Molecular Hydrogen as a Lewis Base in Hydrogen Bonds and Other Interactions
Abstract
1. Introduction
2. Results and Discussion
2.1. The Strength of Interactions in Complexes of Dihydrogen
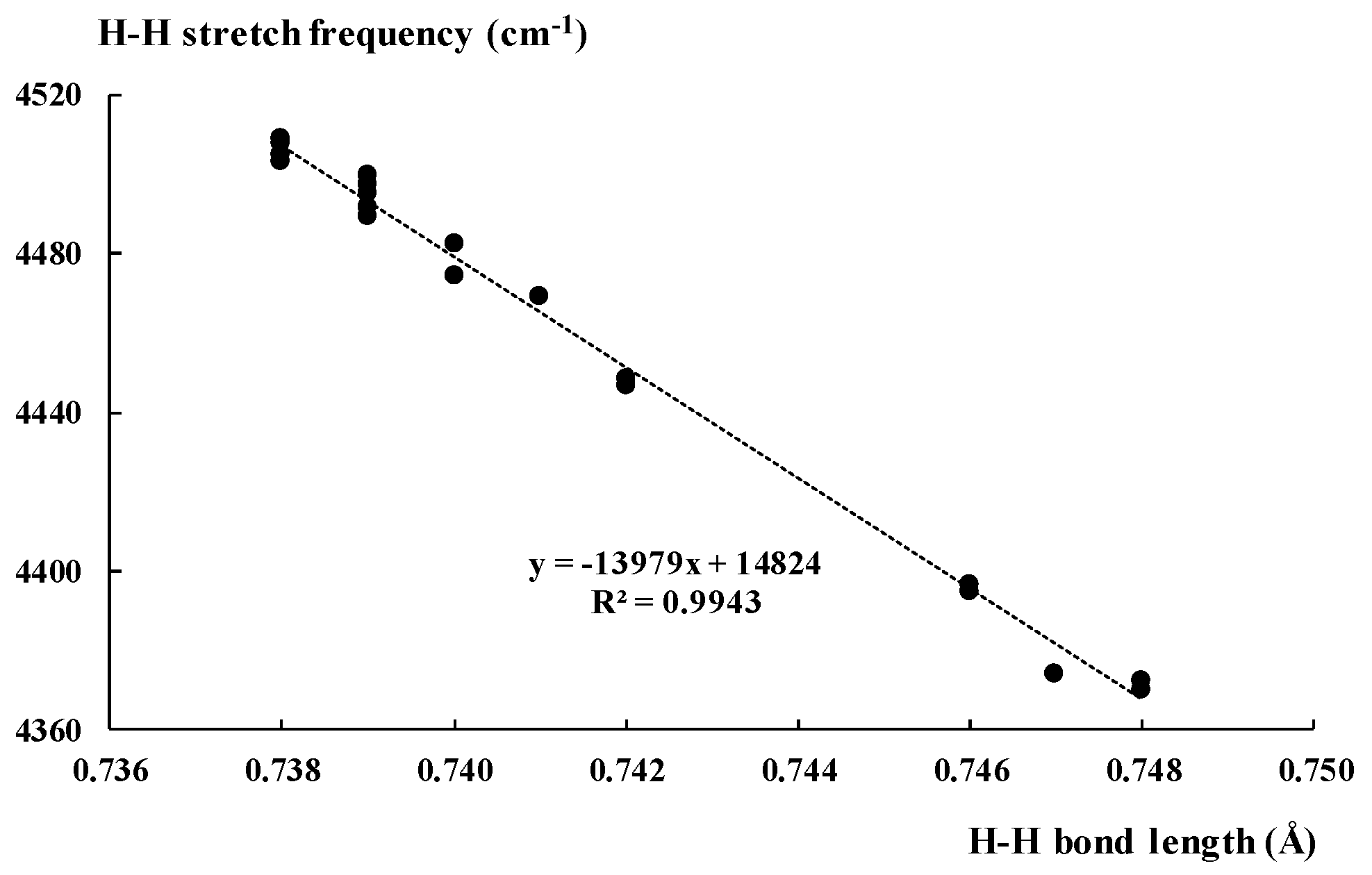
2.2. The H2 Stretch Mode in Complexes of Dihydrogen
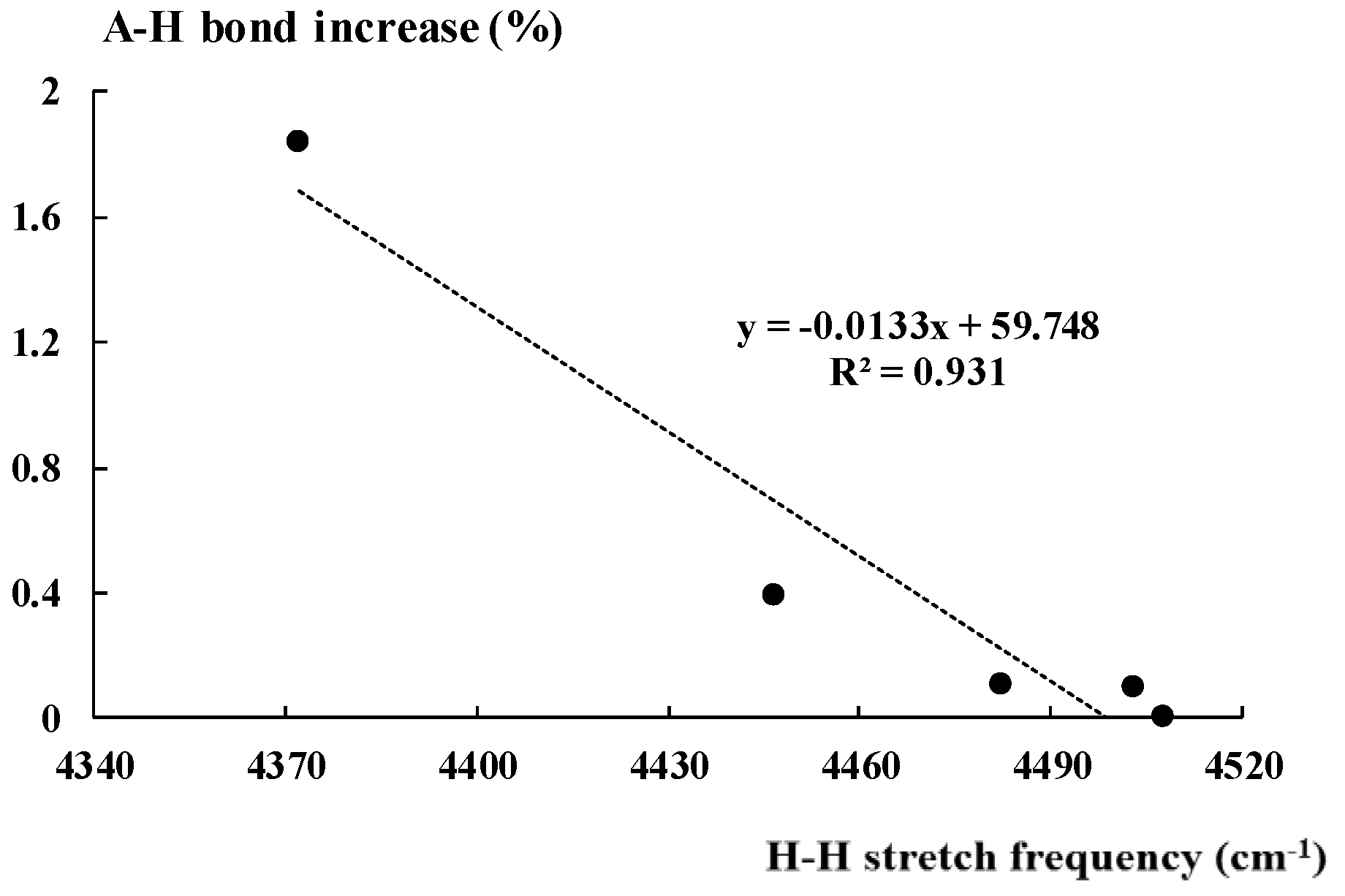
2.3. The A-H Stretching Mode in Hydrogen Bonded Complexes
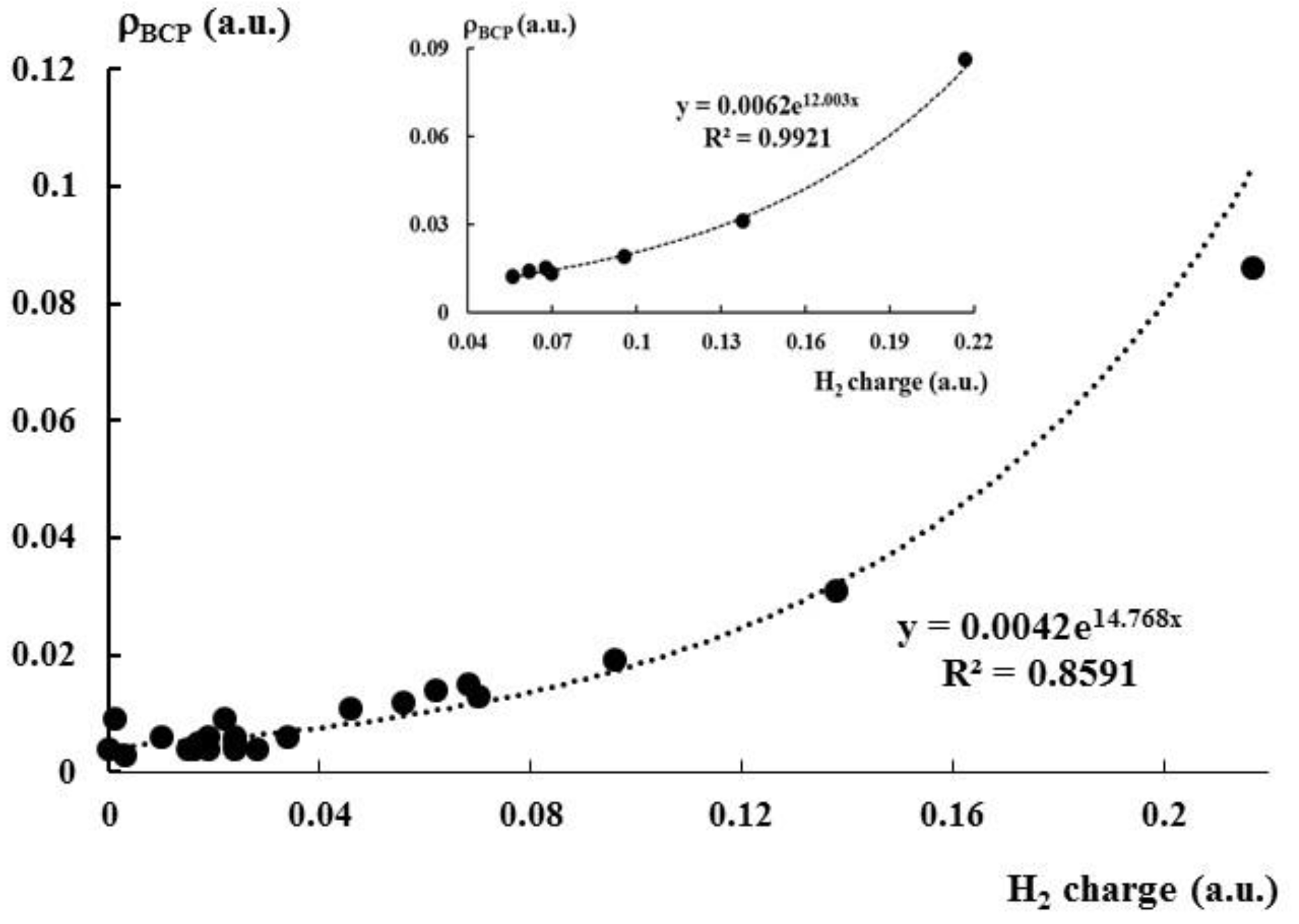
2.4. The Topological Parameters
2.5. The Decomposition of the Energy of Interaction
3. Conclusions
4. Computational Details
Funding
Acknowledgments
Conflicts of Interest
References
- Grabowski, S.J. What is the Covalency of Hydrogen Bonding? Chem. Rev. 2011, 11, 2597–2625. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Dihydrogen bond and X-H···σ interaction as sub-classes of hydrogen bond. J. Phys. Org. Chem. 2013, 26, 452–459. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C. Valency and Bonding, A Natural Bond Orbital Donor—Acceptor Perspective; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Weinhold, F.; Klein, R.A. What is a hydrogen bond? Mutually consistent theoretical and experimental criteria for characterizing H-bonding interactions. Mol. Phys. 2012, 110, 565–579. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press Inc.: New York, NY, USA, 1999. [Google Scholar]
- Scheiner, S.; Grabowski, S.J. Acetylene as a potential hydrogen-bond proton acceptor. J. Mol. Struct. 2002, 615, 209–218. [Google Scholar] [CrossRef]
- Baiocchi, F.A.; Williams, J.H.; Klemperer, W. Molecular beam studies of hexafluorobenzene, trifluorobenzene, and benzene complexes of hydrogen fluoride. The rotational spectrum of benzene-hydrogen fluoride. J. Phys. Chem. 1983, 87, 2079–2084. [Google Scholar] [CrossRef]
- Nishio, M.; Hirota, M.; Umezawa, Y. The CH/π Interaction: Evidence, Nature and Consequences; Wiley-VCH, Inc.: New York, NY, USA, 1998. [Google Scholar]
- Szymczak, J.J.; Grabowski, S.J.; Roszak, S.; Leszczynski, J. H···σ interactions—An ab initio and ‘atoms in molecules’ study. Chem. Phys. Lett. 2004, 393, 81–86. [Google Scholar] [CrossRef]
- Grabowski, S.J. Hydrogen Bonds with π and σ Electrons as the Multicenter Proton Acceptors: High Level Ab Initio Calculations. J. Phys. Chem. A 2007, 111, 3387–3393. [Google Scholar] [CrossRef]
- Rozas, I.; Alkorta, I.; Elguero, J. Unusual hydrogen bonds: H···pi interactions. J. Phys. Chem. A 1997, 101, 9457–9463. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J.R.; Vivier-Bunge, A. Non-alkane behavior of cyclopropane and its derivatives: Characterization of unconventional hydrogen bond interactions. Theor. Chem. Acc. 2007, 118, 597–606. [Google Scholar] [CrossRef]
- Grabowski, S.J. A-H···σ Hydrogen Bonds: Dihydrogen and Cycloalkanes as Proton Acceptors. ChemPhysChem 2019, 20, 565–574. [Google Scholar] [CrossRef]
- Grabowski, S.J.; Sokalski, W.A.; Leszczynski, J. Is a π···H+···π Complex Hydrogen Bonded? J. Phys. Chem. A 2004, 108, 1806–1812. [Google Scholar] [CrossRef]
