Combined Effect of Modified Atmosphere Packaging and UV-C Radiation on Pathogens Reduction, Biogenic Amines, and Shelf Life of Refrigerated Tilapia (Oreochromis niloticus) Fillets
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Packaging Treatments
2.3. UV-C Treatment
2.4. Inoculation Experiment
2.4.1. Preparation of Inoculum and Application into Tilapia Fillets
2.4.2. Bacteriological Analyses
2.5. Shelf Life Experiment
2.5.1. Bacteriological Analyses
2.5.2. Quantification of Biogenic Amines
2.5.3. Determination of pH and Ammonia Levels
2.5.4. Measurement of Lipid Oxidation
2.5.5. Determination of Instrumental Color Parameters
2.5.6. Determination of Texture Profile
2.6. Statistical Analyses
3. Results and Discussion
3.1. Effect of MAP and UV-C on Reduction of Pathogenic Bacteria
3.2. Effect of MAP and UV-C on Biogenic Amines and Overall Quality Parameters of Tilapia Fillets During Refrigerated Storage
3.2.1. Bacterial Growth
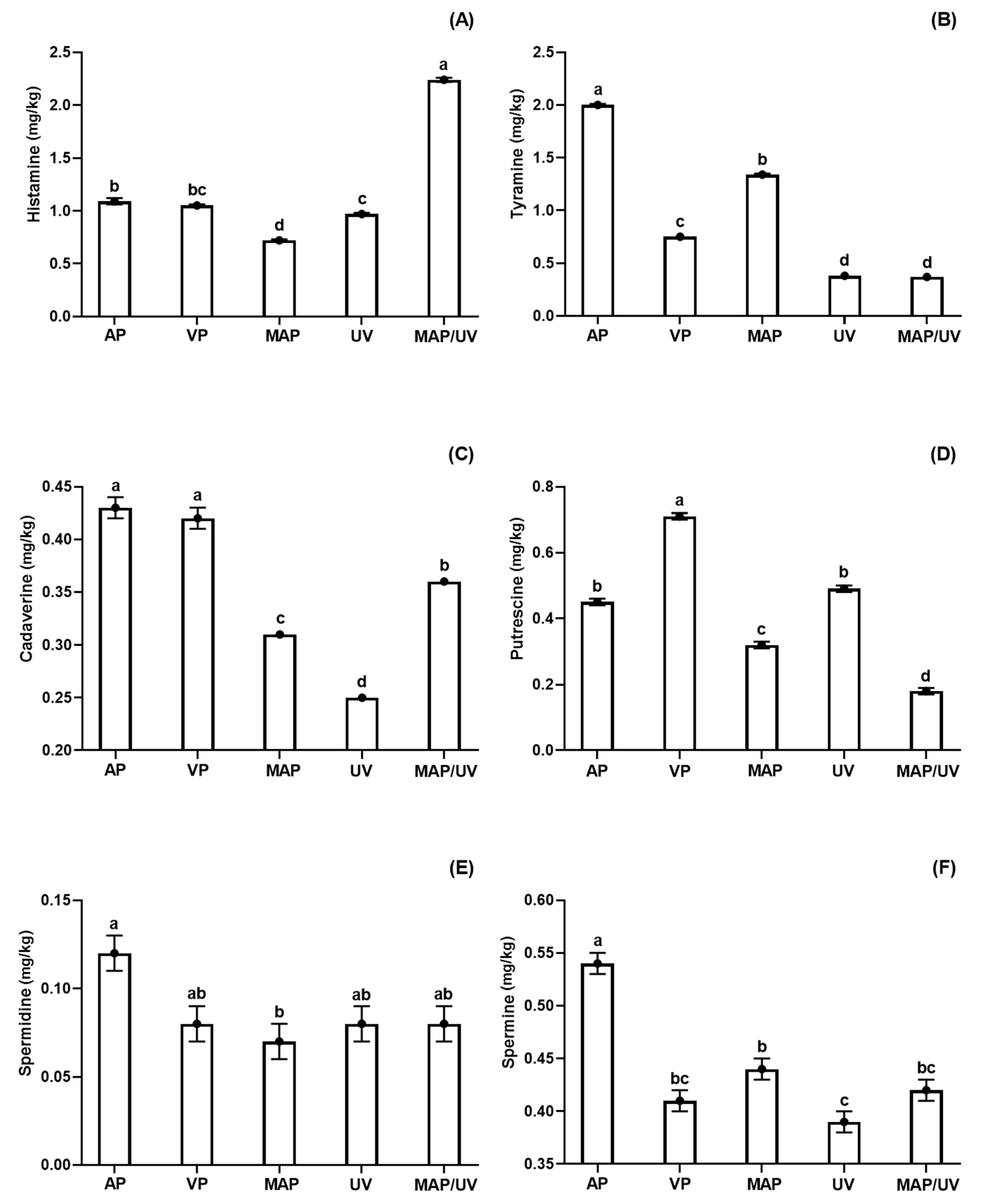
3.2.2. Biogenic Amines
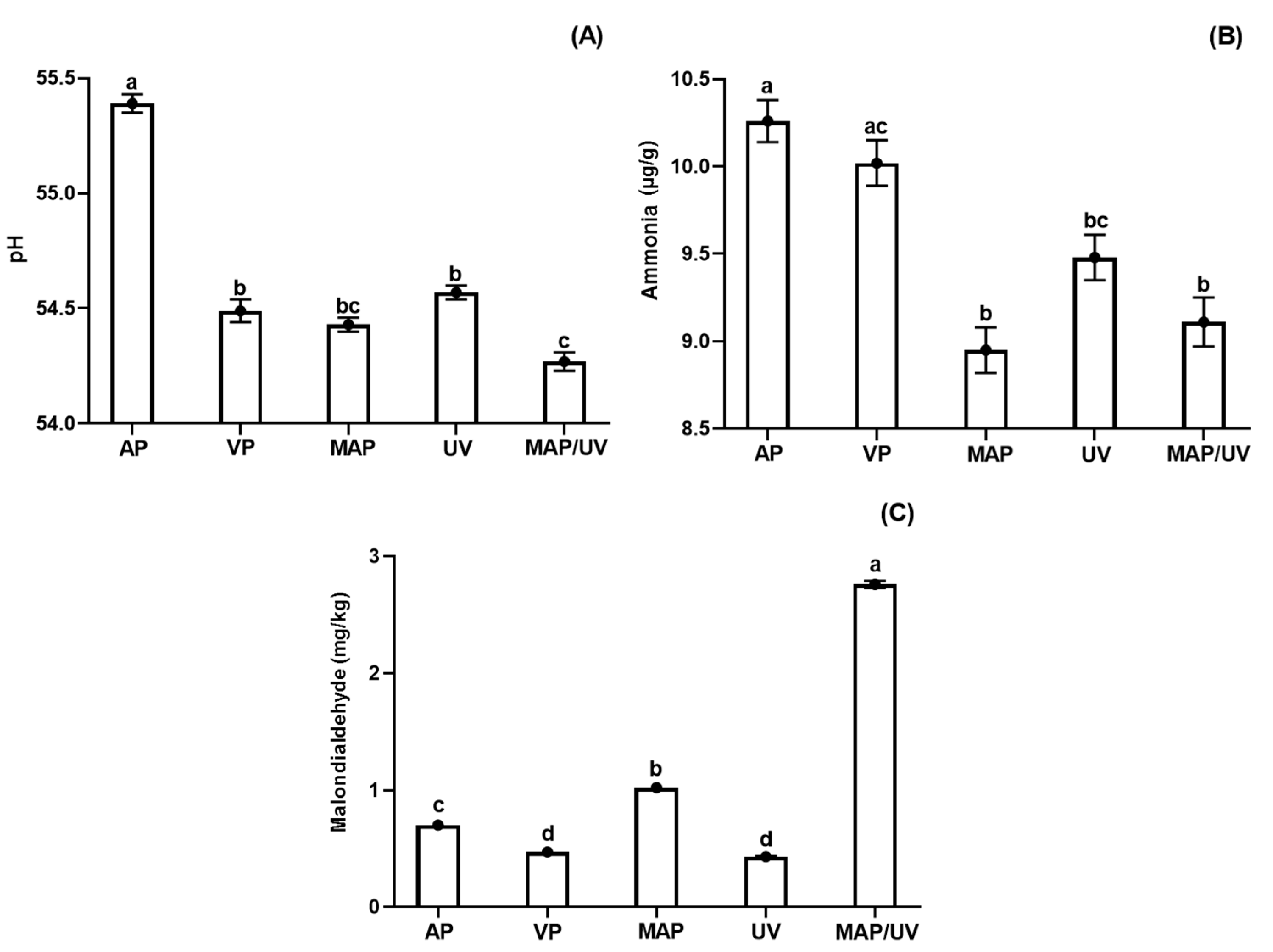
3.2.3. Fish pH
3.2.4. Ammonia
3.2.5. Lipid Oxidation
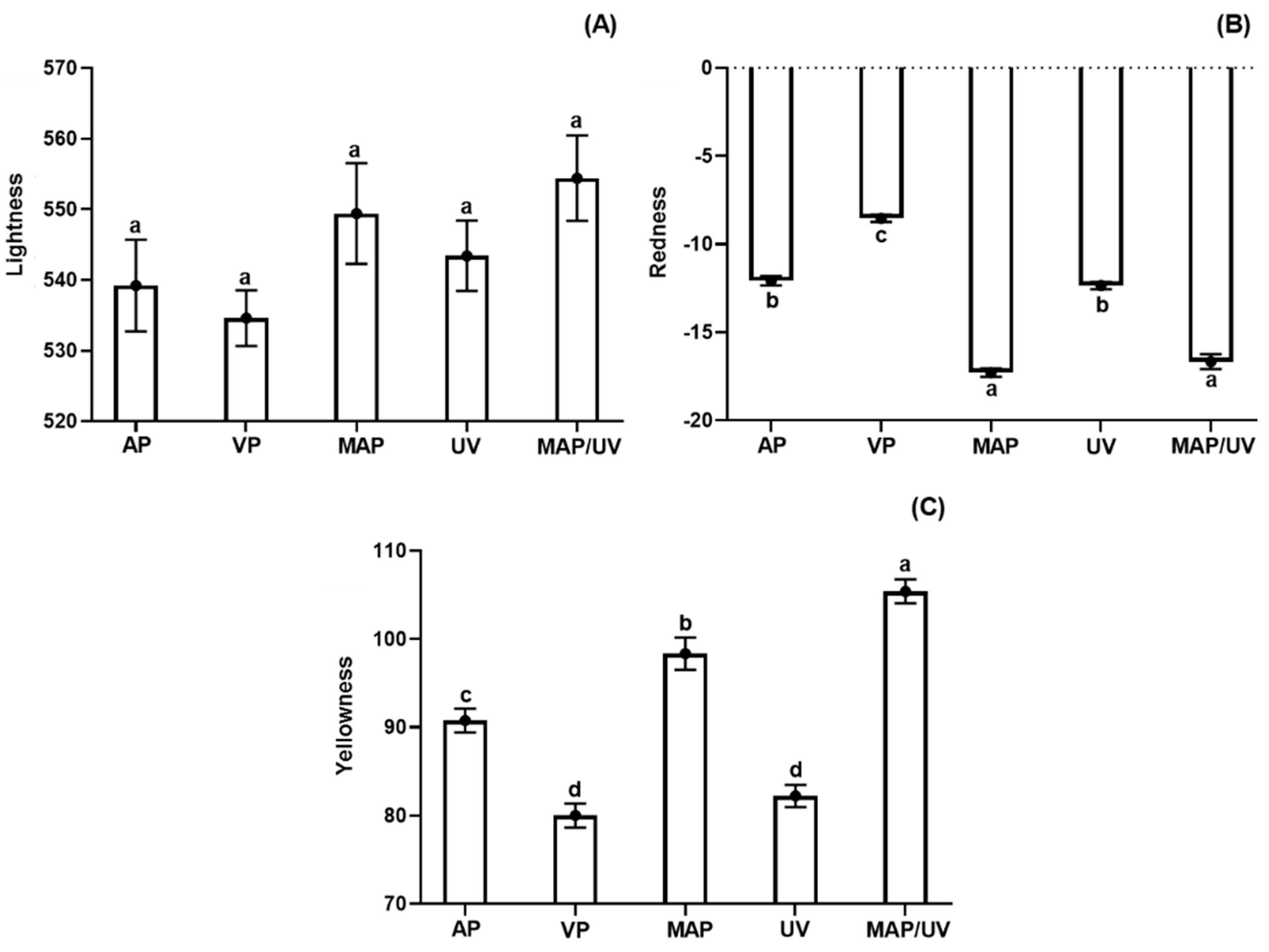
3.2.6. Instrumental Color Parameters
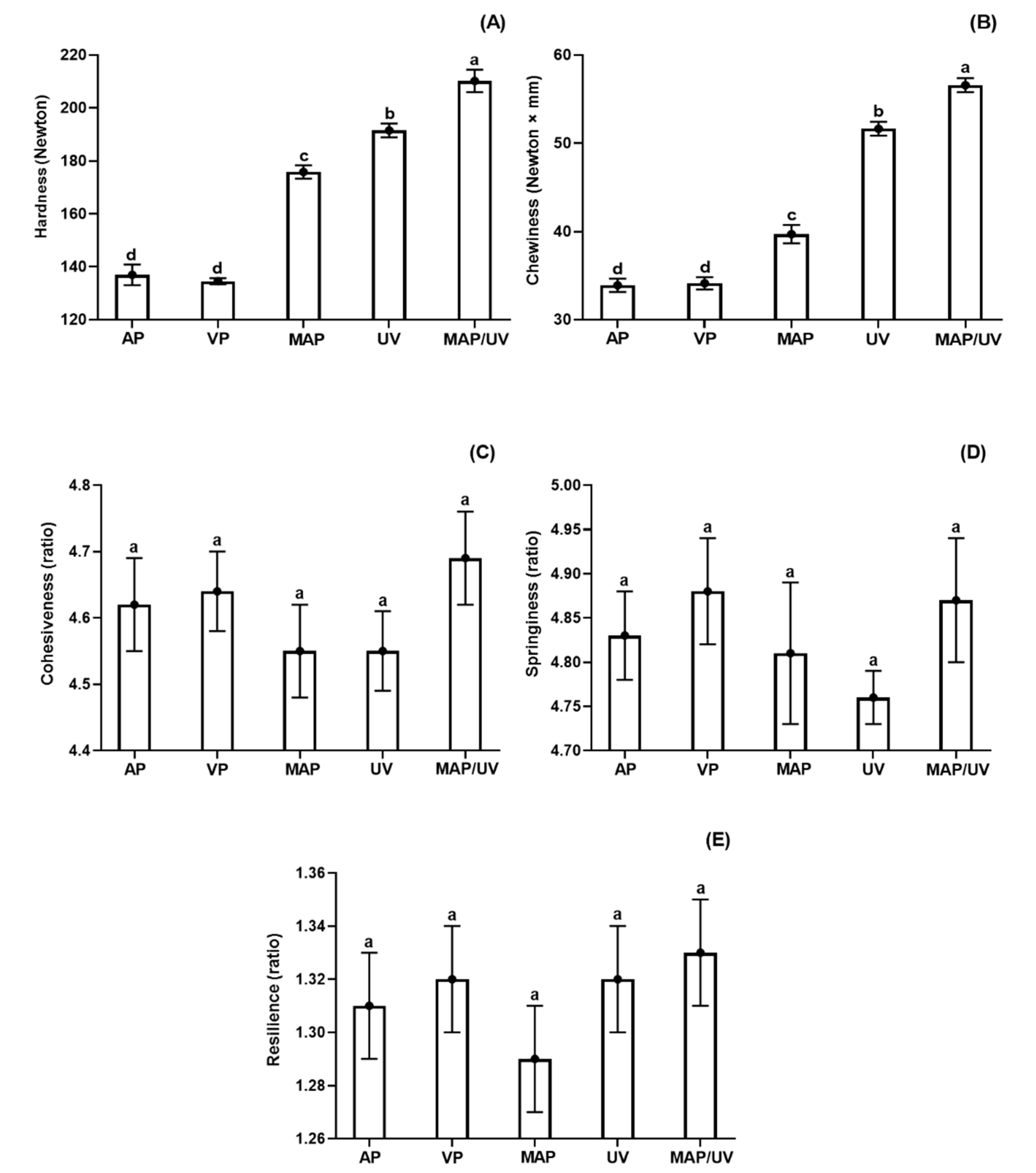
3.2.7. Instrumental Texture Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Rodrigues, B.L.; Alvares, T.S.; Sampaio, G.S.L.; Cabral, C.C.; Araujo, J.V.A.; Franco, R.M.; Mano, S.B.; Conte Junior, C.A. Influence of vacuum and modified atmosphere packaging in combination with UV-C radiation on the shelf life of rainbow trout (Oncorhynchus mykiss) fillets. Food Control 2016, 60, 596–605. [Google Scholar] [CrossRef]
- Monteiro, M.L.G.; Mársico, E.T.; Canto, A.C.V.C.S.; Costa-Lima, B.R.C.; Costa, M.P.; Viana, F.M.; Silva, T.J.P.; Conte-Junior, C.A. Impact of UV-C light on the fatty acid profile and oxidative stability of Nile tilapia (Oreochromis niloticus) fillets. J. Food Sci. 2017, 82, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Nowsad, A.A.K.M.; Hossain, M.M.; Hassan, M.N.; Sayem, S.M.; Polanco, J.F. Assessment of post harvest loss of wet fish: A novel approach based on sensory indicator assessment. SAARC J. Agric. 2015, 13, 75–89. [Google Scholar] [CrossRef]
- Budiati, T.; Rusul, G.; Wan-Abdullah, W.N.; Chuah, L.; Ahmad, R.; Thong, K.L. Genetic relatedness of Salmonella serovars isolated from catfish (Clarias gariepinus) and tilapia (Tilapia mossambica) obtained from wet markets and ponds in Penang, Malaysia. J. Food Prot. 2016, 79, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Deering, A.J.; Kim, H. The occurrence of shiga toxin-producing E. coli in aquaponic and hydroponic systems. Horticulturae 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Dewey-Mattia, D.; Kisselburgh, H.; Manikonda, K.; Silver, R.; Subramhanya, S.; Sundararaman, P.; Whitham, H.; Crowe, S. Annual Report 2017 of the Center for Disease Control and Prevention (CDC). 2017. Available online: https://www.cdc.gov/fdoss/pdf/2017_FoodBorneOutbreaks_508.pdf (accessed on 10 April 2020).
