Five-Coordinated Geometries from Molecular Structures to Solutions in Copper(II) Complexes Generated from Polydentate-N-Donor Ligands and Pseudohalides
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthetic Aspects
2.2. IR Spectra of the Complexes
2.3. Description of the Structures
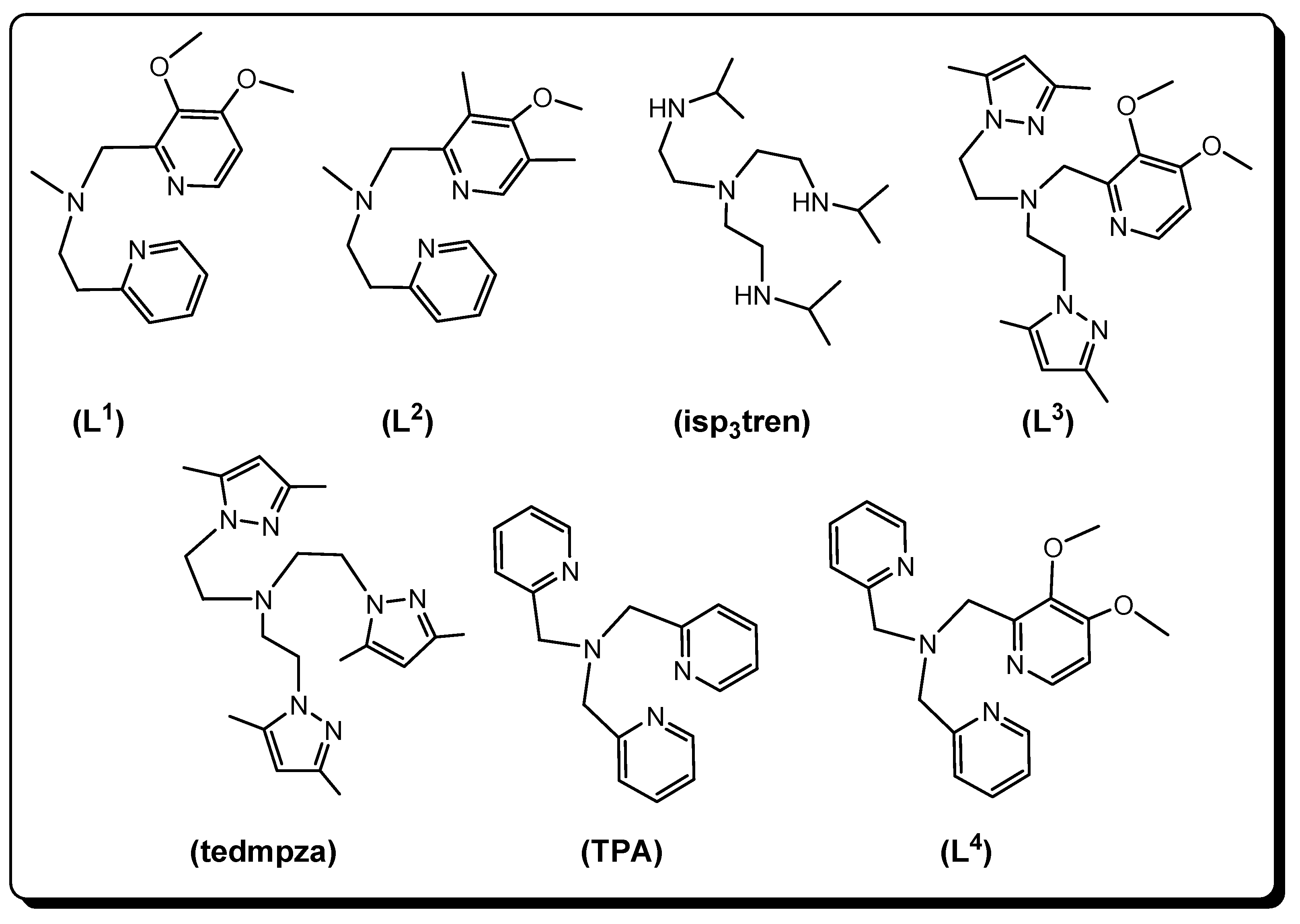
2.3.1. [Cu(L1)(NCS)2] (1), [Cu(L2)(NCS)2] (2) and [Cu(L3)(NCS)]ClO4 (5)
2.3.2. [Cu(isp3tren)(N3)]ClO4 (3), [Cu(isp3tren)(dca)]ClO4 (4), and [Cu(tedmpza)(dca)]ClO4∙0.67H2O (6)
2.4. UV-Vis Spectra of the Complexes
2.5. Molecular vs. Solution Structures in Five-Coordinated Cu(II) Complexes
3. Materials and Methods
3.1. Materials and Physical Measurements
3.2. Synthesis of the Compounds
3.3. X-ray Crystal Structure Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kelland, L.R. Preclinical perspectives on platinum resistance. Drugs 2000, 59, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kaim, W.; Schwederski, B.; Klein, A. Bioinorganic Chemistry-Inorganic Elements in the Chemistry of Life: An Introduction and Guide, 2nd ed.; Wiley: Chichester, UK, 2013. [Google Scholar]
- Kitajima, N.; Moro-oka, Y. Copper-Dioxygen complexes. Inorganic and bioinorganic perspectives. Chem. Rev. 1994, 94, 737–757. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in copper complexes as anticancer agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef] [PubMed]
- Jopp, M.; Becker, J.; Becker, S.; Miska, A.; Gandin, V.; Marzano, C.; Schindler, S. Anticancer activity of a series of copper(II) complexes with tripodal ligands. Eur. J. Med. Chem. 2017, 132, 274–281. [Google Scholar] [CrossRef]
- Massoud, S.S.; Louka, F.R.; Ducharme, G.T.; Fischer, R.C.; Mautner, F.A.; Vančo, J.; Herchel, R.; Dvořák, Z.; Trávníček, Z. Copper(II) complexes based on tripodal pyrazolyl amines: Synthesis, structure, magnetic properties and anticancer activity. J. Inorg. Biochem. 2018, 180, 39–46. [Google Scholar] [CrossRef]
- Kettenmann, S.D.; Nossol, Y.; Louka, F.R.; Legrande, J.R.; Marine, E.; Fischer, R.C.; Mautner, F.A.; Hergl, V.; Kulak, N.; Massoud, S.S. DNA Cleavage and cytotoxicity of Cu(II) complexes with piperazine-bearing symmetrical pyridyl arms. J. Inorg. Biochem. 2020, submitted. [Google Scholar]
- Massoud, S.S.; Louka, F.R.; Tusa, A.F.; Bordelon, N.E.; Fischer, R.C.; Mautner, F.A.; Vančo, J.; Hošek, J.; Dvořákd, Z.; Trávníček, Z. Copper(II) complexes based on tripodal pyridyl-amine derivatives as efficient anticancer agents. New J. Chem. 2019, 43, 6186–6196. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Park, G.Y.; Lippard, S.J. Understanding and improving platinum anticancer drugs—Phenanthriplatin. Anticancer Res. 2014, 34, 471–476. [Google Scholar]
- Ribeiro, N.; Roy, S.; Butenko, N.; Cavaco, I.; Pinheiro, T.; Alho, I.; Marques, F.; Avecilla, F.; Pessoa, J.C.; Correi, I. New Cu(II) complexes with pyrazolyl derived Schiff base ligands: Synthesis and biological evaluation. J. Inorg. Biochem. 2017, 174, 63–75. [Google Scholar] [CrossRef]
- Kettenmann, S.D.; Louka, F.R.; Marine, E.; Fischer, R.C.; Mautner, F.A.; Kulak, N.; Massoud, S.S. Efficient artificial nucleases for mediating DNA cleavage based on tuning the steric effect in the pyridyl derivatives of tripod tetraamine-cobalt(II) complexes. Eur. J. Inorg. Chem. 2018, 2018, 2322–2338. [Google Scholar] [CrossRef]
- Massoud, S.S.; Perkins, R.S.; Louka, F.R.; Xu, W.; Le Roux, A.; Dutercq, Q.; Fischer, R.C.; Mautner, F.A.; Handa, M.; Hiraoka, Y.; et al. Efficient hydrolytic cleavage of plasmid DNA by chloro-cobalt(II) complexes based on sterically hindered pyridyl tripod tetraamine ligands: Synthesis, crystal structure and DNA cleavage activity. Dalton Trans. 2014, 43, 10086–10103. [Google Scholar] [CrossRef] [PubMed]
