Lignin Refinery Using Organosolv Process for Nanoporous Carbon Synthesis
Abstract
1. Introduction
2. Results and Discussion
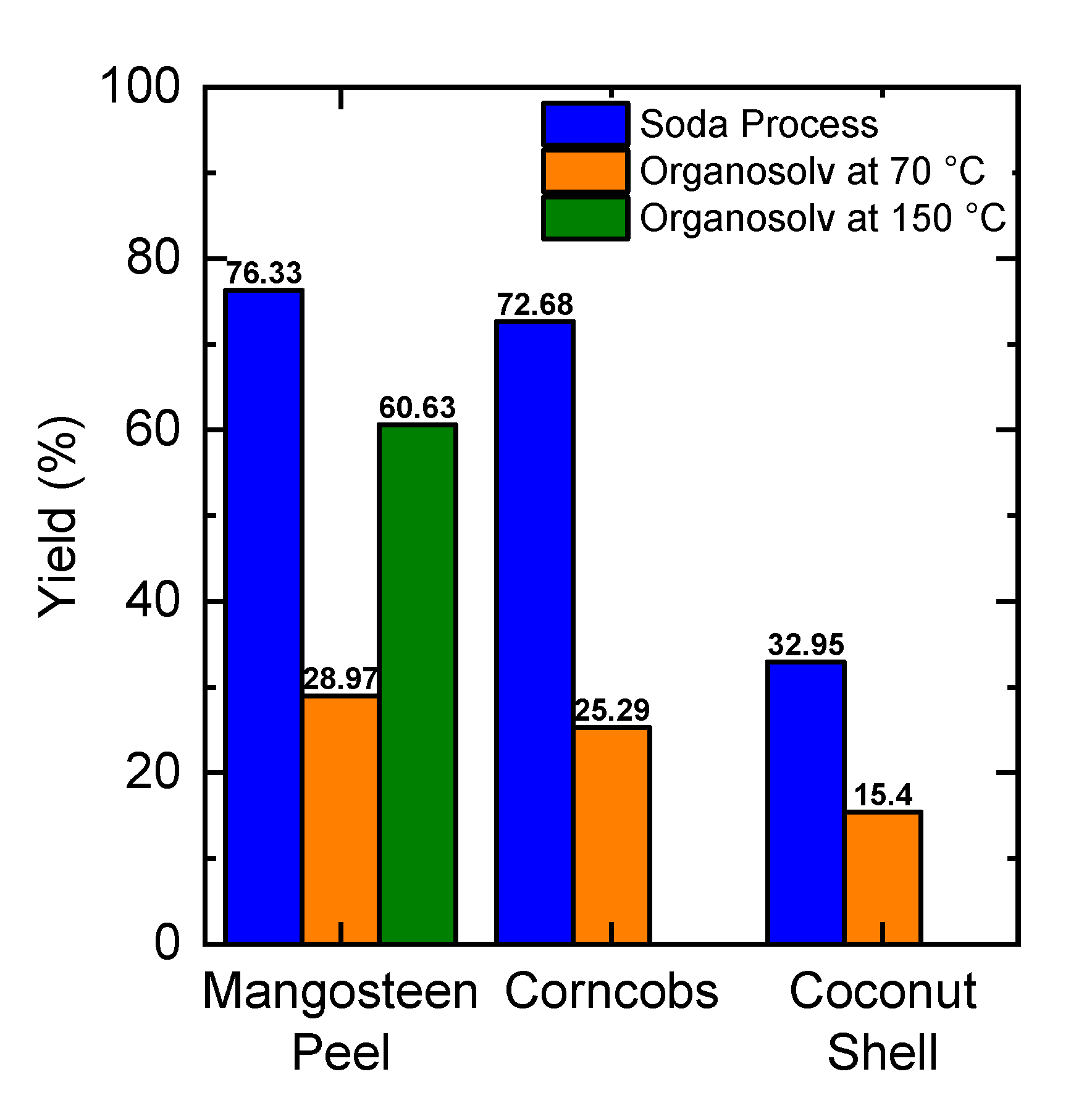
2.1. Lignin Yield
2.2. Characteristics of Lignin
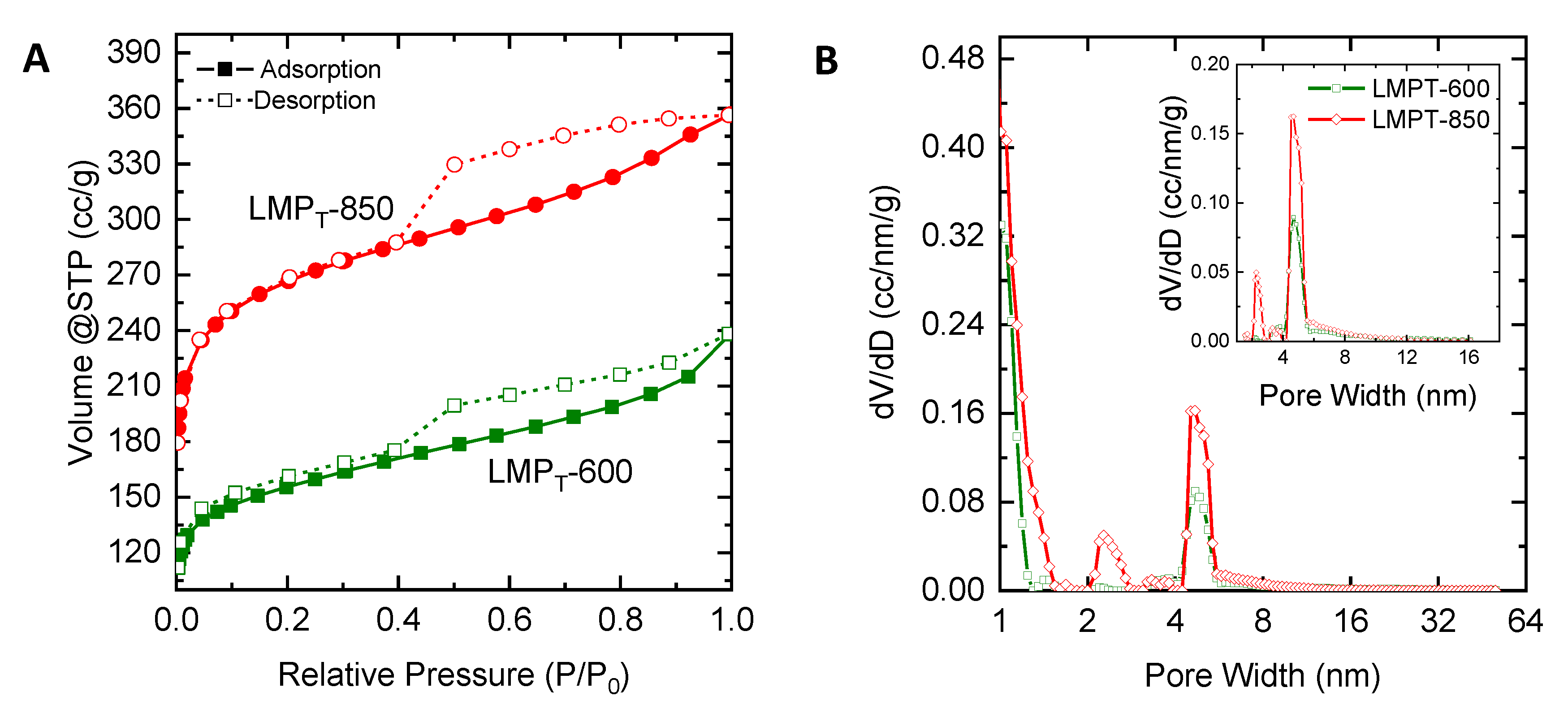
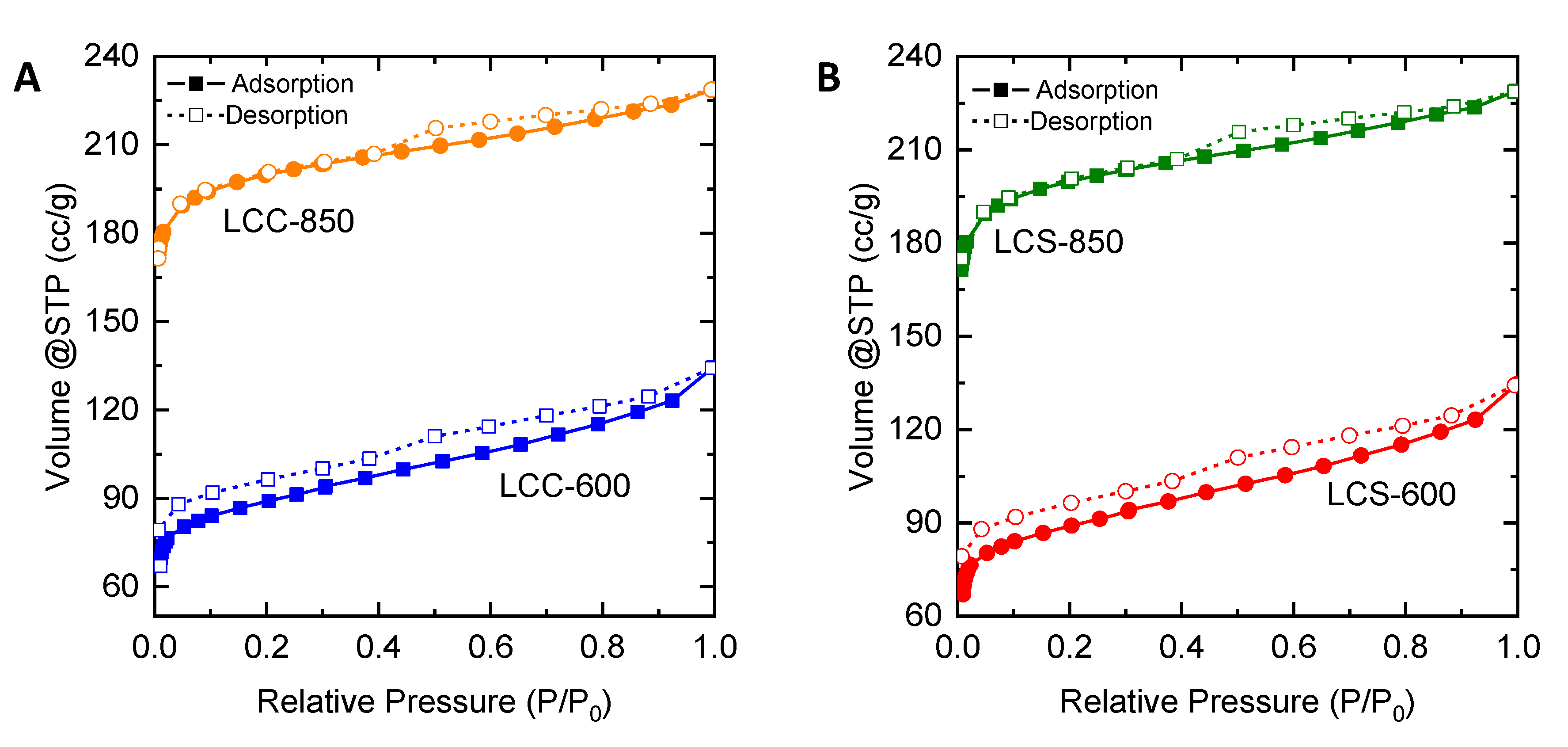
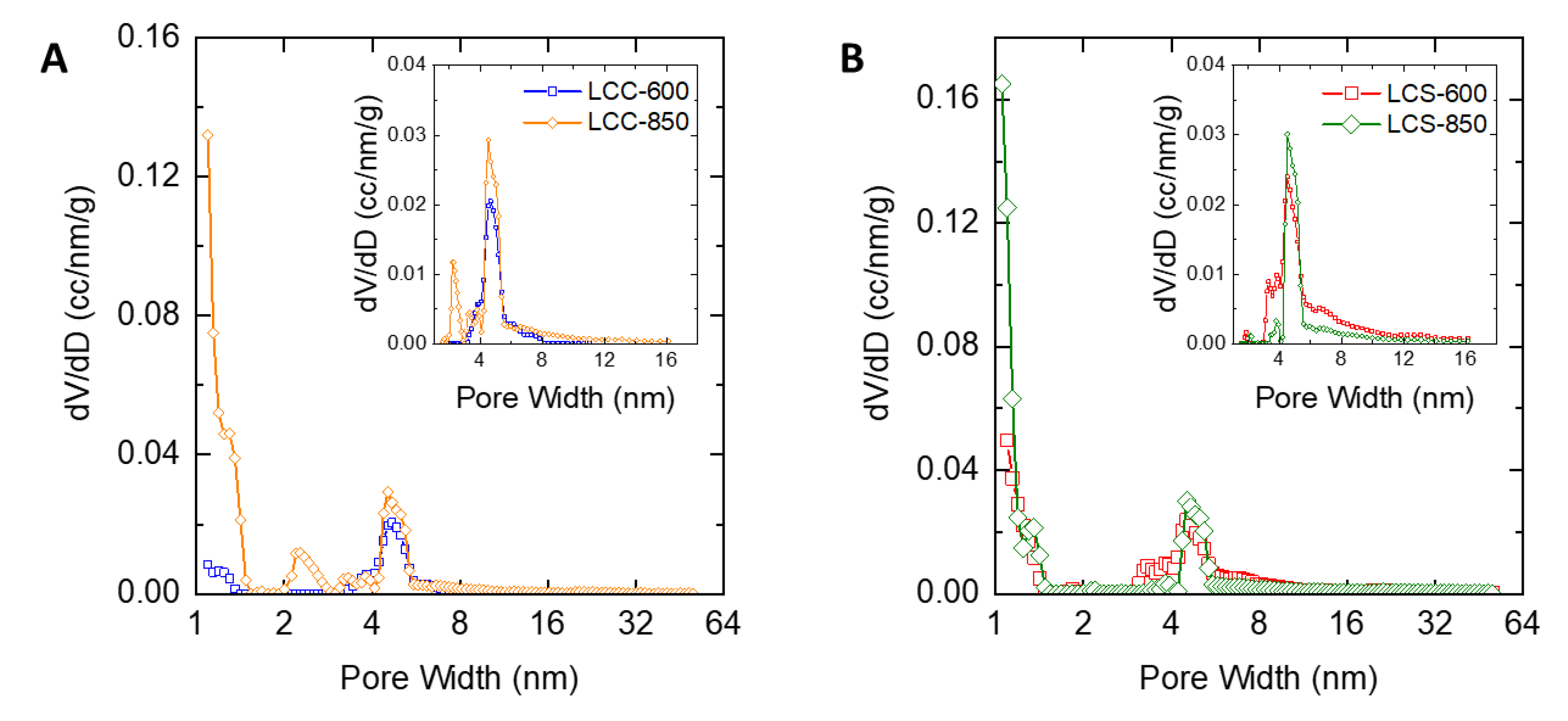
2.3. Characteristics of Porous Carbon Produced from Lignin
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prasetyo, I.; Mukti, N.I.F.; Ariyanto, T. Ethylene adsorption using cobalt oxide-loaded polymer-derived nanoporous carbon and its application to extend shelf life of fruit. Molecules 2019, 24, 1507. [Google Scholar] [CrossRef] [PubMed]
