Increased Recovery of Gold Thiosulfate Alkaline Solutions by Adding Thiol Groups in the Porous Structure of Activated Carbon
Abstract
1. Introduction
2. Results and Discussion
2.1. Gold Adsorption Studies
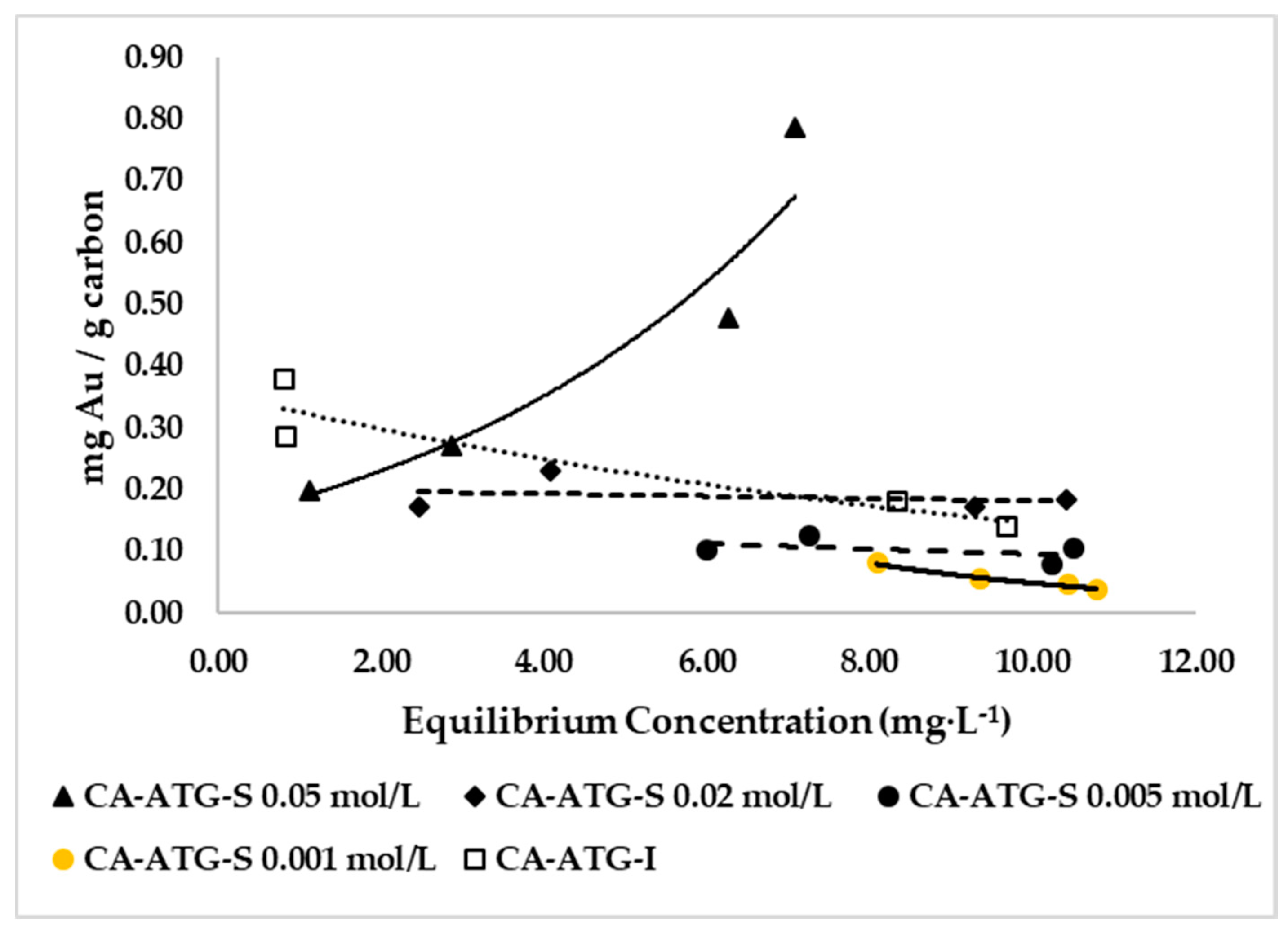
2.1.1. Influence of ATG in Solution on Gold Adsorption
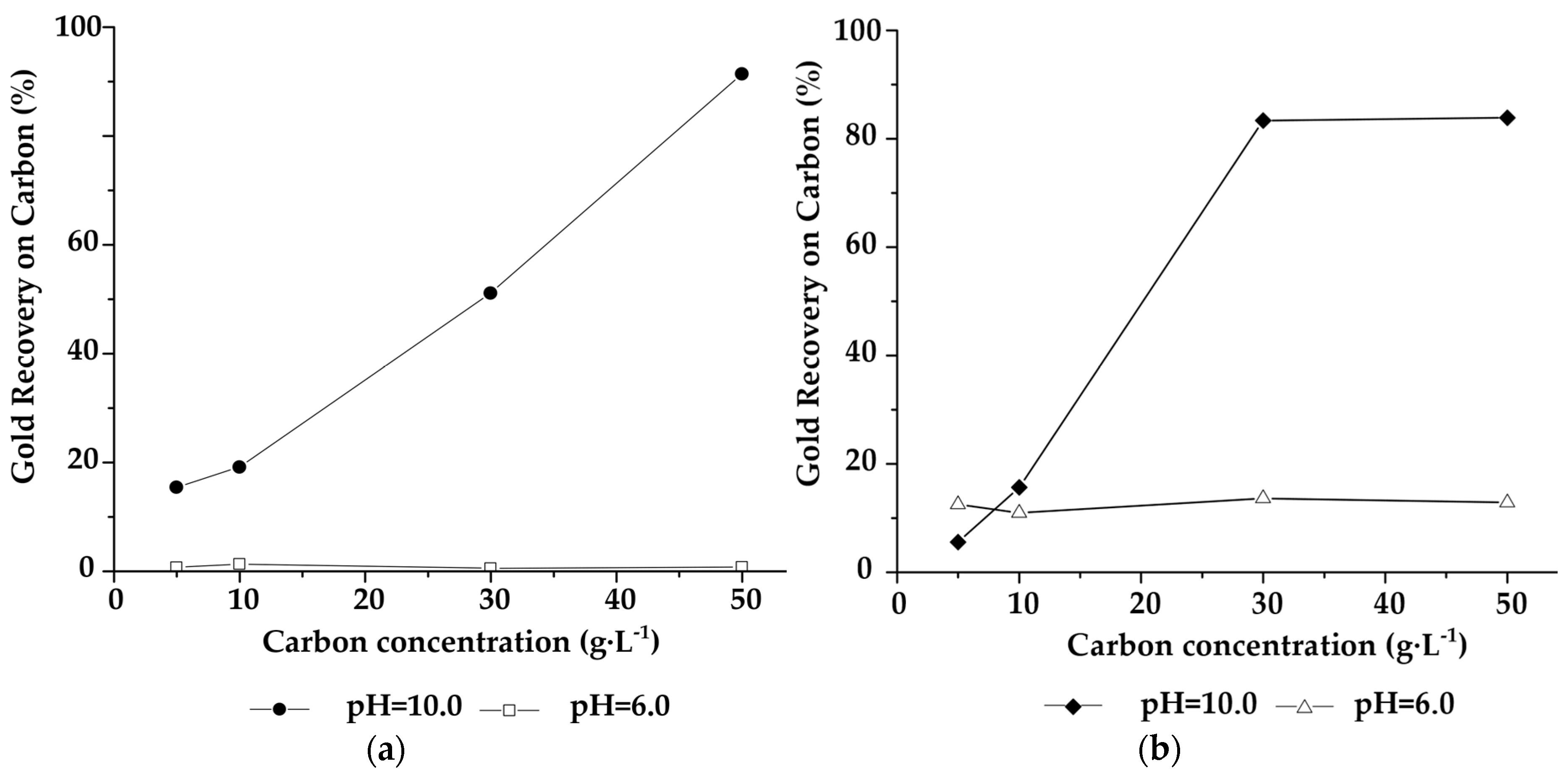
2.1.2. Influence of Activated Carbon Impregnated with ATG on Gold Adsorption
2.1.3. Influence of pH on the ATG Adsorption Tests
2.1.4. Experiment for Gold Stripping from ATG-Impregnated Carbon
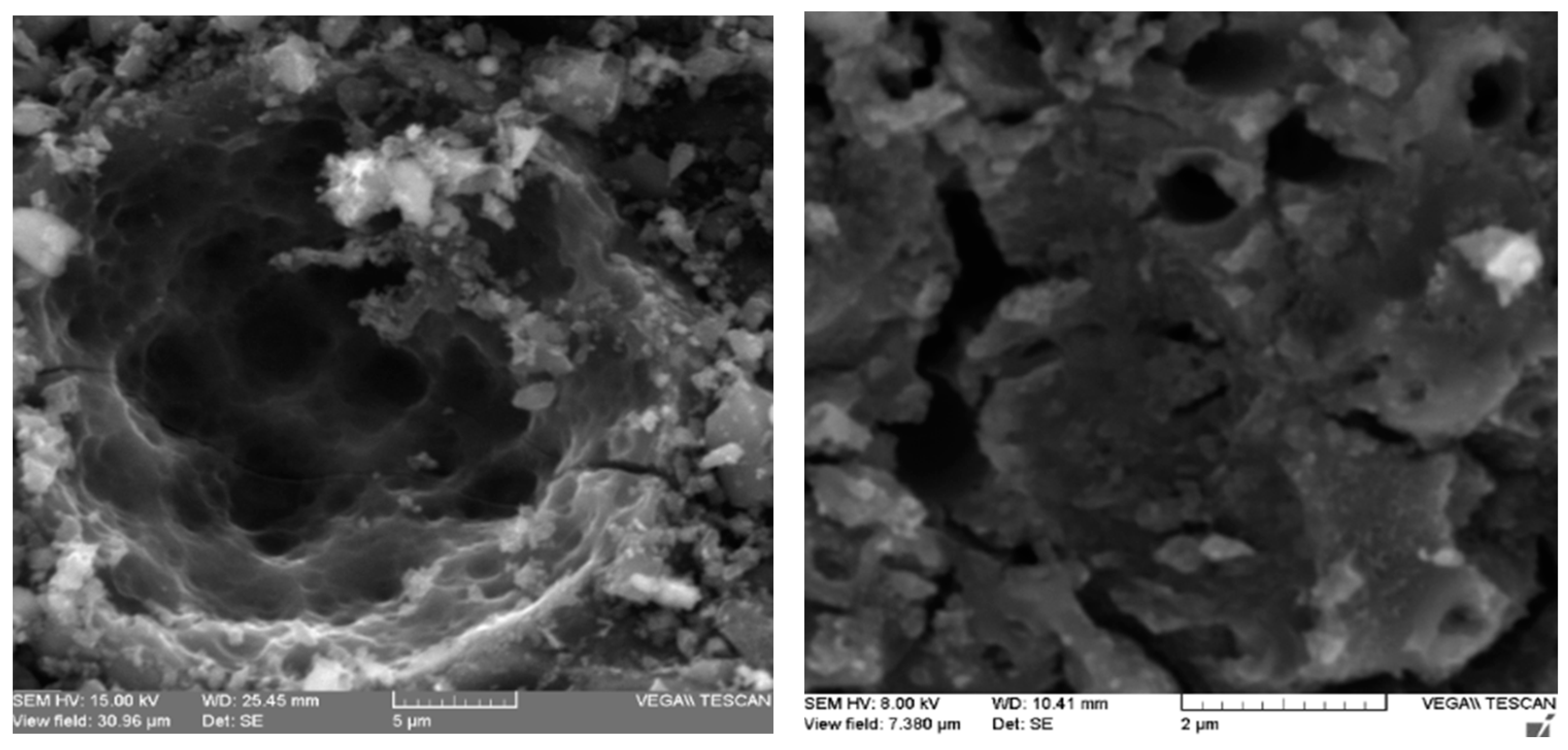
2.2. Specific Surface, Volatile Matter Content, and Zero Charge pH of Activated Carbons
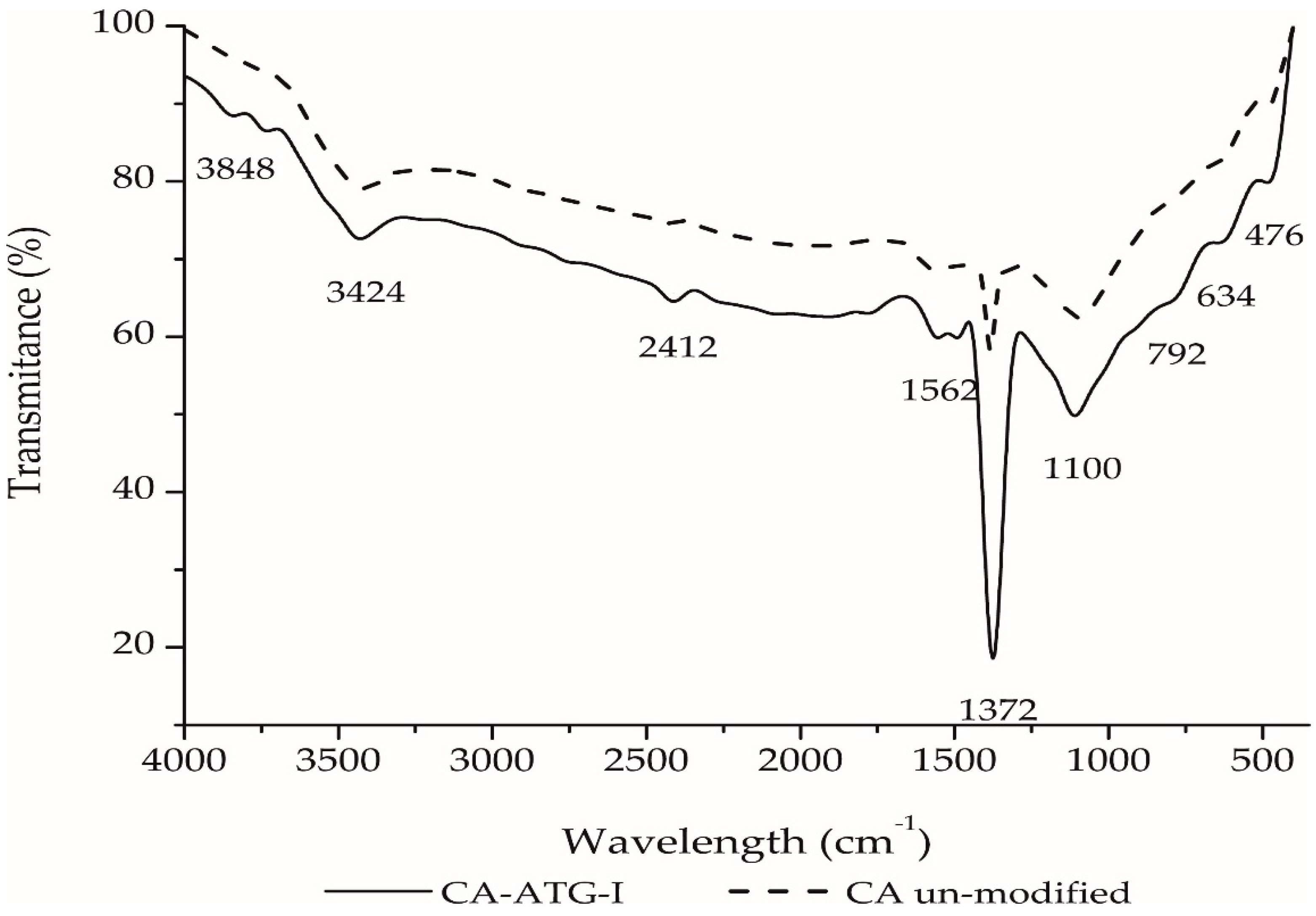
2.3. FTIR Characterization of Activated Carbon Impregnated with ATG
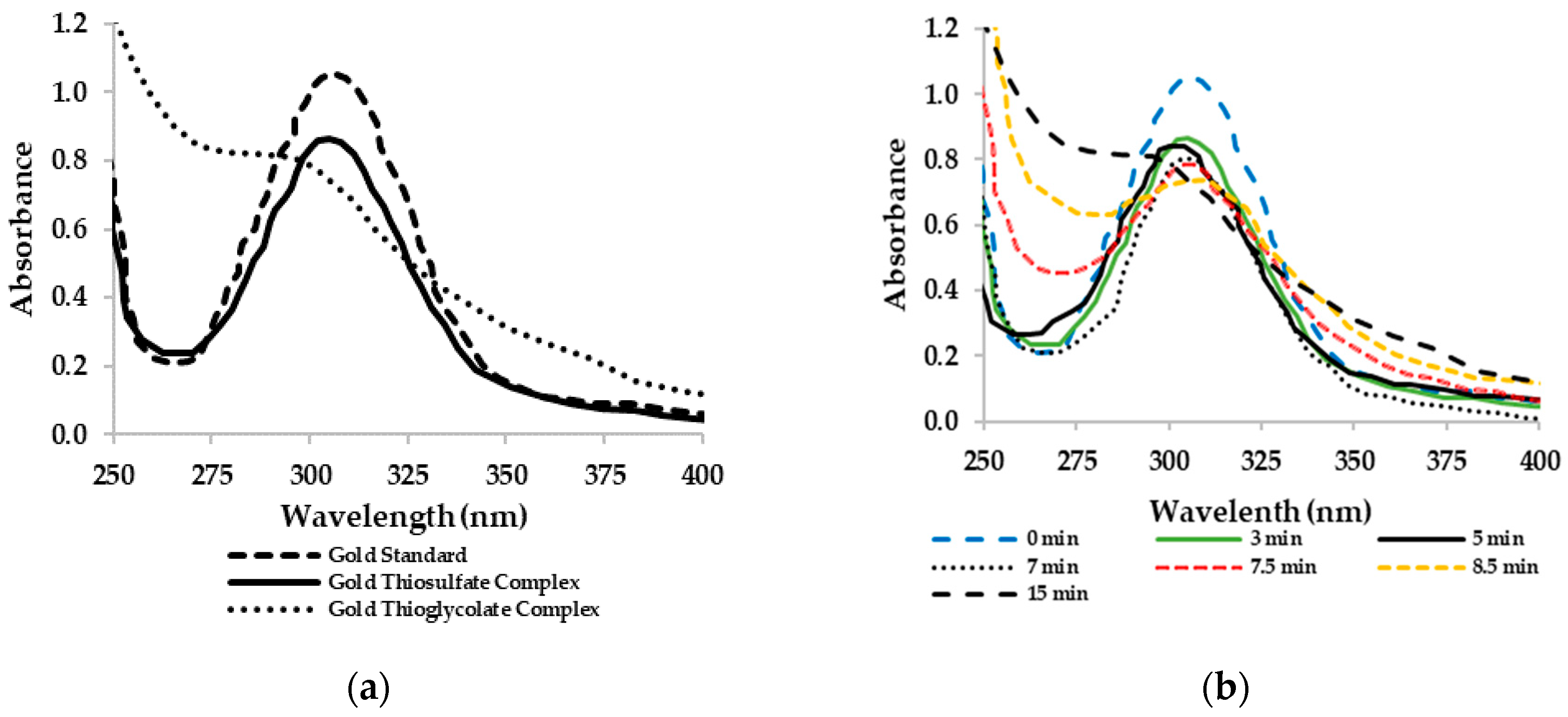
2.4. Cyclic Voltammetry of Gold Thiosulphate and Gold Thiolate Synthetic Solutions
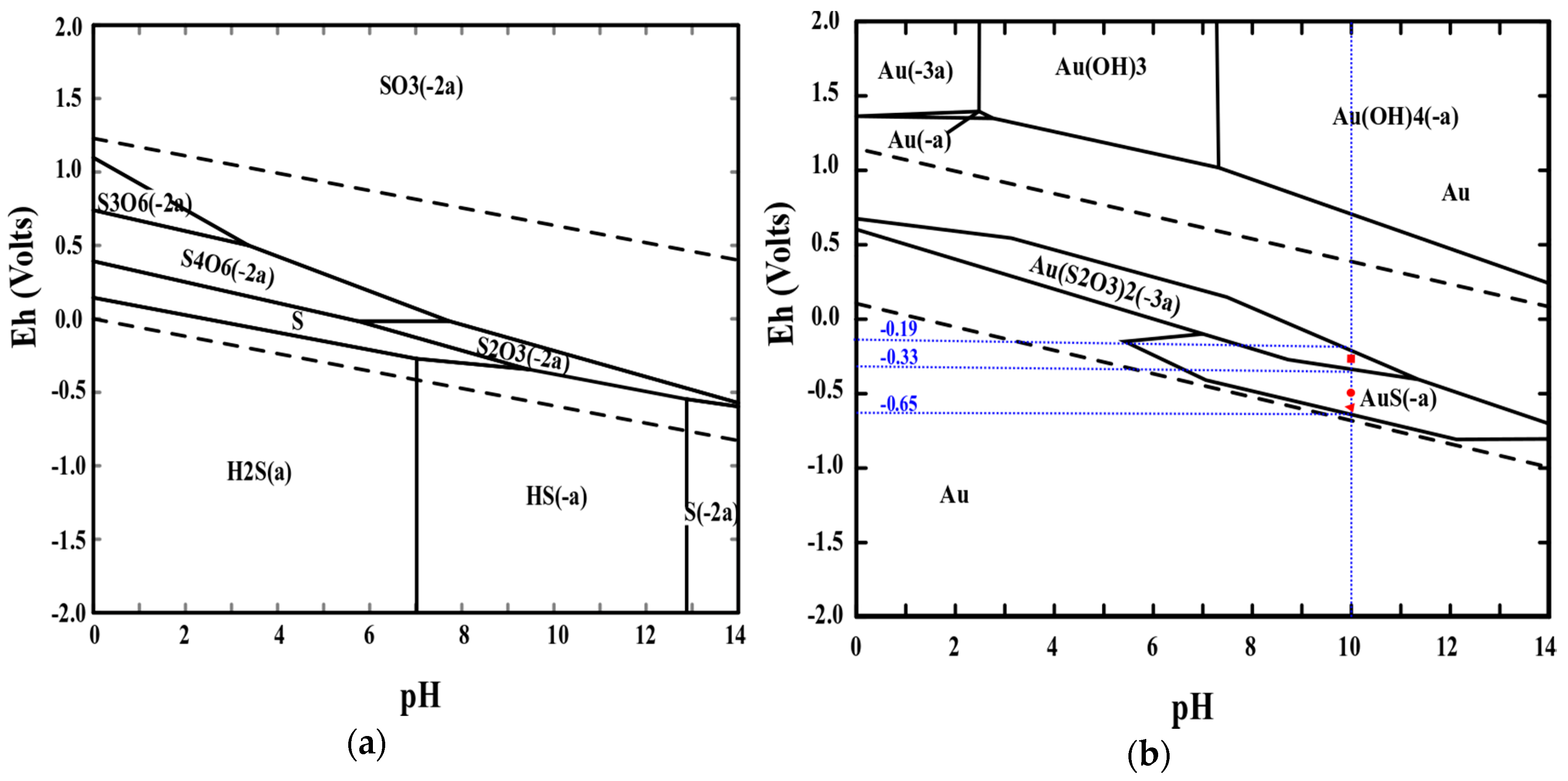
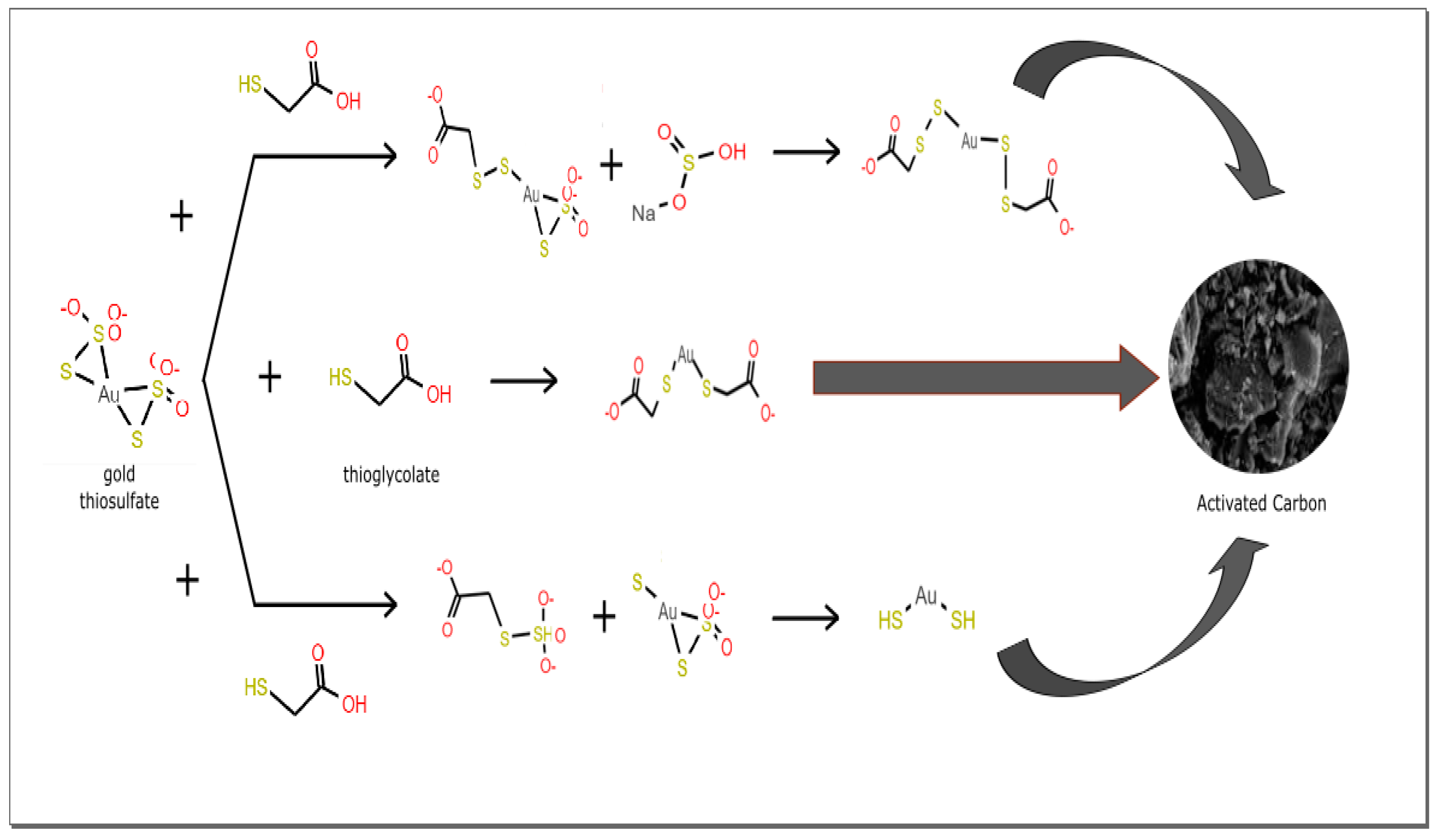
2.5. Adsorption Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparation of Activated Carbons
3.3. Gold Adsorption Studies
3.3.1. Preparation of the Adsorption Solution
3.3.2. Adsorption Tests
3.3.3. Elution Tests
3.4. Characterization of Activated Carbons
3.5. Characterization of Gold Thiosulphate and Gold Thiolate Synthetic Solutions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Main Results of the BET Analysis of Unmodified Activated Carbon

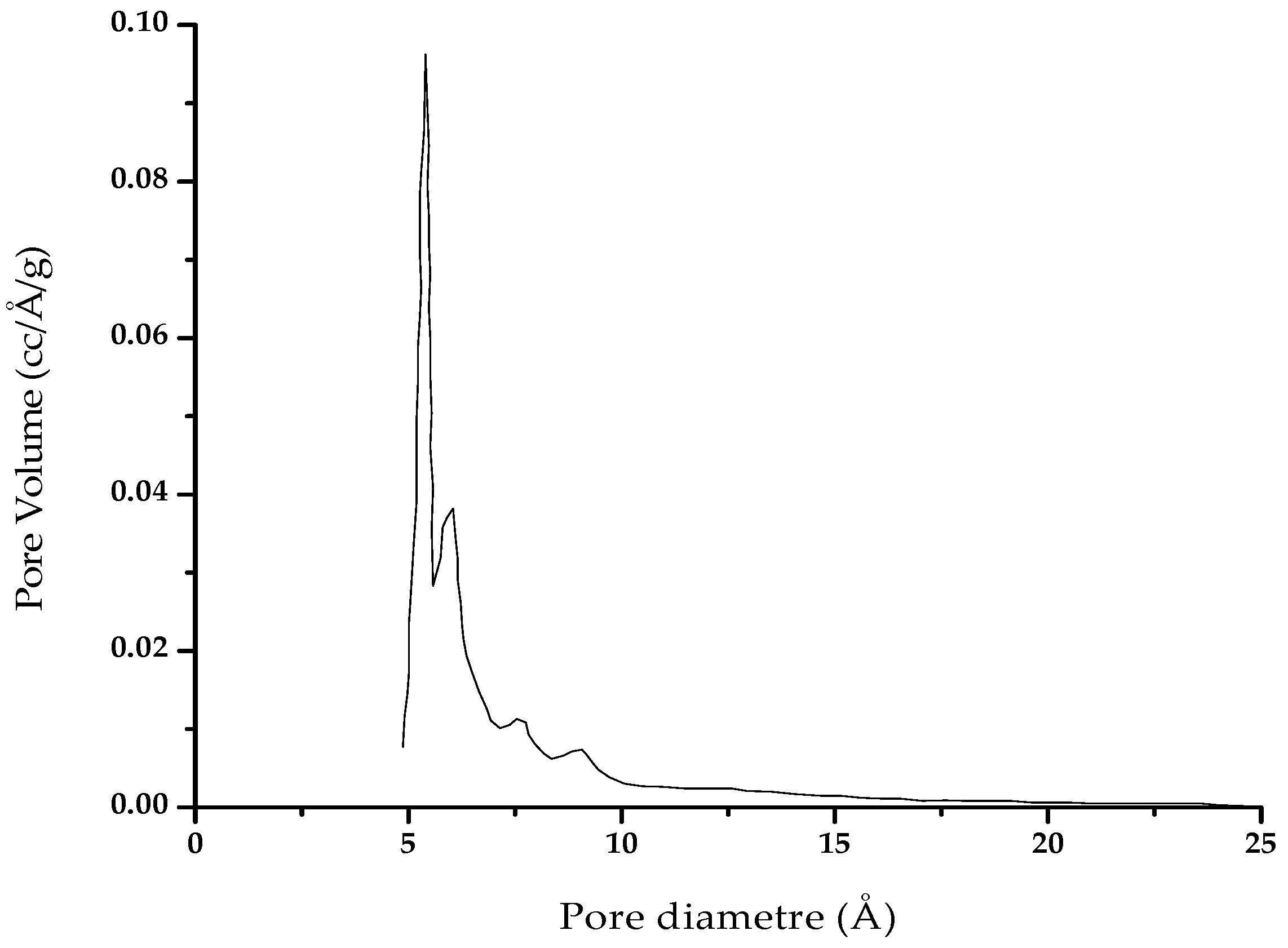
Appendix B
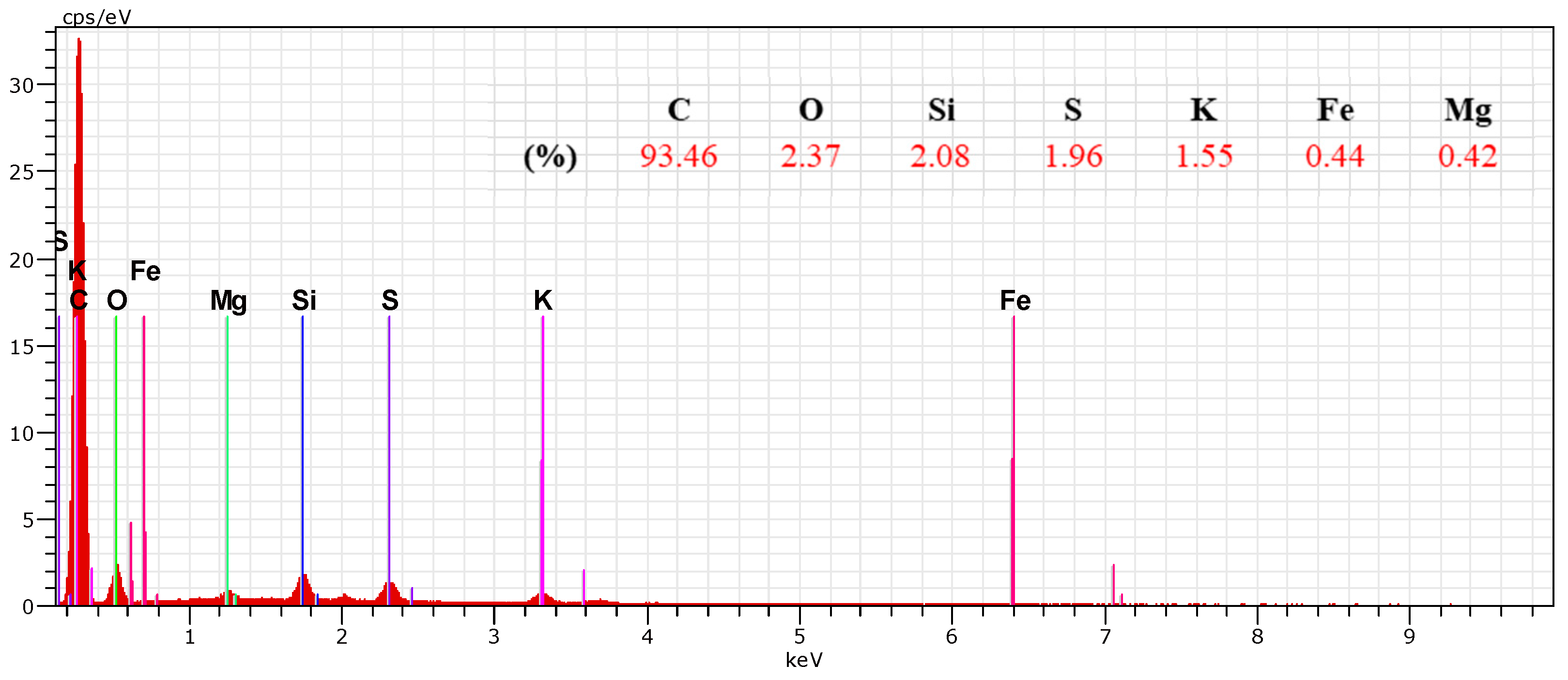
Elemental Analysis of the thiol-impregnated Activated Carbon by Scanning Electron Microscope (SEM-EDS)
| Activated Carbon | C | O | Si | S | K | Fe | Mg |
|---|---|---|---|---|---|---|---|
| (%) | 93.36 | 2.37 | 2.08 | 1.96 | 1.55 | 0.44 | 0.42 |
| Description | Au (ppm) | Dilution | Recovered Volume (mL) | Au (mg) | Au (%) |