- Świtlicka, A.; Czerwińska, K.; Machura, B.; Penkala, M.; Bieńko, A.; Bieńkod, D.; Zierkiewiczd, W. Thiocyanate copper complexes with pyrazole-derived ligands–synthesis, crystal structures, DFT calculations and magnetic properties. CrystEngComm 2016, 18, 9042–9055. [Google Scholar] [CrossRef]
- Mautner, F.A.; Fischer, R.C.; Torvisco, A.; Henary, M.M.; Milner, A.; De Villier, H.; Karsili, T.N.V.; Louka, F.R.; Massoud, S.S. Steric effects of alkyl substituents at N-donor bidentate amines direct the nuclearity, bonding and bridging modes in isothiocyanato-copper(II) coordination compounds. Crystals 2019, 9, 38. [Google Scholar] [CrossRef]
- Batten, R.S.; Murray, K.S. Structure and magnetism of coordination polymers containing dicyanamide and tricyanomethanide. Coord. Chem. Rev. 2003, 246, 103–130. [Google Scholar] [CrossRef]
- Mautner, F.A.; Jantscher, P.; Fischer, R.C.; Torvisco, A.; Vicente, R.; Karsili, T.N.V.; Massoud, S.S. Structure, DFT calculations and magnetic characterization of coordination polymers of bridged dicyanamido-metal(II) complexes. Magnetochemistry 2019, 5, 41. [Google Scholar] [CrossRef]
- Adhikary, C.; Koner, S. Structural and magnetic studies on copper(II) azido complexes. Coord. Chem. Rev. 2010, 254, 2933–2958. [Google Scholar] [CrossRef]
- Massoud, S.S.; Louka, F.R.; Obaid, Y.K.; Vicente, R.; Ribas, J.; Fischer, R.C.; Mautner, F.A. Metal ions directing the geometry and nuclearity of azido-metal(II) complexes derived from bis(2-(3,5-dimethyl-1H-pyrazol-1-yl)ethyl)amine. Dalton Trans. 2013, 42, 3968–3978. [Google Scholar] [CrossRef] [PubMed]
- Massoud, S.S.; Henary, M.M.; Maxwell, L.; Martín, A.; Ruiz, E.; Vicente, R.; Fischer, R.C.; Mautner, F.A. Structure, magnetic properties and DFT calculations of azido-copper(II) complexes with different azido-bonding, nuclearity and dimensionality. New J. Chem. 2018, 42, 2627–2639. [Google Scholar] [CrossRef]
- Escuer, A.; Font-Bardia, M.; Massoud, S.S.; Mautner, F.A.; Penalba, E.; Solans, X.; Vicente, R. Three new dinuclear copper(II) complexes with [Cu(μ1,3-N3)2Cu]2+ and [Cu(μ1,1-N3)2Cu]2+ assymetrical cores: Syntheses, structure and magnetic behavior. New J. Chem. 2004, 28, 681–686. [Google Scholar] [CrossRef]
- Mautner, F.A.; Fischer, R.C.; Rashmawi, L.G.; Louka, F.R.; Massoud, S.S. Structural characterization of metal(II) thiocyanato complexes derived from bis(2-(1-H-pyrazol-1-yl)ethyl)amine. Polyhedron 2017, 124, 237–242. [Google Scholar] [CrossRef]
- Massoud, S.S.; Louka, F.R.; David, R.N.; Dartez, M.J.; Nguyn, Q.L.; Labry, N.J.; Fischer, R.C.; Mautner, F.R. Five-coordinated metal(II) complexes based pyrazolyl ligands. Polyhedron 2015, 90, 258–265. [Google Scholar] [CrossRef]
- Mautner, F.A.; Albering, J.H.; Harrelson, E.V.; Gallo, A.A.; Massoud, S.S. N-bonding vs. S-bonding in thiocyanato-copper(II) complexes. J. Mol. Struct. 2011, 1006, 570–575. [Google Scholar] [CrossRef]
- Massoud, S.S.; Le Quan, L.; Gatterer, K.; Albering, J.H.; Fischer, R.C.; Mautner, F.A. Structural characterization of five-coordinated copper(II), nickel(II) and cobalt(II) thiocyanato complexes derived from bis(2-(3,5-dimethyl-1-pyrazolyl)ethyl)amine. Polyhedron 2012, 31, 601–606. [Google Scholar] [CrossRef]
- Mautner, F.A.; Albering, J.H.; Vicente, R.; Louka, F.R.; Gallo, A.A.; Massoud, S.S. Copper(II) complexes derived from tripodal tris[(2-ethyl-(1H-pyrazol-1-yl)]amine. Chim. Acta 2011, 365, 290–296. [Google Scholar]
- Machura, B.; Świtlicka, A.; Penkala, M. N- and S-bonded thiocyanate copper(II) complexes of 2,6-bis-(benzimidazolyl)pyridine–Synthesis, spectroscopic characterization, X-ray structure and DFT calculations. Polyhedron 2012, 45, 221–228. [Google Scholar] [CrossRef]
