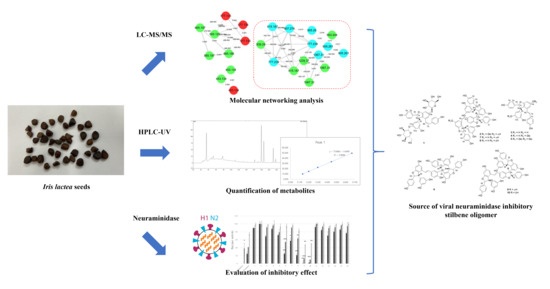
Combined MS/MS-NMR Annotation Guided Discovery of Iris lactea var. chinensis Seed as a Source of Viral Neuraminidase Inhibitory Polyphenols
Abstract
1. Introduction
2. Results and Discussion
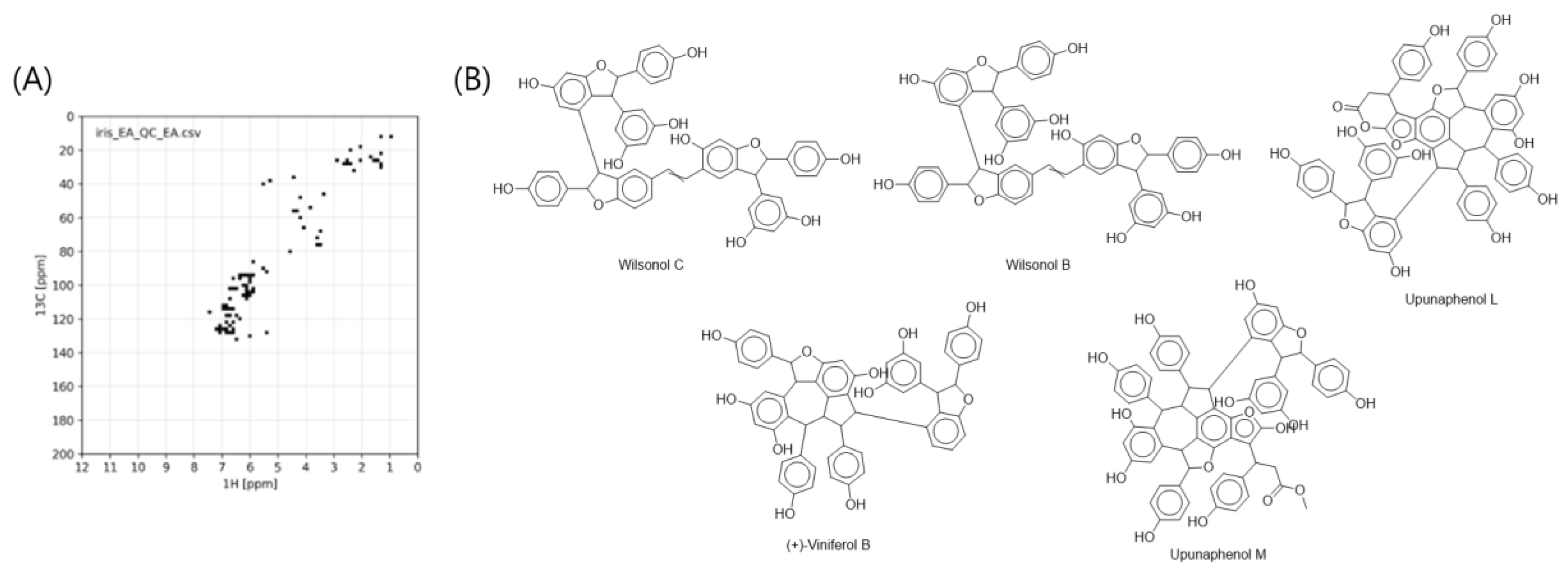
2.1. Annotation of the Stilbenoids from I. lactea var. Chinensis Using MS/MS-NMR Analyses
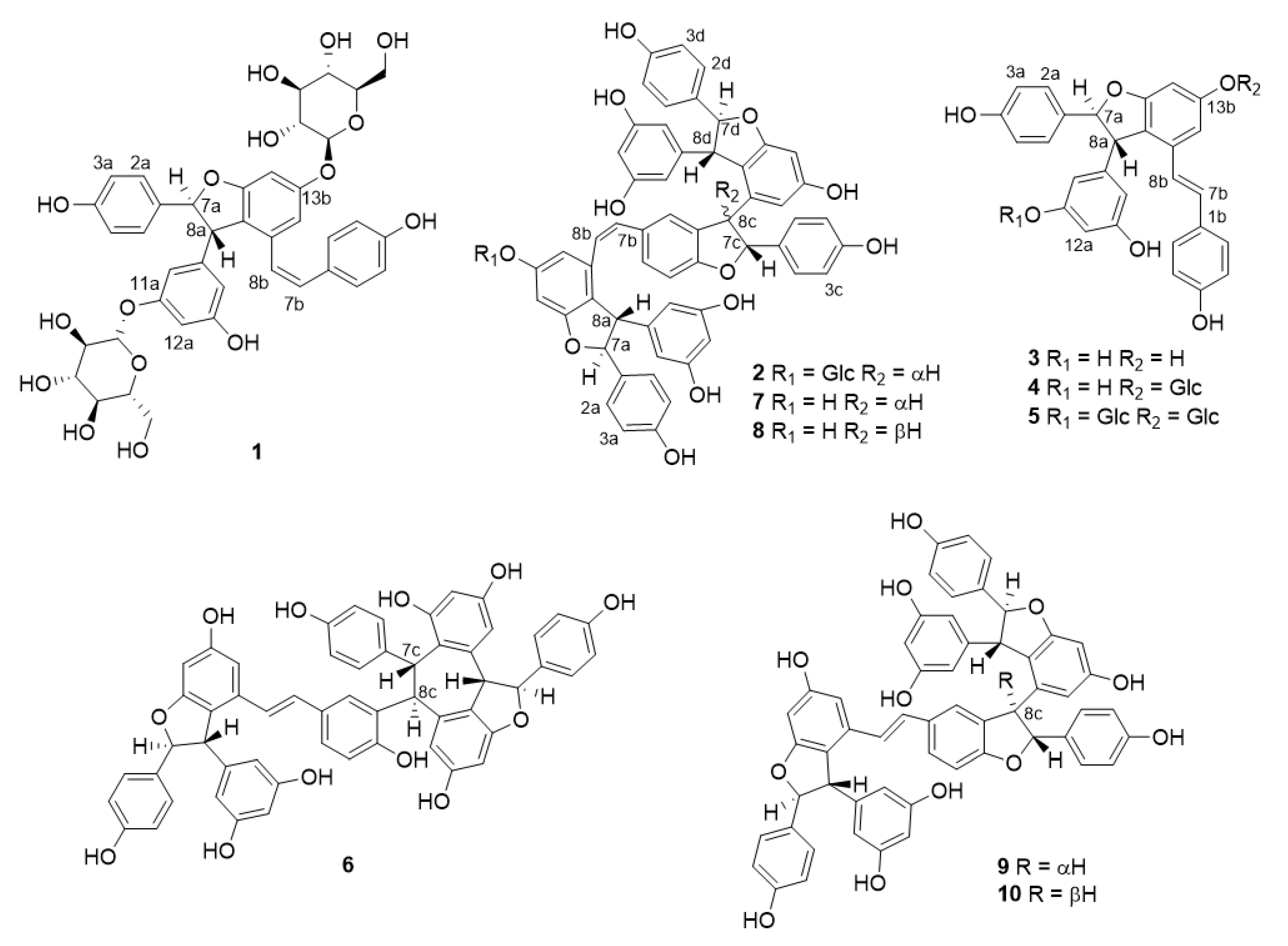
2.2. Structure Elucidation of Isolated Stilbenoids from I. Lactea var. Chinensis Seeds
2.3. Quantification of Stilbenoids in I. lactea var. Chinensis Seed Extract
2.4. Viral Neuraminidase Inhibitory Effects of Isolated Stilbenoids from I. lactea var. Chinensis Seeds
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Plant Materials
3.3. Sample Extraction
3.4. LC-MS/MS-Based Metabolite Profiling
3.4.1. Sample Preparation
3.4.2. Data Acquisition and Molecular Networking Analysis
3.5. SMART Analysis
3.6. Isolation of Stilbene Oligomers
3.7. Quantification of Major Stilbene Oligomers Using HPLC-UV Analysis
3.7.1. Preparation of Standard and Sample Solution
3.7.2. HPLC-UV Chromatographic System
3.7.3. Method Validation
3.8. Evaluation of Viral Neuraminidase Inhibitory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sobolev, V.S.; Horn, B.W.; Potter, T.L.; Deyrup, S.T.; Gloer, J.B. Production of Stilbenoids and Phenolic Acids by the Peanut Plant at Early Stages of Growth. J. Agric. Food Chem. 2006, 54, 3505–3511. [Google Scholar] [CrossRef] [PubMed]
- Rühmann, S.; Pfeiffer, J.; Brunner, P.; Szankowski, I.; Fischer, T.C.; Forkmann, G.; Treutter, D. Induction of stilbene phytoalexins in grapevine (Vitis vinifera) and transgenic stilbene synthase-apple plants (Malus domestica) by a culture filtrate of Aureobasidium pullulans. Plant Physiol. Biochem. 