- Canto, A.C.V.C.S.; Monteiro, M.L.G.; Costa-Lima, B.R.C.; Lázaro, C.A.; Marsico, E.T.; Silva, T.J.P.; Conte-Junior, C.A. Effect of UV-C radiation on Salmonella spp. reduction and oxidative stability of caiman (Caiman crocodilus yacare) meat. J. Food Saf. 2019, 39, e12604. [Google Scholar] [CrossRef]
- Byelashov, O.A.; Sofos, J.N. Strategies for on-line decontamination of carcasses. In Safety of Meat and Processed Meat, 1st ed.; Toldrá, F., Ed.; Springer: New York, NY, USA, 2009; pp. 149–182. [Google Scholar]
- Koutchma, T. Principles and applications of UV light technology. In Ultraviolet Light in Food Technology: Principles and Applications, 2nd ed.; Koutchma, T., Ed.; CRC Press: New York, NY, USA, 2019; pp. 1–48. [Google Scholar]
- Skowron, K.; Bauza-Kaszewska, J.; DobrzaNski, Z.; Paluszak, Z.; Skowron, K.J. UV-C radiation as a factor reducing microbiological contamination of fish meal. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Silva, H.L.A.; Costa, M.P.; Frasao, B.S.; Mesquita, E.F.M.; Mello, S.C.R.P.; Conte-Junior, C.A.; Franco, R.M.; Miranda, Z.B. Efficacy of ultraviolet-C light to eliminate on precooked shredded bullfrog back meat. J. Food Saf. 2015, 35, 318–323. [Google Scholar] [CrossRef]
- Sommers, C.H.; Scullen, O.J.; Sheen, S. Inactivation of uropathogenic Escherichia coli in ground chicken meat using high pressure processing and gamma radiation, and in purge and chicken meat surfaces by ultraviolet light. Front. Microbiol. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Bottino, F.O.; Rodrigues, B.L.; Ribeiro, J.D.N.; Lazaro, C.A.; Conte-Junior, C.A. Influence of UV-C radiation on shelf life of vacuum package tambacu (Colossoma macropomum x Piaractus mesopotamicus) fillets. J. Food Process. Preserv. 2017, 41, e13003. [Google Scholar] [CrossRef]
- Monteiro, M.L.G.; Mársico, E.T.; Mano, S.B.; Alvares, T.S.; Rosenthal, A.; Lemos, M.; Ferrari, E.; Lázaro, C.A.; Conte-Junior, C.A. Combined effect of high hydrostatic pressure and ultraviolet radiation on quality parameters of refrigerated vacuum-packed tilapia (Oreochromis niloticus) fillets. Sci. Rep. 2018, 8, e9524. [Google Scholar] [CrossRef] [PubMed]