- Das, S.; Bhar, K.; Fun, H.-K.; Chantrapromma, S.; Ghosh, B.K. Syntheses, characterizations and structures of complexes of the types mononuclear [MII (tren)(dca)]ClO4 [M = Cu and Zn; tren = tris(2-aminoethyl)amine; dca = dicyanamide] and dinuclear [CdII2(tren)2(dca)](ClO4)3: Variation of nuclearities and architectures with metal-ion templates. Inorg. Chim. Acta 2010, 363, 784–792. [Google Scholar]
- Mautner, F.A.; Soileau, J.B.; Bankole, P.K.; Gallo, A.; Massoud, S.S. Synthesis and spectroscopic characterization of dicyanamido-Cu(II) Complexes. Part 2. Crystal structure of the complexes of tris[2-(2-pyridylethyl)]amine, tris(2-pyridylmethyl)amine and 1,4-bis[2-(2-pyridylethyl)]piperazine. J. Mol. Struct. 2008, 889, 271–278. [Google Scholar] [CrossRef]
- Mautner, F.A.; Landry, K.N.; Gallo, A.A.; Massoud, S.S. Molecular structure of mononuclear azido- and diacyanamido-Cu(II) complexes. J. Mol. Struct. 2007, 837, 72–78. [Google Scholar] [CrossRef]
- Sadhu, M.H.; Kumar, S.B. Synthesis, characterization and structures of copper(II) and cobalt(II) complexes involving N3S-coordinated tetradentate ligand and azide/thiocyanate/nitrite ion. J. Mol. Struct. 2018, 1164, 239–247. [Google Scholar] [CrossRef]
- Berry, R.S. Correlation of rates of intermolecular tunneling process, with application to some group V compounds. J. Chem. Phys. 1960, 32, 933–937. [Google Scholar] [CrossRef]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Rounaghi, G.; Mohajeri, M.; Atashi, Z.; Kakhki, R.M. Conductometric study of complexation reaction between15-crown-5 and Cr3+, Mn2+ and Zn2+ metal cations in pure and binary mixed organic solvents. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 435–441. [Google Scholar] [CrossRef]
- Ghosh, T.; Chattopadhyay, T.; Das, S.; Mondal, S.; Sureh, E.; Zangrando, E.; Das, D. Thiocyanate and dicyanamide anion controlled nuclearity in Mn, Co, Ni, Cu, and Zn metal complexes with hemilabile ligand 2-benzoylpyridine. Cryst. Growth Des. 2011, 11, 3198–3205. [Google Scholar] [CrossRef]
- Machura, B.; Palion, J.; Penkala, M.; Groń, T.; Duda, H.; Kruszynski, R. Thiocyanate manganese(II) and cobalt(II) complexes of bis(pyrazol-1-yl)methane and bis(3,5-dimethylpyrazol-1-yl)methane—Syntheses, spectroscopic characterization, X-ray structure and magnetic properties. Polyhedron 2013, 56, 189–199. [Google Scholar] [CrossRef]
- Massoud, S.S.; Broussard, K.T.; Mautner, F.A.; Vicente, R.; Saha, M.K.; Bernal, I. Five-coordinated cobalt(II) complexes of tris(2-pyridylmethyl)amine (TPA): Synthesis, structural and magnetic characterization of a terephthalato-bridged dinuclear cobalt(II) complex. Inorg. Chim. Acta 2008, 361, 123–131. [Google Scholar] [CrossRef]
- Kohler, H.; Kolbe, A.; Lux, G. Metall-Pseudohalogenide. 27. Zur struktur der dicyanamide zweiwertiger 3d-metalle M(N(CN)2)2. Z. Anorg. Allg. Chem. 1977, 428, 103–112. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Rijin, J.V.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Hathaway, B.J. Comprehensive Coordination Chemistry; Wilkinson, G., Gillard, R.D., McCleverty, J.A., Eds.; Pergamon Press: Oxford, UK, 1987; Volume 5, p. 533. [Google Scholar]
- Mautner, F.A.; Louka, F.R.; Gallo, A.A.; Saber, M.R.; Burham, N.B.; Albering, J.H.; Massoud, S.S. Thiocyanato-copper(II) complexes derived from a tridentate amine ligand and from alanine. Transit. Met. Chem. 2010, 35, 613–619. [Google Scholar] [CrossRef]
- Mukhopadhyay, U.; Bernal, I.; Massoud, S.S.; Mautner, F.A. Syntheses, structures and some electrochemistry of Cu(II) complexes with tris[(2-pyridyl)methyl]amine: [Cu{N(CH2-py)3}(N3)]ClO4 (I), [Cu{N(CH2-py)3}(O–NO)]ClO4 (II) and [Cu{N(CH2-py)3}(NCS)]ClO4 (III). Inorg. Chim. Acta 2004, 357, 3673–3683. [Google Scholar] [CrossRef]
