Microencapsulated Pomegranate Modifies the Composition and Function of High-Density Lipoproteins (HDL) in New Zealand Rabbits
Abstract
1. Introduction
2. Results
2.1. Biochemical Characteristic and HDL Lipids Profile
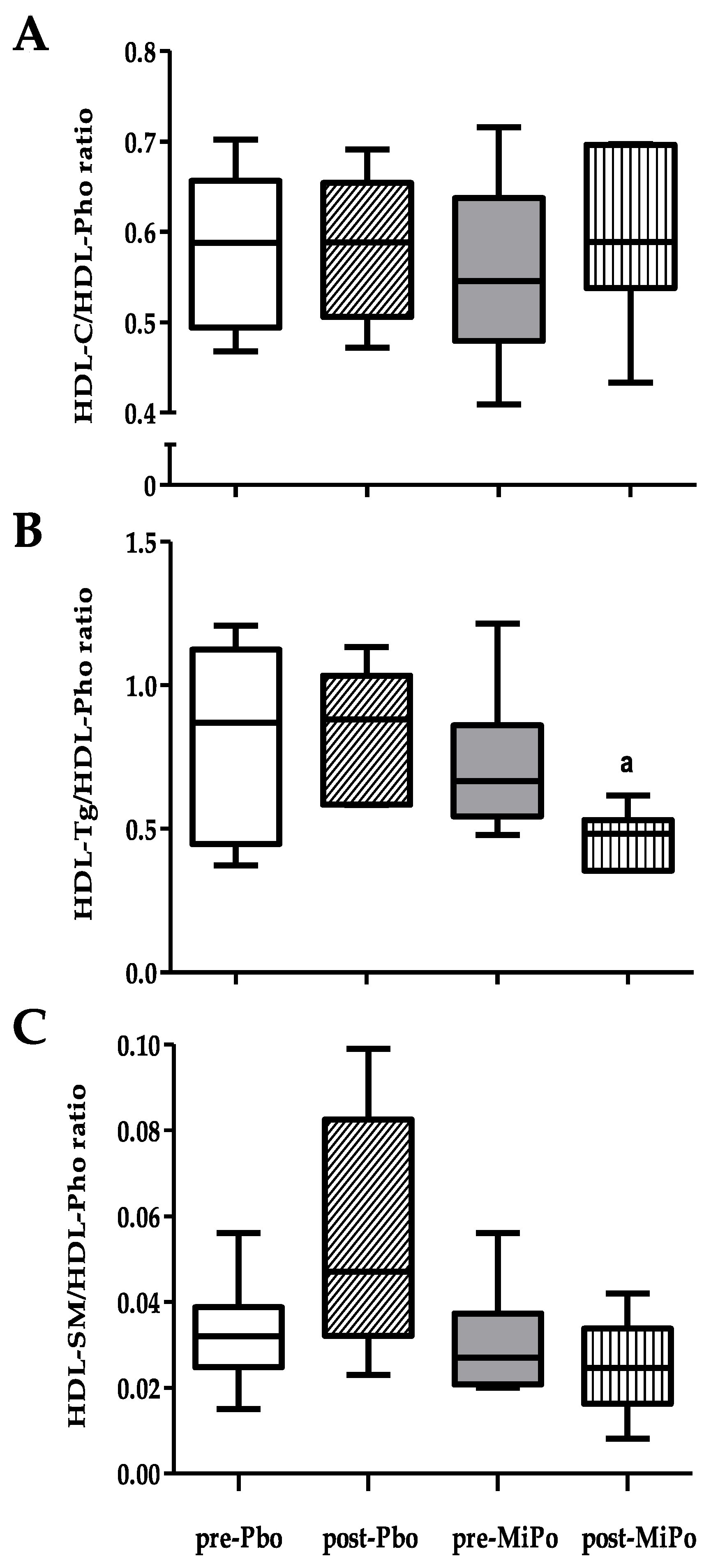
2.2. Size and Lipid Composition of HDL Subclasses
2.3. Kinetics of Conjugated Dienes Formation in HDL (Oxidation)
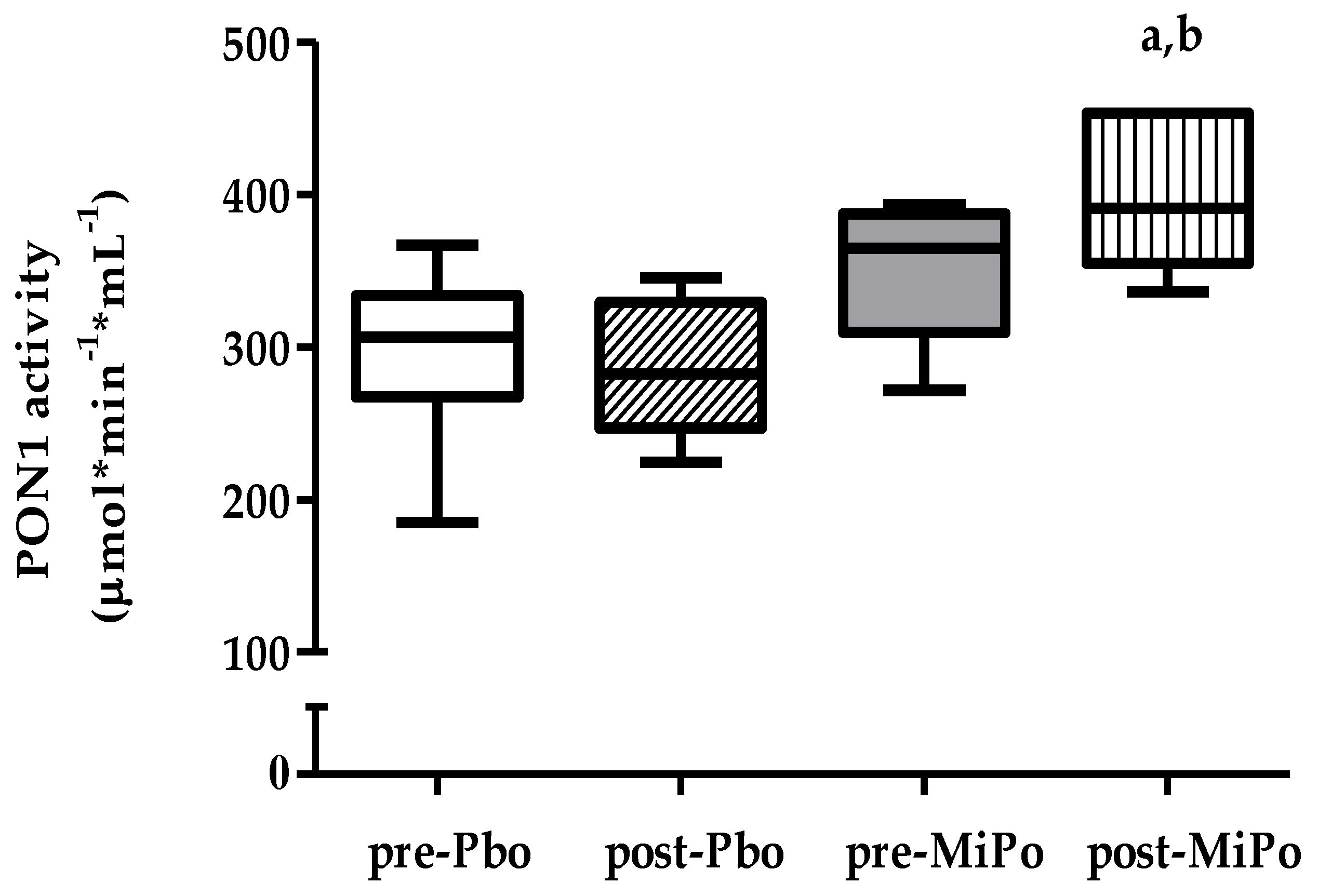
2.4. Paraoxonase-1 (PON1) Activity
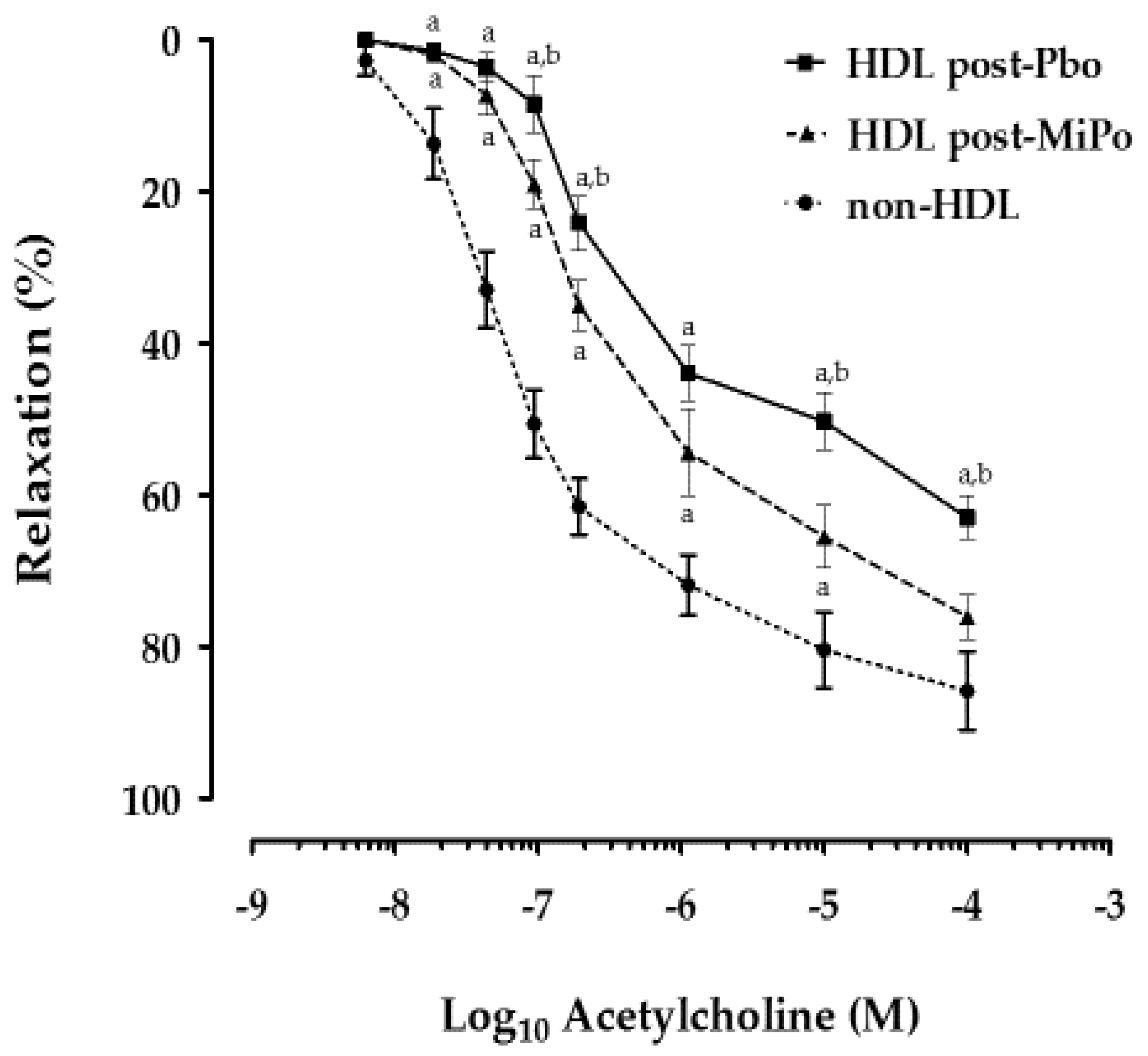
2.5. Effect of HDL on Endothelial Function
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Microencapsulated Pomegranate (MiPo)
4.3. Blood Samples
4.4. Biochemical Analyses
4.5. HDL Subclasses Composition Assessment
4.6. Kinetics of HDL Conjugated Dienes Formation (Oxidation)
4.7. Paraoxonase-1 (PON1) Activity
4.8. Vascular Reactivity of Aorta Rings
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Assmann, G.; Schulte, H.; von Eckardstein, A.; Huang, Y. Thematic review series: High density lipoprotein structure, function, and metabolism cardioprotective functions of HDLs 1. J. Lipid Res. 1996, 32, S11–S20. [Google Scholar] [CrossRef]
- Fisher, E.A.; Feig, J.E.; Hewing, B.; Hazen, S.L.; Smith, J.D. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arter. Thromb. Vasc. Biol. 2012, 32, 2813–2820. [Google Scholar] [CrossRef] [PubMed]
- Rye, K.A.; Barter, P.J. Cardioprotective functions of HDLs. J. Lipid Res. 2014, 55, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Jensen, M.K. From High-Density Lipoprotein Cholesterol to Measurements of Function: Prospects for the Development of Tests for High-Density Lipoprotein Functionality in Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2018, 38, 487–499. [Google Scholar] [CrossRef]
- Passaro, A.; Vigna, G.B.; Romani, A.; Sanz, J.M.; Cavicchio, C.; Bonaccorsi, G.; Valacchi, G.; Cervellati, C. Distribution of Paraoxonase-1 (PON-1) and Lipoprotein Phospholipase A2 (Lp-PLA2) across Lipoprotein Subclasses in Subjects with Type 2 Diabetes. Oxid. Med. Cell. Longev. 2018, 2018, 1752940. [Google Scholar] [CrossRef]
- Martínez-Beamonte, R.; Lou-Bonafonte, J.M.; Martínez-Gracia, M.V.; Osada, J. Sphingomyelin in high-density lipoproteins: Structural role and biological function. Int. J. Mol. Sci. 2013, 14, 7716–7741. [Google Scholar] [CrossRef]
- Kontush, A.; Lindahl, M.; Lhomme, M.; Calabresi, L.; Chapman, M.J.; Davidson, W.S. Structure of HDL: Particle subclasses and molecular components. Handb. Exp. Pharm. 2015, 224, 3–51. [Google Scholar] [CrossRef]