|---|---|---|---|---|---|
| Initial Solution | 11.78 | 1 | 50 | 0.589 | NA |
| Strong Solution | 1.34 | 1 | 43 | 0.057 | 9.5% |
| Wash solution | 0.23 | 1 | 45 | 0.010 | 1.7% |
| Carbon | NA | NA | NA | 0.530 | 88.7% |
| Re-calculated feed | 0.597 | 100.0% | |||
Appendix C
| Description | r2 | qm | K(ka/kd) |
|---|---|---|---|
| CA-ATG-S 0.001 mol/L | 0.7938 | 0.0016 | 0.2458 |
| CA-ATG-S 0.005 mol/L | 0.8064 | 0.0002 | 0.1576 |
| CA-ATG-S 0.02 mol/L | 0.9396 | 0.0063 | 1.1049 |
| CA-ATG-S 0.05 mol/L | 0.7887 | 0.0103 | 3.5244 |
| CA-ATG-I | 0.8429 | 0.0111 | 1.7839 |
References
- de la Torre, E.; Gámez, S.; Pazmiño, E. Improvements to the cyanidation process for precious metal recovery from WPCBs. In Waste Electrical and Electronic Equipment Recycling; Francesco, V., Ionela, B., Eds.; Woodhead Publishing: Duxford, UK, 2018; pp. 115–137. ISBN 9780081020579. [Google Scholar]
- Xu, B.; Kong, W.; Li, Q.; Yang, Y.; Jiang, T.; Liu, X. A Review of Thiosulfate Leaching of Gold: Focus on Thiosulfate Consumption and Gold Recovery from Pregnant Solution. Metal 2017, 7, 222. [Google Scholar] [CrossRef]
- O’Malley, G. Recovery of Gold from Thiosulfate Solutions and Pulps with Ion-Exchange Resins. Ph.D. Thesis, Murdoch University, Western Australia, Australia, 2002. [Google Scholar]
- Gámez, S.; Garcés, K.; De La Torre, E.; Guevara, A. Precious metals recovery from waste printed circuit boards using thiosulfate leaching and ion exchange resin. Hydrometallurgy 2019, 186, 1–11. [Google Scholar] [CrossRef]
- Liu, X.; Xu, B.; Min, X.; Li, Q.; Yang, Y.; Jiang, T.; He, Y.; Zhang, X. Effect of Pyrite on Thiosulfate Leaching of Gold and the Role of Ammonium Alcohol Polyvinyl Phosphate (AAPP). Metals 2017, 7, 278. [Google Scholar] [CrossRef]