- Massoud, S.S.; Guilbeau, A.E.; Luong, H.T.; Vicente, R.; Albering, J.H.; Fischer, R.C.; Mautner, F.A. Mononuclear, dinuclear and polymeric 1D thiocyanato-and dicyanamido–copper(II) complexes based on tridentate coligands. Polyhedron 2013, 54, 26–33. [Google Scholar] [CrossRef]
- Mautner, F.A.; Louka, F.R.; LeGuet, T.; Massoud, S.S. Pseudohalide copper(II) complexes derived from polypyridyl ligands: Synthesis and characterization. J. Mol. Struct. 2009, 919, 196–203. [Google Scholar] [CrossRef]
- Samim Khan, S.; Al Masum, A.A.; Islam, M.M.; Michael, G.B.; Drew, M.G.B.; Bauzá, A.; Frontera, A.; Chattopadhyay, S. Observation of π-hole interactions in the solid state structures of three new copper(II) complexes with a tetradentate N4 donor Schiff base: Exploration of their cytotoxicity against MDA-MB 468 cells. Polyhedron 2017, 123, 334–343. [Google Scholar] [CrossRef]
- Biswas, P.; Dutta, S.; Ghosh, M. Influence of counter anions on structural, spectroscopic and electrochemical behaviours of copper(II) complexes of dipyrido[3,2-f: 20,30-h]-quinoxaline (dpq). Polyhedron 2008, 27, 2105–2112. [Google Scholar] [CrossRef]
- Rahaman, S.H.; Fun, H.-K.; Ghosh, B.K. A study on copper(II)-Schiff base-azide coordination complexes: Synthesis, X-ray structure and luminescence properties of [Cu(L)(N3)]X (L = Schiff bases; X = ClO4, PF6). Polyhedron 2005, 24, 3091–3097. [Google Scholar] [CrossRef]
- Bhadra, M.; Lee, J.Y.C.; Cowley, R.; Kim, S.; Siegler, M.A.; Solomon, E.I.; Karlin, K.D. Intramolecular hydrogen bonding enhances stability and reactivity of mononuclear cupric superoxide complexes. J. Am. Chem. Soc. 2018, 140, 9042–9045. [Google Scholar] [CrossRef] [PubMed]
- Massoud, S.S.; Mautner, F.A.; Abu-Youssef, M.; Shuaib, N.M. Synthesis and characterization of binuclear and polymeric five-coordinated copper(II) complexes derived from 3,3′,3″-triaminotripropylamine (trpn): Crystal structure of [Cu(trpn)(N3)]ClO4 (I) and [Cu2(trpn)(tren)(NO2)(H2O)](ClO4)3 (II). Polyhedron 1999, 18, 2061–2067. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Dixit, A.; Banerjee, S.; Roy, B.; Kumar, A.; Karande, A.A.; Chakravarty, A.R. BODIPY appended copper(II) complexes for cellular imaging and singlet oxygen mediated anticancer activity in visible light. RSC Adv. 2016, 6, 104474–104482. [Google Scholar] [CrossRef]
- Angel, N.R.; Khatib, R.M.; Jenkins, J.; Smith, M.; Rubalcava, J.M.; Le, B.K.; Lussier, D.; Chen, Z.; Tham, F.S.; Wilson, E.H.; et al. Copper(II) complexes possessing alkyl-substituted polypyridyl ligands: Structural Characterization and in vitro antitumor activity. J. Inorg. Biochem. 2017, 166, 12–25. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Nie, Y.; Sang, H.; Suo, J.-J.; Li, Z.-J.; Gu, W.; Tian, J.-L.; Liu, X.; Yan, S.-P. Three structurally related copper complexes with two isomers: DNA/BSA binding ability, DNA cleavage activity and excellent cytotoxicity. Inorg. Chim. Acta 2016, 457, 7–18. [Google Scholar] [CrossRef]
- Xu, W.; Craft, J.A.; Fontenot, P.R.; Barens, M.; Knierim, K.D.; Albering, J.H.; Mautner, F.A.; Massoud, S.S. Effect of the central metal ion on the cleavage of DNA by [M(TPA)Cl]ClO4 complexes (M = CoII, CuII and ZnII, TPA = tris(2-pyridylmethyl)amine): An efficient artificial nuclease for DNA cleavage. Inorg. Chim. Acta 2011, 373, 159–166. [Google Scholar] [CrossRef]
- Corsi, D.M.; Murthy, N.N.; Young, V.G.; Karlin, K.D. Synthesis, structure, and solution NMR studies of cyanide−copper(II) and cyanide-bridged iron(III)−copper(II) complexes. Inorg. Chem. 1999, 38, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, R.R.; Tyeklar, Z.; Karlin, K.D.; Zubieta, J. Fluoride as a terminal and bridging ligand for copper: Isolation and X-ray crystallographic characterization of copper monomeric and dimeric complexes [CuII(TMPA)F]nn+ (n = 1 or 2; TMPA = tris[(2-pyridyl)methyl]amine). Inorg. Chem. 1991, 30, 2035–2040. [Google Scholar] [CrossRef]