- Kontush, A.; Chantepie, S.; Chapman, M.J. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arter. Thromb. Vasc. Biol. 2003, 23, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Fruhwürth, S.; Pavelka, M.; Bittman, R.; Kovacs, W.J.; Walter, K.M.; Röhrl, C.; Stangl, H. High-density lipoprotein endocytosis in endothelial cells. World J. Biol. Chem. 2013, 4, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Perisa, D.; Rohrer, L.; Kaech, A.; Von Eckardstein, A. Itinerary of high density lipoproteins in endothelial cells. Biochim. Biophys. Acta 2016, 1861, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Vega, M.; Massó, F.; Páez, A.; Vargas-Alarcón, G.; Coral-Vázquez, R.; Mas-Oliva, J.; Carreón-Torres, E.; Pérez-Méndez, Ó. HDL-Mediated Lipid Influx to Endothelial Cells Contributes to Regulating Intercellular Adhesion Molecule (ICAM)-1 Expression and eNOS Phosphorylation. Int. J. Mol. Sci. 2018, 19, 3394. [Google Scholar] [CrossRef] [PubMed]
- Lemaire-Ewing, S.; Lagrost, L.; Néel, D. Lipid rafts: A signalling platform linking lipoprotein metabolism to atherogenesis. Atherosclerosis. 2012, 221, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Bonacina, F.; Pirillo, A.; Catapano, A.L.; Norata, G.D. Cholesterol membrane content has a ubiquitous evolutionary function in immune cell activation: The role of HDL. Curr. Opin. Lipidol. 2019, 30, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Ertek, S. High-density Lipoprotein (HDL) Dysfunction and the Future of HDL. Curr. Vasc. Pharm. 2018, 16, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; de Beer, M.C.; Wroblewski, J.M.; Charnigo, R.J.; Ji, A.; Webb, N.R.; de Beer, F.C.; van der Westhuyzen, D.R. Impact of individual acute phase serum amyloid A isoforms on HDL metabolism in mice. J. Lipid Res. 2016, 57, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Annema, W.; von Eckardstein, A. High-density lipoproteins. Multifunctional but vulnerable protections from atherosclerosis. Circ. J. 2013, 77, 2432–2448. [Google Scholar] [CrossRef]
- Annema, W.; von Eckardstein, A. Dysfunctional high-density lipoproteins in coronary heart disease: Implications for diagnostics and therapy. Transl. Res. 2016, 173, 30–57. [Google Scholar] [CrossRef]
- Quintanilla-Cantú, A.; Peña-de-la-Sancha, P.; Flores-Castillo, C.; Mejía-Domínguez, A.M.; Posadas-Sánchez, R.; Pérez-Hernández, N.; Bautista-Pérez, R.; Enriquez-Calderón, R.E.; Juárez-Oropeza, M.A.; Fragoso, J.M.; et al. Small HDL subclasses become cholesterol-poor during postprandial period after a fat diet intake in subjects with high triglyceridemia increases. Clin. Chim. Acta 2017, 464, 98–105. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Tahbaz, F.; Gaieni, I.; Alavi-Majd, H.; Azadbakht, L. Concentrated pomegranate juice improves lipid profiles in diabetic patients with hyperlipidemia. J. Med. Food 2004, 7, 305–308. [Google Scholar] [CrossRef]