2013, 72, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; De Rosso, M.; Bavaresco, L. Study of Grape Polyphenols by Liquid Chromatography-High-Resolution Mass Spectrometry (UHPLC/QTOF) and Suspect Screening Analysis. J. Anal. Methods Chem. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Vannozzi, A.; Dry, I.B.; Fasoli, M.; Zenoni, S.; Lucchin, M. Genome-wide analysis of the grapevine stilbene synthase multigenic family: Genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Boil. 2012, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Regev-Shoshani, G.; Shoseyov, O.; Bilkis, I.; Kerem, Z. Glycosylation of resveratrol protects it from enzymic oxidation. Biochem. J. 2003, 374, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Wang, X.-N.; Lou, H.-X. Natural stilbenes: An overview. Nat. Prod. Rep. 2009, 26, 916. [Google Scholar] [CrossRef]
- Na, M.K.; Min, B.S.; Bae, K.H. Protective effect of stilbenes on oxidative damage. Nat. Prod. Sci. USA 2007, 13, 369–372. [Google Scholar]
- Błaszczyk, A.; Sady, S.; Sielicka, M. The stilbene profile in edible berries. Phytochem. Rev. 2018, 18, 37–67. [Google Scholar] [CrossRef]
- Mäkinen, S.; Hellström, J.; Mäki, M.; Korpinen, R.; Mattila, P.H. Bilberry and Sea Buckthorn Leaves and Their Subcritical Water Extracts Prevent Lipid Oxidation in Meat Products. Foods 2020, 9, 265. [Google Scholar] [CrossRef]
- Pan, J.; Shi, M.; Li, L.; Liu, J.; Guo, F.; Feng, Y.; Ma, L.; Fu, P. Pterostilbene, a bioactive component of blueberries, alleviates renal fibrosis in a severe mouse model of hyperuricemic nephropathy. Biomed. Pharmacother. 2019, 109, 1802–1808. [Google Scholar] [CrossRef]
- Goldblatt, P. Phylogeny and classification of the Iridaceae and the relationships of Iris. Annali di Botanica 2000, 58, 13–28. [Google Scholar]