- Nagao, H.; Komeda, N.; Mukaida, M.; Suzuki, M.; Tanaka, K. Structural and electrochemical comparison of copper(II) complexes with tripodal ligands. Inorg. Chem. 1996, 35, 6809–6815. [Google Scholar] [CrossRef] [PubMed]
- Schatz, M.; Becker, M.; Thaler, F.; Hampel, F.; Schindler, S.; Jacobson, R.R.; Tyeklár, Z.; Murthy, N.N.; Ghosh, P.; Chen, Q.; et al. Copper(I) complexes, copper(I)/O2 reactivity, and copper(II) complex adducts, with a series of tetradentate tripyridylalkylamine tripodal ligands. Inorg. Chem. 2001, 40, 2312–2322. [Google Scholar] [CrossRef]
- Karlin, K.D.; Hayes, J.C.; Juen, S.; Hutchinson, J.P.; Zubieta, J. Tetragonal vs. trigonal coordination in copper(II) complexes with tripod ligands: Structures and properties of [Cu(C21H24N4)Cl]PF6 and [Cu(C18H18N4)Cl]PF6. Inorg. Chem. 1982, 21, 4106–4108. [Google Scholar] [CrossRef]
- Eckenhoff, W.T.; Pintauer,T. Atom transfer radical addition (ATRA) catalyzed by copper complexes with tris[2-(dimethylamino)ethyl]amine (Me6TREN) ligand in the presence of free-radical diazo initiator AIBN. Dalton Trans. 2011, 40, 4909–4917. [Google Scholar] [CrossRef]
- Massoud, S.S.; Louka, F.R.; Fischer, R.C.; Mautner, F.A.; Trávníček, Z. Five-coordinate cobalt(II) complexes showing slow magnetic relaxation. Inorg. Chim. Acta 2020. submitted. [Google Scholar]
- Massoud, S.S.; Perez, Z.E.; Courson, J.R.; Fischer, R.C.; Mautner, F.A.; Vančo, J.; Čajan, M.; Trávníček, Z. Slow magnetic relaxation in penta-coordinate cobalt(II) field-induced single-ion magnets (SIMs) with easy-axis magnetic anisotropy. Dalton Trans. 2020, submitted. [Google Scholar]
- Maiti, M.; Thakurta, S.; Sadhukhan, D.; Pilet, G.; Rosair, G.M.; Nonat, A.; Charbonnière, L.J.; Mitra, S. Thermally stable luminescent zinc–Schiff base complexes: A thiocyanato bridged 1D coordination polymer and a supramolecular 1D polymer. Polyhedron 2013, 65, 6–15. [Google Scholar] [CrossRef]
- Coropceanu, E.B.; Croitor, L.; Siminel, A.V.; Fonari, M.S. Preparation, structural characterization and luminescence studies of mono-and binuclear Zn(II) and Cd(II) acetates with pyridine-4-aldoxime and pyridine-4-amidoxime ligands. Polyhedron 2014, 75, 73–80. [Google Scholar] [CrossRef]
- Bruker (2005). SAINT v. 7.23; Bruker AXS Inc.: Madison, WI, USA, 2005. [Google Scholar]
- Bruker (2006). APEX 2, v. 2.0-2; Bruker AXS Inc.: Madison, WI, USA, 2005. [Google Scholar]
- Sheldrick, G.M. SADABS v. 2; University of Goettingen: Goettingen, Germany, 2001. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure with SHELX. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Macrae, C.F.; Edington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, T.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Speck, A.L. PLATON, a Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 2001. [Google Scholar]
- Duggan, M.; Ray, N.; Hathaway, B.; Tomlinson, G.; Brint, P.; Pelin, K.J. Crystal structure and electronic properties of ammine[tris(2-aminoethyl)amine]copper(II) diperchlorate and potassium penta-amminecopper(II) tris(hexafluorophosphate). J. Chem. Soc. Dalton Trans. 1980, 1342–1348. [Google Scholar] [CrossRef]
- Dittler-Klingemann, A.M.; Hahn, F.E. Trigonal-bipyramidal copper(II) complexes with symmetric and unsymmetric tripodal tetramine ligands. Inorg. Chem. 1996, 35, 1996–1999. [Google Scholar] [CrossRef]
- Fischmann, A.J.; Warden, A.C.; Black, J.; Spiccia, L. Synthesis, characterization, and structures of copper(II)—Thiosulfate complexes incorporating tripodal tetraamine ligands. Inorg. Chem. 2004, 43, 6568–6578. [Google Scholar] [CrossRef] [PubMed]