- Estrada-Luna, D.; Martínez-Hinojosa, E.; Cancino-Diaz, J.C.; Belefant-Miller, H.; López-Rodríguez, G.; Betanzos-Cabrera, G. Daily supplementation with fresh pomegranate juice increases paraoxonase 1 expression and activity in mice fed a high-fat diet. Eur. J. Nutr. 2018, 57, 383–389. [Google Scholar] [CrossRef]
- Estrada-Luna, D.; Carreón-Torres, E.; Bautista-Pérez, R.; Betanzos-Cabrera, G.; Dorantes-Morales, A.; Luna-Luna, M.; Vargas-Barrón, J.; Mejía, A.M.; Fragoso, J.M.; Carvajal-Aguilera, K.; et al. Microencapsulated Pomegranate Reverts High-Density Lipoprotein (HDL)-Induced Endothelial Dysfunction and Reduces Postprandial Triglyceridemia in Women with Acute Coronary Syndrome. Nutrients 2019, 11, 1710. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Challah, M.; Watanabe, T. Transgenic rabbit models for biomedical research: Current status, basic methods and future perspectives. Pathol. Int. 1999, 49, 583–594. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, C.; Torres-Tamayo, M.; Juárez-Meavepeña, M.; López-Osorio, C.; Toledo-Ibelles, P.; Monter-Garrido, M.; Cruz-Robles, D.; Carreón-Torres, E.; Vargas-Alarcón, G.; Pérez-Méndez, O. Lipid plasma concentrations of HDL subclasses determined by enzymatic staining on polyacrylamide electrophoresis gels in children with metabolic syndrome. Clin. Chim. Acta 2011, 412, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Schlitt, A.; Blankenberg, S.; Yan, D.; von Gizycki, H.; Buerke, M.; Werdan, K.; Bickel, C.; Lackner, K.J.; Meyer, J.; Rupprecht, H.J.; et al. Further evaluation of plasma sphingomyelin levels as a risk factor for coronary artery disease. Nutr. Metab. 2006, 3, 5. [Google Scholar] [CrossRef]
- Ding, M.; Rexrode, K.M. A Review of Lipidomics of Cardiovascular Disease Highlights the Importance of Isolating Lipoproteins. Metabolites 2020, 10, 163. [Google Scholar] [CrossRef]
- Martínez-Ramírez, M.; Madero, M.; Vargas-Alarcón, G.; Vargas-Barrón, J.; Fragoso, J.M.; Rodríguez-Pérez, J.M.; Martínez-Sánchez, C.; González-Pacheco, H.; Bautista-Pérez, R.; Carreón-Torres, E.; et al. HDL-sphingomyelin reduction after weight loss by an energy-restricted diet is associated with the improvement of lipid profile, blood pressure, and decrease of insulin resistance in overweight/obese patients. Clin. Chim. Acta 2016, 454, 77–81. [Google Scholar] [CrossRef]