- Lv, H.; Wang, H.; He, Y.; Ding, C.; Wang, X.; Suo, Y. Separation and purification of four oligostilbenes from Iris lactea Pall. var. chinensis (Fisch.) Koidz by high-speed counter-current chromatography. J. Chromatogr. B 2015, 988, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Idelbayev, Y.; Roberts, N.; Tao, Y.; Nannapaneni, Y.; Duggan, B.M.; Min, J.; Lin, E.C.; Gerwick, E.C.; Cottrell, G.W.; et al. Small Molecule Accurate Recognition Technology (SMART) to Enhance Natural Products Research. Sci. Rep. 2017, 7, 14243. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.-H.M. The Strengths and Weaknesses of NMR Spectroscopy and Mass Spectrometry with Particular Focus on Metabolomics Research. Adv. Struct. Safe. Stud. 2015, 1277, 161–193. [Google Scholar] [CrossRef]
- .Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.A.; Kapono, C.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Ernst, M.; Bin Kang, K.; Caraballo-Rodríguez, A.M.; Nothias, L.-F.; Wandy, J.; Chen, C.; Wang, M.; Rogers, S.; Medema, M.H.; Dorrestein, P.C.; et al. MolNetEnhancer: Enhanced Molecular Networks by Integrating Metabolome Mining and Annotation Tools. Metabolisms 2019, 9, 144. [Google Scholar] [CrossRef]
- Crüsemann, M.; O’Neill, E.C.; Larson, C.B.; Melnik, A.V.; Floros, D.J.; Da Silva, R.R.; Jensen, P.R.; Dorrestein, P.C.; Moore, B.S. Prioritizing Natural Product Diversity in a Collection of 146 Bacterial Strains Based on Growth and Extraction Protocols. J. Nat. Prod. 2016, 80, 588–597. [Google Scholar] [CrossRef]
- Boya P., C.A.; Fernández-Marín, H.; Mejía, L.C.; Spadafora, C.; Dorrestein, P.C.; Gutiérrez, M. Imaging mass spectrometry and MS/MS molecular networking reveals chemical interactions among cuticular bacteria and pathogenic fungi associated with fungus-growing ants. Sci. Rep. 2017, 7, 5604. [Google Scholar] [CrossRef]
- Woo, S.; Bin Kang, K.; Kim, J.; Sung, S.H. Molecular Networking Reveals the Chemical Diversity of Selaginellin Derivatives, Natural Phosphodiesterase-4 Inhibitors from Selaginella tamariscina. J. Nat. Prod. 2019, 82, 1820–1830. [Google Scholar] [CrossRef]
