Design, Synthesis and Bioactive Evaluation of Oxime Derivatives of Dehydrocholic Acid as Anti-Hepatitis B Virus Agents
Abstract
1. Introduction
2. Results and Discussion
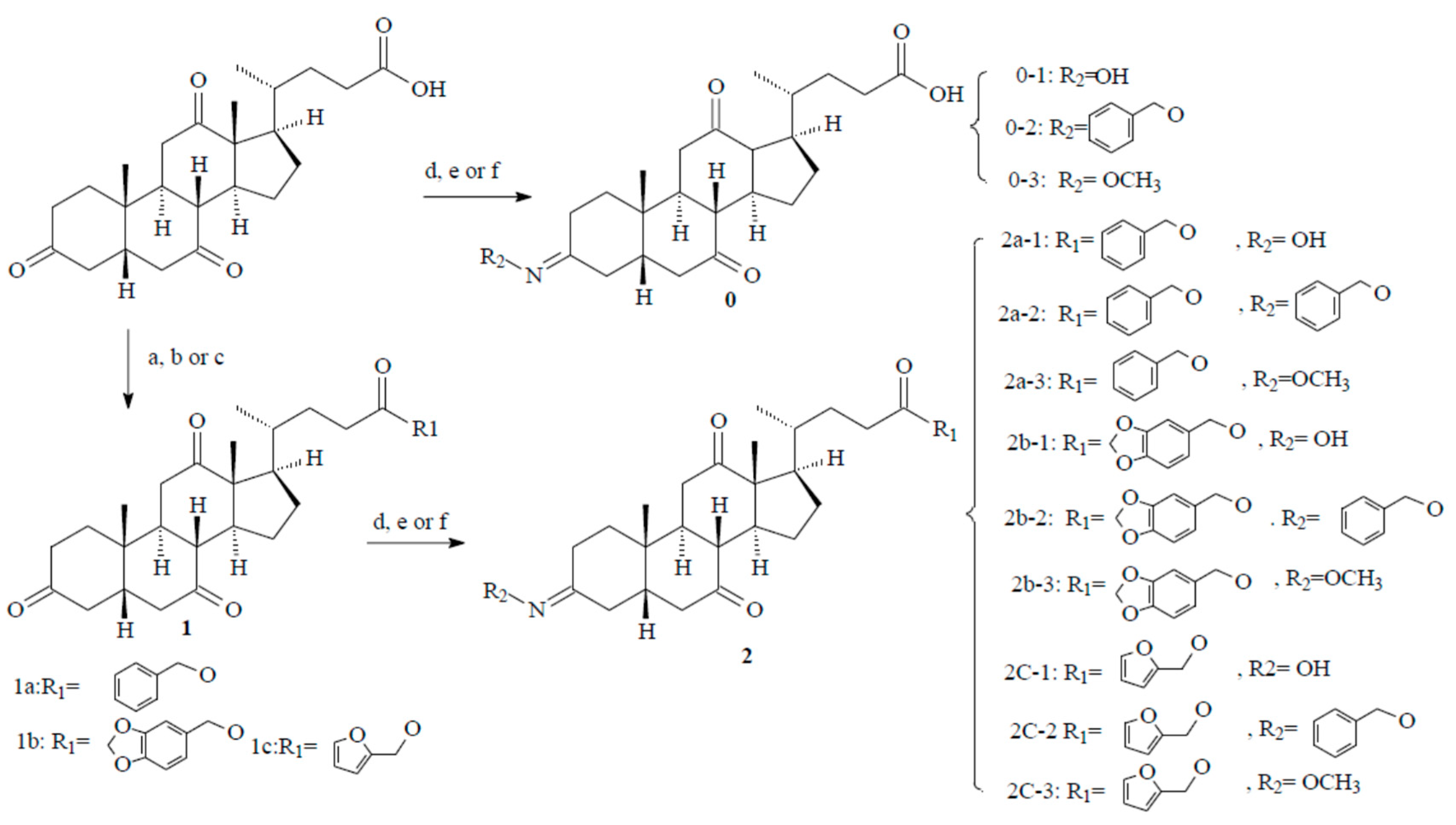
2.1. Chemistry
2.2. Anti-Hepatitis B Virus (HBV) Activity
2.3. Structure–Activity Relationship (SAR)
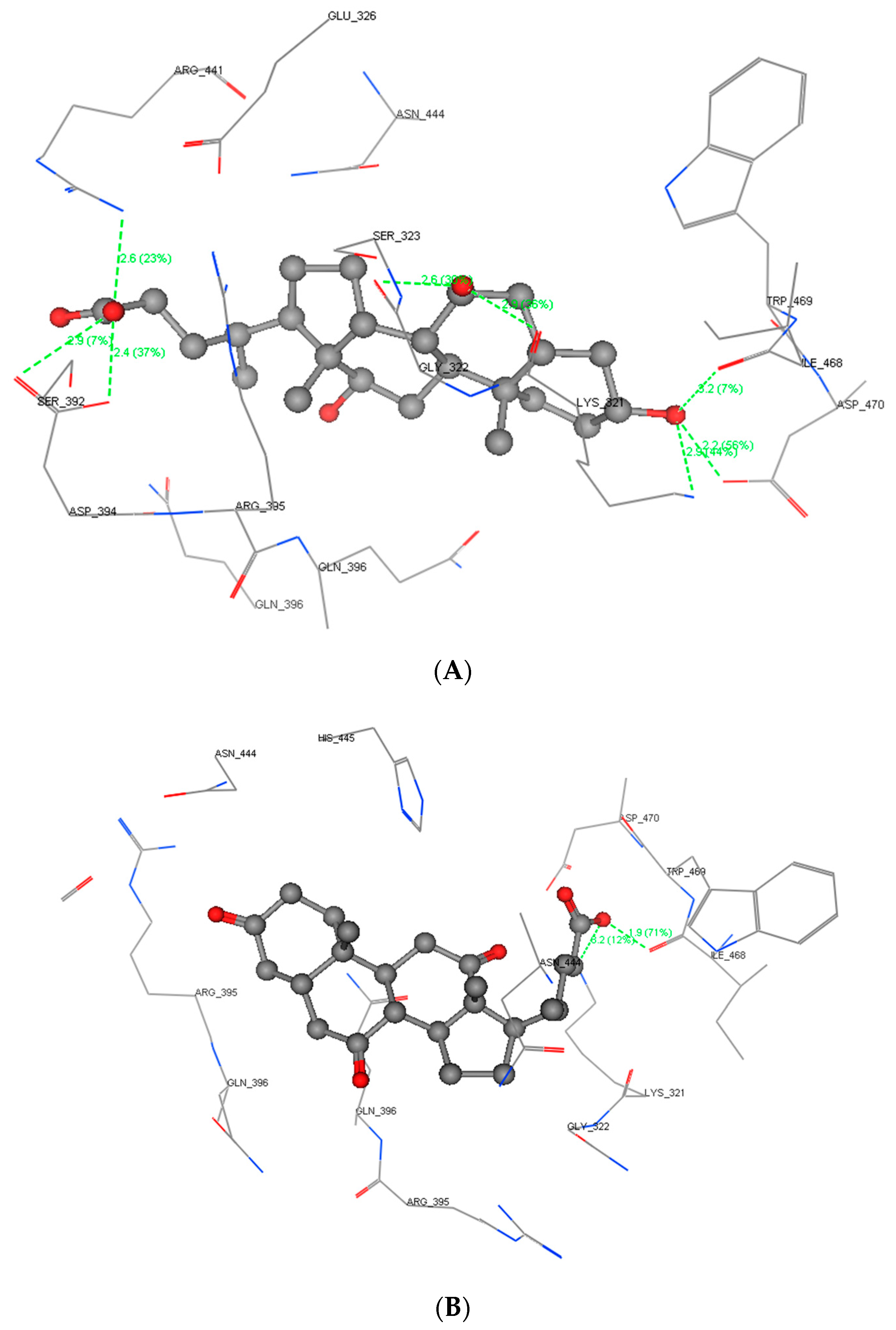
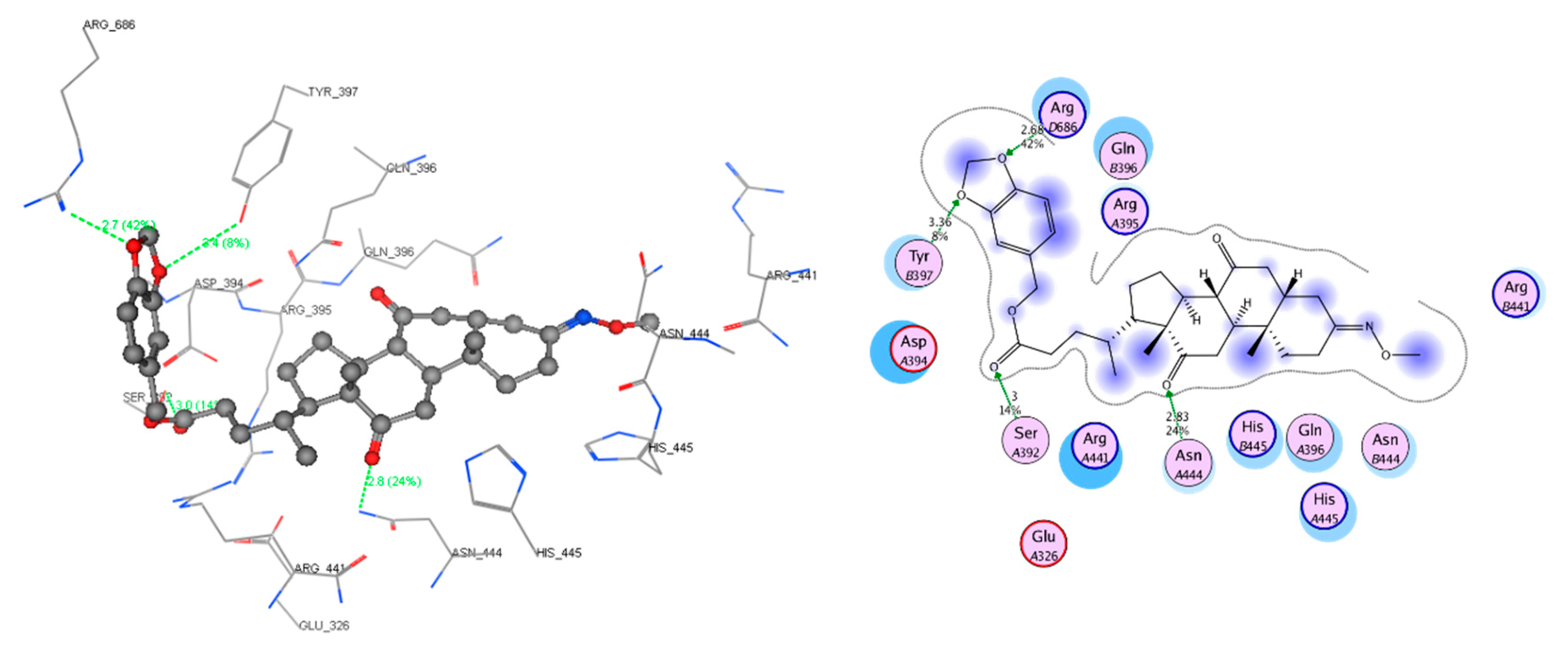
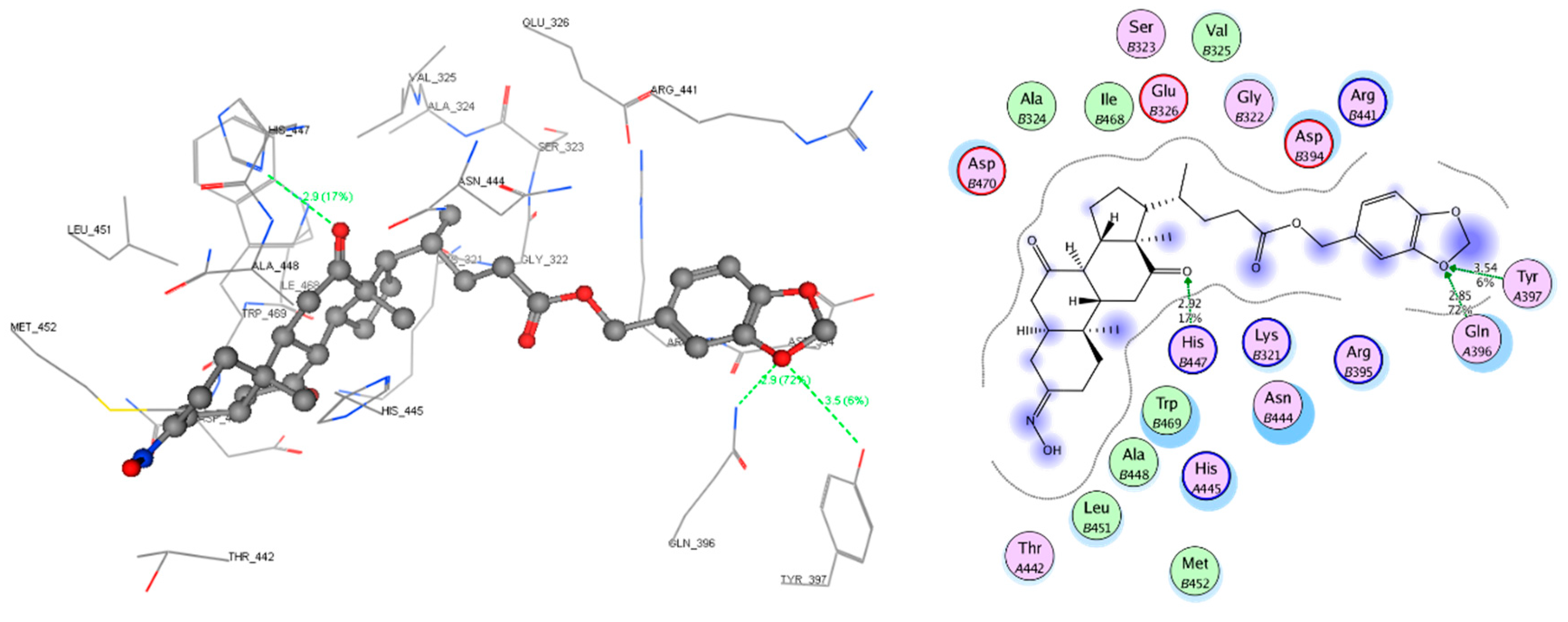
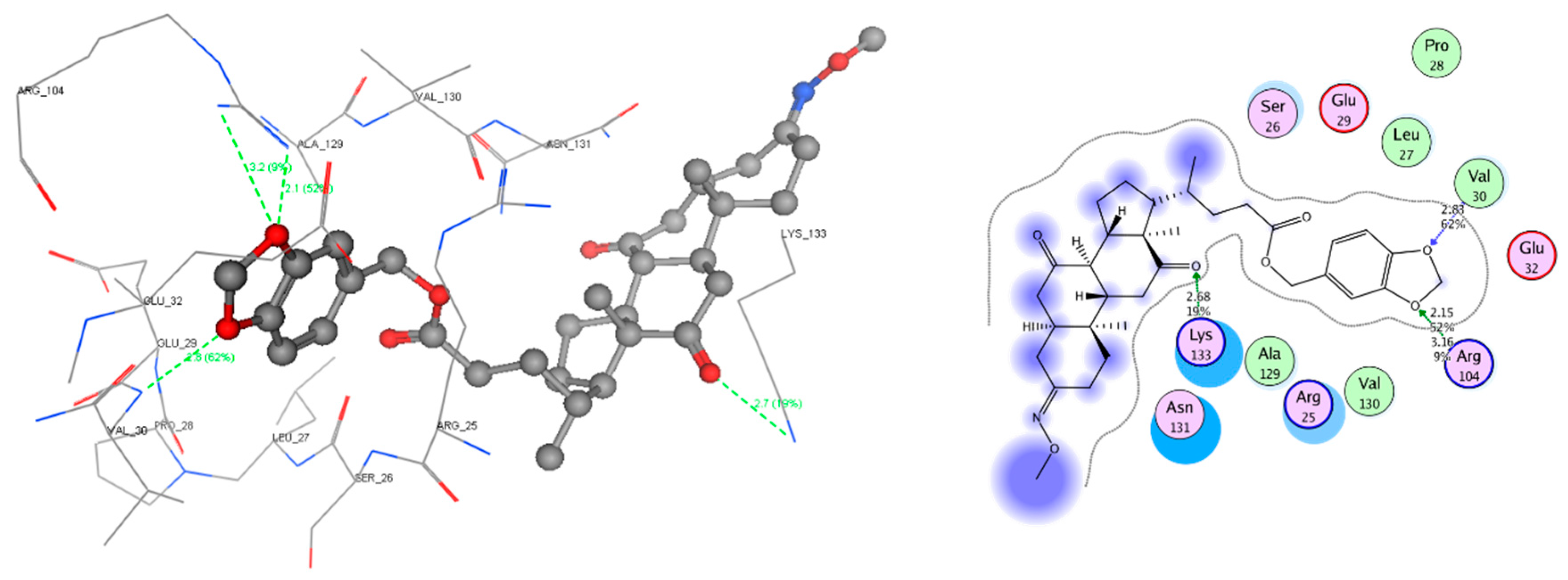
2.4. Molecular Docking Study
3. Methods
3.1. Synthesis Methods
3.1.1. Chemistry and Chemical Methods
3.1.2. General Procedure for the Intermediate Compounds (1a, 1b, and 1c)
3.1.3. General Procedure for the Target Compounds (2a-1~2a-3, 2b-1~2b-3, 2c-1~2c-3, and 0-1~0-3)
3.2. Biological Evaluation Methods
3.2.1. Cell Culture and Drug Treatment
3.2.2. Method for Cell Toxicity and HBsAg and HBeAg Inhibition Assays
3.3. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- You, C.R.; Lee, S.W.; Jang, J.W.; Yoon, S.K. Update on hepatitis B virus infection. World J. Gastroenterol. 2014, 20, 13293–13305. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Kao, J.H. Risk stratification for hepatitis B virus related hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2013, 28, 10–17. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Hepatitis Report 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf (accessed on 4 July 2017).