- Aroua, M.K.; Daud, W.M.A.W.; Yin, C.Y.; Adinata, D. Adsorption capacities of carbon dioxide, oxygen, nitrogen and methane on carbon molecular basket derived from polyethyleneimine impregnation on microporous palm shell activated carbon. Sep. Purif. Technol. 2008, 62, 609–613. [Google Scholar] [CrossRef]
- Prasetyo, I.; Mukti, N.I.F.; Fahrurrozi, M.; Ariyanto, T. Removing ethylene by adsorption using cobalt oxide-loaded nanoporous carbon. ASEAN J. Chem. Eng. 2018, 18, 9–16. [Google Scholar] [CrossRef]
- Yeon, S.-H.; Osswald, S.; Gogotsi, Y.; Singer, J.P.; Simmons, J.M.; Fischer, J.E.; Lillo-Rodenas, M.A.; Linares-Solano, A. Enhanced methane storage of chemically and physically activated carbide-derived carbon. J. Power Sources 2009, 191, 560–567. [Google Scholar] [CrossRef]
- Tee, E.; Tallo, I.; Kurig, H.; Thomberg, T.; Jänes, A.; Lust, E. Huge enhancement of energy storage capacity and power density of supercapacitors based on the carbon dioxide activated microporous SiC-CDC. Electrochimica Acta 2015, 161, 364–370. [Google Scholar] [CrossRef]
- Gläsel, J.; Diao, J.; Feng, Z.; Hilgart, M.; Wolker, T.; Su, D.S.; Etzold, B.J. Mesoporous and graphitic carbide-derived carbons as selective and stable catalysts for the dehydrogenation reaction. Chem. Mater. 2015, 27, 5719–5725. [Google Scholar] [CrossRef]
- Prasetyo, I.; Rochmadi, R.; Wahyono, E.; Ariyanto, T. controlling synthesis of polymer-derived carbon molecular sieve and its performance for CO2/CH4 separation. Eng. J. 2017, 21, 83–94. [Google Scholar] [CrossRef]
- Petkovich, N.D.; Stein, A. Controlling macro- and mesostructures with hierarchical porosity through combined hard and soft templating. Chem. Soc. Rev. 2013, 42, 3721–3739. [Google Scholar] [CrossRef] [PubMed]
