:1) | 12a (11%) |
| (E)-prop-1-en-1-yl | 10c | 11c (89%, (S)-/(R) = 1.3:1) | |
| Thiophen-2-yl | 10d | 11d (78%, (S)-isomer) | 12d, 13d (5%, 7:1) |
| 3-Methylthiophen-2-yl | 10e | 11e (86%, (S)-isomer) | 12e (2%) |
| 5-Methylthiophen-2-yl | 10f | 11f (85%, (S)-isomer) | 12f, 13f (2%, 3:1) |
| 4-Bromothiophen-2-yl | 10g | - | 12g (20%) |
| 5-Bromothiophen-2-yl | 10h | 11h (73%, (S)-isomer) | 12h (15%) |
| 5-Nitrothiophen-2-yl | 10i | - | 12i, 13i (18%, 5:1) |
| 5-Nitrofuran-2-yl | 10j | - | 12j (18%) |
| Thiophen-3-yl | 10k | 11k (91%, (S)-/(R) = 1.5:1) | 12k (21%) |
| R | Compound 11 | IC501, μM | Compound 12 | IC501, μM |
|---|---|---|---|---|
| 4-Hydroxy-3-methoxyphenyl | 11a | >20 | 12a | >20 |
| (E)-prop-1-en-1-yl | 11c | >20 | - | - |
| Thiophen-2-yl | 11d | 4.85 ± 1.06 | 12d | 3.35 ± 1.06 |
| 3-Methylthiophen-2-yl | 11e | 3.6 ± 1.7 | 12e | 2.25 ± 0.63 |
| 5-Methylthiophen-2-yl | 11f | 4.7 ± 2.0 | - | - |
| 4-Bromothiophen-2-yl | - | - | 12g | 0.65 ± 0.22 |
| 5-Bromothiophen-2-yl | 11h | 0.75 ± 0.07 | 12h | 1.75 ± 0.78 |
| 5-Nitrothiophen-2-yl | - | - | 12i | 14 ± 1 |
| 5-Nitrofuran-2-yl | - | - | 12j | 28 ± 10 |
| Thiophen-3-yl | 11k | 1.60 ± 0.56 | 12k | 1.20 ± 0.14 |
| Furamidine | 1.2 ± 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Il’ina, I.V.; Dyrkheeva, N.S.; Zakharenko, A.L.; Sidorenko, A.Y.; Li-Zhulanov, N.S.; Korchagina, D.V.; Chand, R.; Ayine-Tora, D.M.; Chepanova, A.A.; Zakharova, O.D.; et al. Design, Synthesis, and Biological Investigation of Novel Classes of 3-Carene-Derived Potent Inhibitors of TDP1. Molecules 2020, 25, 3496. https://doi.org/10.3390/molecules25153496
Il’ina IV, Dyrkheeva NS, Zakharenko AL, Sidorenko AY, Li-Zhulanov NS, Korchagina DV, Chand R, Ayine-Tora DM, Chepanova AA, Zakharova OD, et al. Design, Synthesis, and Biological Investigation of Novel Classes of 3-Carene-Derived Potent Inhibitors of TDP1. Molecules. 2020; 25(15):3496. https://doi.org/10.3390/molecules25153496
Chicago/Turabian StyleIl’ina, Irina V., Nadezhda S. Dyrkheeva, Alexandra L. Zakharenko, Alexander Yu. Sidorenko, Nikolay S. Li-Zhulanov, Dina V. Korchagina, Raina Chand, Daniel M. Ayine-Tora, Arina A. Chepanova, Olga D. Zakharova, and et al. 2020. "Design, Synthesis, and Biological Investigation of Novel Classes of 3-Carene-Derived Potent Inhibitors of TDP1" Molecules 25, no. 15: 3496. https://doi.org/10.3390/molecules25153496
APA StyleIl’ina, I. V., Dyrkheeva, N. S., Zakharenko, A. L., Sidorenko, A. Y., Li-Zhulanov, N. S., Korchagina, D. V., Chand, R., Ayine-Tora, D. M., Chepanova, A. A., Zakharova, O. D., Ilina, E. S., Reynisson, J., Malakhova, A. A., Medvedev, S. P., Zakian, S. M., Volcho, K. P., Salakhutdinov, N. F., & Lavrik, O. I. (2020). Design, Synthesis, and Biological Investigation of Novel Classes of 3-Carene-Derived Potent Inhibitors of TDP1. Molecules, 25(15), 3496. https://doi.org/10.3390/molecules25153496