- Molina, B.; Sáez, M.I.; Martínez, T.F.; Guil-Guerrero, J.L.; Suárez, M.D. Effect of ultraviolet light treatment on microbial contamination, some textural and organoleptic parameters of cultured sea bass fillets (Dicentrarchus labrax). Innov. Food Sci. Emerg. Technol. 2014, 26, 205–213. [Google Scholar] [CrossRef]
- Prasad, P.; Kochhar, A. Active packaging in food industry. J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 1–7. [Google Scholar]
- Di Pierro, P.; Sorrentino, A.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Chitosan/whey protein film as active coating to extend ricotta cheese shelf-life. Lwt-Food Sci. Technol. 2011, 44, 2324–2327. [Google Scholar] [CrossRef]
- Giosafatto, C.V.L.; Fusco, A.; Al-Asmar, A.; Mariniello, L. Microbial transglutaminase as a tool to improve the features of hydrocolloid-based bioplastics. Int. J. Mol. Sci. 2020, 21, 3656. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.; Alvares, T.; Costa, M.; Sampaio, G.; Lázaro, C.A.; Panzenhagen, P.; Mársico, E.; Mano, S.; Conte-Junior, C. Combined effect of modified atmosphere package and short-wave ultraviolet does not affect Proteus mirabilis growth on rainbow trout fillets (Oncorhynchus mykiss). J. Food Nutr. Res. 2019, 7, 342–346. [Google Scholar] [CrossRef]
- Conte-Junior, C.A.; Fernández, M.; Mano, S. Use of carbon dioxide to control the microbial spoilage of bullfrog (Rana catesbeiana) meat. In Modern Multidisciplinary Applied Microbiology: Exploiting Microbes and Their Interactions, 1st ed.; Mendez-Vilas, A., Ed.; Wiley-VCH Verlag GmbH &and Co. KGaA: Weinheim, Germany, 2006; pp. 356–361. [Google Scholar]
- Soccol, M.C.H.; Oetterer, M. Use of modified atmosphere in seafood preservation. Braz. Arch. Biol. Technol. 2003, 46, 569–580. [Google Scholar] [CrossRef]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef]
- Monteiro, M.L.G.; Mársico, E.T.; Mano, S.B.; Teixeira, C.E.; Canto, A.C.V.C.S.; Vital, H.C.; Conte-Junior, C.A. Influence of good manufacturing practices on the shelf life of refrigerated fillets of tilapia (Oreochromis niloticus) packed in modified atmosphere and gamma-irradiated. Food Sci. Nutr. 2013, 1, 298–306. [Google Scholar] [CrossRef]
- Reddy, N.R.; Villanueva, M.; Kautter, D.A. Shelf-Life of modified atmosphere packaged fresh tilapia fillets stored under refrigeration and temperature abuse conditions. J. Food Prot. 1995, 58, 908–914. [Google Scholar] [CrossRef]
- Lázaro, C.A.; Conte-Júnior, C.A.; Monteiro, M.L.G.; Canto, A.C.V.S.; Costa-Lima, B.R.C.; Mano, S.B.; Franco, R.M. Effects of ultraviolet light on biogenic amines and other quality indicators of chicken meat during refrigerated storage. Poult. Sci. 2014, 93, 2304–2313. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.L.G.; Mársico, E.T.; Mutz, Y.S.; Castro, V.S.; Moreira, R.V.B.P.; Álvares, T.S.; Conte-Junior, C.A. Combined effect of oxygen scavenger packaging and UV-C radiation on shelf life of refrigerated tilapia (Oreochromis niloticus) fillets. Sci. Rep. 2020, 10, e4243. [Google Scholar] [CrossRef] [PubMed]