- Giraud, C.; Tournadre, A.; Pereira, B.; Dutheil, F.; Soubrier, M.; Lhomme, M.; Kontush, A.; Sébédio, J.L.; Capel, F. Alterations of HDL particle phospholipid composition and role of inflammation in rheumatoid arthritis. J. Physiol. Biochem. 2019, 75, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tian, L. Diverse Phytochemicals and Bioactivities in the Ancient Fruit and Modern Functional Food Pomegranate (Punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Di Giosia, P.; De Feo, M.; Fellini, E.; Cheli, P.; Ferri, L.; Ferri, C. Tea, flavonoids, and cardiovascular health: Endothelial protection. Am. J. Clin. Nutr. 2013, 98, 1660S–1666S. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Ferri, C. Flavonoids: Antioxidants against atherosclerosis. Nutrients 2010, 2, 889–902. [Google Scholar] [CrossRef]
- Turrini, E.; Ferruzzi, L.; Fimognari, C. Potential Effects of Pomegranate Polyphenols in Cancer Prevention and Therapy. Oxid. Med. Cell. Longev. 2015, 2015, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.L.; Duclos, Q.; Blesso, C.N. Effects of Dietary Flavonoids on Reverse Cholesterol Transport, HDL Metabolism, and HDL Function. Adv. Nutr. 2017, 8, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, D.; Yang, Y.; Xia, M.; Li, D.; Li, G.; Zhu, Y.; Xiao, Y.; Ling, W. Cyanidin-3-O-β-glucoside improves obesity and triglyceride metabolism in KK-Ay mice by regulating lipoprotein lipase activity. J. Sci. Food Agric. 2011, 91, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; Saedisomeolia, A.; Wood, L.G.; Yaseri, M.; Tavasoli, S. Effects of pomegranate extract supplementation on inflammation in overweight and obese individuals: A randomized controlled clinical trial. Complement. Ther. Clin. Pr. 2016, 22, 44–50. [Google Scholar] [CrossRef]
- Taheri Rouhi, S.Z.; Sarker, M.M.R.; Rahmat, A.; Alkahtani, S.A.; Othman, F. The effect of pomegranate fresh juice versus pomegranate seed powder on metabolic indices, lipid profile, inflammatory biomarkers, and the histopathology of pancreatic islets of Langerhans in streptozotocin-nicotinamide induced type 2 diabetic. BMC Complement. Altern. Med. 2017, 17, 1–13. [Google Scholar] [CrossRef]
- Arao, K.; Wang, Y.M.; Inoue, N.; Hirata, J.; Cha, J.Y.; Nagao, K.; Yanagita, T. Dietary effect of pomegranate seed oil rich in 9cis, 11trans, 13cis conjugated linolenic acid on lipid metabolism in obese, hyperlipidemic OLETF rats. Lipids Health Dis. 2004, 3, 1–7. [Google Scholar] [CrossRef]
- Arao, K.; Yotsumoto, H.; Han, S.-Y.; Nagao, K.; Yanagita, T. The 9cis,11trans,13cis isomer of conjugated linolenic acid reduces apolipoprotein B100 secretion and triacylglycerol synthesis in HepG2 cells. Biosci. Biotechnol. Biochem. 2004, 68, 2643–2645. [Google Scholar] [CrossRef]