- Grabowski, S.J. Hydrogen bonds, and σ-hole and π-hole bonds—Mechanisms protecting doublet and octet electron structures. Phys. Chem. Chem. Phys. 2017, 19, 29742–29759. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Triel bond and coordination of triel centres – Comparison with hydrogen bond interaction. Coord. Chem. Rev. 2020, 407, 213171. [Google Scholar] [CrossRef]
- Hamilton, C.W.; Baker, R.T.; Staubitz, A.; Manners, I. B-N compounds for chemical hydrogen storage. Chem. Soc. Rev. 2009, 38, 279–293. [Google Scholar] [CrossRef]
- Keaton, R.J.; Blacquiere, J.M.; Baker, R.T. Base Metal Catalyzed Dehydrogenation of Ammonia−Borane for Chemical Hydrogen Storage. J. Am. Chem. Soc. 2007, 129, 1844–1845. [Google Scholar] [CrossRef]
- Staubitz, A.; Besora, M.; Harvey, J.N.; Manners, I. Computational Analysis of Amine− Borane Adducts as Potential Hydrogen Storage Materials with Reversible Hydrogen Uptake. Inorg. Chem. 2008, 47, 5910–5918. [Google Scholar] [CrossRef]
- Grabowski, S.J.; Ruipérez, F. Dihydrogen bond interactions as a result of H2 cleavage at Cu, Ag and Au centres. Phys. Chem. Chem. Phys. 2016, 18, 12810–12818. [Google Scholar] [CrossRef]
- Kubas, G.J. Metal Dihydrogen and σ-Bond Complexes—Structure, Theory, and Reactivity; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2001. [Google Scholar]
- Crabtree, R.H. The Organometallic Chemistry of the Transition Metals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Stephan, D.W.; Erker, G. Frustrated Lewis pair chemistry of carbon, nitrogen and sulfur oxides. Chem. Sci. 2014, 5, 2625–2641. [Google Scholar] [CrossRef]
- Stephan, D.W. Frustrated Lewis pairs: A new strategy to small molecule activation and hydrogenation catalysis. Dalton Trans. 2009, 3129–3136. [Google Scholar] [CrossRef]
- Rokob, T.A.; Bakó, I.; Stirling, A.; Hamza, A.; Pápai, I. Reactivity models of hydrogen activation by frustrated Lewis pairs: Synergistic electron transfers or polarization by electric field? J. Am. Chem. Soc. 2013, 135, 4425–4437. [Google Scholar] [CrossRef]
- Pérez, P.; Yepes, D.; Jaque, P.; Chamorro, E.; Domingo, L.R.; Rojas, R.S.; Toro-Labbé, A. A computational and conceptual DFT study on the mechanism of hydrogen activation by novel frustrated Lewis pairs. Phys. Chem. Chem. Phys. 2015, 17, 10715–10725. [Google Scholar] [CrossRef] [PubMed]
- Frey, G.D.; Lavallo, V.; Donnadieu, B.; Schoeller, W.W.; Bertrand, G. Facile splitting of hydrogen and ammonia by nucleophilic activation at a single carbon center. Science 2007, 316, 439–441. [Google Scholar] [CrossRef]
- Grabowski, S.J. Cleavage of hydrogen by activation at a single non-metal centre towards new hydrogen storage materials. Phys. Chem. Chem. Phys. 2015, 17, 13539–13546. [Google Scholar] [CrossRef]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Lane, P.; Concha, M.C.; Ma, Y.; Murray, J.S. An overview of halogen bonding. J. Mol. Model. 2007, 13, 305–311. [Google Scholar] [CrossRef]
- Politzer, P.; Riley, K.E.; Bulat, F.A.; Murray, J.S. Perspectives on halogen bonding and other σ-hole interactions: Lex parsimoniae (Occam’s Razor). Comput. Theor. Chem. 2012, 998, 2–8. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7758. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other σ-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef]
