Evaluation of the Immunosafety of Cucurbit[n]uril on Peripheral Blood Mononuclear Cells In Vitro
Abstract
1. Introduction
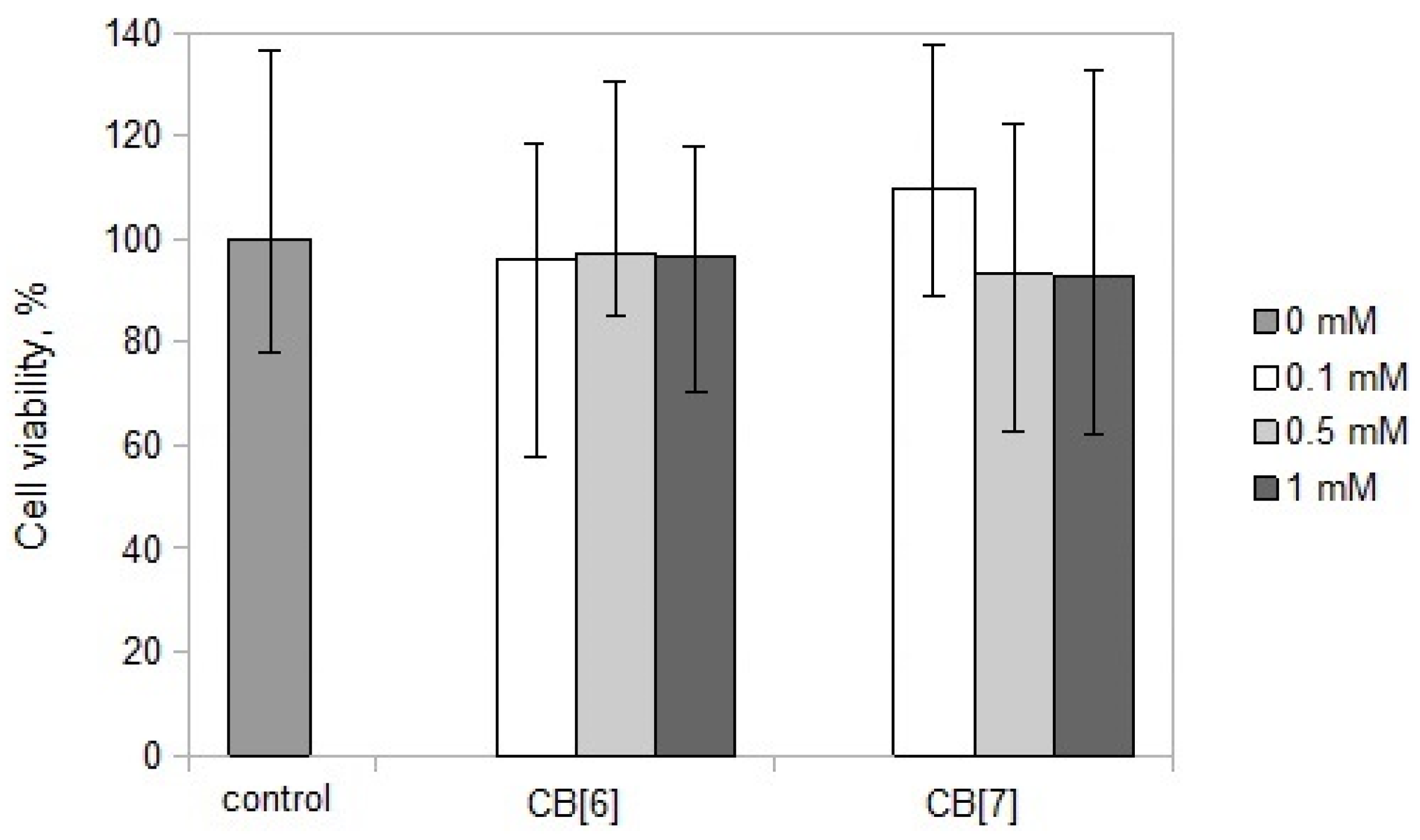
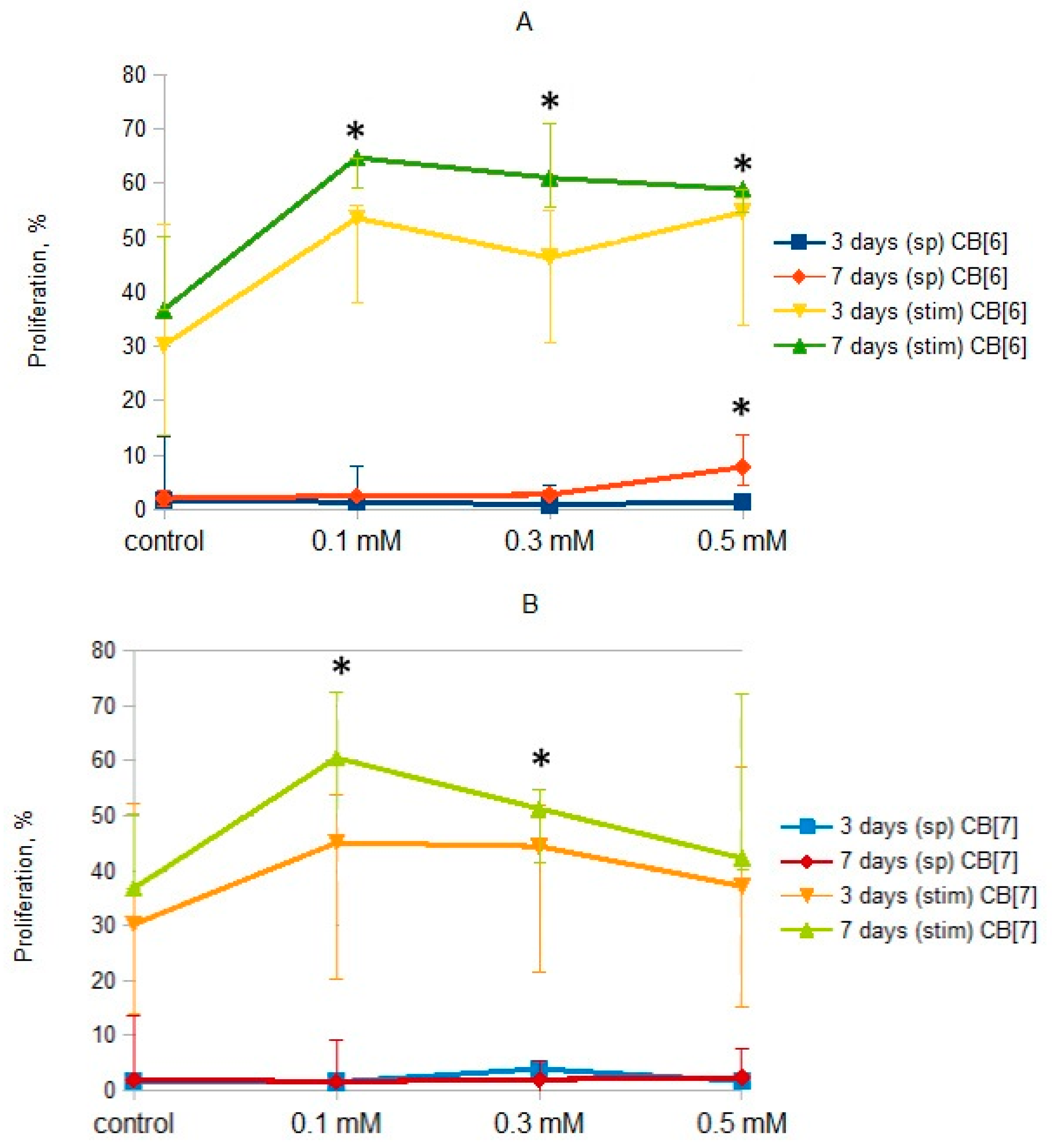
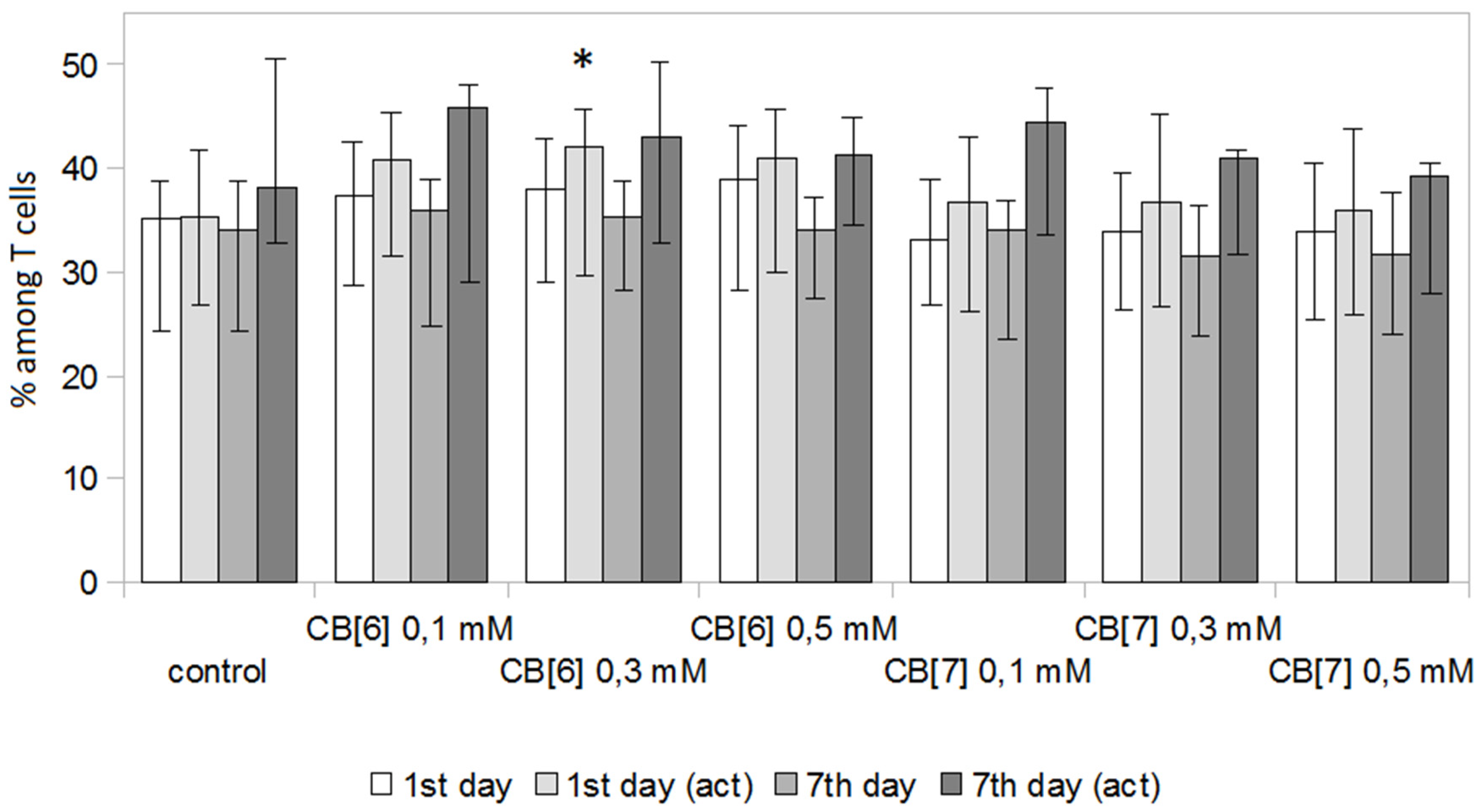
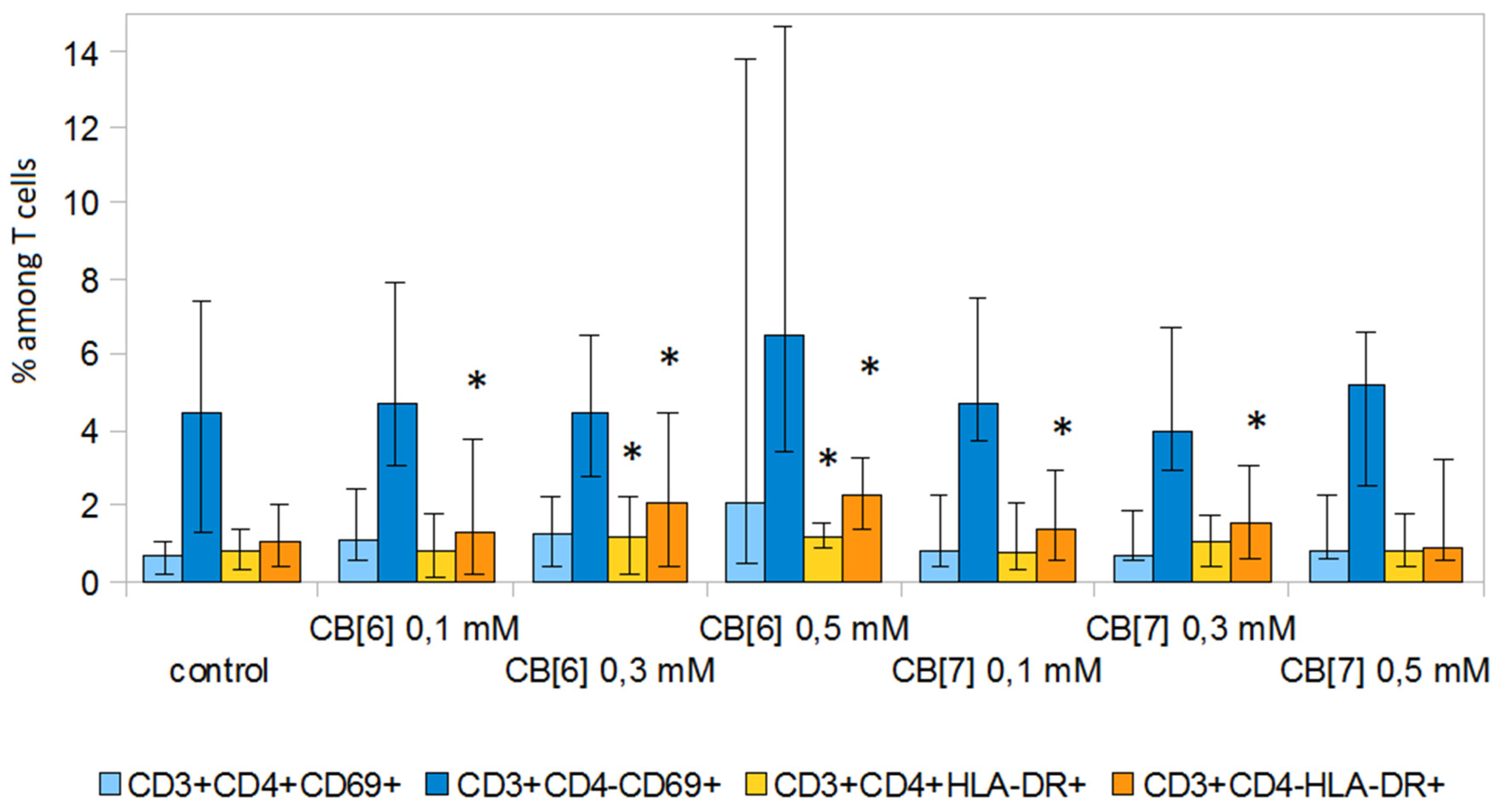
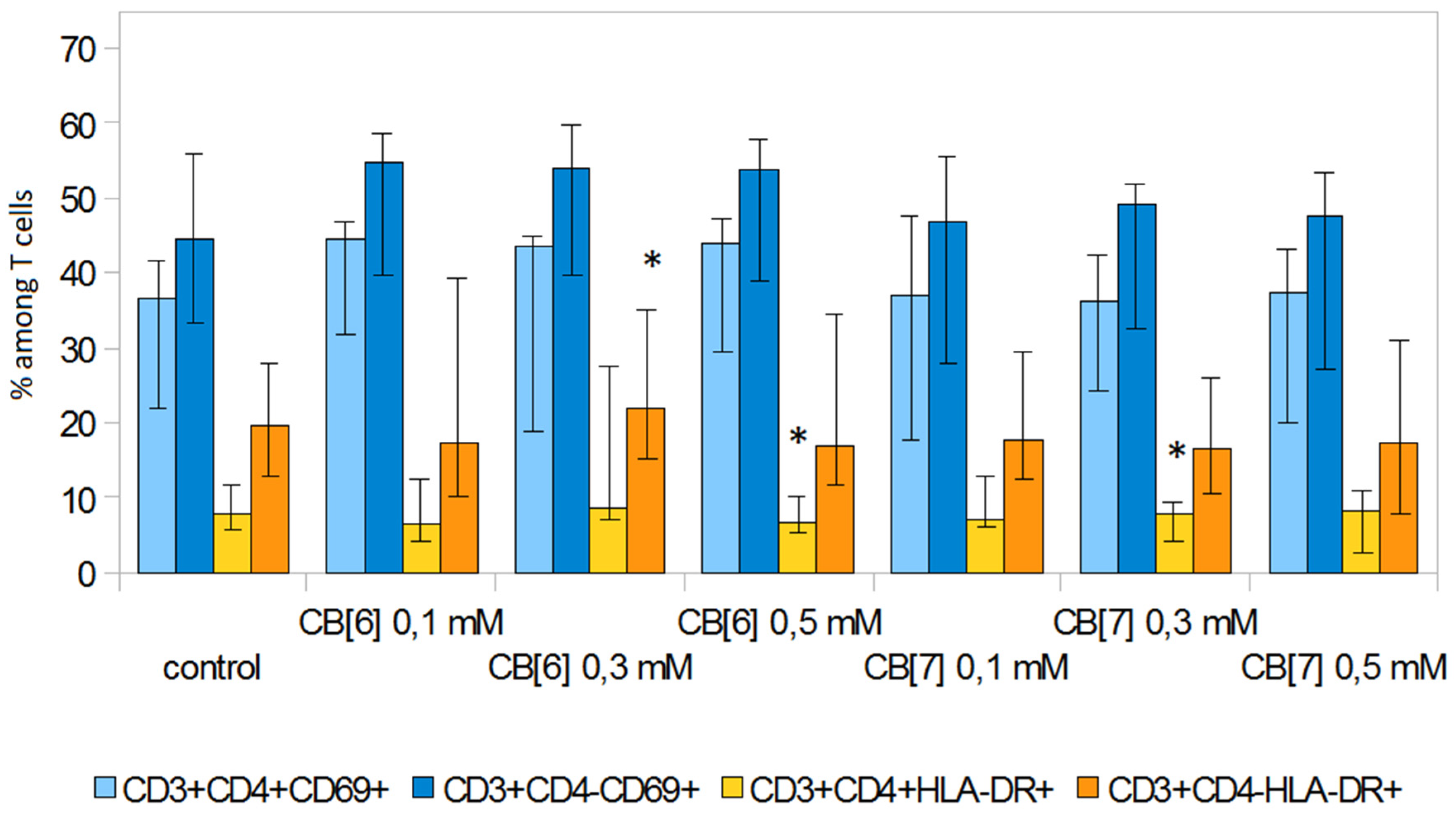
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Isolation and Cultivation of Peripheral Blood Mononuclear Cells (PBMCs)
3.3. Cell Viability
3.4. Cell Cycle Analysis
3.5. T Cell Analysis
3.6. Expression of Activation Molecules
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ye, C.; Chi, H. A review of recent progress in drug and protein encapsulation: Approaches, applications and challenges. Mater. Sci. Eng. C. 2018, 83, 233–246. [Google Scholar] [CrossRef]
- Geng, W.C.; Sessler, J.L.; Guo, D.S. Supramolecular prodrugs based on host–guest interactions. Chem. Soc. Rev. 2020, 49, 2303–2315. [Google Scholar] [CrossRef] [PubMed]
- McInnes, F.J.; Anthony, N.G.; Kennedy, A.R.; Wheate, N.J. Solid state stabilisation of the orally delivered drugs atenolol, glibenclamide, memantine and paracetamol through