- Dienstag, J.L.; Schiff, E.R.; Wright, T.L.; Perrillo, R.P.; Hann, H.W.L.; Goodman, Z.; Crowther, L.; Condreay, L.D.; Woessner, M.; Rubin, M.; et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N. Engl. J. Med. 1999, 341, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Torresi, J.; Locarnini, S. Antiviral chemotherapy for the treatment of hepatitis b virus infections. Gastroenterology 2000, 118, S83–S103. [Google Scholar] [CrossRef]
- Gish, R.G.; Lai, C.; Yuen, M. Entecavir: A Review of its Use in the Treatment of Chronic Hepatitis B in Patients with Decompensated Liver Disease. Drugs 2011, 71, 2511–2529. [Google Scholar]
- Seifer, M.; Hamatake, R.K.; Colonno, R.J.; Standring, D.N. In Vitro Inhibition of Hepadnavirus Polymerases by the Triphosphates of BMS–200475 and Lobucavir, Antimicrob. Agents Chemoth. 1998, 42, 3200–3208. [Google Scholar] [CrossRef]
- Ghany, M.G.; Doo, E.C. Antiviral Resistance and Hepatitis B Therapy. Hepatlogy 2009, 49, S174–S184. [Google Scholar] [CrossRef]
- Zoulim, F.; Poynard, T.; Degos, F.; Slama, A.; Hasnaoui El, A.; Blin, P.; Mercier, F.; Deny, P.; Landais, P.; Parvaz, P.; et al. A prospective study of the evolution of lamivudine resistance mutations in patients with chronic hepatitis B treated with lamivudine. J. Viral Hepat. 2006, 13, 278–288. [Google Scholar] [CrossRef]
- Yang, X.Y.; Xu, X.Q.; Guan, H.; Wang, L.L.; Wu, Q.; Wu, G.M.; Li, S. A new series of HAPs as anti-HBV agents targeting at capsid assembly. Bioorg. Med. Chem. Lett. 2014, 24, 4247–4249. [Google Scholar] [CrossRef]
