Electrospinning Ag-TiO2 Nanorod-Loaded Air Treatment Filters and Their Applications in Air Purification
Abstract
1. Introduction
2. Result and Discussion
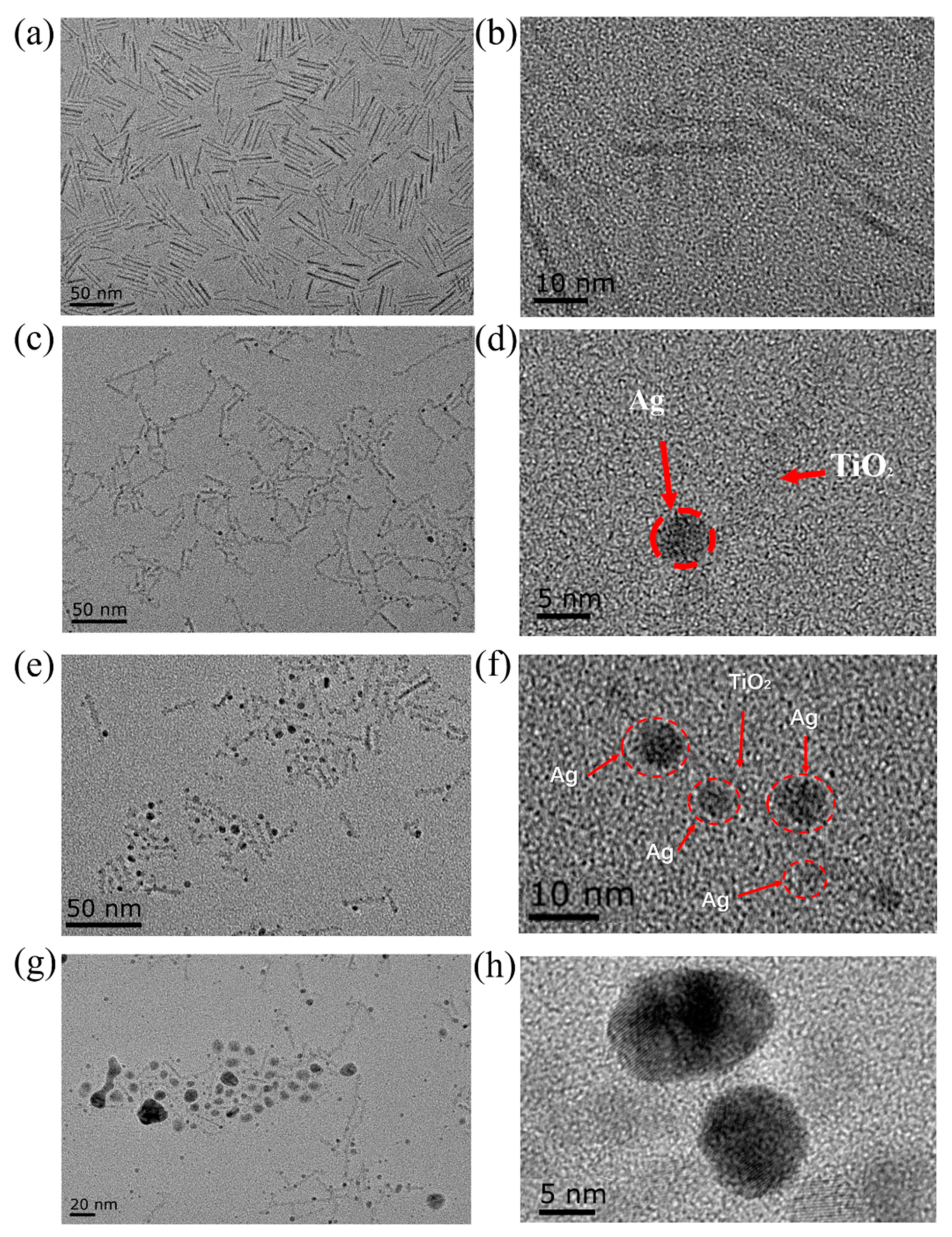
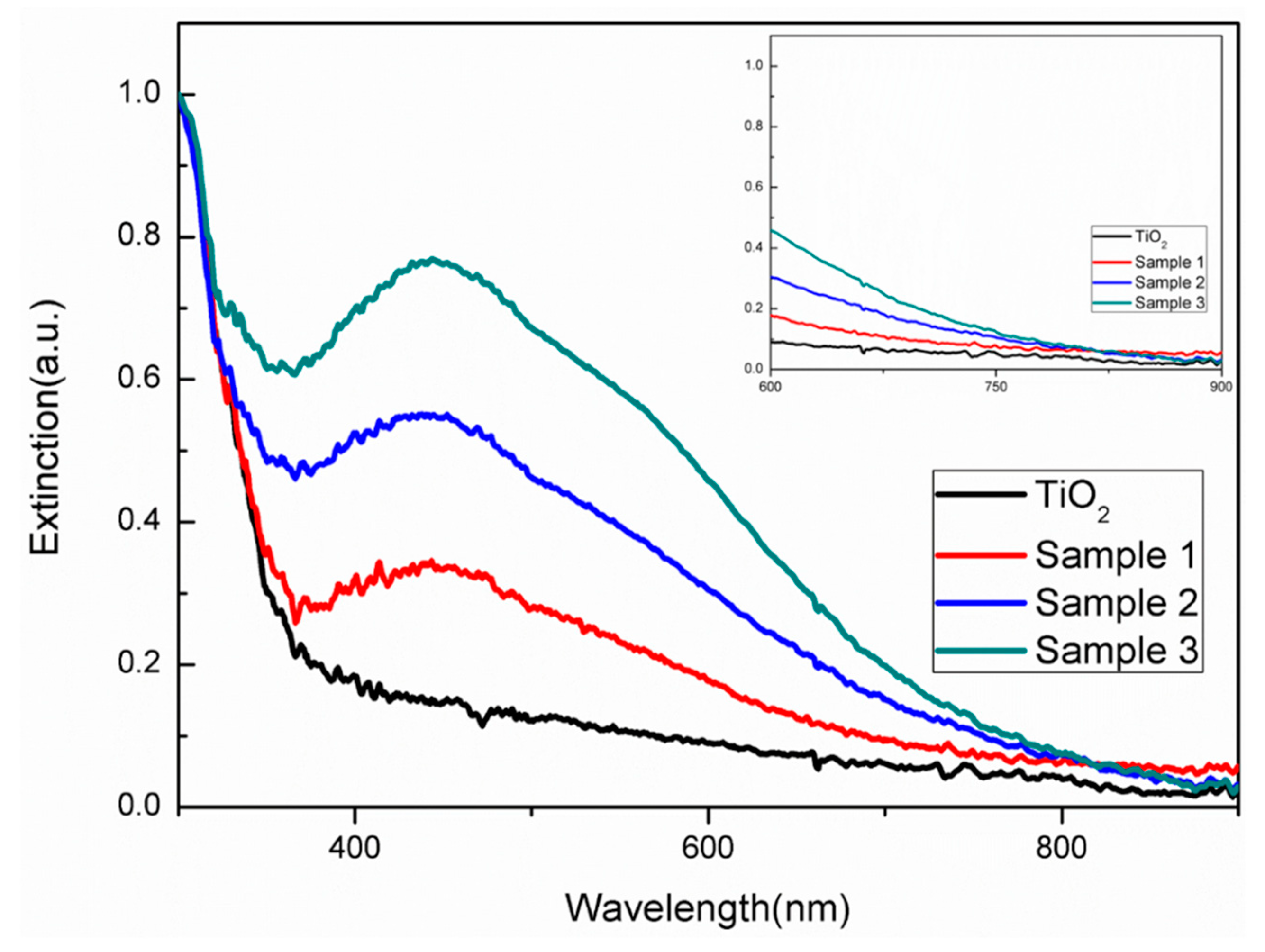
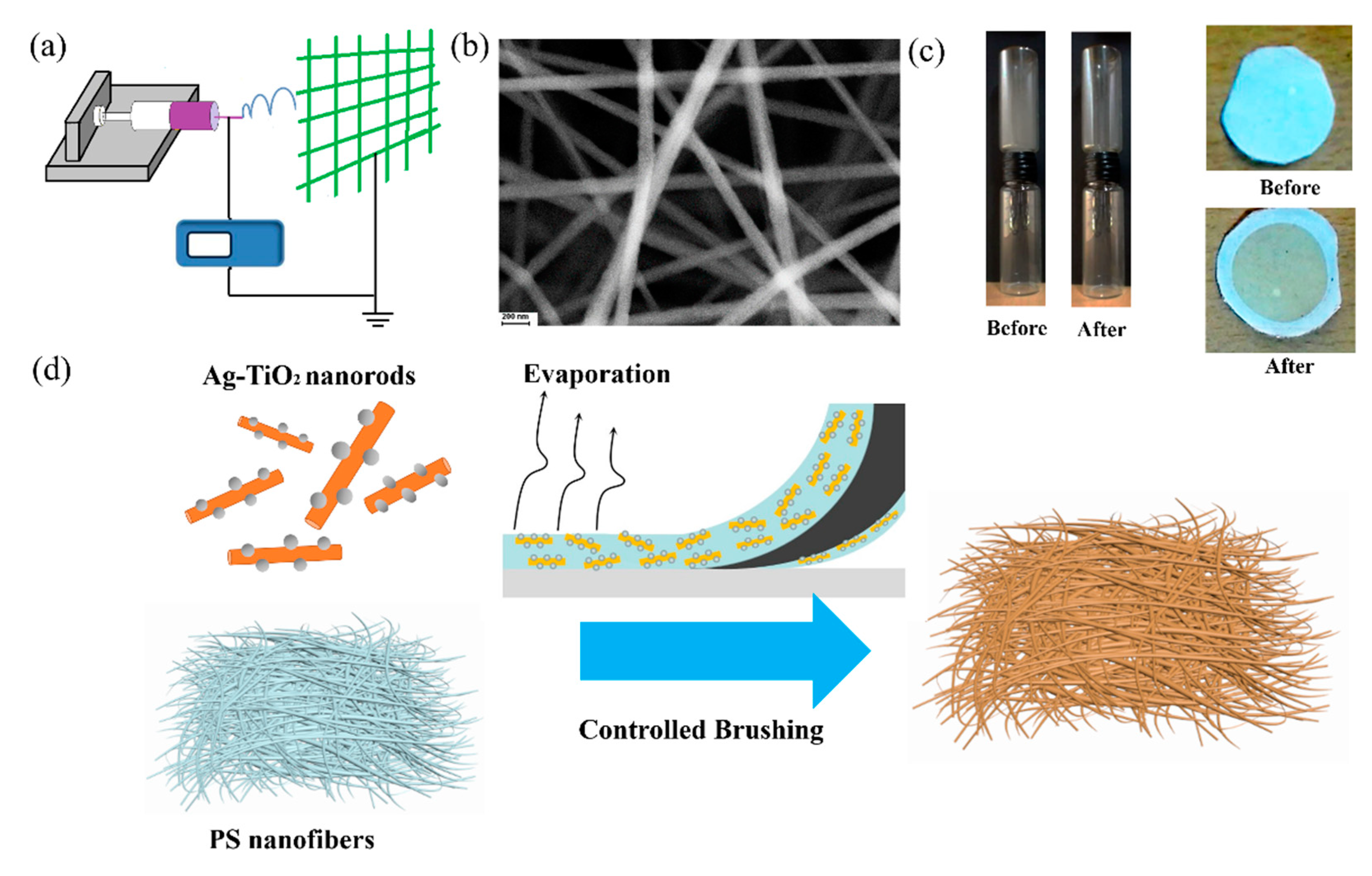
2.1. Characterization of Ag-TiO2 Nanorod Composites and Electrospinning Nanofibers
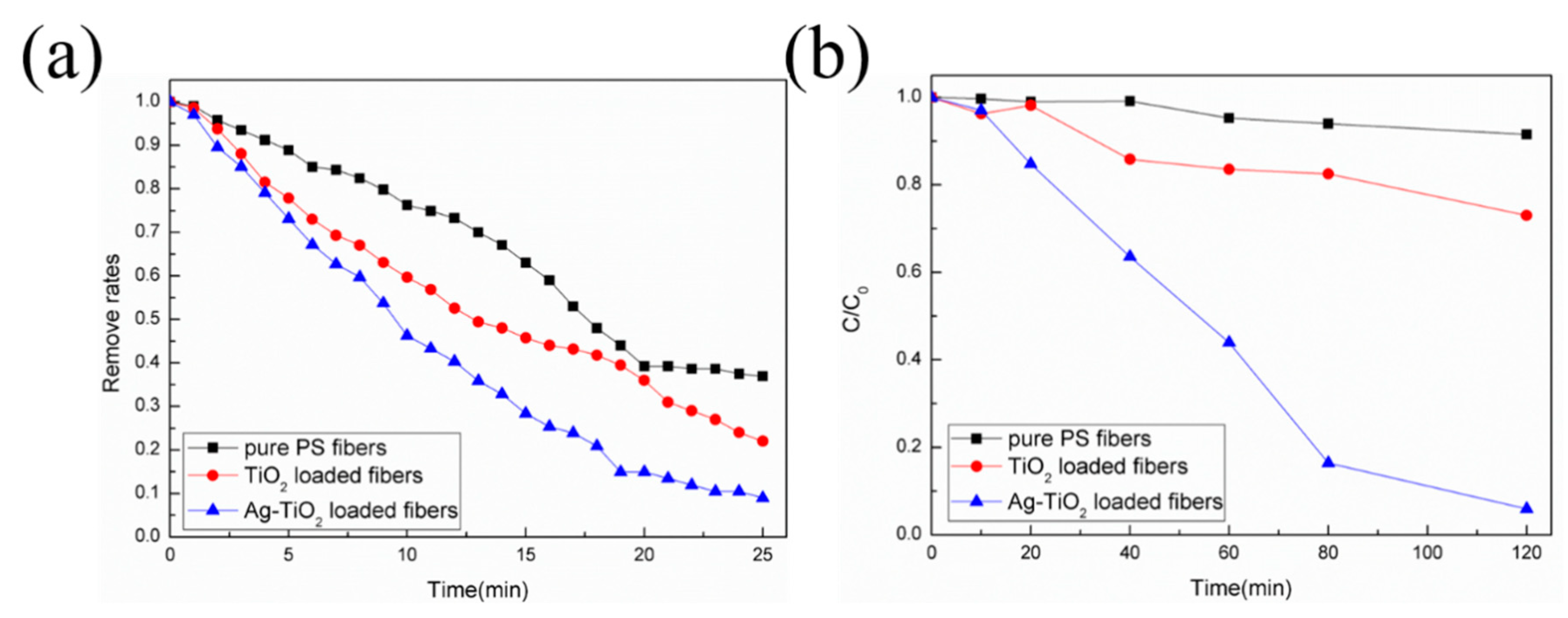
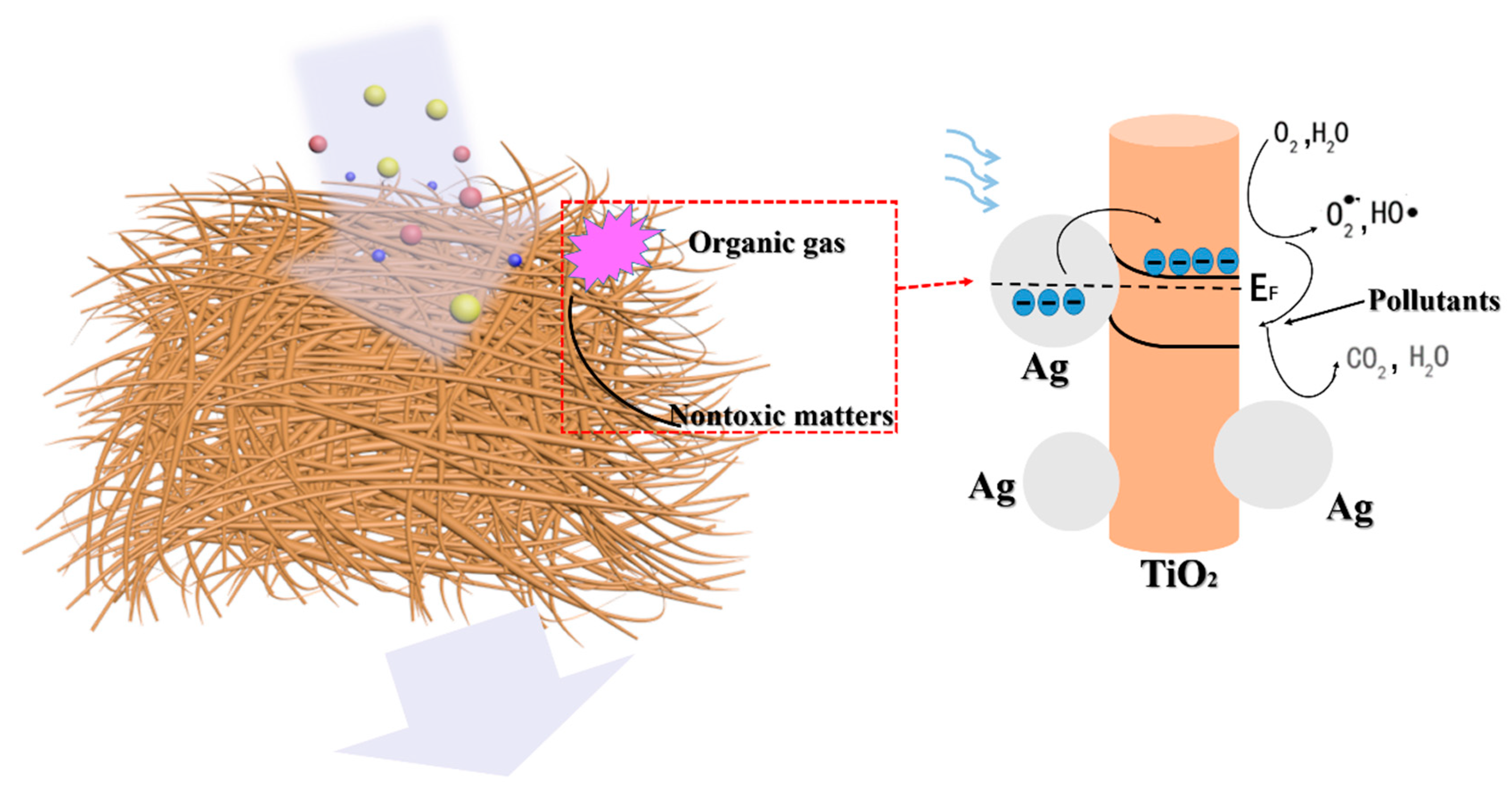
2.2. Photodegradation and PM Particulate Filteration Test
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Silver/Titanium Dioxide Nanorod Composites
4.2. Synthesis of Polystyrene Nanofibers Loaded with Silver/Titanium Dioxide Nanorod Composites
4.3. Characterization
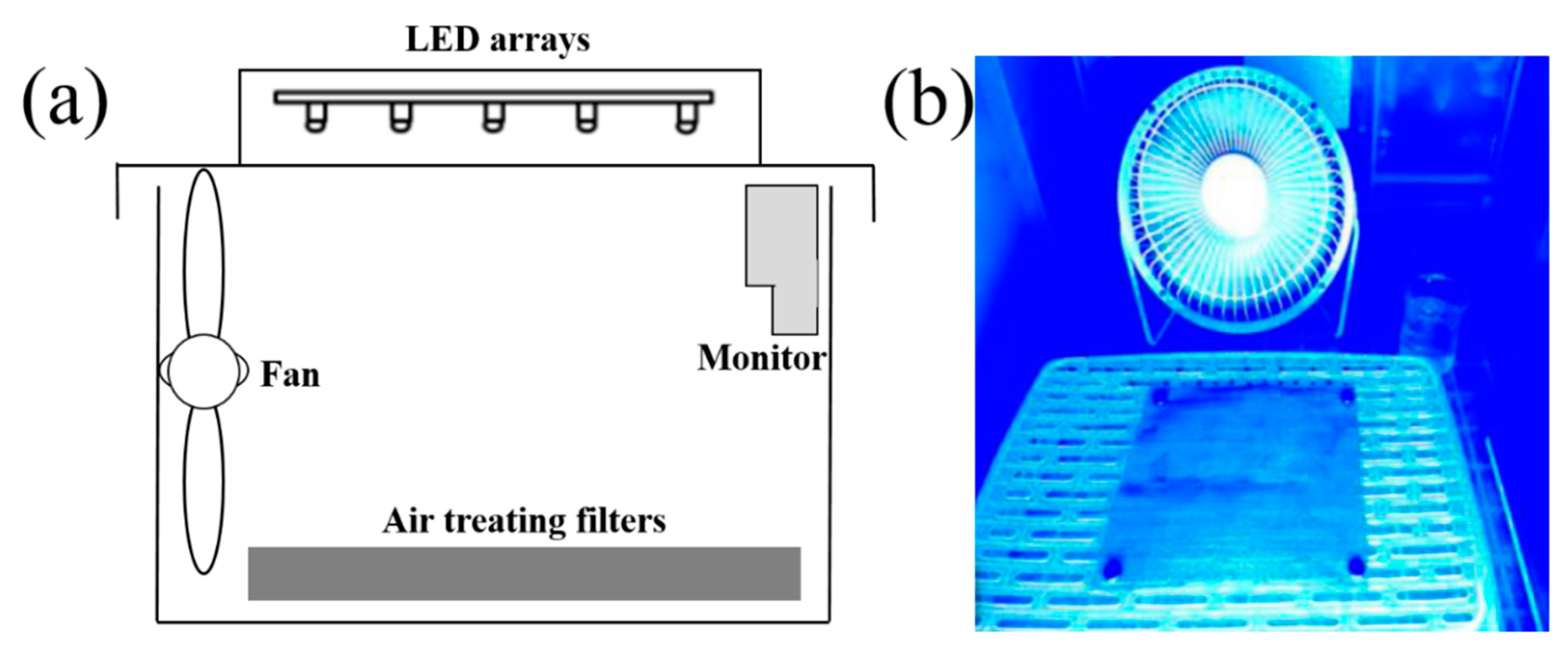
4.4. Filtration and Photodegradation Measurement
Author Contributions
Funding
Conflicts of Interest
References
- Boeck, M.D.; Hoet, P.; Lombaert, N.; Nemery, B.; Kirsch-Volders, M.; Lison, D. In vivo genotoxicity of hard metal dust: Induction of micronuclei in rat type II epithelial lung cells. Carcinogenesis 2003, 24, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Hoek, G.; Krishnan, R.M.; Beelen, R.; Peters, A.; Ostro, B.; Brunekreef, B.; Kaufman, J.D. Long-term