their complexation with cucurbit[7]uril. Org. Biomol. Chem. 2010, 8, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.L.; Zavalij, P.Y.; Isaacs, L. Synthesis of a disulfonated derivative of cucurbit[7]uril and investigations of its ability to solubilize insoluble drugs. Supramol. Chem. 2015, 27, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.; Koner, A.L.; Nau, W.M. Activation and stabilization of drugs by supramolecular pKa shifts: Drug delivery applications tailored for cucurbiturils. Angew. Chem. Int. Ed. 2008, 47, 5398–5401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Isaacs, L. Acyclic cucurbit[n]uriltype molecular containers: Influence of aromatic walls on their function as solubilizing excipients for insoluble drugs. J. Med. Chem. 2014, 57, 9554–9563. [Google Scholar] [PubMed]
- Knauer, N.E.; Pashkina, E. Apartsin topological aspects of the design of nanocarriers for therapeutic peptides and proteins. Pharmaceutics 2019, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Mirzaeva, I.V.; Andrienko, I.V.; Kovalenko, E.A.; Pashkina, E.A.; Aktanova, A.A. 1H NMR study of the effect of cucurbit[7]uril on the hydrolysis of carboplatin in biologically relevant media. Appl. Magn. Reson. 2019, 50, 1267–1276. [Google Scholar] [CrossRef]