- Zembower, D.E.; Lin, Y.M.; Flavin, M.T.; Chen, F.C.; Korba, B.E. Robustaflavone, a potential non-nucleoside anti-hepatitis B agent. Antivir. Res. 1998, 39, 81–88. [Google Scholar] [CrossRef]
- Wei, W.X.; Li, X.R.; Wang, K.W.; Zheng, Z.W.; Zhou, M. Lignans with anti-hepatitis B virus activities from Phyllanthus niruri L. Phytother. Res. 2012, 26, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wei, W.X.; Shi, K.C.; Cao, X.; Zhou, M.; Liu, Z.P. In vitro and in vivo anti-hepatitis B virus activities of the lignan niranthin isolated from Phyllanthus niruri L. J. Eethnopharmacol. 2014, 155, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Jiang, X.M.; Liu, X.Y. Naturally Occurring and Synthetic Bioactive Molecules as Novel Non-Nucleoside HBV Inhibitors. Mini-Rev. Med. Chem. 2010, 10, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, G. A review of non-nucleoside anti-hepatitis B virus agents. Eur. J. Med. Chem. 2014, 75, 267–281. [Google Scholar] [CrossRef]
- Yang, W.; Peng, Y.M.; Wang, J.W.; Song, C.J.; Yu, W.Q.; Zhou, Y.B.; Jiang, J.H.; Wang, Q.D.; Wu, J.; Chang, J.B. Design, synthesis, and biological evaluation of novel 2′-deoxy-2′-fluoro-2′-C-methyl 8-azanebularine derivatives as potent anti-HBV agents. Bioorg. Med. Chem. Lett. 2019, 29, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Ivashchenko, A.V.; Mitkin, O.D.; Kravchenko, D.V.; Kuznetsova, I.V.; Kovalenko, S.M.; Bunyatyan, N.D.; Langer, T. Synthesis, X-Ray Crystal Structure, Hirshfeld Surface Analysis, and Molecular Docking Study of Novel Hepatitis B (HBV) Inhibitor: 8-Fluoro-5-(4-fluorobenzyl)-3-(2-methoxybenzyl)-3,5-dihydro -4H-pyrimido[5, 4-b]indol-4-one. Heliyon 2019, 2019, e02738. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.B.; Wei, W.X.; Wang, K.W.; Wang, L.S.; Wang, J.Y. Design, Synthesis, Molecular Docking Studies and Anti-HBV Activity of Phenylpropanoid Derivatives. Chemico-Biol. Interact. 2016, 25, 1–9. [Google Scholar] [CrossRef]
- Zhao, S.M.; Zhen, Y.Q.; Fu, L.L.; Gao, F.; Zhou, X.L.; Huang, S.; Zhang, L. Design, synthesis and biological evaluation of benzamide derivatives as novel NTCP inhibitors that induce apoptosis in HepG2 cells. Bioorg. Med. Chem. Lett. 2019, 29, 126623. [Google Scholar] [CrossRef]
- Vandyck, K.; Rombouts, G.; Stoops, B.; Tahri, A.; Vos, A.; Verschueren, W.; Wu, Y.; Yang, J.; Hou, F.; Huang, B.; et al. Synthesis and evaluation of N-phenyl-3-sulfamoyl-benzamide derivatives as capsid assembly modulators inhibiting hepatitis B virus (HBV). J. Med. Chem. 2018, 61, 6247–6260. [Google Scholar]
- Li, T.; Li, J.; Yang, Y.; Han, Y.L.; Wu, D.R.; Xiao, T.; Wang, Y.; Liu, T.; Zhao, Y.L.; Li, Y.J.; et al. Synthesis, pharmacological evaluation, and mechanistic study of adefovir mixed phosphonate derivatives bearing cholic acid and L-amino acid moieties for the treatment of HBV. Bioorg. Med. Chem. 2019, 27, 3707–3721. [Google Scholar] [CrossRef] [PubMed]