- Ha, V.H.; Lee, J.-C.; Huynh, T.H.; Jeong, J.; Pandey, B. Optimizing the thiosulfate leaching of gold from printed circuit boards of discarded mobile phone. Hydrometallurgy 2014, 149, 118–126. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, T.; Xu, B.; Yang, Y.; Li, Q. An eco-friendly and efficient process of low potential thiosulfate leaching-resin adsorption recovery for extracting gold from a roasted gold concentrate. J. Clean. Prod. 2019, 229, 387–398. [Google Scholar] [CrossRef]
- Sitando, O.; Senanayake, G.; Dai, X.; Breuer, P.L. The adsorption of gold(I) on minerals and activated carbon (preg-robbing) in non-ammoniacal thiosulfate solutions - effect of calcium thiosulfate, silver(I), copper(I) and polythionate ions. Hydrometallurgy 2019, 184, 206–217. [Google Scholar] [CrossRef]
- Aylmore, M.; Muir, D. Thiosulfate leaching of gold—A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Navarro, P.; Vargas, C.; Alonso, M.; Alguacil, F.J. Towards a more environmentally friendly process for gold: Models on gold adsorption onto activated carbon from ammoniacal thiosulfate solutions. Desalination 2007, 211, 58–63. [Google Scholar] [CrossRef]
- Navarro, P.; Vargas, C.; Alonso, M.; Alguacil, F.J. The adsorption of gold on activated carbon from thiosulfate-ammoniacal solutions. Gold Bull. 2006, 39, 93–97. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, T.; Xu, B.; Yang, Y.; Li, Q. Recovery of Gold from Pregnant Thiosulfate Solutions by the Resin Adsorption Technique. Metals 2017, 7, 555. [Google Scholar] [CrossRef]
- Muñoz, M.; Aller, A.-J.; Littlejohn, D. The bonding of heavy metals on nitric acid-etched coal fly ashes functionalized with 2-mercaptoethanol or thioglycolic acid. Mater. Chem. Phys. 2014, 143, 1469–1480. [Google Scholar] [CrossRef]
- Chen, Y.; Zi, F.; Hu, X.; Yang, P.; Ma, Y.; Cheng, H.; Wang, Q.; Qin, X.; Liu, Y.; Chen, S.; et al. The use of new modified activated carbon in thiosulfate solution: A green gold recovery technology. Sep. Purif. Technol. 2020, 230, 115834. [Google Scholar] [CrossRef]