- Gerasko, O.A.; Kovalenko, E.A.; Fedin, V.P. Macrocyclic cavitands cucurbit[n]urils: Application prospects in biochemistry, medicine, and nanotechnology. Russ. Chem. Rev. 2016, 85, 795–816. [Google Scholar] [CrossRef]
- Wheate, N.J.; Buck, D.P.; Day, A.I.; Collins, J.G. Cucurbit[n]uril binding of platinum anticancer complexes. Dalton trans. 2006, 451–458. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Kim, S.Y.; Ko, Y.H.; Sakamoto, S.; Yamaguchi, K.; Kim, K. Novel molecular drug carrier: Encapsulation of oxaliplatin in cucurbit[7]uril and its effects on stability and reactivity of the drug. Org. Biomol. Chem. 2005, 3, 2122–2125. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, E.A.; Pashkina, E.A.; Kanazhevskaya, L.Y.; Masliy, A.N.; Kozlov, V.A. Chemical and biological properties of a supramolecular complex of tuftsin and cucurbit[7]uril. Int. Immunopharmacol. 2017, 47, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Assaf, K.I.; Nau, W.M. Applications of cucurbiturils in medicinal chemistry and chemical biology. Front. Chem. 2019, 7, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Z.; Niu, Y.; Chen, X.; Lee, S.M.Y.; Wang, R. Influence of supramolecular encapsulation of camptothecin by cucurbit[7]uril: Reduced toxicity and preserved anti-cancer activity. MedChemComm 2016, 7, 1392–1397. [Google Scholar] [CrossRef]
- Li, S.; Chan, J.Y.-W.; Li, Y.; Bardelang, D.; Zheng, J.; Yew, W.W.; Chan, D.P.-C.; Lee, S.M.Y.; Wang, R. Complexation of clofazimine by macrocyclic cucurbit[7]uril reduced its cardiotoxicity without affecting the antimycobacterial efficacy. Org. Biomol. Chem. 2016, 14, 7563–7569. [Google Scholar] [CrossRef]
- Hettiarachchi, G.; Nguyen, D.; Wu, J.; Lucas, D.; Ma, D.; Isaacs, L.; Briken, V. Toxicology and drug delivery by cucurbit[n]uril type molecular containers. PLoS ONE 2010, 6, e10514. [Google Scholar] [CrossRef]
- Uzunova, V.D.; Cullinane, C.; Brix, K.; Nau, W.M.; Day, A.I. Toxicity of cucurbit[7]uril and cucurbit[8]uril: An exploratory in vitro and in vivo study. Org. Biomol. Chem. 2010, 8, 2037–2042. [Google Scholar] [CrossRef]