- Li, W. The Hepatitis B Virus Receptor. Ann. Rev. Cell Develop. Biol. 2015, 31, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Magnusson, B.M.; Burczynski, F.J.; Weiss, M. Enterohepatic Circulation: Physiological, pharmacokinetic and clinical implications. Clin. Pharmacokinet. 2002, 41, 751–790. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F. The enterohepatic circulation of bile acids in man. Adv. Intern. Med. 1976, 21, 501–534. [Google Scholar] [PubMed]
- Erlinger, S. Review article: New insights into the mechanisms of hepatic transport and bile secretion. J. Gastroenterol. Hepatol. 1996, 11, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cai, H.X.; Liu, Z.J.; Yao, P. Effective Enhancement of Hypoglycemic Effect of Insulin by Liver-Targeted Nanoparticles Containing Cholic Acid-Modified Chitosan Derivative. Mol. Pharm. 2016, 137, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Mitamura, K.; Higashi, T. Gas chromatography and high-performance liquid chromatography of natural steroids. J. Chromatog. A 2001, 935, 141–172. [Google Scholar] [CrossRef]
- Tan, J.; Zhou, M.; Cui, X.H.; Wei, Z.C.; Wei, W.X. Discovery of Oxime Ethers as Hepatitis B Virus (HBV) Inhibitors by Docking, Screening and In Vitro Investigation. Molecules 2018, 23, 637. [Google Scholar] [CrossRef]