- Røssvoll, E.; Hauge, S.J.; Skjerve, E.; Johannessen, G.; Økland, M.; Røtterud, O.; Nesbakken, T.; Alvseike, O. Experimental evaluation of performance of sampling techniques for microbiological quantification on carcass surfaces. Food Prot. Trends 2017, 37, 419–429. [Google Scholar]
- American Public Health Association (APHA). Compendium of Methods for the Microbiological Examination of Foods, 4th ed.; APHA: Washington, DC, USA, 2001. [Google Scholar]
- Lázaro, C.A.; Conte-Júnior, C.A.; Cunha, F.L.; Mársico, E.T.; Mano, S.B.; Franco, R.M. Validation of an HPLC methodology for the identification and quantification of biogenic amines in chicken meat. Food Anal. Methods 2013, 6, 1024–1032. [Google Scholar] [CrossRef]
- Mccullough, H. The determination of ammonia in whole blood by a direct colorimetric method. Clin. Chim. Acta 1967, 17, 297–304. [Google Scholar] [CrossRef]
- Tarladgis, B.; Watts, B.; Younathan, M.; Dugan, L. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J. Am. Oil Chem. Soc. 1960, 37, 44–48. [Google Scholar] [CrossRef]
- Monteiro, M.L.G.; Mársico, E.T.; Teixeira, C.E.; Mano, S.B.; Conte Júnior, C.A.; Vital, H.C. Validade comercial de filés de tilápia do Nilo (Oreochromis niloticus) resfriados embalados em atmosfera modificada e irradiados. Cienc. Rural 2012, 42, 737–743. [Google Scholar] [CrossRef]
- American Meat Science Association (AMSA). Meat Color Measurement Guidelines, 2nd ed.; AMSA: Champaign, IL, USA, 2012. [Google Scholar]
- Sun, T.; Hao, W.; Li, J.; Dong, Z.; Wu, C. Preservation properties of in situ modified CaCO3–chitosan composite coatings. Food Chem. 2015, 183, 217–226. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Conte-Junior, C.A.; Monteiro, M.L.G.; Patrícia, R.; Mársico, E.T.; Lopes, M.M.; Alvares, T.S.; Mano, S.B. The Effect of different packaging systems on the shelf life of refrigerated ground beef. Foods 2020, 9, 495. [Google Scholar] [CrossRef]
- Sivertsvik, M.; Jeksrud, W.K.; Rosnes, J.T. A review of modified atmosphere packaging of fish and fishery products—significance of microbial growth, activities and safety. Int. J. Food Sci. Technol. 2002, 37, 107–127. [Google Scholar] [CrossRef]
- McClelland, M.; Florea, L.; Sanderson, K.; Clifton, S.W.; Parkhill, J.; Churcher, C.; Dougan, G.; Wilson, R.K.; Miller, W. Comparison of the Escherichia coli K-12 genome with sampled genomes of a Klebsiella pneumoniae and three salmonella enterica serovars, Typhimurium, Typhi and Paratyphi. Nucleic Acids Res. 2000, 28, 4974–4986. [Google Scholar] [CrossRef] [PubMed]