- Chen, H.; Chan, J.Y.W.; Yang, X.; Wyman, I.W.; Macartney, D.H.; Bardelang, D.; Lee, S.M.Y.; Wang, R. Developmental and organspecific toxicity of cucurbit[7]uril: In vivo study on zebrafish models. RSC Adv. 2015, 5, 30067–30074. [Google Scholar] [CrossRef]
- Chen, H.; Chan, J.Y.W.; Li, S.; Liu, J.J.; Wyman, I.W.; Lee, S.M.Y.; Macartney, D.H.; Wang, R. In vivo reversal of general anesthesia by cucurbit[7]uril with zebrafish models. RSC Adv. 2015, 5, 63745–63752. [Google Scholar] [CrossRef]
- Miao, X.; Li, Y.; Wyman, I.W.; Lee, S.M.Y.; Macartney, D.H.; Zheng, Y.; Wang, R. Enhanced in vitro and in vivo uptake of a hydrophobic model drug coumarin-6-in the presenceof cucurbit[7]uril. Med. Chem. Commun. 2015, 6, 1370–1374. [Google Scholar] [CrossRef]
- Oun, R.; Floriano, R.S.; Isaacs, L.; Rowana, E.G.; Wheate, N.J. The ex vivo neurotoxic, myotoxic and cardiotoxic activity of cucurbiturilbased macrocyclic drug delivery vehicles. Toxicol. Res. 2014, 3, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, X.; Li, S.; Wang, L.H.; Zhang, J.; Wang, R. A systematic evaluation of the biocompatibility of cucurbit[7]uril in mice. Sci. Rep. 2018, 8, 8819–8825. [Google Scholar] [CrossRef] [PubMed]