- Presser, V.; Heon, M.; Gogotsi, Y. Carbide-derived carbons—from porous networks to nanotubes and graphene. Adv. Funct. Mater. 2011, 21, 810–833. [Google Scholar] [CrossRef]
- Han, J.; Kwon, J.H.; Lee, J.-W.; Lee, J.H.; Roh, K.C. An effective approach to preparing partially graphitic activated carbon derived from structurally separated pitch pine biomass. Carbon 2017, 118, 431–437. [Google Scholar] [CrossRef]
- Tagami, A.; Gioia, C.; Lauberts, M.; Budnyak, T.M.; Moriana, R.; Lindström, M.E.; Sevastyanova, O. Solvent fractionation of softwood and hardwood kraft lignins for more efficient uses: Compositional, structural, thermal, antioxidant and adsorption properties. Ind. Crop. Prod. 2019, 129, 123–134. [Google Scholar] [CrossRef]
- Mohit, N.; Agarwal, B.; Porwal, A.; Yadav, B.; Dhannajay, K. Manufacturing of paper by sulphate (Kraft) process. Int. J. Sci. Res. Rev. 2014, 3, 106–120. [Google Scholar]
- Wang, F.-L.; Li, S.; Sun, Y.-X.; Han, H.-Y.; Zhang, B.-X.; Hu, B.; Gao, Y.-F.; Hu, X. Ionic liquids as efficient pretreatment solvents for lignocellulosic biomass. RSC Adv. 2017, 7, 47990–47998. [Google Scholar] [CrossRef]
- Shiflett, M.B. Commercial Applications of Ionic Liquids. In Green Chemistry and Sustainable Technology; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- McDonough, T. The chemistry of organosolv delignification. IPST Tech. Pap. Ser. 1992, 455, 1–17. [Google Scholar]
- ICIS. Indicative Chemical Prices A-Z. Available online: https://www.icis.com/explore/chemicals/channel-info-chemicals-a-z/ (accessed on 2 July 2020).