- Scheiner, S. Detailed Comparison of the Pnicogen Bond with Chalcogen, Halogen, and Hydrogen Bonds. Int. J. Quantum Chem. 2013, 113, 1609–1620. [Google Scholar] [CrossRef]
- Scheiner, S. The Pnicogen Bond: Its Relation to Hydrogen, Halogen, and Other Noncovalent Bonds. Acc. Chem. Res. 2013, 46, 280–288. [Google Scholar] [CrossRef]
- Jucks, K.W.; Miller, R.E. Infrared Stark spectroscopy of the hydrogen–HF binary complex. J. Chem. Phys. 1987, 87, 5629–5633. [Google Scholar] [CrossRef]
- Moore, D.T.; Miller, R.E. Dynamics of hydrogen–HF complexes in helium nanodroplets. J. Chem. Phys. 2003, 118, 9629–9636. [Google Scholar] [CrossRef]
- Moore, D.T.; Miller, R.E. Solvation of HF by molecular hydrogen: Helium nanodroplet vibrational spectroscopy. J. Phys. Chem. A 2003, 107, 10805–10812. [Google Scholar] [CrossRef]
- Moore, D.T.; Miller, R.E. Rotationally Resolved Infrared Laser Spectroscopy of (H2)n-HF and (D2)n-HF (n = 2−6) in Helium Nanodroplets. J. Phys. Chem. A 2004, 108, 1930–1937. [Google Scholar] [CrossRef]
- Bieske, E.J.; Nizkorodov, S.A.; Bennett, F.R.; Maier, J.P. The infrared spectrum of the H2–HCO+ complex. J. Chem. Phys. 1995, 102, 5152–5164. [Google Scholar] [CrossRef]
- Grabowski, S.J.; Alkorta, I.; Elguero, J. Complexes between Dihydrogen and Amine, Phosphine, and Arsine Derivatives. Hydrogen Bond versus Pnictogen Interaction. J. Phys. Chem. A 2013, 117, 3243–3251. [Google Scholar] [CrossRef]
- Fau, S.; Frenking, G. Theoretical investigation of the weakly bonded donor—Acceptor complexes X3B—H2, X3B—C2H4, and X3B—C2H2 (X = H, F, Cl). Mol. Phys. 1999, 96, 519–527. [Google Scholar]
- Piela, L. Ideas of Quantum Chemistry; Elsevier Science Publishers: Amsterdam, The Netherlands, 2007; pp. 684–691. [Google Scholar]
- Grabowski, S.J.; Sokalski, W.A. Different types of hydrogen bonds: Correlation analysis of interaction energy components. J. Phys. Org. Chem. 2005, 18, 779–784. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–561. [Google Scholar] [CrossRef]
- Yáñez, M.; Sanz, P.; Mó, O.; Alkorta, I.; Elguero, J. Beryllium Bonds, Do They Exist? J. Chem. Theory Comput. 2009, 5, 2763–2771. [Google Scholar] [CrossRef]
- Eskandari, K. Characteristics of beryllium bonds; a QTAIM study. J. Mol. Model. 2012, 18, 3481–3487. [Google Scholar] [CrossRef] [PubMed]
- Mó, O.; Yáñez, M.; Alkorta, I.; Elguero, J. Modulating the Strength of Hydrogen Bonds through Beryllium Bonds. J. Chem. Theory Comput. 2012, 8, 2293–2300. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Cheng, J.; Li, W. A new interaction mechanism of LiNH2 with MgH2: Magnesium bond. J. Mol. Model. 2013, 19, 247–253. [Google Scholar] [CrossRef] [PubMed]
- McDowell, S.A.C.; Maynard, S.J. A computational study of model hydrogen-, halogen-, beryllium-and magnesium-bonded complexes of aziridine derivatives. Mol. Phys. 2016, 114, 1609–1618. [Google Scholar] [CrossRef]
- Li, S.Y.; Wu, D.; Li, Y.; Yu, D.; Liu, J.Y.; Li, Z.R. Insight into structural and π–magnesium bonding characteristics of the X2Mg···Y (X = H, F.; Y = C2H2, C2H4 and C6H6) complexes. RSC Adv. 2016, 6, 102754–102761. [Google Scholar] [CrossRef]