- Reher, R.; Kim, H.W.; Zhang, C.; Mao, H.H.; Wang, M.; Nothias, L.-F.; Caraballo-Rodriguez, A.M.; Glukhov, E.; Teke, B.; Leao, T.; et al. A Convolutional Neural Network-Based Approach for the Rapid Annotation of Molecularly Diverse Natural Products. J. Am. Chem. Soc. 2020, 142, 4114–4120. [Google Scholar] [CrossRef]
- Yuk, H.J.; Ryu, H.W.; Jeong, S.H.; Curtis-Long, M.J.; Kim, H.J.; Wang, Y.; Song, Y.H.; Park, K. Profiling of neuraminidase inhibitory polyphenols from the seeds of Paeonia lactiflora. Food Chem. Toxicol. 2013, 55, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Ito, T.; Ohguchi, K.; Nasu, M.; Masuda, Y.; Oyama, M.; Nozawa, Y.; Ito, M.; Iinuma, M. Resveratrol Oligomers fromVatica albiramis. J. Nat. Prod. 2010, 73, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Gabaston, J.; Leborgne, C.; Waffo-Teguo, P.; Fonayet, J.V.; Pinto, A.P.; Richard, T.; Cluzet, S.; Merillon, J.-M. Wood and roots of major grapevine cultivars and rootstocks: A comparative analysis of stilbenes by UHPLC-DAD-MS/MS and NMR. Phytochem. Anal. 2019, 30, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Takaya, Y.; Oshima, Y.; Niwa, M. New oligostilbenes having a benzofuran from Vitis vinifera ‘Kyohou’. Tetrahedron 1999, 55, 2529–2544. [Google Scholar] [CrossRef]
- Ito, J.; Niwa, M. Absolute structures of new hydroxystilbenoids, vitisin C and viniferal, from Vitis vinifera ‘Kyohou’. Tetrahedron 1996, 52, 9991–9998. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Karplus, M. Vicinal Proton Coupling in Nuclear Magnetic Resonance. J. Am. Chem. Soc. 1963, 85, 2870–2871. [Google Scholar] [CrossRef]
- Adrian, M.; Jeandet, P.; Douillet-Breuil, A.C.; Tesson, L.; Bessis, R. Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. J. Agric. Food Chem. 2000, 48, 6103–6105. [Google Scholar] [CrossRef]