- Mirmiran, P.; Fazeli, M.R.; Asghari, G.; Shafiee, A.; Azizi, F. Effect of pomegranate seed oil on hyperlipidaemic subjects: A double-blind placebo-controlled clinical trial. Br. J. Nutr. 2010, 104, 402–406. [Google Scholar] [CrossRef]
- Plochberger, B.; Röhrl, C.; Preiner, J.; Rankl, C.; Brameshuber, M.; Madl, J.; Bittman, R.; Ros, R.; Sezgin, E.; Eggeling, C.; et al. HDL particles incorporate into lipid bilayers—A combined AFM and single molecule fluorescence microscopy study. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; DiGuardo, M.; Climent, M.; Vives, C.; Carbo, A.; Jouni, Z.E.; Einerhand, A.W.; O’Shea, M.; Hontecillas, R. Activation of PPARγ and δ by dietary punicic acid ameliorates intestinal inflammation in mice. Br. J. Nutr. 2011, 106, 878–886. [Google Scholar] [CrossRef]
- Huang, T.H.; Peng, G.; Kota, B.P.; Li, G.Q.; Yamahara, J.; Roufogalis, B.D.; Li, Y. Anti-diabetic action of Punica granatum flower extract: Activation of PPAR-gamma and identification of an active component. Toxicol. Appl. Pharm. 2005, 207, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Khateeb, J.; Gantman, A.; Kreitenberg, A.J.; Aviram, M.; Fuhrman, B. Paraoxonase 1 (PON1) expression in hepatocytes is upregulated by pomegranate polyphenols: A role for PPAR-gamma pathway. Atherosclerosis 2010, 208, 119–125. [Google Scholar] [CrossRef]
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef] [PubMed]
- Sola, R.; Baudet, M.F.; Motta, C.; Maillé, M.; Boisnier, C.; Jacotot, B. Effects of dietary fats on the fluidity of human high-density lipoprotein: Influence of the overall composition and phospholipid fatty acids. Biochim. Biophys. Acta 1990, 1043, 43–51. [Google Scholar] [CrossRef]
- Jové, M.; Naudi, A.; Portero-Otin, M.; Cabre, R.; Rovira-Llopis, S.; Banuls, C.; Rocha, M.; Hernandez-Mijares, A.; Victor, V.M.; Pamplona, R. Plasma lipidomics discloses metabolic syndrome with a specific HDL phenotype. FASEB J. 2014, 28, 5163–5171. [Google Scholar] [CrossRef]
- Skeggs, J.W.; Morton, R.E. LDL and HDL enriched in triglyceride promote abnormal cholesterol transport. J. Lipid Res. 2002, 43, 1264–1274. [Google Scholar] [CrossRef]
- Davidson, W.S.; Gillotte, K.L.; Lund-Katz, S.; Johnson, W.J.; Rothblat, G.H.; Phillips, M.C. The effect of high-density lipoprotein phospholipid acyl chain composition on the efflux of cellular free cholesterol. J. Biol. Chem. 1995, 270, 5882–5890. [Google Scholar] [CrossRef]
- Rock, W.; Rosenblat, M.; Miller-Lotan, R.; Levy, A.P.; Elias, M.; Aviram, M. Consumption of wonderful variety pomegranate juice and extract by diabetic patients increases paraoxonase 1 association with high-density lipoprotein and stimulates its catalytic activities. J. Agric. Food Chem. 2008, 56, 8704–8713. [Google Scholar] [CrossRef]