- Chen, Y.; Zi, F.; Hu, X.; Yu, H.; Nie, Y.; Yang, P.; Cheng, H.; Wang, Q.; Qin, X.; Chen, S.; et al. Grafting of organic sulfur-containing functional groups on activated carbon for gold(I) adsorption from thiosulfate solution. Hydrometallurgy 2019, 185, 102–110. [Google Scholar] [CrossRef]
- Zhou, Z.; Anderson, C.M.; Butler, S.K.; Thompson, S.K.; Whitty, K.J.; Shen, T.-C.; Stowers, K.J. Stability and efficiency of CO 2 capture using linear amine polymer modified carbon nanotubes. J. Mater. Chem. A 2017, 5, 10486–10494. [Google Scholar] [CrossRef]
- Fotoohi, B.; Mercier, L. Some insights into the chemistry of gold adsorption by thiol and amine functionalized mesoporous silica in simulated thiosulfate system. Hydrometallurgy 2015, 156, 28–39. [Google Scholar] [CrossRef]
- Ewecharoen, A.; Thiravetyan, P.; Wendel, E.; Bertagnolli, H. Nickel adsorption by sodium polyacrylate-grafted activated carbon. J. Hazard. Mater. 2009, 171, 335–339. [Google Scholar] [CrossRef]
- Jang, M.; Cannon, F.S.; Parette, R.B.; Yoon, S.-J.; Chen, W. Combined hydrous ferric oxide and quaternary ammonium surfactant tailoring of granular activated carbon for concurrent arsenate and perchlorate removal. Water Res. 2009, 43, 3133–3143. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Bandosz, T.; Ania, C.O. Chapter 4 Surface chemistry of activated carbons and its characterization. Interface Sci. Technol. 2006, 7, 159–229. [Google Scholar] [CrossRef]
- Yu, H.; Zi, F.; Hu, X.; Nie, Y.; Chen, Y.; Cheng, H. Adsorption of gold from thiosulfate solutions with chemically modified activated carbon. Adsorpt. Sci. Technol. 2017, 36, 408–428. [Google Scholar] [CrossRef]
- Melashvili, M.; Fleming, C.; Dymov, I.; Matthews, D.; Dreisinger, D. Equation for thiosulphate yield during pyrite oxidation. Miner. Eng. 2015, 74, 105–111. [Google Scholar] [CrossRef]