- Tama, R.; Mó, O.; Yáñez, M.; Montero-Campillo, M.M. Characterizing magnesium bonds: Main features of a non-covalent interaction. Theor. Chem. Acc. 2017, 136, 36. [Google Scholar] [CrossRef]
- Montero-Campillo, M.M.; Sanz, P.; Mó, O.; Yáñez, M.; Alkorta, I.; Elguero, J. Alkaline-earth (Be, Mg and Ca) bonds at the origin of huge acidity enhancements. Phys. Chem. Chem. Phys. 2018, 20, 2413–2420. [Google Scholar] [CrossRef]
- Kollman, P.A.; Liebman, J.F.; Allen, L.C. The Lithium Bond. J. Am. Chem. Soc. 1970, 92, 1142–1150. [Google Scholar] [CrossRef]
- McDowell, S.A.C.; Hill, J.A.S.S. A theoretical study of hydrogen- and lithium-bonded complexes of F–H/Li and Cl–H/Li with NF3, NH3, and NH2(CH3). J. Chem. Phys. 2011, 135, 164303. [Google Scholar] [CrossRef]
- Lipkowski, P.; Grabowski, S.J. Could the lithium bond be classified as the σ-hole bond—QTAIM and NBO analysis. Chem. Phys. Lett. 2014, 591, 113–118. [Google Scholar] [CrossRef]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Scheiner, S. Hydrogen Bonding: A Theoretical Perspective; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Bundhun, A.; Ramasami, P.; Murray, J.S.; Politzer, P. Trends in σ-hole Strengths and Interactions of F3MX Molecules (M = C, Si, Ge and X = F, Cl, Br, I). J. Mol. Model. 2013, 19, 2739–2746. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Tetrel-Bonding Interaction: Rediscovered Supramolecular Force? Angew. Chem. Int. Ed. 2013, 52, 12317–12321. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Tetrel bond σ-hole bond as a preliminary stage of the SN2 reaction. Phys. Chem. Chem. Phys. 2014, 16, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Comparison between Tetrel Bonded Complexes Stabilized by σ and π Hole Interactions. Molecules 2018, 23, 1416. [Google Scholar] [CrossRef]
- Sundberg, M.R.; Uggla, R.; Viñas, C.; Teixidor, F.; Paavola, S.; Kivekäs, R. Nature of intramolecular interactions in hypercoordinate C-substituted 1, 2-dicarba-closo-dodecaboranes with short P···P distances. Inorg. Chem. Commun. 2007, 10, 713–716. [Google Scholar]
- Bauer, S.; Tschirschwitz, S.; Lönnecke, P.; Franck, R.; Kirchner, B.; Clark, M.L.; Hey-Hawkins, E. Enantiomerically pure bis (phosphanyl) carbaborane (12) compounds. Eur. J. Inorg. Chem. 2009, 2009, 2776–2788. [Google Scholar] [CrossRef]
- Del Bene, J.E.; Alkorta, I.; Sanchez-Sanz, G.; Elguero, J. Structures, Energies, Bonding, and NMR Properties of Pnicogen Complexes H2XP:NXH2 (X = H, CH3, NH2, OH, F, Cl). J. Phys. Chem. A 2011, 115, 13724–13731. [Google Scholar] [CrossRef]
- Scheiner, S. Can two trivalent N atoms engage in a direct N···N noncovalent interaction? Chem. Phys. Lett. 2011, 514, 32–35. [Google Scholar] [CrossRef]
- Scheiner, S.; Wysokiński, R.; Michalczyk, M.; Zierkiewicz, W. Pnicogen Bonds Pairing Anionic Lewis Acid With Neutral and Anionic Bases. J. Phys. Chem. A 2020, 124, 4998–5006. [Google Scholar] [CrossRef] [PubMed]