- Lü, H.Z.; Zhu, A.Y.; Chen, Y.; Tang, J.; Li, B.Q. Lower Concentrations of Methyl-β-Cyclodextrin Combined With interleukin-2 Can Preferentially Induce Activation and Proliferation of Natural Killer Cells in Human. Peripheral Blood. Hum. Immunol. 2011, 72, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Yun, C.-H.; Han, S.H. Induction of Dendritic Cell Maturation and Activation by a Potential Adjuvant, 2-Hydroxypropyl-β-Cyclodextrin. Front. Immunol. 2016, 7, 435–444. [Google Scholar] [CrossRef]
- Day, A.; Arnold, A.P.; Blanch, R.J.; Snushall, B. Controlling factors in the synthesis of cucurbituril and its homologues. J. Org. Chem. 2001, 66, 8094–8100. [Google Scholar] [CrossRef] [PubMed]
- Böyum, A. Separation of leukocytes from blood and bone marrow. Scand. J. Clin. Lab. Investig. Suppl. 1968, 97, 77–89. [Google Scholar] [PubMed]
Sample Availability: Samples of all compounds are available from the authors. |


| Sub-G1/G0 | G1/G0 | S | G2 | |
|---|---|---|---|---|
| Control | 0.6 (0.4–1.6) | 98.7 (95.7–99.3) | 0.6 (0.3–2.5) | 0.2 (0.1–0.4) |
| CB[6] 0.1 mM | 1.0 (0.3–2.0) | 97.9 (96.7–98.9) | 0.8 (0.6–1.4) | 0.1 (0.1–0.2) |