- Cui, X.H.; Zhou, M.; Tan, J.; Wei, Z.C.; Wei, W.X.; Luo, P.; Lin, C.W. Design, Synthesis, and Bioactive Screen In Vitro of Cyclohexyl (E)-4-(Hydroxyimino)-4-Phenylbutanoates and Their Ethers for Anti-Hepatitis B Virus Agents. Molecules 2019, 24, 2063. [Google Scholar] [CrossRef]
- Liu, S.; Wei, W.X.; Li, Y.B.; Liu, X.; Cao, X.; Lei, K.C.; Zhou, M. Design, synthesis, biological evaluation and molecular docking studies of phenylpropanoid derivatives as potent anti-hepatitis B virus agents. Eur. J. Med. Chem. 2015, 95, 473–482. [Google Scholar] [CrossRef]
- Moiteiro, C.; Marcelo Curto, M.J.; Mohamed, N. Biovalorization of Friedelane Triterpenes Derived from Cork Processing Industry Byproducts. J. Agric. Food Chem. 2006, 54, 3566–3571. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.S.; Almeida, P.; Santos, L. Functionalisation of terpenoids at C-4 via organopalladium dimers: Cyclopropane formation during oxidation of homoallylic σ-organopalladium intermediates with lead tetraacetate. Tetrahedron 2007, 63, 12608–12615. [Google Scholar] [CrossRef]
- Giorgio, C.; Russo, S.; Incerti, M.; Lodola, A.; Tognolini, M. Biochemical characterization of EphA2 antagonists with improved physico-chemical properties by cell-based assays and surface plasmon resonance analysis. Biochem. Pharmacol. 2016, 99, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.G.; Huang, L.L.; Huang, Y.M.; Fan, J.C. A Facile Synthesis of a Cholic Acid Derivative with Anticancer Activity. Chin. J. Org. Chem. 2009, 6, 971–974. [Google Scholar]