- Melashvili, M.; Fleming, C.; Dymov, I.; Matthews, D.; Dreisinger, D. Dissolution of gold during pyrite oxidation reaction. Miner. Eng. 2016, 87, 2–9. [Google Scholar] [CrossRef]
- Grosse, A.C.; Dicinoski, G.W.; Shaw, M.J.; Haddad, P.R. Leaching and recovery of gold using ammoniacal thiosulfate leach liquors (a review). Hydrometallurgy 2003, 69, 1–21. [Google Scholar] [CrossRef]
- Staunton, W. Carbon-in-Pulp; Elsevier BV: Amsterdam, The Netherlands, 2016; ISBN 9780444636584. [Google Scholar]
- Toledo, R.B.C.; Aragón-Tobar, C.F.; Gámez, S.; De La Torre, E. Reactivation Process of Activated Carbons: Effect on the Mechanical and Adsorptive Properties. Molecules 2020, 25, 1681. [Google Scholar] [CrossRef]
- Houshmand, A.; Shafeeyan, M.S.; Arami-Niya, A.; Daud, W.M.A.W. Anchoring a halogenated amine on the surface of a microporous activated carbon for carbon dioxide capture. J. Taiwan Inst. Chem. Eng. 2013, 44, 774–779. [Google Scholar] [CrossRef]
- Mohammadnejad, S.; Provis, J.L.; Van Deventer, J.S.J. Computational modelling of gold complexes using density functional theory. Comput. Theor. Chem. 2015, 1073, 45–54. [Google Scholar] [CrossRef]
- Abechi, S.E.; Gimba, C.E.; Uzairu, A.; Dallatu, Y.A. Preparation and Characterization of Activated Carbon from Palm Kernel Shell by Chemical Activation. Res. J. Chem. Sci. 2013, 3, 54–61. [Google Scholar]
- Kasper, A.C.; Carrillo-Abad, J.; García-Gabaldón, M.; Veit, H.M.; Pérez-Herranz, V. Determination of the potential gold electrowinning from an ammoniacal thiosulphate solution applied to recycling of printed circuit board scraps. Waste Manag. Res. 2015, 34, 47–57. [Google Scholar] [CrossRef]