- Mautner, F.A.; Vicente, R.; Massoud, S.S. Structure determination of nitrito- and thiocyanato-copper(II) complexes: X-ray structures of [Cu(Medpt)(ONO)(H2O)]ClO4 (1), [Cu(dien)(ONO)]ClO4 (2) and [Cu2(Medpt)2(μN,S-NCS)2](ClO4)2 (3) (Medpt = 3,3′-diamino-N-methyldipropylamine and dien = diethylenetriamine). Polyhedron 2006, 25, 1673–1680. [Google Scholar]
Sample Availability: Not available. |
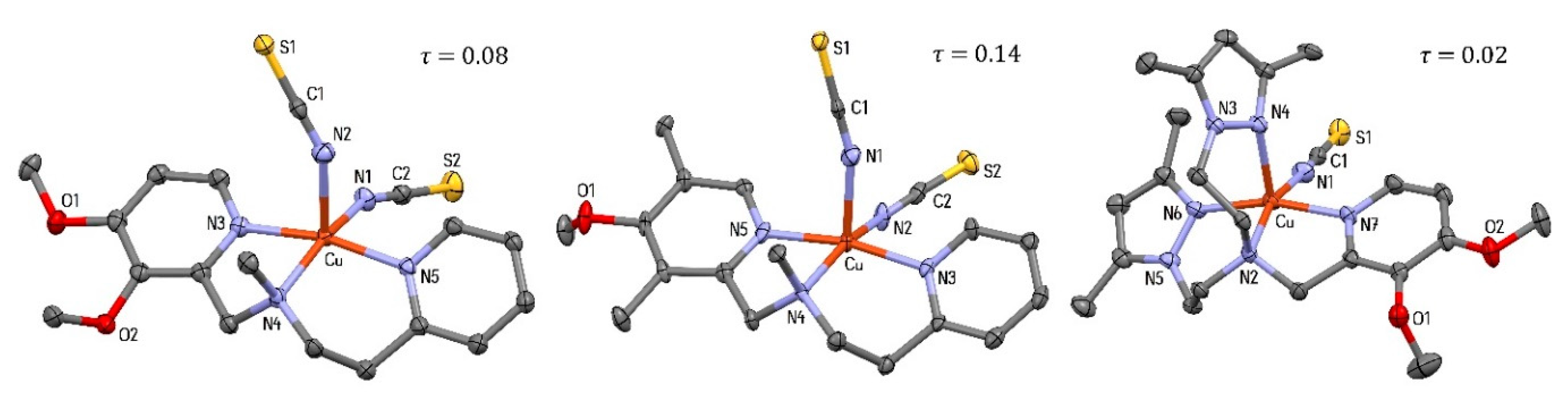


| Complex | τ Value | Geometry b | Ref. | |
|---|---|---|---|---|
| [Cu(L1)(NCS)2] | (1) | 0.08 | dist. SP | This work |
| [Cu(L2)(NCS)2] | (2) | 0.14 | dist. SP | This work |
| [Cu(bdmpzpy)(NCS)2] | (8) | 0.25 | dist. SP | [23] |
| [Cu(Mebedmpza)(NCS)2] | (9) | 0.06 | dist. SP | [22] |
| [Cu(Bzbedmpza)(NCS)2] | (10) | 0.26 | dist. SP | [22] |
| [Cu(DPA)(NCS)(ClO4)] | (11) | 0.21 | dist. SP | [23] |
| [Cu(mpipy)(NCS)2] | (12) | 0.11 | dist. SP | [42] |
| [Cu(mpiq)(NCS)2] | (13) | 0.33 | dist. SP | [42] |
| [Cu(pzdepy)(NCS)]ClO4 | (14) | 0.11 | dist. SP | [43] |
| [Cu(Me3dpt)(NCS)2] c | (15) | 0.37, 0.49 | dist. SP, intermed. | [40] |
| [Cu(bedmpza)(NCS)2] c | (16) | 0.24, 0.46 | dist. SP, intermed. | [24] |
| [Cu(tepza)(NCS)]ClO4 | (17) | 0.47 | Intermed. | [25] |
| [Cu(bdmpe)(NCS)]ClO4 | (18) | 0.17 | dist. SP | [30] |
| [Cu(L3)(NCS)]ClO4 | (5) | 0.02 | SP | This work |
| [Cu(cyclen-tpam)(NCS)]ClO4·3H2O | (19) | 0.00 | SP | [23] |
| [Cu(L5)(NCS)]ClO4 | (20) | 0.43 | dist. SP | [44] |
| [Cu(L5)(N3)]ClO4 c | (21) | 0.57, 0.65, 0.69 | dist. TBP | [44] |
| [Cu(dpq)2(N3)]ClO4 | (22) | 0.22 | dist. SP | [45] |
| [Cu(pbpd)(N3)]PF6 | (23) | 0.60 | dist. SP | [46] |
| [Cu(pfbd)(N3)]ClO4 | (24) | 0.50 | Intermed. | [46] |
| [Cu(Me3tren)(N3)]ClO4 | (25) | 0.80 | dist. TBP | [29] |
| [Cu(isp3tren)(N3)]ClO4 | (3) | 0.78 | dist. TBP | This work |
| [Cu(pdpa)(N3)]ClO4 | (26) | 0.60 | dist. TBP | [29] |
| [Cu(TPA)(N3)]ClO4 | (27) | 0.91 | dist. TBP | [41] |
| [Cu(BA)(N3)]ClO4 | (28) | 0.65 | dist. TBP | [47] |
| [Cu(F5BA)(N3)]CF3SO3 c,d | (29) | 0.73; 0.68; 0.50; 0.61; 0.31 | dist. TBP, dist. SP, Intermed. | [47] |
| [Cu(pmap)(N3)]ClO4 | (30) | 0.10 | dist. SP | [43] |
| [Cu(tedmpza)(N3)]ClO4 | (31) | 0.06 | dist. SP | [23] |
| [Cu(trpn)(N3)]ClO4 | (32) | 0.23 | dist. SP | [48] |
| [Cu(pmedien)(dca)2] | (33) | 0.23 | dist. SP | [29] |
| [Cu(pzdepy)(dca)]ClO4 | (34) | 0.24 | dist. SP | [28] |
| [Cu(tren)(dca)]ClO4 | (35) | 0.87 | dist. TBP | [27] |
| [Cu(isp3tren)(dca)]ClO4 | (4) | 0.77 | dist. TBP | This work |
| [Cu(TPA)(dca)]ClO4 | (36) | 0.95 | dist. TBP | [28] |
| [Cu(tepa)(dca)]ClO4 | (37) | 0.21 | dist. SP | [28] |
| [Cu(tepza)(dca)]ClO4 c | (38) | 0.61, 0.63 | dist. TBP | [25] |
| [Cu(tedmpza)(dca)]ClO4·0.67H2O c | (6) | 0.79, 0.89, 0.82 | dist. TBP | This work |
| Complex | Solvent | λmax, nm (εmax, M−1cm−1) | Predicted Geometry | Ref. | |
|---|---|---|---|---|---|
| [Cu(L1)(NCS)2] | (1) | CH3CN | 676 (190) | dist. SP | This work |
| [Cu(L2)(NCS)2] | (2) | CH3CN | 676 (196) | dist. SP | This work |
| [Cu(bdmpzpy)(NCS)2] | (8) | CH3OH | 670 (sat) | dist. SP | [23] |
| [Cu(Mebedmpza)(NCS)2] | (9) | CH3CN | 731 (155, br) | dist. SP | [22] |
| [Cu(Bzbedmpza)(NCS)2] | (10) | CH3CN | 704 (155, br) | dist. SP | [22] |
| [Cu(mpipy)(NCS)2] | (12) | CH3CN | 729 (288) | dist. SP | [42] |
| [Cu(mpiq)(NCS)2] | (13) | CH3CN | 775 (230) | dist. SP | [42] |
| [Cu(pzdepy)(NCS)]ClO4 | (14) | H2O | 591 (304) | dist. SP | [43] |
| [Cu(Me3dpt)(NCS)2] | (15) | CH3CN | 686 (204), 932 (141) | dist. SP | [40] |
| [Cu(bedmpza)(NCS)2] | (16) | CH3CN | 725 (149) | dist. SP | [24] |
| [Cu(tepza)(NCS)]ClO4 | (17) | CH3CN | 704 (174, br) | dist. SP | [25] |
| [Cu(bdmpe)(NCS)]ClO4 | (18) | CH3CN | 663 (174) | dist. SP | [30] |
| [Cu(L3)(NCS)]ClO4 | (5) | CH3CN | ~630 (sh), ~940 (76) | dist. SP | This work |
| [Cu(cyclen-tpam)(NCS)]ClO4·3H2O | (19) | H2O | 623 (435) | dist. SP | [23] |
| [Cu(L5)(NCS)]ClO4 | (20) | CH3CN | 720 (203) | dist. SP | [44] |