- Knöppel, A.; Knopp, M.; Albrecht, L.M.; Lundin, E.; Lustig, U.; Näsvall, J.; Andersson, D.I. Genetic adaptation to growth under laboratory conditions in Escherichia coli and Salmonella enterica. Front. Microbiol. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Djordjević, J.; Bošković, M.; Starčević, M.; Ivanović, J.; Karabasil, N.; Dimitrijević, M.; Lazić, I.B.; Baltić, M.Ž. Survival of Salmonella spp. in minced meat packaged under vacuum and modified atmosphere. Braz. J. Microbiol. 2018, 49, 607–613. [Google Scholar] [CrossRef]
- Stahlke, E.R.; Rossa, L.S.; Silva, G.M.; Sotomaior, C.S.; Pereira, A.J.; Luciano, F.B.; Borges, T.D.; Macedo, R.E.F. Effects of modified atmosphere packaging (MAP) and slaughter age on the shelf life of lamb meat. Food Sci. Technol. 2019, 39, 328–335. [Google Scholar] [CrossRef]
- International Commission on Microbiological Specifications for Foods (ICMSF). Microorganisms in Foods, 2nd ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2001. [Google Scholar]
- Ahmed, I.; Lin, H.; Zou, L.; Brody, A.L.; Li, Z.; .Qazi, I.M.; Pavase, T.R.; Lv, L. A comprehensive review on the application of active packaging technologies to muscle foods. Food Control 2017, 82, 163–178. [Google Scholar] [CrossRef]
- Izumi, H.; Inoue, A. Viability of sublethally injured coliform bacteria on fresh-cut cabbage stored in high CO2 atmospheres following rinsing with electrolyzed water. Int. J. Food Microbiol. 2018, 266, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Pakingking, R.; Palma, P.; Usero, R. Quantitative and qualitative analyses of the bacterial microbiota of tilapia (Oreochromis niloticus) cultured in earthen ponds in the Philippines. World J. Microbiol. Biotechnol. 2015, 31, 265–275. [Google Scholar] [CrossRef]
- Jagger, J. Physiological effects of near-ultraviolet radiation on bacteria. In Photochemical and Photobiological Reviews, 1st ed.; Smith, K.C., Ed.; Springer: Boston, MA, USA, 1983; pp. 1–75. [Google Scholar]
- López-Caballero, M.E.; Gonçalves, A.; Nunes, M.L. Effect of CO2/O2-containing modified atmospheres on packed deepwater pink shrimp (Parapenaeus longirostris). Eur. Food Res. Technol. 2002, 214, 192–197. [Google Scholar] [CrossRef]
- Noseda, B.; Vermeulen, A.; Ragaert, P.; Devlieghere, K. Packaging of fish and fishery products. In Seafood Processing: Technology, Quality and Safety, 1st ed.; Boziaris, I.S., Ed.; John Wiley & Sons: Chichester, UK, 2014; pp. 237–255. [Google Scholar]