- Dimitrijevic, S.; Rajčić-Vujasinović, M.; Alagić, S.Č.; Grekulović, V.; Trujic, V. Formulation and characterization of electrolyte for decorative gold plating based on mercaptotriazole. Electrochimica Acta 2013, 104, 330–336. [Google Scholar] [CrossRef]
- Sitando, O.; Senanayake, G.; Dai, X.; Nikoloski, A.; Breuer, P.L. A review of factors affecting gold leaching in non-ammoniacal thiosulfate solutions including degradation and in-situ generation of thiosulfate. Hydrometallurgy 2018, 178, 151–175. [Google Scholar] [CrossRef]
- Osorio, H.M.; Cea, P.; Ballesteros, L.M.; Gascón, I.; Marqués-González, S.; Low, P.J.; Nichols, R.J.; Perez, F.; Martín, S. Preparation of nascent molecular electronic devices from gold nanoparticles and terminal alkyne functionalised monolayer films. J. Mater. Chem. C 2014, 2, 7348–7355. [Google Scholar] [CrossRef]
- Laurentius, L.B.; Stoyanov, S.R.; Gusarov, S.; Kovalenko, A.; Du, R.; Lopinski, G.P.; McDermott, M. Diazonium-Derived Aryl Films on Gold Nanoparticles: Evidence for a Carbon–Gold Covalent Bond. ACS Nano 2011, 5, 4219–4227. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-B.; Zhang, X.; Xu, B.; Li, Q.; Jiang, T.; Wang, Y. Effect of arsenopyrite on thiosulfate leaching of gold. Trans. Nonferrous Met. Soc. China 2015, 25, 3454–3460. [Google Scholar] [CrossRef]
- Jia, W.-G.; Dai, Y.-C.; Zhang, H.-N.; Lu, X.; Sheng, E.-H. Synthesis and characterization of gold complexes with pyridine-based SNS ligands and as homogeneous catalysts for reduction of 4-nitrophenol. RSC Adv. 2015, 5, 29491–29496. [Google Scholar] [CrossRef]
- Zawadzki, H.J. Synthesis and spectral studies of gold(III) complexes with guanidine derivatives. Transit. Met. Chem. 2003, 28, 820–826. [Google Scholar] [CrossRef]