- Millati, R.; Wikandari, R.; Ariyanto, T.; Putri, R.U.; Taherzadeh, M.J. Pretreatment technologies for anaerobic digestion of lignocelluloses and toxic feedstocks. Bioresour. Technol. 2020, 304, 122998. [Google Scholar] [CrossRef]
- Permatasari, P.R.; Prasetyo, I.; Ariyanto, T. Synthesis of nanoporous carbon from mangosteen peel lignin extracted by using organosolv and soda processes. In AIP Conference Proceedings; AIP Publishing: College Park, MD, USA, 2019; Volume 2085, p. 020051. [Google Scholar]
- Marton, R.; Granzow, S. Ethanol-alkali pulping (Spruce chips, paper, pulp industry). J. Tech. Assoc. Pulp Pap. Ind. 1982, 65, 103–106. [Google Scholar]
- Balogh, D.; Curvelo, A.; De Groote, R. Solvent effects on organosolv lignin from Pinus caribaea hondurensis. Holzforsch. 1992, 46, 343–348. [Google Scholar] [CrossRef]
- Ni, Y.; Hu, Q. Alcell® lignin solubility in ethanol–water mixtures. J. Appl. Polym. Sci. 1995, 57, 1441–1446. [Google Scholar] [CrossRef]
- Singh, S.K.; Dhepe, P.L. Isolation of lignin by organosolv process from different varieties of rice husk: Understanding their physical and chemical properties. Bioresour. Technol. 2016, 221, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Kline, L.M.; Hayes, D.G.; Womac, A.R.; Labbé, N. Simplified determination of lignin content in hard and soft woods via UV-spectrophotometric analysis of biomass dissolved in ionic liquids. BioResources 2010, 5, 1366–1383. [Google Scholar] [CrossRef]
- Ramakoti, B.; Dhanagopal, H.; Deepa, K.; Rajesh, M.; Ramaswamy, S.; Tamilarasan, K. Solvent fractionation of organosolv lignin to improve lignin homogeneity: Structural characterization. Bioresour. Technol. Rep. 2019, 7, 100293. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Zhang, L.; Hou, Q. Characterization and comparison of lignin derived from corncob residues to better understand its potential applications. Int. J. Boil. Macromol. 2019, 134, 20–27. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Q.; Leng, W.; Li, J.; Zhang, J.; Cai, Z.; Hassan, E.B. Carbon nanostructure of kraft lignin thermally treated at 500 to 1000 °C. Materials 2017, 10, 975. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Perspective: Jatropha cultivation in southern India: Assessing farmers’ experiences. Biofuels Bioprod. Biorefining 2012, 6, 246–256. [Google Scholar] [CrossRef]
- Xue, B.-L.; Wen, J.-L.; Sun, S.-L. Producing lignin-based polyols through microwave-assisted liquefaction for rigid polyurethane foam production. Materials 2015, 8, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Mukti, N.I.F.; Prasetyo, I.; Mindaryani, A.; Septarini, S. Preparation of porous carbon as ethylene adsorbent by pyrolysis of extraction waste Mangosteen rinds. MATEC Web Conf. 2018, 154, 01032. [Google Scholar] [CrossRef][Green Version]
- Sych, N.; Trofymenko, S.; Poddubnaya, O.; Tsyba, M.; Sapsay, V.; Klymchuk, D.; Puziy, A.M. Porous structure and surface chemistry of phosphoric acid activated carbon from corncob. Appl. Surf. Sci. 2012, 261, 75–82. [Google Scholar] [CrossRef]
- Ariyanto, T.; Sarwendah, R.A.G.; Amimmal, Y.M.N.; Laksmana, W.T.; Prasetyo, I. Modifying nanoporous carbon through hydrogen peroxide oxidation for removal of metronidazole antibiotics from simulated wastewater. Processes 2019, 7, 835. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Do, D.D. Adsorption Analysis: Equilibria and Kinetics, 2nd ed.; Imperial College Press: London, UK, 1998; ISBN 1860941303. [Google Scholar]