- Deakin, S.; Leviev, I.; Gomaraschi, M.; Calabresi, L.; Franceschini, G.; James, R.W. Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high affinity, saturable, desorption mechanism. J. Biol. Chem. 2002, 277, 4301–4308. [Google Scholar] [CrossRef]
- Zarfeshany, A.; Asgary, S.; Javanmard, S.H. Potent health effects of pomegranate. Adv. Biomed. Res. 2014, 3, 1–8. [Google Scholar] [CrossRef]
- Hurtado, I.; Fiol, C.; Gracia, V.; Caldú, P. In vitro oxidised HDL exerts a cytotoxic effect on macrophages. Atherosclerosis 1996, 125, 39–46. [Google Scholar] [CrossRef]
- Niesor, E. Will Lipidation of ApoA1 through Interaction with ABCA1 at the Intestinal Level Affect the Protective Functions of HDL. Biology 2015, 4, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health Benefits of Anthocyanins and Their Encapsulation for Potential Use in Food Systems: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.; Bretz, M.; Hubbermann, E.M.; Schwarz, K. Influence of different pectins on powder characteristics of microencapsulated anthocyanins and their impact on drug retention of shellac coated granulate. J. Food Eng. 2012, 108, 158–165. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 1996; 146p. [CrossRef]
- Huesca-Gómez, C.; Carreón-Torres, E.; Nepomuceno-Mejía, T.; Sánchez-Solorio, M.; Galicia-Hidalgo, M.; Mejía, A.M.; Montaño, L.F.; Franco, M.; Posadas-Romero, C.; Pérez-Méndez, O. Contribution of cholesteryl ester transfer protein and lecithin:cholesterol acyltransferase to HDL size distribution. Endocr. Res. 2004, 30, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Ibelles, P.; García-Sánchez, C.; Ávila-Vazzini, N.; Carreón-Torres, E.; Posadas-Romero, C.; Vargas-Alarcón, G.; Pérez-Méndez, O. Enzymatic assessment of cholesterol on electrophoresis gels for estimating HDL size distribution and plasma concentrations of HDL subclasses. J. Lipid Res. 2010, 51, 1610–1617. [Google Scholar] [CrossRef]
- Schilcher, I.; Ledinski, G.; Radulović, S.; Hallström, S.; Eichmann, T.; Madl, T.; Zhang, F.; Leitinger, G.; Kolb-Lenz, D.; Darnhofer, B.; et al. Endothelial lipase increases antioxidative capacity of high-density lipoprotein. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Gan, K.N.; Smolen, A.; Eckerson, H.W.; La Du, B.N. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab. Dispos. 1991, 19, 100–106. [Google Scholar]
- Bautista, R.; Carreón-Torres, E.; Luna-Luna, M.; Komera-Arenas, Y.; Franco, M.; Fragoso, J.M.; López-Olmos, V.; Cruz-Robles, D.; Vargas-Barrón, J.; Vargas-Alarcón, G.; et al. Early endothelial nitrosylation and increased abdominal adiposity in Wistar rats after long-term consumption of food fried in canola oil. Nutrition 2014, 30, 1055–1060. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Placebo n = 6 | MiPo n = 6 | |