- Bleiholder, C.; Gleiter, R.; Werz, D.B.; Köppel, H. Theoretical Investigations on Heteronuclear Chalcogen—Chalcogen Interactions: On the Nature of Weak Bonds between Chalcogen Centers. Inorg. Chem. 2007, 46, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Bleiholder, C.; Werz, D.B.; Köppel, H.; Gleiter, R. Theoretical investigations on chalcogen− chalcogen interactions: What makes these nonbonded interactions bonding? J. Am. Chem. Soc. 2006, 128, 2666–2674. [Google Scholar] [CrossRef]
- Sanz, P.; Yañez, M.; Mó, O. Competition between X···H···Y Intramolecular Hydrogen Bonds and X····Y (X = O, S, and Y = Se, Te) Chalcogen−Chalcogen Interactions. J. Phys. Chem. A 2002, 106, 4661–4668. [Google Scholar] [CrossRef]
- Wang, W.; Ji, B.; Zhang, Y. Chalcogen bond: A sister noncovalent bond to halogen bond. J. Phys. Chem. A 2009, 113, 8132–8135. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, E.; Fuster, F.; Madebene, B.; Grabowski, S.J. Topological reaction sites–very strong chalcogen bonds. Phys. Chem. Chem. Phys. 2014, 16, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Scilabra, P.; Terraneo, G.; Resnati, G. The chalcogen bond in crystalline solids: A world parallel to halogen bond. Acc. Chem. Res. 2019, 52, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Resnati, G. Halogen bonding: A paradigm in supramolecular chemistry. Chem. Eur. J. 2001, 7, 2511–2519. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]
- Grabowski, S.J. Boron and other triel lewis acid centers: From hypovalency to hypervalency. ChemPhysChem 2014, 15, 2985–2993. [Google Scholar] [CrossRef]
- Grabowski, S.J. π-hole bonds: Boron and aluminium lewis acid centers. ChemPhysChem 2015, 16, 1470–1479. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Halogen Bonding: An Interim Discussion. ChemPhysChem 2013, 14, 278–294. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the σ-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Li, Q.; Scheiner, S. Comparative Strengths of Tetrel, Pnicogen, Chalcogen, and Halogen Bonds and Contributing Factors. Molecules 2018, 23, 1681. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Yang, S.; Li, Q.; Cheng, J.; Li, H.; Liu, S. Tetrel Bond between 6-OTX3-Fulvene and NH3: Substituents and Aromaticity. Molecules 2019, 24, 10. [Google Scholar] [CrossRef]
- Könczöl, L.; Turczel, G.; Szpisjak, T.; Szieberth, D. The stability of η2-H2 borane complexes—A theoretical investigation. Dalton Trans. 2014, 43, 13571–13577. [Google Scholar] [CrossRef] [PubMed]
- Hiberty, P.C.; Ohanessian, G. Comparison of minimal and extended basis sets in terms of resonant formulas. Application to 1,3 dipoles. J. Am. Chem. Soc. 1982, 104, 66–70. [Google Scholar] [CrossRef]
- Dickenson, G.D.; Niu, M.L.; Salumbides, E.J.; Komasa, J.; Eikema, K.S.E.; Pachucki, K.; Ubachs, W. Fundamental Vibration of Molecular Hydrogen. Phys. Rev. Lett. 2013, 110, 193601. [Google Scholar] [CrossRef]
- Crabtree, R.H.; Siegbahn, P.E.M.; Eisenstein, O.; Rheingold, A.L.; Koetzle, T.F.A. A New Intermolecular Interaction: Unconventional Hydrogen Bonds with Element-Hydride Bonds as Proton Acceptor. Acc. Chem. Res. 1996, 29, 348–354. [Google Scholar] [CrossRef]