- Vergara, C.; Von Baer, D.; Mardones, C.; Wilkens, A.; Wernekinck, K.; Damm, A.; Macke, S.; Gorena, T.; Winterhalter, P. Stilbene Levels in Grape Cane of Different Cultivars in Southern Chile: Determination by HPLC-DAD-MS/MS Method. J. Agric. Food Chem. 2012, 60, 929–933. [Google Scholar] [CrossRef]
- McNicholl, I.R.; McNicholl, J.J. Neuraminidase inhibitors: Zanamivir and oseltamivir. Ann. Pharmacother. 2001, 35, 57–70. [Google Scholar] [CrossRef]
- Nguyen, T.N.A.; Dao, T.T.; Tung, B.T.; Choi, H.; Kim, E.; Park, J.; Lim, S.-I.; Oh, W.K. Influenza A (H1N1) neuraminidase inhibitors from Vitis amurensis. Food Chem. 2011, 124, 437–443. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds studied are available/are not available from the authors. |

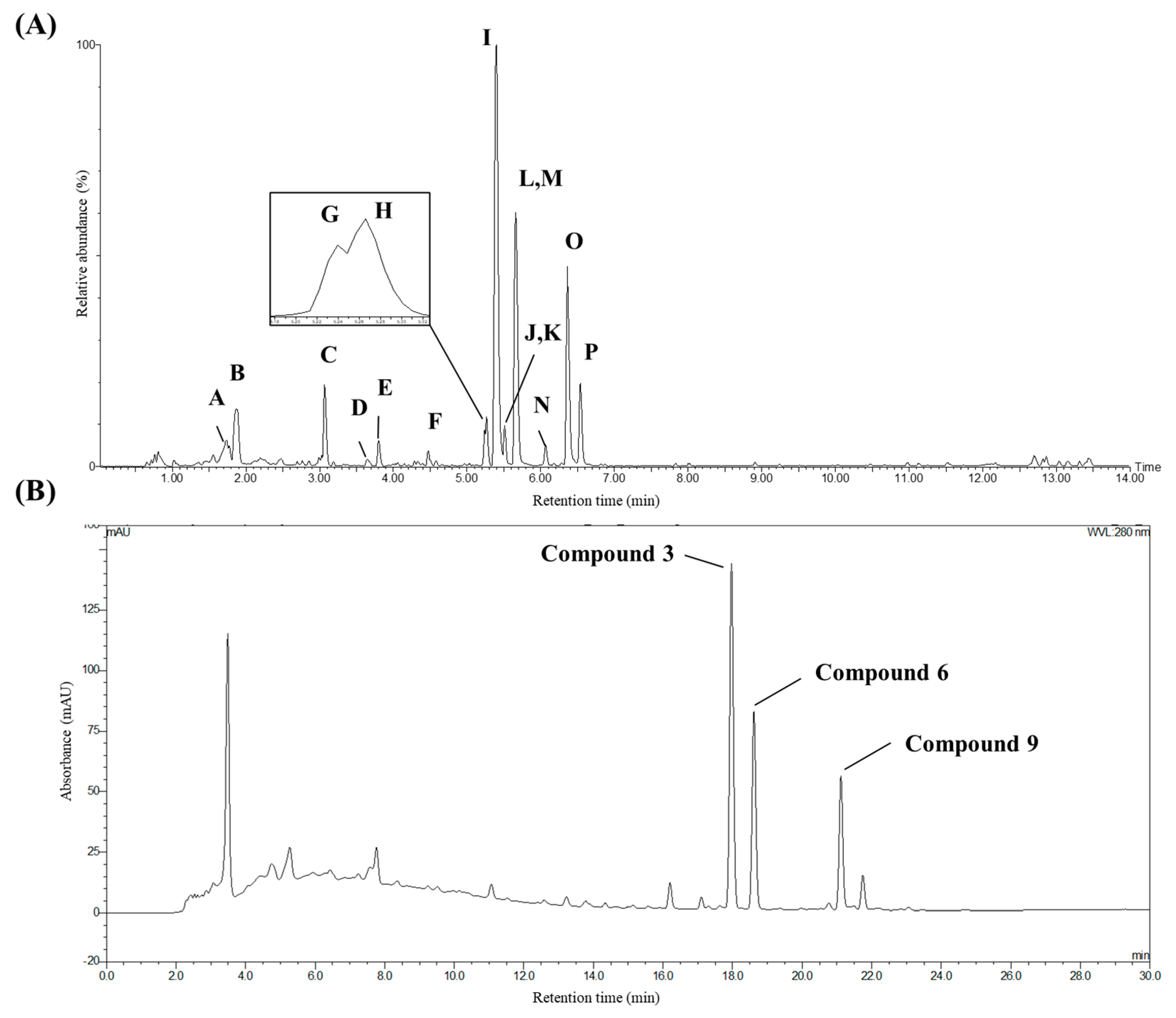
| Peak | Retention Time (min) | Precursor Ion m/z ([M−H]-) | Molecular Formula | Error (ppm) | MS/MS Fragments | Compounds |
|---|---|---|---|---|---|---|
| A | 1.74 | 577.1344 | C30H26O12 | −0.3 | 425, 407, 289, 125 | procyanidin B3 |
| B | 1.86 | 289.0701 | C15H14O6 | −3.8 | 245, 203, 125 | catechin |
| C | 3.06 | 777.2402 | C40H42O16 | 0.9 | 615, 453, 359, 347 | vatalbinoside C (5) |
| D | 3.64 | 777.2381 | C40H42O16 | −1.8 | 633, 575, 453 | cis-ε-viniferin-11a,13b-O-β-d-diglucopyranoside (1) |
| E | 3.80 | 615.1884 | C34H32O11 | 2.9 | 475, 453, 359, 347 | trans-ε-viniferin-13b-O-β-d-glucopyranose (4) |
| F | 4.48 | 615.1891 | C34H32O11 | 4.1 | 537, 475, 453, 435, 359, 347 | Stilbene dimer glycoside |
| G | 5.24 | 1067.3105 | C62H52O17 | 1.3 | 905, 799, 663, 573, 453, 359, 347, 253 | cis-vitisin B-13b-O-β-d-glucopyranoside (2) |
| H | 5.27 | 453.1336 | C28H22O6 | −0.4 | 435, 411, 359, 347, 253, 225 | Stilbene dimer |
| I | 5.40 | 453.1335 | C28H22O6 | −0.7 | 435, 411, 385, 369, 359, 347, 253, 225 | trans-ε-viniferin (3) |