- Han, Y.Q.; Huang, Z.M.; Yang, X.B.; Liu, H.Z.; Wu, G.X. In vivo and in vitro anti-hepatitis B virus activity of total phenolics from Oenanthe javanica. J. Ethnopharmacol. 2008, 118, 148–153. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, Y.M.; Lu, J.J.; Wang, J.W.; Ma, H.R.; Song, C.J.; Liu, B.J.; Qiao, Y.; Yu, W.Q.; Wu, J.; et al. Design, synthesis, and biological evaluation of new 1, 2, 3-triazolo-2′-deoxy -2′-f luoro-4′-azido nucleoside derivatives as potent anti-HBV agents. Eur. J. Med. Chem. 2018, 143, 137–149. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 01–03 are available from the authors. |

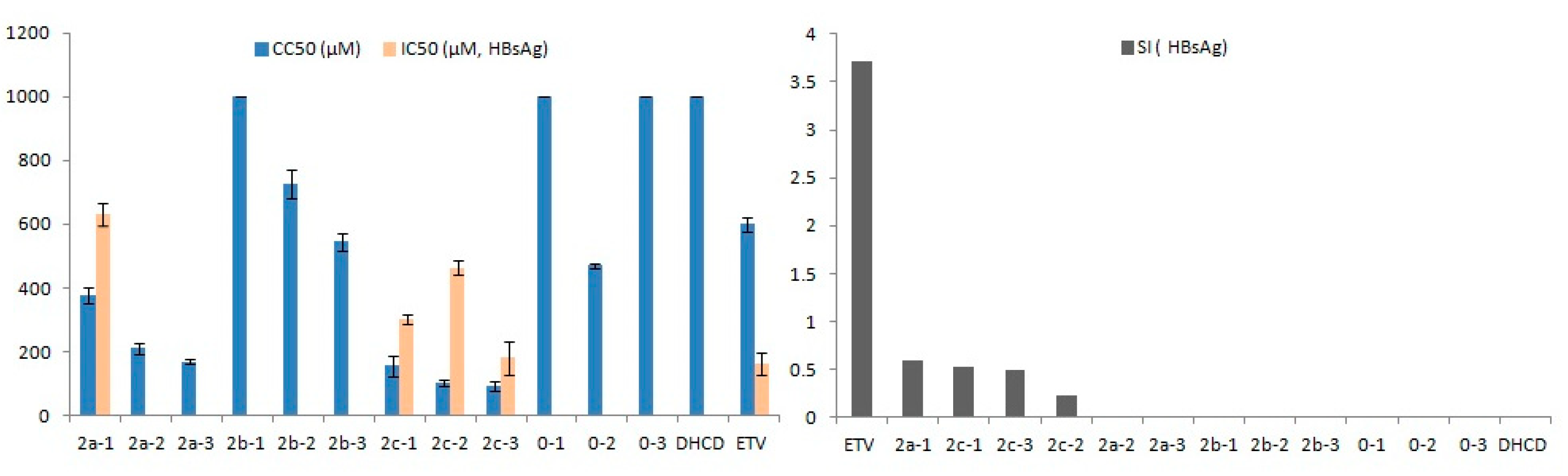

| Compound | CC50 a (μM) | HBeAg d | HBsAg e | ||
|---|---|---|---|---|---|
| IC50 b (μM) | SI c | IC50 b (μM) | SI c | ||
| 2a-1 | 377.88 ± 25.31 ** | 229.34 ± 12.78 | 1.65 | 630.32 ± 34.95 ** | 0.60 |
| 2a-2 | 210.69 ± 17.11 ** | 248.66 ± 47.61 | 0.85 | - f | - |
| 2a-3 | 169.10 ± 5.75 ** | 187.76 ± 9.51 | 0.90 | - | - |
| 2b-1 | >1000 ** | 96.64 ± 28.99 ** | 10.35 | - | - |
| 2b-2 | 728.15 ± 45.22 * | - | - | - | - |
| 2b-3 | 544.73 ± 28.92 | 49.39 ± 12.78 ** | 11.03 | - | - |
| 2c-1 | 155.05 ± 30.83 ** | 110.61 ± 28.30 ** | 1.40 | 300.00 ± 15.30 ** | 0.52 |
| 2c-2 | 101.04 ± 10.66 ** | 151.23 ± 32.11 * | 0.67 | 464.29 ± 20.10 ** | 0.22 |
| 2c-3 | 90.85 ± 15.59 ** | 105.19 ± 22.20 ** | 0.86 | 180.57 ± 52.83 | 0.50 |