|---|---|---|---|
| Weight (kg) | Baseline After 30 days | 2.2 (2.0–2.3) a 3.1 (2.9–3.2) | 2.1 (2.0–2.2) b 3.0 (2.9–3.0) |
| Glucose (mg/dL) | Baseline After 30 days | 76.6 (66.9–84.5) 80.9 (75.1–90.1) | 85.0 (76.8–99.4) 79.9 (73.5–83.2) |
| Total cholesterol (mg/dL) | Baseline After 30 days | 52.5 (45.2–59.2) 54.7 (47.4–62.4) | 52.3 (45.3–71.7) b 41.4 (33.9–54.8) |
| Triglycerides (mg/dL) | Baseline After 30 days | 75.8 (60.3–85.1) a 85.9 (68.6–94.4) | 65.9 (59.2–85.8)b 50.5 (46.2–56.8) c |
| Non-HDL-Cholesterol (mg/dL) | Baseline After 30 days | 20.9 (16.5–28.9) 27.8 (21.3–32.0) | 29.2 (17.7–40.6) b 9.4 (4.3–15.9)c |
| Sphingomyelin (mg/dL) | Baseline After 30 days | 5.6 (4.8–7.1) 7.7 (4.2–8.9) | 4.6 (3.9–6.5) 5.8 (3.9–7.6) |
| Non-HDL-SM (mg/dL) | Baseline fter 30 days | 3.8(3.3–4.5) 4.0 (2.7–4.8) | 3.6 (3.2–4.4) b 2.6 (2.0–3.0)c |
| HDL-C (mg/dL) | Baseline After 30 days | 30.4 (27.2–33.6) a 27.2 (24.8–29.2) | 32.6 (30.6–34.6) b 37.6 (34.0–40.3) c |
| HDL-Tg (mg/dL) | Baseline After 30 days | 45.2 (23.6–55.4) 40.8 (31.7–46.2) | 41.1 (33–49.6) b 30.2 (23.5–33.6) |
| HDL-Pho (mg/dL) | Baseline After 30 days | 52.0 (44.4–61.0) 46.1 (42.1–52.3) | 60.4 (56.1–64.0) b 64.8 (59.3–68.0) |
| HDL-SM (mg/dL) | Baseline After 30 days | 1.6 (1.2–1.9) a 2.5 (1.5–4.2) | 1.5 (1.1–2.2) 1.4 (0.9–2.1) |
| HDL Lipids Subclass | Placebo n = 6 | MiPo n = 6 | |
|---|---|---|---|
| Cholesterol (mg/dL) | |||
| HDL 2b | Baseline After 30 days | 12.1 (8.7–15.6) 11.7 (6.8–17.8) | 11.8 (6.9–16.7) 13.9 (10.0–18.0) |
| HDL 2a | Baseline After 30 days | 4.7 (3.4–6.0) a 3.8 (2.7–5.2) | 4.6 (3.9–5.2) a 4.6 (3.9–5.3) c |
| HDL 3a | Baseline After 30 days | 6.7 (5.5–7.9) 5.7 (4.9–6.6) | 8.3 (6.1–10.6) b 7.6 (6.0–9.0) |
| HDL 3b | Baseline After 30 days | 2.8 (2.1–3.5) 2.5 (1.3–4.7) | 3.6 (2.3–4.9) 4.1 (2.4–5.8) |
| HDL 3c | Baseline After 30 days | 3.5 (1.1–5.9) 3.4 (1.1–5.8) | 4.0 (1.4–6.5) b 6.9 (3.2–9.7)c |
| Triglycerides (mg/dL) | |||
| HDL 2b | Baseline After 30 days | 17.5 (4.9–30.1) 16.7 (9.6–23.9) | 16.6 (8.0–25.3) 12.4 (6.7–18.1)c |
| HDL 2a | Baseline After 30 days | 6.1 (2.2–10.1) 5.6 (3.1–8.1) | 5.7 (3.3–8.4) b 4.1 (2.1–6.1) |
| HDL 3a | Baseline After 30 days | 9.0 (2.9–15.1) 8.6 (7.0–9.4) | 9.6 (4.8–14.4) b 6.7 (4.1–9.2) |
| HDL 3b | Baseline After 30 days | 4.3 (2.9–7.6) 3.9 (2.6–4.7) | 5.0 (2.1–7.9) b 2.8 (1.8–3.9) |
| HDL 3c | Baseline After 30 days | 4.5 (3.1–6.3) 4.3 (2.3–7.0) | 4.9 (1.1–8.7) b 3.1 (0.5–6.6) |
| Phospholipids (mg/dL) | |||