| J | 5.50 | 1067.3103 | C62H52O17 | 1.3 | 905, 799, 663, 573, 453, 359, 347, 253 | Stilbene tetramer glycoside |
| K | 5.51 | 905.2580 | C56H42O12 | −2.0 | 811, 799, 453, 359, 347 | cis-vitisin B (7) |
| L | 5.63 | 1067.3110 | C62H52O17 | 1.5 | 905, 799, 663, 573, 453, 359, 347, 253 | Stilbene tetramer glycoside |
| M | 5.66 | 905.2591 | C56H42O12 | −0.8 | 811, 799, 675, 545, 451, 439, 359, 347, 333 | vitisin A (6) |
| N | 6.06 | 905.2562 | C56H42O12 | −4.0 | 811, 799, 679, 573, 545, 477, 451, 359, 347 | cis-vitisin C (8) |
| O | 6.36 | 905.2609 | C56H42O12 | 1.2 | 811, 799, 693, 545, 359, 347 | vitisin B (9) |
| P | 6.54 | 905.2591 | C56H42O12 | −0.8 | 811, 799, 693, 545, 451, 439, 359, 347, 333 | vitisin C (10) |
| 1 | 2 | |||
|---|---|---|---|---|
| Position | δC | δH (J in Hz) | δC | δH (J in Hz) |
| 1a | 133.5 | 133.5 | ||
| 2a | 128.5 | 6.96 (d, 8.6) | 128.5 | 7.00 (d, 8.6) |
| 3a | 116.3 | 6.73 (d, 8.6) | 116.3 | 6.74 (d, 8.6) |
| 4a | 158.5 | 158.6 | ||
| 5a | 116.3 | 6.73 (d, 8.6) | 116.3 | 6.74 (d, 8.6) |
| 6a | 128.5 | 6.96 (d, 8.6) | 128.5 | 7.00 (d, 8.6) |
| 7a | 94.9 | 5.24 (d, 6.1) | 95.2 | 5.26 (d, 6.5) |
| 8a | 57.6 | 3.33 (d, 6.1) | 57.9 | 3.91 (d, 6.5) |
| 9a | 147.0 | 146.6 | ||
| 10a | 108.4 | 6.22 (brs) | 107.4 | 5.92 (d, 2.2) |
| 11a | 160.3 | 159.6 | ||
| 12a | 103.4 | 6.41 (t, 2.1) | 102.2 | 6.10 (t, 2.1) |
| 13a | 159.6 | 159.6 | ||
| 14a | 110.0 | 6.12 (brs) | 107.4 | 5.92 (d, 2.2) |
| 1b | 130.0 | 131.5 | ||
| 2b | 131.2 | 6.92 (d, 8.6) | 126.8 | 6.51 (m) |
| 3b | 116.1 | 6.60 (d, 8.6) | 127.9 | |
| 4b | 157.9 | 159.6 | ||
| 5b | 116.1 | 6.60 (d, 8.6) | 110.0 | 6.56 (d, 8.3) |
| 6b | 131.2 | 6.92 (d, 8.6) | 130.2 | 6.91 (dd, 8.3, 1.3) |
| 7b | 132.2 | 6.25 (d, 12.0) | 131.8 | 6.08 (d, 12.2) |
| 8b | 126.4 | 6.07 (d, 12.0) | 126.3 | 5.96 (d, 12.2) |
| 9b | 137.5 | 137.6 | ||
| 10b | 123.2 | 123.3 | ||