| [Cu(L5)(N3)]ClO4 | (21) | CH3CN | 383 (155) | dist. SP | [44] |
| [Cu(dpq)2(N3)]ClO4 | (22) | DMSO | 680 (110), 1050 (sh) | dist. SP | [45] |
| [Cu(pbpd)(N3)]PF6 | (23) | MeOH | 718 | dist. SP | [46] |
| [Cu(pfbd)(N3)]ClO4 | (24) | MeOH | 720 | dist. SP | [46] |
| [Cu(Me3tren)(N3)]ClO4 | (25) | H2O | 665 (~162), 855 (300, br) | dist. TBP | [29] |
| [Cu(isp3tren)(N3)]ClO4 | (3) | CH3CN | 695 (364, br), 870 (397, br) | dist. TBP | This work |
| [Cu(pdpa)(N3)]ClO4 | (26) | H2O | 656 (132, sh), 852 (200) | dist. TBP | [29] |
| [Cu(TPA)(N3)]ClO4 | (27) | CH3OH | ~650, 836 (201) | dist. TBP | [41] |
| [Cu(BA)(N3)]ClO4 | (28) | CH3CN | 650, ~880 | ~dist. TBP | [47] |
| [Cu(F5BA)(N3)]ClO4 | (29) | CH3CN | 655, ~870 | ~dist. TBP | [47] |
| [Cu(pmap)(N3)]ClO4 | (30) | H2O | 634 (268) | dist. SP | [43] |
| [Cu(tedmpza)(N3)]ClO4 | (31) | CH3CN | 713 (354, br) | dist. SP | [23] |
| [Cu(trpn)(N3)]ClO4 | (32) | H2O | 685 (118), 900 (sh) | dist. SP | [48] |
| [Cu(trpn)(N3)]ClO4 | (32) | DMSO | 670 (275), 920 (sh) | dist. SP | [48] |
| [Cu(pmedien)(dca)2] | (33) | H2O | 638 (265) | dist. SP | [29] |
| [Cu(pzdepy)(dca)]ClO4 | (34) | H2O | 591 (304) | dist. SP | [28] |
| [Cu(tren)(dca)]ClO4 | (35) | CH3CN | 665, 828 | dist. TBP | [27] |
| [Cu(isp3tren)(dca)]ClO4 | (4) | CH3CN | ~660 (sh), 837 (397, br) | dist. TBP | This work |
| [Cu(TPA)(dca)]ClO4 | (36) | H2O | ~650, 872 (239) | dist. TBP | [28] |
| [Cu(tepa)(dca)]ClO4 | (37) | H2O | 650 (114), ~739 (sh) | dist. SP | [28] |
| [Cu(tepa)(dca)]ClO4 | (37) | CH3OH | 640 (187) | dist. SP | [28] |
| [Cu(tepa)(dca)]ClO4 | (37) | CH3CN | 645 (217) | dist. SP | [28] |
| [Cu(tepa)(dca)]ClO4 | (37) | CH3NO2 | 640 (229) | dist. SP | [28] |
| [Cu(tepza)(dca)]ClO4 | (38) | H2O | 676 (93, br) | dist. SP | [25] |
| [Cu(tedmpza)(dca)]ClO4·0.67H2O | (6) | CH3CN | ~730 (90, sh), ~940 (110, br) | dist. TBP | This work |
| [Cu(L4)(dca)](ClO4)·2H2O | (7) | CH3CN | ~665 (sh), 880 (256, br) | dist. TBP | This work |
| Complex | τ Value | Geometry | λmax, nm (εmax, M−1cm−1) | Assigned Geometry | Ref. | |
|---|---|---|---|---|---|---|
| [Cu(TPA)Cl]ClO4·½H2O b,c | (39) | 0.98, 0.94 | TBP | ~730 (sh), 950 (br) | dist TBP | [52] |
| [Cu(TPA)Cl]PF6 | (40) | ≈1.0 | TBP | 962 (210), 632 (88, sh) | TBP | [53,54] |
| [Cu(TPA)F]PF6·CH2Cl2 | (41) | 0.91 | dist. TBP | 710 (sh), 872 | dist. TBP | [54] |
| [Cu(6-MeTPA)Cl]PF6 | (42) | 0.12 | dist. SP | ~670 (sh), 885 (132, br) | dist TBP | [8] |
| [Cu(6-MeTPA)Cl]ClO4 c | (43) | 0.16, 0.24 | dist. SP | - | [55] | |
| [Cu(6-Me2TPA)Cl]PF6 | (44) | - | - | 685 (148), ~860 (sh) | dist TBP | [8] |
| [Cu(6-Me2TPA)Cl]ClO4 | (45) | 0.07 | SP | - | - | [55] |
| [Cu(6-Me3TPA)Cl]ClO4 | (46) | 0.43 | Intermed. | - | - | [55] |
| [Cu(pmea)Cl]ClO4·H2O | (47) | ≈0 | SP | - | [56] | |
| [Cu(pmapCl]ClO4 | (48) | ≈0 | SP | - | [56,57] | |
| [Cu(pmap)Cl]PF6·¼CH2Cl2 | (49) | - | dist. SP | 639 (192), 898 (sh, 23) | dist SP | [56] |
| [Cu(tepa)Cl]PF6 | (50) | ≈0 | SP | 665 (200), 967 (48) | SP | [57] |
| [Cu(BPQA)Cl]ClO4 | (51) | 0.16 | dist. SP | ~640, 880 (151, br) | dist TBP | [8] |
| [Cu(BPQA)Cl]PF6 | (52) | 0.13 | dist. SP | ~700 (sh), 900 (159, br) | dist TBP | [8] |
| [Cu(BQPA)Cl]ClO4 | (53) | - | - | 730 (138, br), ~880 (143, br) | Intermed. | [8] |
| [Cu(BQPA)Cl]PF6 | (54) | 0.64 | dist. TBP | ~660 (sh), 880 (141, br) | dist TBP | [8] |
| [Cu(L6)Cl]ClO4 | (55) | - | - | ~725 (sh), 955 (221) | dist TBP | [8] |
| [Cu(L6)Cl]PF6 c | (56) | 0.96 (mean) | TBP | ~700, ~850 (sh), 970 (355) | dist TBP | [8] |
| [Cu(L7)Cl]ClO4 | (57) | 0.86 | dist. TBP | ~715 (sh), 960 (230, br) | dist TBP | [8] |
| [Cu(L8)Cl]ClO4 | (58) | 0.80 | dist. TBP | ~710, ~880 (sh), 970 (371) | dist TBP | [8] |
| [Cu(Me6tren)Cl]Cl | (59) | 1.0 | TBP | 938 (451) | dist TBP | [58] |
| [Cu(Me6tren)Br]Br | (60) | 1.0 | TBP | 973 (426) | dist TBP | [58] |