| CB[6] 0.3 mM | 1.1 (0.4–5.0) | 97.2 (88.6–97.9) | 1.3 (1.0–6.1) | 0.6 (0.3–0.8) |
| CB[6] 0.5 mM | 0.6 (0.3–11.7) | 97.7(79.8–98.6) | 1.3 (1.1–7.7) | 0.5 (0.2–1.0) |
| CB[7] 0.1 mM | 0.2 (0.1–0.3) | 99.2 (99.0–99.5) | 0.3 (0.3–0.6) | 0.1 (0.1–0.3) |
| CB[7] 0.3 mM | 1.2 (0.2–6.4) | 96.6 (91.0–98.6) | 1.9 (1.1–2.4) | 0.2 (0.2–0.3) |
| CB[7] 0.5 mM | 1.2 (0.3–2.4) | 98.0 (93.1–99.2) | 0.7 (0.5–4.1) | 0.2 (0.1–0.3) |
| Sub-G1/G0 | G1/G0 | S | G2 | |
|---|---|---|---|---|
| Control | 2.8 (1.3–5.7) | 56.4 (55.1–69.6) | 30.4 (20.6–31.9) | 9.5 (7.8–10.1) |
| CB[6] 0.1 mM | 4.4 (2.3–5.4) | 58.6 (55.5–72.7) | 27.8 (17.7–31.7) | 8.4 (6.4–9.2) |
| CB[6] 0.3 mM | 3.3 (2.0–4.6) | 58.4 (52.5–74.4) | 28.0 (16.9–33.4) | 9.1 (5.4–10.9) |
| CB[6] 0.5 mM | 3.5 (2.0–4.5) | 58.5 (52.9–72.5) | 28.7 (19.0–33.1) | 8.6 (6.5–9.7) |
| CB[7] 0.1 mM | 2.3 (1.1–3.5) | 61.3 (54.1–76.8) | 28.1 (16.5–31.7) | 8.3 (5.3–11.2) |
| CB[7] 0.3 mM | 2.8 (1.4–3.5) | 59.6 (54.0–74.1) | 28.2 (18.2–32.1) | 8.8 (6.1–10.7) |