| 11b | 162.6 | 162.6 | ||
| 12b | 98.3 | 6.54 (d, 1.7) | 98.1 | 6.47 (d, 2.1) |
| 13b | 160.4 | 160.0 | ||
| 14b | 110.6 | 6.55 (d, 1.7) | 110.8 | 6.48 (d, 2.1) |
| 13b-Glc | ||||
| 1′ | 102.4 | 4.73 (d, 7.1) | 102.4 | 4.72 (d, 7.2) |
| 2′ | 74.8 | 3.40–3.50 (m) | 74.9 | 3.30–3.50 (m) |
| 3′ | 77.9 | 3.40–3.50 (m) | 77.8 | 3.30–3.50 (m) |
| 4′ | 71.1 | 3.40–3.50 (m) | 71.0 | 3.30–3.50 (m) |
| 5′ | 78.0 | 3.40–3.50 (m) | 77.8 | 3.30–3.50 (m) |
| 6′ | 62.3 | 3.81 (dd, 12.1, 2.2) 3.70 (dd, 12.1, 5.1) | 62.2 | 3.78 (dd, 12.2, 2.3) 3.70 (dd, 12.2, 4.8) |
| 11a-Glc | ||||
| 1′′ | 102.6 | 4.76 (d, 7.1) | ||
| 2′′ | 74.8 | 3.40–3.50 (m) | ||
| 3′′ | 77.8 | 3.40–3.50 (m) | ||
| 4′′ | 71.1 | 3.40–3.50 (m) | ||
| 5′′ | 78.0 | 3.40–3.50 (m) | ||
| 6′′ | 62.2 | 3.84 (dd, 12.1, 2.2) 3.65 (dd, 12.1, 5.1) | ||
| 1c | 132.6 | |||
| 2c | 127.9 | 6.60 (d, 8.6) | ||
| 3c | 116.2 | 6.55 (d, 8.6) | ||
| 4c | 158.0 | |||
| 5c | 116.2 | 6.55 (d, 8.6) | ||
| 6c | 127.9 | 6.60 (d, 8.6) | ||
| 7c | 92.5 | 5.44 (d, 5.8) | ||
| 8c | 52.9 | 4.22 (d, 5.8) | ||
| 9c | 142.3 | |||
| 10c | 120.4 | |||
| 11c | 162.5 | |||
| 12c | 96.8 | 6.30 (t, 2.1) | ||
| 13c | 160.3 | |||
| 14c | 107.5 | 6.13 (d, 2.1) | ||
| 1d | 134.2 | |||
| 2d | 128.0 | 7.12 (d, 8.4) | ||
| 3d | 116.5 | 6.77 (d, 8.4) | ||
| 4d | 158.5 | |||
| 5d | 116.5 | 6.77 (d, 8.4) | ||
| 6d | 128.0 | 7.12 (d, 8.4) | ||
| 7d | 95.0 | 5.30 (d, 5.2) | ||
| 8d | 57.8 | 4.26 (d, 5.2) | ||
| 9d | 147.6 | |||
| 10d | 107.2 | 5.97 (brs) | ||
| 11d | 159.9 | |||
| 12d | 102.5 | 6.05 (t, 2.1) | ||
| 13d | 159.9 | |||
| 14d | 107.2 | 5.97 (brs) | ||
| Compound | Regression Equation | R2 | LOD | LOQ | Rec 1 (High) | Rec 2 (Medium) | Rec 3 (Low) |
|---|---|---|---|---|---|---|---|
| 3 | y = 73.868x + 0.4495 | 0.9999 | 0.0009 | 0.0028 | 95.9 | 96.4 | 97.6 |
| 6 | y = 17.892x + 0.4495 | 1.0000 | 0.0040 | 0.0122 | 90.4 | 92.7 | 94.9 |