air pollution exposure and cardio-respiratory mortality: A review. Environ. Health 2013, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.I.; Ghoshal, A.K. Removal of volatile organic compounds from polluted air. J. Loss Prevent. Proc. 2000, 13, 527–545. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, B.P.; Punia, M.; Singh, D.; Kumar, K.; Jain, V.K. Determination of volatile organic compounds and associated health risk assessment in residential homes and hostels within an academic institute, New Delhi. Indoor Air 2014, 24, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Shindell, D.; Kuylenstierna, J.C.; Vignati, E.; Dingenen, R.V.; Amann, M.; Klimont, Z.; Anenberg, S.C.; Muller, N.; Janssens-Maenhout, G.; Raes, F.; et al. Simultaneously mitigating near-term climate change and improving human health and food security. Science 2012, 335, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Ren, H.; Koshy, P.; Chen, W.F.; Qi, S.H.; Sorrell, C.C. Photocatalytic materials and technologies for air purification. J. Hazard. Mater. 2017, 325, 340–366. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F. Photocatalytic air cleaners and materials technologies-abilities and limitations. Build. Environ. 2015, 91, 191–203. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photochem. Photobiol. C 2018, 13, 247–262. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.G.; Pan, J.; Yang, Y.Q.; Lu, G.Q.; Cheng, H.M. Titanium dioxide crystals with tailored facets. Chem. Rev. 2014, 114, 9559–9612. [Google Scholar]
- Wang, F.; Li, Q.; Xu, D. Recent progress in semiconductor-based nanocomposite photocatalysts for solar-to-chemical Energy conversion. Adv. Energy Mater. 2017, 7, 1700529. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-plasmon-driven hot electron photochemistry. Chem. Rev. 2017, 118, 2927–2954. [Google Scholar] [CrossRef] [PubMed]
- Phillip, C.; Moskovits, M. Hot charge carrier transmission from plasmonic nanostructures. Annu. Rev. Phys. Chem. 2017, 68, 379–398. [Google Scholar]
- Zhang, T.; Wang, S.J.; Zhang, X.Y.; Su, D.; Yang, Y.; Wu, J.Y.; Xu, Y.Y.; Zhao, N. Progress in the utilization efficiency improvement of hot carriers in plasmon-mediated heterostructure photocatalysis. Appl. Sci. Basel 2019, 9, 2093. [Google Scholar] [CrossRef]