| HDL 2b | Baseline After 30 days | 22.6 (10.2–35.8) 19.5 (11.7–31.4) | 24.6 (15.8–33.3) 27.5 (23.1–31.8) |
| HDL 2a | Baseline After 30 days | 8.2 (6.3–10.1) 6.3 (4.8–8.1) | 8.5 (6.4–10.7) 9.8 (7.6–12.0) |
| HDL 3a | Baseline After 30 days | 11.8 (8.7–14.8) 12.2 (8.2–16.3) | 15.6 (8.2–23.0) 14.3 (9.5–19.2) |
| HDL 3b | Baseline After 30 days | 5.1 (1.5–8.6) 6.1 (2.1–9.5) | 6.7 (2.1–11.3) 6.9 (2.9–10.9) |
| HDL 3c | Baseline After 30 days | 4.9 (1.9–10.7) 2.9 (1.2–3.9) | 4.1 (1.2–8.4) 4.7 (2.1–7.4) |
| Sphingomyelin (mg/dL) | |||
| HDL 2b | Baseline After 30 days | 0.7 (0.3–1.7) 1.3 (0.8–2.6) | 0.8 (0.6–1.6) 0.6 (0.2–0.9) |
| HDL 2a | Baseline After 30 days | 0.3 (0.2–0.4) 0.4 (0.2–0.5) | 0.3 (0.1–0.5) 0.2 (0.1–0.5) |
| HDL 3a | Baseline After 30 days | 0.4 (0.2–0.6) 0.5 (0.4–0.7) | 0.4 (0.2–0.7) 0.4 (0.3–.0.9) |
| HDL 3b | Baseline After 30 days | 0.1(0.1–0.2) 0.2 (0.0–0.4) | 0.2 (0.0–0.3) 0.2 (0.0–0.4) |
| HDL 3c | Baseline After 30 days | 0.2 (0.1–0.2) 0.2 (0.0–0.3) | 0.1 (0.1–0.2) 0.1 (0.1–0.2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorantes-Morales, A.; Estrada-Luna, D.; Bautista-Pérez, R.; Betanzos-Cabrera, G.; Luna-Luna, M.; Flores-Castillo, C.; Vargas-Alarcón, G.; Fragoso, J.M.; Pérez-Méndez, Ó.; Carreón-Torres, E. Microencapsulated Pomegranate Modifies the Composition and Function of High-Density Lipoproteins (HDL) in New Zealand Rabbits. Molecules 2020, 25, 3297. https://doi.org/10.3390/molecules25143297
Dorantes-Morales A, Estrada-Luna D, Bautista-Pérez R, Betanzos-Cabrera G, Luna-Luna M, Flores-Castillo C, Vargas-Alarcón G, Fragoso JM, Pérez-Méndez Ó, Carreón-Torres E. Microencapsulated Pomegranate Modifies the Composition and Function of High-Density Lipoproteins (HDL) in New Zealand Rabbits. Molecules. 2020; 25(14):3297. https://doi.org/10.3390/molecules25143297
Chicago/Turabian StyleDorantes-Morales, Alan, Diego Estrada-Luna, Rocío Bautista-Pérez, Gabriel Betanzos-Cabrera, María Luna-Luna, Cristóbal Flores-Castillo, Gilberto Vargas-Alarcón, José Manuel Fragoso, Óscar Pérez-Méndez, and Elizabeth Carreón-Torres. 2020. "Microencapsulated Pomegranate Modifies the Composition and Function of High-Density Lipoproteins (HDL) in New Zealand Rabbits" Molecules 25, no. 14: 3297. https://doi.org/10.3390/molecules25143297
APA StyleDorantes-Morales, A., Estrada-Luna, D., Bautista-Pérez, R., Betanzos-Cabrera, G., Luna-Luna, M., Flores-Castillo, C., Vargas-Alarcón, G., Fragoso, J. M., Pérez-Méndez, Ó., & Carreón-Torres, E. (2020). Microencapsulated Pomegranate Modifies the Composition and Function of High-Density Lipoproteins (HDL) in New Zealand Rabbits. Molecules, 25(14), 3297. https://doi.org/10.3390/molecules25143297