| 0-1 | >1000 ** | - | - | - | - |
| 0-2 | 470.47 ± 6.35 * | 119.03 ± 1.86 ** | 3.95 | - | - |
| 0-3 | >1000 ** | - | - | - | - |
| DHCD g | >1000 ** | - | - | - | - |
| ETV h | 600.12 ± 23.44 | 246.87 ± 50.03 | 2.43 | 161.24 ± 35.94 | 3.72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Tan, J.; Cui, X.; Zhou, M.; Huang, Y.; Zang, N.; Chen, Z.; Wei, W. Design, Synthesis and Bioactive Evaluation of Oxime Derivatives of Dehydrocholic Acid as Anti-Hepatitis B Virus Agents. Molecules 2020, 25, 3359. https://doi.org/10.3390/molecules25153359
Wei Z, Tan J, Cui X, Zhou M, Huang Y, Zang N, Chen Z, Wei W. Design, Synthesis and Bioactive Evaluation of Oxime Derivatives of Dehydrocholic Acid as Anti-Hepatitis B Virus Agents. Molecules. 2020; 25(15):3359. https://doi.org/10.3390/molecules25153359
Chicago/Turabian StyleWei, Zhuocai, Jie Tan, Xinhua Cui, Min Zhou, Yunhou Huang, Ning Zang, Zhaoni Chen, and Wanxing Wei. 2020. "Design, Synthesis and Bioactive Evaluation of Oxime Derivatives of Dehydrocholic Acid as Anti-Hepatitis B Virus Agents" Molecules 25, no. 15: 3359. https://doi.org/10.3390/molecules25153359
APA StyleWei, Z., Tan, J., Cui, X., Zhou, M., Huang, Y., Zang, N., Chen, Z., & Wei, W. (2020). Design, Synthesis and Bioactive Evaluation of Oxime Derivatives of Dehydrocholic Acid as Anti-Hepatitis B Virus Agents. Molecules, 25(15), 3359. https://doi.org/10.3390/molecules25153359