- Aslam, U.; Rao, V.G.; Chavez, S.; Linic, S. Catalytic conversion of solar to chemical energy on plasmonic metal nanostructures. Nat. Catal. 2018, 1, 656–665. [Google Scholar] [CrossRef]
- Zhan, C.; Chen, X.J.; Yi, J.; Li, J.F.; Wu, D.Y.; Tian, Z.Q. From plasmon-enhanced molecular spectroscopy to plasmon-mediated chemical reactions. Nat. Rev. Chem. 2018, 2, 216–230. [Google Scholar] [CrossRef]
- Khan, M.R.; Chuan, T.W.; Yousuf, A.; Chowdhury, M.N.K.; Cheng, C.K. Schottky barrier and surface plasmonic resonance phenomena towards the photocatalytic reaction: Study of their mechanisms to enhance photocatalytic activity. Catal. Sci. Technol. 2015, 5, 2522–2531. [Google Scholar] [CrossRef]
- Govorov, A.O.; Zhang, H.; Demir, H.V.; Gun’ko, Y.K. Photogeneration of hot plasmonic electrons with metal nanocrystals: Quantum description and potential applications. Nano Today 2014, 9, 85–101. [Google Scholar] [CrossRef]
- Wu, K.; Chen, J.; Mcbride, J.R.; Lian, T. Efficient hot-electron transfer by a plasmon-induced interfacial charge-transfer transition. Science 2015, 349, 632–635. [Google Scholar] [CrossRef] [PubMed]