- Shalaby, A.R. Significance of biogenic amines to food safety and human health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- Lázaro, C.A.; Conte-Júnior, C.A.; Canto, A.C.; Monteiro, M.L.G.; Costa-Lima, B.; Cruz, A.G.; Mársico, E.T.; Franco, R.M. Biogenic amines as bacterial quality indicators in different poultry meat species. LWT-Food Sci. Technol. 2015, 60, 15–21. [Google Scholar]
- Masniyom, P. Deterioration and shelf-life extension of fish and fishery products by modified atmosphere packaging. Songklanakarin J. Sci. Technol. 2011, 33, 181–192. [Google Scholar]
- Fan, S.W.; Srivastava, A.K.; Dravid, V.P. UV-activated room-temperature gas sensing mechanism of poly crystalline ZnO. Appl. Phys. Lett. 2009, 95, 106–142. [Google Scholar] [CrossRef]
- Nashalian, O.; Yaylayan, V.A. Thermally induced oxidative decarboxylation of copper complexes of amino acids and formation of strecker aldehyde. J. Agric. Food Chem. 2014, 62, 8518–8523. [Google Scholar] [CrossRef] [PubMed]
- Enes Dapkevicius, M.L.N.; Robert Nout, M.S.; Pombouts, F.M.; Houben, J.H.; Wymenga, W. Biogenic amine formation and degradation by potential fish silage starter microorganisms. Int. J. Food Microbiol. 2000, 57, 107–114. [Google Scholar] [CrossRef]
- Klein, G.; Pack, A.; Bonaparte, C.; Reuter, G. Taxonomy and physiology of probiotic lactic acid bacteria. Int. J. Food Microbiol. 1998, 41, 103–125. [Google Scholar] [CrossRef]
- Vieira, C.P.; Da Costa, M.P.; Da Silva Frasão, B.; De Melo Silva, V.L.; De Barros Pinto Moreira, R.V.; De Oliveira Nunes, Y.E.C.; Conte-Junior, C.A. Nondestructive prediction of the overall quality of cow milk yogurt by correlating a biogenic amine index with traditional quality parameters using validated nonlinear models. J. Food Compos. Anal. 2019, 84, 103328. [Google Scholar] [CrossRef]
- Yew, C.C.; Bakar, F.A.; Rahman, R.A.; Bakar, J.; Zaman, M.Z.; Velu, S.; Shariat, M. Effects of modified atmosphere packaging with various carbon dioxide composition on biogenic amines formation in Indian mackerel (Rastrelliger kanagurta) stored at 5 ± 1 °C. Packag. Technol. Sci. 2014, 27, 249–254. [Google Scholar] [CrossRef]
- Santos, J.S.L.; Mársico, E.T.; Lemos, M.; Cinquini, M.A.; Silva, F.A.; Dutra, Y.B.; Franco, R.M.; Conte Junior, C.A.; Monteiro, M.L.G. Effect of the UV-C radiation on shelf life of vacuum-packed refrigerated pirarucu (Arapaima gigas) fillets. J. Aquat. Food Prod. Technol. 2018, 27, 48–60. [Google Scholar] [CrossRef]
- European Comission. Commission Regulation (EC) n° 2073/2005 of 15 November 2005. Microbiological Criteria for Foodstuffs. Available online: https://www.fsai.ie/uploadedFiles/Consol_Reg2073_2005.pdf (accessed on 15 April 2020).