- Al-Maythalony, B.A.; Wazeer, M.I.; Isab, A.A. 1H, 13C NMR and UV spectroscopy studies of gold(III)-tetracyanide complex with l-cysteine, glutathione, captopril, l-methionine and dl-seleno-methionine in aqueous solution. Inorg. Chim. Acta 2010, 363, 3244–3253. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; He, Y.; Song, P.; Wang, R. Preparation of Keratin-Glycine Metal Complexes and Their Scavenging Activity for Superoxide Anion Radicals. Int. J. Polym. Sci. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- De Guzman, R.; Tsuda, S.M.; Ton, M.-T.N.; Zhang, X.; Esker, A.R.; Van Dyke, M.E. Binding Interactions of Keratin-Based Hair Fiber Extract to Gold, Keratin, and BMP-2. PLoS ONE 2015, 10, e0137233. [Google Scholar] [CrossRef]
- Parker, A.J.; Kharasch, N. The Scission of the Sulfur-Sulfur Bond. Chem. Rev. 1959, 59, 583–628. [Google Scholar] [CrossRef]
- Bachrach, S.M.; Woody, J.T.; Mulhearn, D.C. Effect of Ring Strain on the Thiolate−Disulfide Exchange. A Computational Study. J. Org. Chem. 2002, 67, 8983–8990. [Google Scholar] [CrossRef]
- Kumar, K.V. Optimum sorption isotherm by linear and non-linear methods for malachite green onto lemon peel. Dye. Pigment. 2007, 74, 595–597. [Google Scholar] [CrossRef]
- Mahmood, T.; Saddique, M.T.; Naeem, A.; Westerhoff, P.; Mustafa, S.; Alum, A. Comparison of Different Methods for the Point of Zero Charge Determination of NiO. Ind. Eng. Chem. Res. 2011, 50, 10017–10023. [Google Scholar] [CrossRef]
- ASTM International. D3802-16 Standard Test Method for Ball-Pan Hardness of Activated Carbon; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM International. D5832-98(2014) Standard Test Method for Volatile Matter Content of Activated Carbon Samples; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM International. D2866-11(2018) Standard Test Method for Total Ash Content of Activated Carbon; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM International. D2867-17(2017) Standard Test Methods for Moisture in Activated Carbon; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]