| [Cu(L9)Cl2] d | (61) | 0.11 | dist. SP | 787 (280) | dist. SP | [49] |
| [Cu(L10)(H2O)(Cl)2] e | (62) | 0.27 | dist. SP | 463 (sh, 503), 806 (135) | dist. SP | [50] |
| [Cu(L11)(Br)2] | (63) | 0.09 | dist. SP | - | [51] | |
| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C18H21CuN5O2S2 | C19H23CuN5OS2 | C15H36ClCuN7O4 |
| Formula mass | 467.01 | 465.09 | 477.51 |
| System | Monoclinic | Monoclinic | Orthorhombic |
| Space group | P21/c | P21/c | P212121 |
| a (Å) | 9.9325(18) | 8.037(3) | 8.2797(3) |
| b (Å) | 7.6958(14) | 28.198(9) | 10.3404(4) |
| c (Å) | 26.491(5) | 9.219(3) | 25.1838(10) |
| α (°) | 90 | 90 | 90 |
| β (°) | 95.923(3) | 93.416(5) | 90 |
| γ (°) | 90 | 90 | 90 |
| V (Å3) | 2014.8(6) | 2085.6(12) | 2156.12(14) |
| Z | 4 | 4 | 4 |
| T (K) | 100(2) | 100(2) | 100(2) |
| μ (mm−1) | 1.316 | 1.267 | 1.173 |
| Dcalc (Mg/m3) | 1.540 | 1.481 | 1.471 |
| θ max (°) | 26.349 | 26.395 | 24.999 |
| Data collected | 14,125 | 12,796 | 71,092 |
| Unique refl./Rint | 4099/0.0268 | 4193/0.1191 | 3756/0.0437 |
| Parameters/Restraints | 256/0 | 257/0 | 260/0 |
| Goodness-of-Fit on F2 | 1.074 | 1.123 | 1.110 |
| R1/wR2 (all data) | 0.0373/0.0956 | 0.0943/0.2051 | 0.0152/0.0388 |
| Residual extrema (e/Å3) | 1.119/−0.465 | 1.215/−0.998 | 0.210/−0.150 |
| Compound | 4 | 5 | 6 |
| Empirical formula | C17H36ClCuN7O4 | C23H32ClCuN7O6S | C69H103Cl3Cu3N30O14 |
| Formula mass | 501.53 | 633.62 | 1873.81 |
| System | Orthorhombic | Monoclinic | Triclinic |
| Space group | Pbca | P21/c | P1 |
| a (Å) | 20.0498(9) | 8.8023(7) | 9.9990(10) |
| b (Å) | 15.1408(6) | 29.700(2) | 15.7623(17) |
| c (Å) | 15.5606(7) | 11.2141(10) | 15.9456(18) |
| α (°) | 90 | 90 | 114.689(5) |
| β (°) | 90 | 93.260(4) | 100.284(5) |
| γ (°) | 90 | 90 | 103.717(5) |
| V (Å3) | 4723.7(4) | 2926.9(4) | 2105.8(4) |
| Z | 8 | 4 | 1 |
| T (K) | 100(2) | 100(2) | 100(2) |
| μ (mm−1) | 1.074 | 0.958 | 0.925 |
| Dcalc (Mg/m3) | 1.410 | 1.438 | 1.478 |
| θ max (°) | 24.997 | 27.025 | 28.276 |
| Data collected | 102,653 | 25,071 | 93,026 |
| Unique refl./Rint | 4125/0.1406 | 6395/0.0647 | 17,329/0.0922 |
| Parameters/Restraints | 415/0 | 395/36 | 1099/12 |
| Goodness-of-Fit on F2 | 1.106 | 1.062 | 1.044 |
| R1/wR2 (all data) | 0.0422/0.1352 | 0.0542/0.1258 | 0.0549/0.1347 |
| Residual extrema (e/Å3) | 1.180/−0.477 | 0.679/−0.526 | 1.249/−0.942 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mautner, F.A.; Fischer, R.C.; Torvisco, A.; Henary, M.M.; Louka, F.R.; Massoud, S.S.; Salem, N.M.H. Five-Coordinated Geometries from Molecular Structures to Solutions in Copper(II) Complexes Generated from Polydentate-N-Donor Ligands and Pseudohalides. Molecules 2020, 25, 3376. https://doi.org/10.3390/molecules25153376
Mautner FA, Fischer RC, Torvisco A, Henary MM, Louka FR, Massoud SS, Salem NMH. Five-Coordinated Geometries from Molecular Structures to Solutions in Copper(II) Complexes Generated from Polydentate-N-Donor Ligands and Pseudohalides. Molecules. 2020; 25(15):3376. https://doi.org/10.3390/molecules25153376
Chicago/Turabian StyleMautner, Franz A., Roland C. Fischer, Ana Torvisco, Maher M. Henary, Febee R. Louka, Salah S. Massoud, and Nahed M. H. Salem. 2020. "Five-Coordinated Geometries from Molecular Structures to Solutions in Copper(II) Complexes Generated from Polydentate-N-Donor Ligands and Pseudohalides" Molecules 25, no. 15: 3376. https://doi.org/10.3390/molecules25153376
APA StyleMautner, F. A., Fischer, R. C., Torvisco, A., Henary, M. M., Louka, F. R., Massoud, S. S., & Salem, N. M. H. (2020). Five-Coordinated Geometries from Molecular Structures to Solutions in Copper(II) Complexes Generated from Polydentate-N-Donor Ligands and Pseudohalides. Molecules, 25(15), 3376. https://doi.org/10.3390/molecules25153376