| CB[7] 0.5 mM | 3.9 (1.7–5.4) | 58.8 (56.5–75.6) | 27.1 (16.3–29.7) | 8.1 (5.1–9.6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pashkina, E.; Aktanova, A.; Blinova, E.; Mirzaeva, I.; Kovalenko, E.; Knauer, N.; Ermakov, A.; Kozlov, V. Evaluation of the Immunosafety of Cucurbit[n]uril on Peripheral Blood Mononuclear Cells In Vitro. Molecules 2020, 25, 3388. https://doi.org/10.3390/molecules25153388
Pashkina E, Aktanova A, Blinova E, Mirzaeva I, Kovalenko E, Knauer N, Ermakov A, Kozlov V. Evaluation of the Immunosafety of Cucurbit[n]uril on Peripheral Blood Mononuclear Cells In Vitro. Molecules. 2020; 25(15):3388. https://doi.org/10.3390/molecules25153388
Chicago/Turabian StylePashkina, Ekaterina, Alina Aktanova, Elena Blinova, Irina Mirzaeva, Ekaterina Kovalenko, Nadezhda Knauer, Aleksandr Ermakov, and Vladimir Kozlov. 2020. "Evaluation of the Immunosafety of Cucurbit[n]uril on Peripheral Blood Mononuclear Cells In Vitro" Molecules 25, no. 15: 3388. https://doi.org/10.3390/molecules25153388
APA StylePashkina, E., Aktanova, A., Blinova, E., Mirzaeva, I., Kovalenko, E., Knauer, N., Ermakov, A., & Kozlov, V. (2020). Evaluation of the Immunosafety of Cucurbit[n]uril on Peripheral Blood Mononuclear Cells In Vitro. Molecules, 25(15), 3388. https://doi.org/10.3390/molecules25153388