- David, F. Performance and cost of particle air filtration technologies. Indoor Air 2001, 12, 34–223. [Google Scholar]
- Bräuner, E.V.; Forchhammer, L.; Møller, P.; Barregard, L.; Gunnarsen, L.; Afshari, A.; Wåhlin, P.; Glasius, M.; Dragsted, L.O.; Basu, S.; et al. Indoor particles affect vascular function in the aged: An air filtration–based intervention study. Am. J. Resp. Crit. Care 2008, 177, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.C.; Hou, H.Q.; Zhao, Y.; Zhu, Z.T.; Fong, H. Electrospun polyimide nanofibers and their applications. Prog. Polym. Sci. 2016, 61, 67–103. [Google Scholar] [CrossRef]
- Zhang, C.L.; Yu, S.H. Nanoparticles meet electrospinning: Recent advances and future prospects. Chem. Soc. Rev. 2014, 43, 4423–4448. [Google Scholar] [CrossRef]
- Lu, X.F.; Wang, C.; Wei, Y. One-dimensional composite nanomaterials: Synthesis by electrospinning and their applications. Small 2009, 5, 2349–2370. [Google Scholar] [CrossRef]
- Xu, J.W.; Liu, C.; Hsu, P.C.; Liu, K.; Zhang, R.F.; Liu, Y.Y.; Cui, Y. Roll-to-roll transfer of electrospun nanofiber film for high-efficiency transparent air filter. Nano Lett. 2016, 16, 1270–1275. [Google Scholar] [CrossRef]