- Belkova, N.V.; Shubina, E.S.; Gutsul, E.I.; Epstein, L.M.; Eremenko, I.L.; Nefedov, S.E. Structural and energetic aspects of hydrogen bonding and proton transfer to ReH2(CO)(NO)(PR3)2 and ReHCl(CO)(NO)(PMe3)2 by IR and X-ray studies. J. Organomet. Chem. 2000, 610, 58–70. [Google Scholar] [CrossRef]
- Custelcean, R.; Jackson, J.E. Topochemical Control of Covalent Bond Formation by Dihydrogen Bonding. J. Am. Chem. Soc. 1998, 120, 12935–12941. [Google Scholar] [CrossRef]
- Grabowski, S.J. A new measure of hydrogen bonding strength–ab initio and atoms in molecules studies. Chem. Phys. Lett. 2001, 338, 361–366. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules, A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Matta, C.; Boyd, R.J. (Eds.) Quantum Theory of Atoms in Molecules: Recent Progress in Theory and Application; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Carrol, M.T.; Chang, C.; Bader, R.F.W. Prediction of the structures of hydrogen-bonded complexes using the Laplacian of the charge density. Mol. Phys. 1988, 63, 387–405. [Google Scholar] [CrossRef]
- Carrol, M.T.; Bader, R.F.W. An analysis of the hydrogen bond in BASE-HF complexes using the theory of atoms in molecules. Mol. Phys. 1988, 65, 695–722. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Subramanian, V.; Sathyamurthy, N. Hydrogen bonding without borders: An atoms-in-molecules perspective. J. Phys. Chem. A 2006, 110, 3349–3351. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. Intermolecular Interactions in Crystals: Fundamentals of Crystal Engineering; Novoa, J.J., Ed.; The Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Ziegler, T.; Rauk, A. CO, CS, N2, PF3, and CNCH3 as σ Donors and π Acceptors. A Theoretical Study by the Hartree-Fock-Slater Transition-State Method. Inorg. Chem. 1979, 18, 1755–1759. [Google Scholar] [CrossRef]
- Velde, G.T.E.; Bickelhaupt, F.M.; Baerends, E.J.; Guerra, C.F.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H., Jr. Gaussian Basis Sets for Use in Correlated Molecular Calculations. III. The second row atoms, Al-Ar. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef]
- Van Duijneveldt, F.B.; Van Duijneveldt-van de Rijdt, J.G.C.M.; Van Lenthe, J.H. State of the Art in Counterpoise Theory. Chem. Rev. 1994, 94, 1873–1885. [Google Scholar] [CrossRef]
- Breneman, C.M.; Wiberg, K.B. Determining atom-centered monopoles from molecular electrostatic potentials.The need for high sampling density in formamide conformational analysis. J. Comp. Chem. 1990, 11, 361–373. [Google Scholar] [CrossRef]
- Szefczyk, B.; Sokalski, W.A.; Leszczynski, J. Optimal methods for calculation of the amount of intermolecular electron transfer. J. Chem. Phys. 2002, 117, 6952. [Google Scholar] [CrossRef]
- Todd, A.; Keith, T.K. AIMAll (Version 11.08.23); Gristmill Software: Overland Park, KS, USA, 2011. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J. Optimized Slater-type basis sets for the elements 1-118. J. Comput. Chem. 2003, 24, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- ADF2013, SCM. Theoretical Chemistry; Vrije Universiteit: Amsterdam, The Netherlands; Available online: http://www.scm.com/news/adf2013-modeling-suite-released/ (accessed on 19 July 2020).