- Inagaki, M.; Tascón, J.D. Chapter 2 Pore formation and control in carbon materials. Interface Sci. Technol. 2006, 7, 49–105. [Google Scholar] [CrossRef]
- Mestre, A.; Carvalho, A.P. Nanoporous Carbon Synthesis: An Old Story with Exciting New Chapters. In Porosity—Process, Technologies and Applications; IntechOpen: London, UK, 2018. [Google Scholar]
- Lee, C.; Ong, Y.; Aroua, M.K.; Daud, W. Impregnation of palm shell-based activated carbon with sterically hindered amines for CO2 adsorption. Chem. Eng. J. 2013, 219, 558–564. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, G.; Zhang, J.; Wang, Y.; Yao, M. Activated carbon preparation from lignin by H3PO4 activation and its application to gas separation. Chem. Eng. Technol. 2011, 35, 309–316. [Google Scholar] [CrossRef]
- Permatasari, P.R. Pembuatan Karbon Berpori dari Lignin Hasil Ekstraksi Berbagai Limbah Biomass. Master’s Thesis, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2019. [Google Scholar]
Sample Availability: Samples of the compounds of lignin-derived carbons are available from the authors. |



| Sample | Wavenumber (cm−1) | Functionality |
|---|---|---|
| LMP; LCC; LCS; LMPT | 1600 | C=C stretching of the aromatic ring |
| LCS; LMPT | 1510 | Aromatic skeleton vibrations |
| LCC; LCS; LMPT | 1460 | C-H deformation (asymmetric in -CH3 and -CH2-) |
| LMP; LCS; LMPT | 1422 | C-H deformation in lignin |
| LMP; LMPT | 1283 | C-H stretching of G units |
| LMP; LCC; LCS; LMPT | 1162 | H, G, and S units of lignin |
| LMP; LCC; LCS; LMPT | 1110 | Aromatic C-H deformation of syringyl units |
| LMP; LMPT | 917 | C-H bending of syringyl units, aromatic ring |
| LCS; LMPT | 840 | C-H bending of syringyl units |
| Lignin Source | Viscosity (cP) | |
|---|---|---|
| Organosolv Process | Soda Process | |
| Mangosteen peel | 1.43/1.97 (at 150 °C) | 1.23 |
| Corn cob | 1.63 | 1.17 |
| Coconut shell | 1.53 | 1.20 |
| Characteristics | LMPT-600 | LMPT-850 | LMP-600 | LMP-850 | LCC-600 | LCC-850 | LCS-600 | LCS-850 |
|---|---|---|---|---|---|---|---|---|
| Surface area (SBET), m2/g | 567 | 974 | 205 | 595 | 349 | 820 | 333 | 781 |
| Mesopore area (Smeso), m2/g | 110 | 153 | 85 | 42 | 28 | 59 | 58 | 50 |
| %Smeso | 19.5 | 15.67 | 41.76 | 7.06 | 8.06 | 7.18 | 17.55 | 6.34 |
| Total pore volume, cm3/g | 0.37 | 0.55 | 0.21 | 0.28 | 0.16 | 0.38 | 0.21 | 0.35 |
| Mesopore volume (Vmeso), cm3/g | 0.18 | 0.22 | 0.16 | 0.07 | 0.03 | 0.09 | 0.09 | 0.07 |
| %Vmeso | 48.4 | 40.21 | 72.40 | 23.8 | 17.03 | 22.61 | 45.27 | 20.64 |
| Mean pore diameter, nm | 2.50 | 2.04 | 3.04 | 1.47 | 1.38 | 1.64 | 1.88 | 1.45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasetyo, I.; Permatasari, P.R.; Laksmana, W.T.; Rochmadi, R.; Oh, W.-C.; Ariyanto, T. Lignin Refinery Using Organosolv Process for Nanoporous Carbon Synthesis. Molecules 2020, 25, 3428. https://doi.org/10.3390/molecules25153428
Prasetyo I, Permatasari PR, Laksmana WT, Rochmadi R, Oh W-C, Ariyanto T. Lignin Refinery Using Organosolv Process for Nanoporous Carbon Synthesis. Molecules. 2020; 25(15):3428. https://doi.org/10.3390/molecules25153428
Chicago/Turabian StylePrasetyo, Imam, Puspita Rahayu Permatasari, William Teja Laksmana, Rochmadi Rochmadi, Won-Chun Oh, and Teguh Ariyanto. 2020. "Lignin Refinery Using Organosolv Process for Nanoporous Carbon Synthesis" Molecules 25, no. 15: 3428. https://doi.org/10.3390/molecules25153428
APA StylePrasetyo, I., Permatasari, P. R., Laksmana, W. T., Rochmadi, R., Oh, W.-C., & Ariyanto, T. (2020). Lignin Refinery Using Organosolv Process for Nanoporous Carbon Synthesis. Molecules, 25(15), 3428. https://doi.org/10.3390/molecules25153428