- Food and Drug Administration (FDA). Scombro Toxin (Histamine) Formation. 2001. Available online: https://www.fda.gov/downloads/food/guidanceregulation/ucm252400.pdf (accessed on 15 April 2020).
- Ministério da Agricultura, Pecuária e Abastecimento. Portaria n° 185 de 13 de maio de 1997. Regulamento Técnico de Identidade e Qualidade de Peixe Fresco (Inteiro e Eviscerado); Diário Oficial da União: Brasília, Brazil, 1997. Available online: http://www.cidasc.sc.gov.br/inspecao/files/2012/08/portaria-185-1997.pdf (accessed on 15 April 2020).
- Rawdkuen, S.; Jongjareonrak, A.; Benjakul, S.; Chaijan, M. Discoloration and lipid deterioration of farmed giant catfish (Pangasianodon gigas). J. Food Sci. 2008, 73, 179–184. [Google Scholar] [CrossRef]
- Rico, C.W.; Kim, G.; Jo, C.; Nam, K.; Kang, H.; Ahn, D.; Kwon, J. Microbiological and physicochemical quality of irradiated ground beef as affected by added garlic or onion. Korean J. Food Sci. Anim. Resour. 2009, 29, 680–684. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Combined Effect of ethanolic coconut husk extract and modified atmospheric packaging (MAP) in extending the shelf life of Asian sea bass slices. J. Aquat. Food Prod. Technol. 2019, 28, 689–702. [Google Scholar] [CrossRef]
- Pivarnik, L.F.; Faustman, C.; Rossi, S.; Suman, S.P.; Palmer, C.; Richard, N.L.; Christopher Ellis, P.; DiLiberti, M. Quality assessment of filtered smoked yellowfin tuna (Thunnus albacores) steaks. J. Food Sci. 2011, 76, 369–379. [Google Scholar] [CrossRef]
- Fernandez, J.; Perez-Alvarez, I.A.; Fernandez-Lopez, J.A. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997, 59, 345–353. [Google Scholar] [CrossRef]
- Abreu, D.P.; Losada, P.P.; Maroto, J.; Cruz, J.M. Lipid damage during frozen storage of Atlantic halibut (Hippoglossus hippoglossus) in active packaging film containing antioxidants. Food Chem. 2011, 126, 315–320. [Google Scholar] [CrossRef]
- Wongwichian, C.; Klomklao, S.; Panpipat, W.; Benjakul, S.; Chaijan, M. Interrelationship between myoglobin and lipid oxidations in oxeye scad (Selar boops) muscle during iced storage. Food Chem. 2015, 174, 279–285. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). National Nutrient Database. Available online: https://ndb.nal.usda.gov/ndb/ (accessed on 10 April 2020).