Sample Availability: Samples of the compounds are not available from the author. |
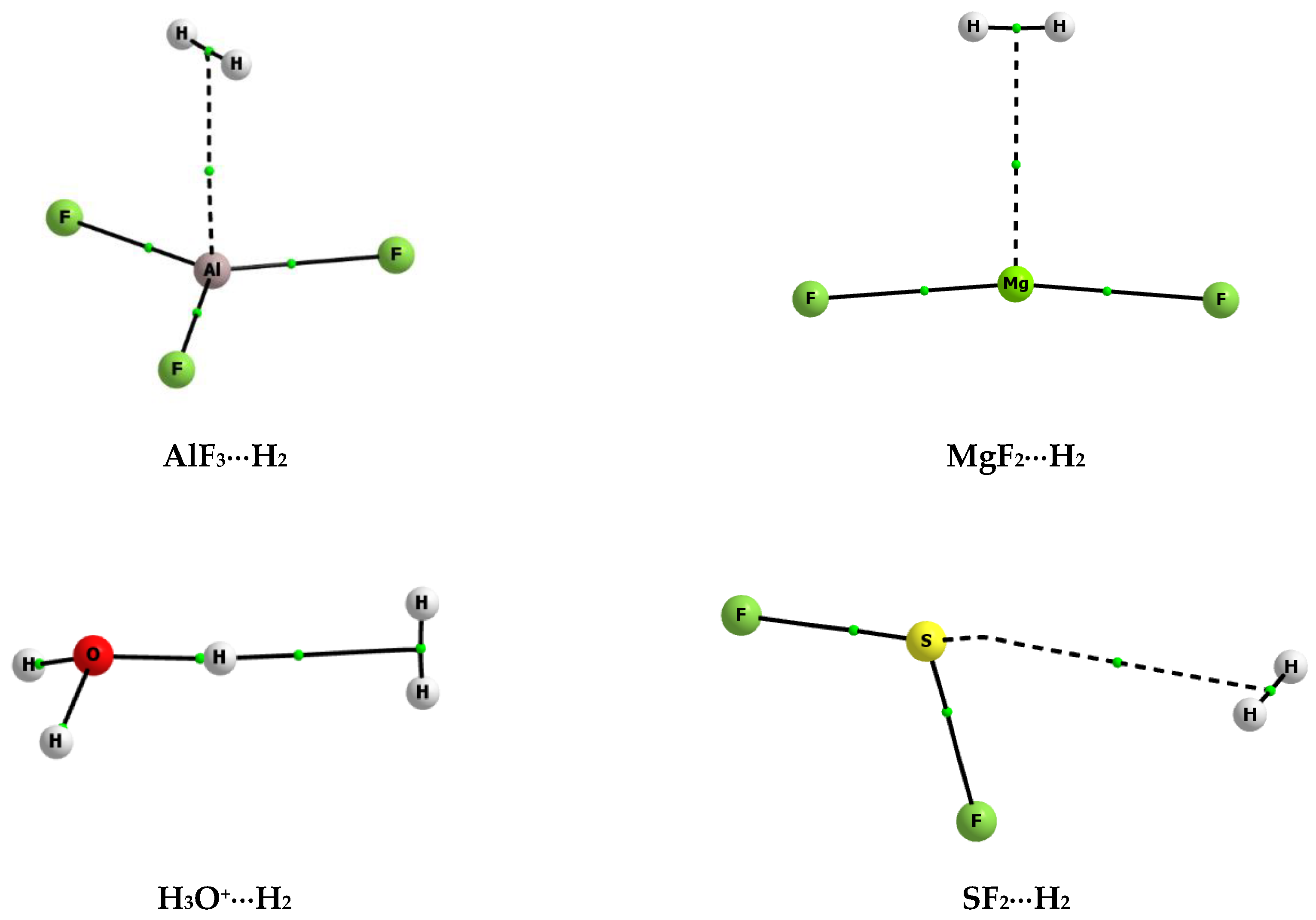

| Lewis Acid | Bond | Eint | Ebin | Edef | BSSE |
|---|---|---|---|---|---|
| AlF3 | Triel | −4.78 | −4.02 | 0.76 | 0.71 |
| AlH3 | Triel | −2.80 | −2.58 | 0.22 | 0.19 |
| BF3 | Triel | −0.67 | −0.66 | 0.01 | 0.39 |
| BH3 | Triel | −12.62 | −5.39 | 7.23 | 0.53 |
| BeF2 | Beryllium | −1.17 | −0.96 | 0.21 | 0.28 |
| BeH2 | Beryllium | −0.48 | −0.46 | 0.02 | 0.06 |
| MgF2 | Magnesium | −3.57 | −3.28 | 0.29 | 0.30 |
| MgH2 | Magnesium | −1.15 | −1.07 | 0.08 | 0.07 |
| Li+ | Lithium | −5.84 | −5.81 | 0.03 | 0.04 |
| HCCH | Hydrogen | −0.30 | −0.30 | 0.00 | 0.12 |
| HF | Hydrogen | −0.94 | −0.94 | 0.00 | 0.18 |
| HCN | Hydrogen | −0.45 | −0.45 | 0.00 | 0.17 |
| NH4+ | Hydrogen | −2.44 | −2.42 | 0.02 | 0.15 |
| H3O+ | Hydrogen | −5.53 | −5.29 | 0.24 | 0.25 |
| SiF4 | Tetrel | −0.41 | −0.41 | 0.00 | 0.38 |
| SiFH3 | Tetrel | −0.57 | −0.57 | 0.00 | 0.22 |
| PFH2 | Pnicogen | −0.92 | −0.91 | 0.01 | 0.30 |
| P(CN)H2 | Pnicogen | −0.54 | −0.54 | 0.00 | 0.16 |
| S(CN)2 | Chalcogen | −0.87 | −0.87 | 0.00 | 0.19 |
| SF2 | Chalcogen | −0.60 | −0.60 | 0.00 | 0.21 |
| Cl2 | Halogen | −0.52 | −0.52 | 0.00 | 0.15 |
| HCCCl | Halogen | −0.37 | −0.37 | 0.00 | 0.1 |
| (NC)CCCl | Halogen | −0.46 | −0.46 | 0.00 | 0.13 |
| NCCl | Halogen | −0.46 | −0.46 | 0.00 | 0.11 |
| CF3Cl | Halogen | −0.32 | −0.32 | 0.00 | 0.12 |
| Lewis Acid | r(H2) | νHH | IHH | q(H2) | ENBO |
|---|---|---|---|---|---|
| AlF3 | 0.748 | 4370.2 | 34.42 | 0.096 | 20.0 |
| AlH3 | 0.747 | 4374.2 | 21.19 | 0.068 | 21.5 |
| BF3 | 0.739 | 4497.46 | 1.95 | 0.01 | 1.1 |
| BH3 | 0.799 | 3630.8 | 19.69 | 0.217 | 282.1 |
| BeF2 | 0.741 | 4469.32 | 4.13 | 0.022 | 3.8 |
| BeH2 | 0.739 | 4491.49 | 2.17 | −0.269 | 1.4 |
| MgF2 | 0.746 | 4396.43 | 11.52 | 0.056 | 11.1 |
| MgH2 | 0.742 | 4448.55 | 6.2 | 0.024 | 4.5 |
| Li+ | 0.746 | 4394.79 | 43.41 | 0.07 | 4.8 |
| HCCH | 0.738 | 4508 | 1.06 | 0 | 0.4 |
| HF | 0.74 | 4482.4 | 4.11 | 0.046 | 1.3 |
| HCN | 0.738 | 4503 | 2.8 | 0.017 | 0.7 |
| NH4+ | 0.742 | 4446.8 | 32.61 | 0.062 | 4 |
| H3O+ | 0.748 | 4372.3 | 45.12 | 0.138 | 16.9 |
| SiF4 | 0.738 | 4505.2 | 0.45 | 0.003 | 0.1 |
| SiFH3 | 0.739 | 4497.3 | 1.16 | −0.005 | 0.7 |