- Khalid, B.; Bai, X.P.; Wei, H.H.; Huang, Y.; Wu, H.; Cui, Y. Direct blow-spinning of nanofifibers on a window screen for highly effiffifficient PM 2.5 removal. Nano Lett. 2017, 17, 1140–1148. [Google Scholar] [CrossRef]
- Xiong, Z.C.; Yang, R.L.; Zhu, Y.J.; Chen, F.F.; Dong, L.Y. Flexible hydroxyapatite ultralong nanowire-based paper for highly efficient and multi-functional air filtration. J. Mater. Chem. A 2017, 5, 17482–17491. [Google Scholar] [CrossRef]
- Altın, İ.; Münevver, S. Preparation of TiO2-polystyrene photocatalyst from waste material and its usability for removal of various pollutants. Appl. Catal. B Environ. 2014, 144, 694–701. [Google Scholar] [CrossRef]
- Joo, J.; Kwon, S.G.; Yu, T.; Cho, M.; Lee, J.; Yoon, J.; Hyeon, T. Large-Scale Synthesis of TiO2 Nanorods via Nonhydrolytic Sol-Gel Ester Elimination Reaction and Their Application to Photocatalytic Inactivation of E. coli. J. Phys. Chem. B 2005, 109, 15297–15302. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Shan, F.; Zhou, H.L.; Su, D.; Xue, X.M.; Wu, J.Y.; Chen, Y.Z.; Zhao, N.; Zhang, T. Silver nanoplate aggregation based multi-functional black metal absorbers for localization, photothermic harnessing enhancement and omnidirectional light antireflection. J. Mater. Chem. C 2018, 6, 989–999. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Xue, X.M.; Zhou, H.L.; Zhao, N.; Shan, F.; Su, D.; Liu, Y.R.; Zhang, T. Seeds screening aqueous synthesis, multiphase interfacial separation and in situ optical characterization of invisible ultrathin silver nanowires. Nanoscale 2018, 10, 15468–15484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, B.; Meng, L.; Bian, R.; Wang, S.; Wang, Y.; Liu, H.; Jiang, L. Ultrasmooth Quantum Dot Micropatterns by a Facile Controllable Liquid-Transfer Approach: Low-Cost Fabrication of High-Performance QLED. J. Am. Chem. Soc. 2018, 140, 8690–8695. [Google Scholar] [CrossRef]
- Lu, Q.; Lu, Z.; Lu, Y.; Lv, L.; Ning, Y.; Yu, H.; Hou, Y.; Yin, Y. Photocatalytic synthesis and photovoltaic application of Ag-TiO2 nanorod composites. Nano Lett. 2013, 13, 5698–5702. [Google Scholar] [CrossRef]
- Su, J.; Yang, G.; Cheng, C.; Huang, C.; Xu, H.; Ke, Q. Hierarchically structured TiO2/PAN nanofibrous membranes for high-efficiency air filtration and toluene degradation. J. Colloid Interf. Sci. 2017, 507, 386–396. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-J.; Zhang, X.-Y.; Su, D.; Wang, Y.-F.; Qian, C.-M.; Zhou, X.-R.; Li, Y.-Z.; Zhang, T. Electrospinning Ag-TiO2 Nanorod-Loaded Air Treatment Filters and Their Applications in Air Purification. Molecules 2020, 25, 3369. https://doi.org/10.3390/molecules25153369
Wang S-J, Zhang X-Y, Su D, Wang Y-F, Qian C-M, Zhou X-R, Li Y-Z, Zhang T. Electrospinning Ag-TiO2 Nanorod-Loaded Air Treatment Filters and Their Applications in Air Purification. Molecules. 2020; 25(15):3369. https://doi.org/10.3390/molecules25153369
Chicago/Turabian StyleWang, Shan-Jiang, Xiao-Yang Zhang, Dan Su, Yun-Fan Wang, Chun-Meng Qian, Xin-Ru Zhou, Yi-Zhi Li, and Tong Zhang. 2020. "Electrospinning Ag-TiO2 Nanorod-Loaded Air Treatment Filters and Their Applications in Air Purification" Molecules 25, no. 15: 3369. https://doi.org/10.3390/molecules25153369
APA StyleWang, S.-J., Zhang, X.-Y., Su, D., Wang, Y.-F., Qian, C.-M., Zhou, X.-R., Li, Y.-Z., & Zhang, T. (2020). Electrospinning Ag-TiO2 Nanorod-Loaded Air Treatment Filters and Their Applications in Air Purification. Molecules, 25(15), 3369. https://doi.org/10.3390/molecules25153369