| Eluent Solutions | Gold Recovery (%) |
|---|---|
| [KSCN] = 1.0 mol·L−1 | 25.8 |
| [CH4N2S] = 0.3 mol·L−1 | 31.8 |
| [Na2S2O3] = 1.0 mol·L−1 | 26.1 |
| [NaCN] = 0.2 mol·L−1; [NaOH] = 0.25 mol·L−1; [CH3CH2OH]= 30% V/V | 44.9 |
| Description | Unmodified Carbon | ATG-Impregnated Carbon |
|---|---|---|
| Diameter (mm) | 4.8 | 4.8 |
| Volatile matter (%) | 10.0 | 13.9 |
| Fixed carbon (%) | 68.9 | 68.0 |
| Specific surface area (m2 × g−1) | 274.0 | - |
| Hardness (%) | 90.3 | 89.1 |
| Acidity (mmol × g−1) | 0.05 | - |
| Point of zero charge (PZC) | 9.80 | 3.20 |
Sample Availability
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar-Ledesma, F.R.; Aragón-Tobar, C.F.; Espinoza-Montero, P.J.; de la Torre-Chauvin, E. Increased Recovery of Gold Thiosulfate Alkaline Solutions by Adding Thiol Groups in the Porous Structure of Activated Carbon. Molecules 2020, 25, 2902. https://doi.org/10.3390/molecules25122902
Escobar-Ledesma FR, Aragón-Tobar CF, Espinoza-Montero PJ, de la Torre-Chauvin E. Increased Recovery of Gold Thiosulfate Alkaline Solutions by Adding Thiol Groups in the Porous Structure of Activated Carbon. Molecules. 2020; 25(12):2902. https://doi.org/10.3390/molecules25122902
Chicago/Turabian StyleEscobar-Ledesma, Freddy R., Carlos F. Aragón-Tobar, Patricio J. Espinoza-Montero, and Ernesto de la Torre-Chauvin. 2020. "Increased Recovery of Gold Thiosulfate Alkaline Solutions by Adding Thiol Groups in the Porous Structure of Activated Carbon" Molecules 25, no. 12: 2902. https://doi.org/10.3390/molecules25122902
APA StyleEscobar-Ledesma, F. R., Aragón-Tobar, C. F., Espinoza-Montero, P. J., & de la Torre-Chauvin, E. (2020). Increased Recovery of Gold Thiosulfate Alkaline Solutions by Adding Thiol Groups in the Porous Structure of Activated Carbon. Molecules, 25(12), 2902. https://doi.org/10.3390/molecules25122902