| PFH2 | 0.74 | 4474.5 | 1.07 | 0.001 | 0.2 |
| P(CN)H2 | 0.739 | 4497.6 | 1.5 | 0.015 | 0.5 |
| S(CN)2 | 0.739 | 4489.2 | 1.8 | 0.024 | 0.4 |
| SF2 | 0.739 | 4495.2 | 1.83 | 0.019 | 0.5 |
| Cl2 | 0.739 | 4499.6 | 2.47 | 0.034 | 0.5 |
| HCCCl | 0.738 | 4507.9 | 0.71 | 0.016 | 0.2 |
| (NC)CCCl | 0.738 | 4505 | 2.15 | 0.019 | 0.2 |
| NCCl | 0.738 | 4505 | 1.78 | 0.028 | 0.2 |
| CF3Cl | 0.738 | 4508.9 | 0.69 | 0.024 | 0.2 |
| L. Acid | νAH | IAH | rAH | AHinc% |
|---|---|---|---|---|
| HCCH | 3430.7 (3433.7) | 118.24 (95.57) | 1.062 (1.062) | 0 |
| HF | 4085.9 (4126.0) | 252.44 (120.59) | 0.923 (0.922) | 0.11 |
| HCN | 3466.2 (3468.5) | 117.44 (77.03) | 1.065 (1.064) | 0.09 |
| NH4+ | 3476.2 (3527.2) | 310.1 (193.57) | 1.026 (1.022) | 0.39 |
| H3O+ | 3253.7 (3686.1) | 1066.52 (481.4) | 0.998 (0.980) | 1.84 |
| Lewis Acid | ρBCP | ▽2ρBCP | HBCP |
|---|---|---|---|
| AlF3 | 0.019 | 0.084 | 0.001 |
| AlH3 | 0.015 | 0.050 | 0 |
| BH3 | 0.086 | 0.073 | −0.061 |
| MgF2 | 0.012 | 0.070 | 0.003 |
| Li+ | 0.013 | 0.072 | 0.004 |
| NH4+ | 0.014 | 0.038 | 0 |
| H3O+ | 0.031 | 0.048 | −0.006 |
| Lewis Acid | ΔEPauli | ΔEelstat | ΔEorb | ΔEdisp | ΔEint |
|---|---|---|---|---|---|
| AlF3 | 15.04 | −8.19 | −10.98 | −1.24 | −5.37 |
| AlH3 | 11.54 | −6.25 | −8.04 | −0.72 | −3.47 |
| BH3 | 83.58 | −33.84 | −66.13 | −1.35 | −17.74 |
| MgF2 | 7.66 | −5.53 | −5.11 | −1.5 | −4.48 |
| Li+ | 3.39 | −2.69 | −6.31 | −2.96 | −8.57 |
| NH4+ | 3.11 | −1.7 | −3.85 | −0.94 | −3.38 |
| H3O+ | 8.01 | −3.52 | −11.21 | −0.69 | −7.41 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowski, S.J. Molecular Hydrogen as a Lewis Base in Hydrogen Bonds and Other Interactions. Molecules 2020, 25, 3294. https://doi.org/10.3390/molecules25143294
Grabowski SJ. Molecular Hydrogen as a Lewis Base in Hydrogen Bonds and Other Interactions. Molecules. 2020; 25(14):3294. https://doi.org/10.3390/molecules25143294
Chicago/Turabian StyleGrabowski, Sławomir J. 2020. "Molecular Hydrogen as a Lewis Base in Hydrogen Bonds and Other Interactions" Molecules 25, no. 14: 3294. https://doi.org/10.3390/molecules25143294
APA StyleGrabowski, S. J. (2020). Molecular Hydrogen as a Lewis Base in Hydrogen Bonds and Other Interactions. Molecules, 25(14), 3294. https://doi.org/10.3390/molecules25143294