- Hernández, E.J.G.P.; Carvalho, R.N., Jr.; Joele, M.R.S.P.; Araújo, C.S.; Lourenço, L.F.H. Effects of modified atmosphere packing over the shelf life of sous vide from captive pirarucu (Arapaima gigas). Innov. Food Sci. Emerg. Technol. 2017, 39, 94–100. [Google Scholar] [CrossRef]
- Suman, S.P.; Joseph, P. Myoglobin chemistry and meat color. Annu. Rev. Food Sci. Technol. 2013, 4, 79–99. [Google Scholar] [CrossRef]
- Wu, W.; Gao, X.G.; Dai, Y.; Fu, Y.; Li, X.M.; Dai, R.T. Post-mortem changes in sarcoplasmic proteome and its relationship to meat color traits in M. semitendinosus of Chinese Luxiyellow cattle. Food Res. Int. 2015, 72, 98–105. [Google Scholar] [CrossRef]
- Lee, E.S.; Park, S.Y.; Ha, S.D. Effect of UV-C light on the microbial and sensory quality of seasoned dried seafood. Food Sci. Technol. Int. 2016, 22, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Jaczynski, J.; Park, J.W. Physicochemical changes in Alaska pollock surimi and surimi gel as affected by electron beam. J. Food Sci. 2004, 69, 53–57. [Google Scholar] [CrossRef]
- Merlo, T.C.; Contreras-Castillo, C.J.; Saldaña, E.; Barancelli, G.V.; Dargelio, M.D.B.; Yoshida, C.M.P.; Ribeiro Junior, E.E.; Massarioli, A.; Venturini, A.C. Incorporation of pink pepper residue extract into chitosan film combined with a modified atmosphere packaging: Effects on the shelf life of salmon fillets. Food Res. Int. 2019, 125, 108633. [Google Scholar] [CrossRef] [PubMed]
- Kovács, E.; Keresztes, Á. Effect of gamma and UV-B/C radiation on plant cells. Micron 2002, 33, 199–210. [Google Scholar] [CrossRef]
- Francis, F.J. Colorimetry of foods. In Physical Properties of Foods, 1st ed.; Peleg, M., Bagley, E.B., Eds.; AVI Publishing: Wesport, CT, USA, 1983; pp. 105–124. [Google Scholar]
- Rodrigues, B.L.; Costa, M.P.; Frasão, B.S.; Silva, F.A.; Mársico, E.T.; Alvares, T.S.; Conte-Junior, C.A. Instrumental texture parameters as freshness indicators in five farmed Brazilian freshwater fish species. Food Anal. Methods 2017, 10, 3589–3599. [Google Scholar] [CrossRef]
- Monteiro, M.L.G.; Mársico, E.T.; Rosenthal, A.; Conte Júnior, C.A. Synergistic effect of ultraviolet radiation and high hydrostatic pressure on texture, color, and oxidative stability of refrigerated tilapia fillets. J. Sci. Food Agric. 2019, 99, 4474–4481. [Google Scholar] [CrossRef]
- Xie, J.; Tang, Y.; Yang, S.; Qian, Y. Effects of whey protein films on the quality of thawed bigeye tuna (Thunnus obesus) chunks under modified atmosphere packaging and vacuum packaging conditions. Food Sci. Biotechnol. 2017, 26, 937–945. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Treatments ¥ | Count € | |
|---|---|---|
| Salmonella typhimurium | Escherichia coli O157:H7 | |
| AP | 5.98 ± 0.24 a | 6.00 ± 0.21 a |
| VP | 5.83 ± 0.21 a,b | 5.89 ± 0.29 a |
| MAP | 5.49 ± 0.20 b | 5.93 ± 0.27 a |
| UV | 4.86 ± 0.43 c | 5.29 ± 0.34 b |
| MAP/UV | 5.48 ± 0.24 b | 5.84 ± 0.39 a |
| Microorganisms £ | Parameters € | Treatments ¥ | ||||
|---|---|---|---|---|---|---|
| AP | VP | MAP | UV | MAP/UV | ||
| TAMC | Lag | 2.89 ± 0.12 c | 3.11 ± 0.18 b,c | 3.31 ± 0.04 b | ND | 5.59 ± 0.20 a |
| GT | 0.52 ± 0.02 c | 0.46 ± 0.02 c | 0.63 ± 0.01 b | 0.72 ± 0.02 a,b | 0.78 ± 0.09 a | |
| NC | ND | ND | ND | ND | ND | |
| TAPC | Lag | ND | ND | 3.87 ± 0.01 | ND | ND |
| GT | 0.44 ± 0.03 d | 0.50 ± 0.01 c | 0.40 ± 0.04 d | 0.56 ± 0.01 b | 0.96 ± 0.09 a | |
| NC | 8.30 ± 0.27 | ND | ND | ND | ND | |
| Enterobacteriaceae | Lag | ND | ND | 2.91 ± 0.04 | ND | ND |
| GT | 0.42 ± 0.01 c | 0.42 ± 0.03 c | 0.26 ± 0.01 d | 0.47 ± 0.02 b | 1.01 ± 0.00 a | |
| NC | ND | ND | 7.88 ± 0.06 | ND | ND | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lázaro, C.A.; Monteiro, M.L.G.; Conte-Junior, C.A. Combined Effect of Modified Atmosphere Packaging and UV-C Radiation on Pathogens Reduction, Biogenic Amines, and Shelf Life of Refrigerated Tilapia (Oreochromis niloticus) Fillets. Molecules 2020, 25, 3222. https://doi.org/10.3390/molecules25143222
Lázaro CA, Monteiro MLG, Conte-Junior CA. Combined Effect of Modified Atmosphere Packaging and UV-C Radiation on Pathogens Reduction, Biogenic Amines, and Shelf Life of Refrigerated Tilapia (Oreochromis niloticus) Fillets. Molecules. 2020; 25(14):3222. https://doi.org/10.3390/molecules25143222
Chicago/Turabian StyleLázaro, César A., Maria Lúcia G. Monteiro, and Carlos A. Conte-Junior. 2020. "Combined Effect of Modified Atmosphere Packaging and UV-C Radiation on Pathogens Reduction, Biogenic Amines, and Shelf Life of Refrigerated Tilapia (Oreochromis niloticus) Fillets" Molecules 25, no. 14: 3222. https://doi.org/10.3390/molecules25143222
APA StyleLázaro, C. A., Monteiro, M. L. G., & Conte-Junior, C. A. (2020). Combined Effect of Modified Atmosphere Packaging and UV-C Radiation on Pathogens Reduction, Biogenic Amines, and Shelf Life of Refrigerated Tilapia (Oreochromis niloticus) Fillets. Molecules, 25(14), 3222. https://doi.org/10.3390/molecules25143222