| 9 | y = 42.076x + 0.4495 | 0.9999 | 0.0024 | 0.0073 | 100.9 | 100.5 | 100.9 |
| Compound | Intra-Day (n = 3) a | Inter-Day (n = 3) a | Contents (mg/g DW) | ||
|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | |||
| 3 | 21.19 (2.1) | 21.56 (0.5) | 21.60 (0.4) | 21.45 (1.1) | 2.32 ± 0.06 |
| 6 | 10.76 (2.8) | 10.83 (0.7) | 10.89 (0.3) | 10.83 (0.6) | 4.95 ± 0.14 |
| 9 | 8.39 (0.3) | 8.48 (0.1) | 8.50 (0.1) | 8.46 (0.7) | 1.64 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.W.; Kim, S.S.; Kang, K.B.; Ryu, B.; Park, E.; Huh, J.; Jeon, W.K.; Chae, H.-S.; Oh, W.K.; Kim, J.; et al. Combined MS/MS-NMR Annotation Guided Discovery of Iris lactea var. chinensis Seed as a Source of Viral Neuraminidase Inhibitory Polyphenols. Molecules 2020, 25, 3383. https://doi.org/10.3390/molecules25153383
Kim HW, Kim SS, Kang KB, Ryu B, Park E, Huh J, Jeon WK, Chae H-S, Oh WK, Kim J, et al. Combined MS/MS-NMR Annotation Guided Discovery of Iris lactea var. chinensis Seed as a Source of Viral Neuraminidase Inhibitory Polyphenols. Molecules. 2020; 25(15):3383. https://doi.org/10.3390/molecules25153383
Chicago/Turabian StyleKim, Hyun Woo, Soo Sung Kim, Kyo Bin Kang, Byeol Ryu, Eunjin Park, Jungmoo Huh, Won Kyung Jeon, Hee-Sung Chae, Won Keun Oh, Jinwoong Kim, and et al. 2020. "Combined MS/MS-NMR Annotation Guided Discovery of Iris lactea var. chinensis Seed as a Source of Viral Neuraminidase Inhibitory Polyphenols" Molecules 25, no. 15: 3383. https://doi.org/10.3390/molecules25153383
APA StyleKim, H. W., Kim, S. S., Kang, K. B., Ryu, B., Park, E., Huh, J., Jeon, W. K., Chae, H.-S., Oh, W. K., Kim, J., Sung, S. H., & Chin, Y.-W. (2020). Combined MS/MS-NMR Annotation Guided Discovery of Iris lactea var. chinensis Seed as a Source of Viral Neuraminidase Inhibitory Polyphenols. Molecules, 25(15), 3383. https://doi.org/10.3390/molecules25153383