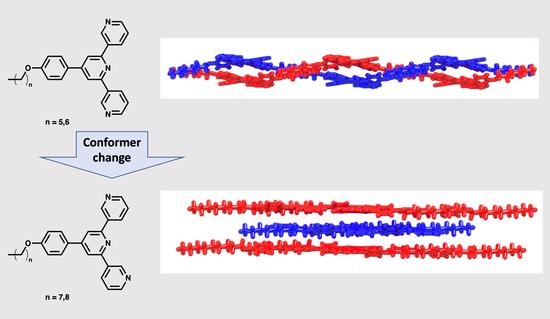
Switching the Conformation of 3,2′:6′,3″-tpy Domains in 4′-(4-n-Alkyloxyphenyl)-3,2′:6′,3″-Terpyridines
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Compounds 8 and 9
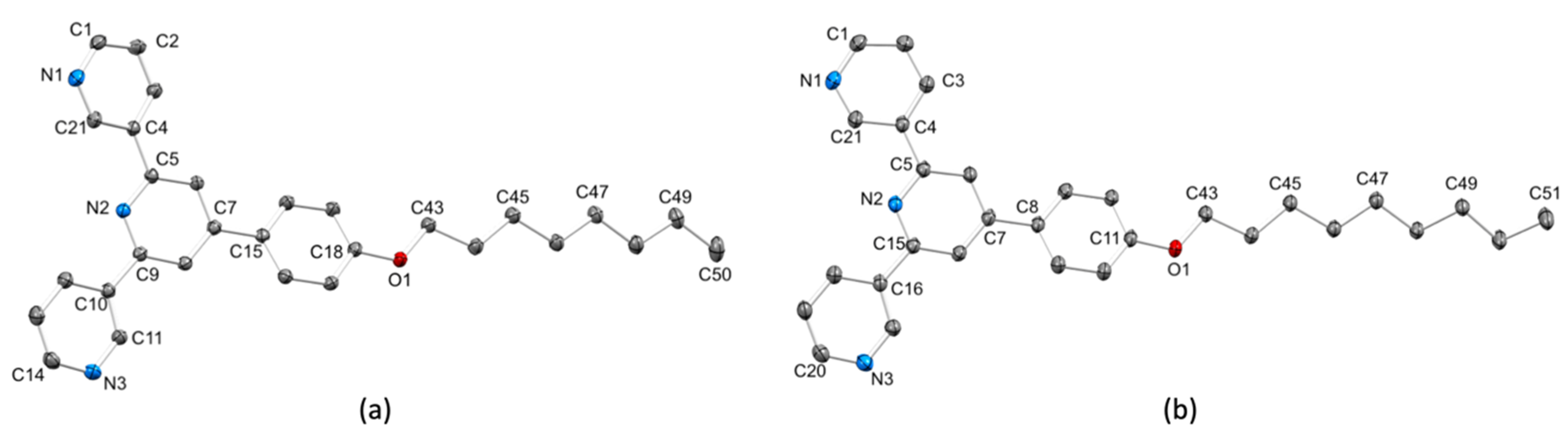
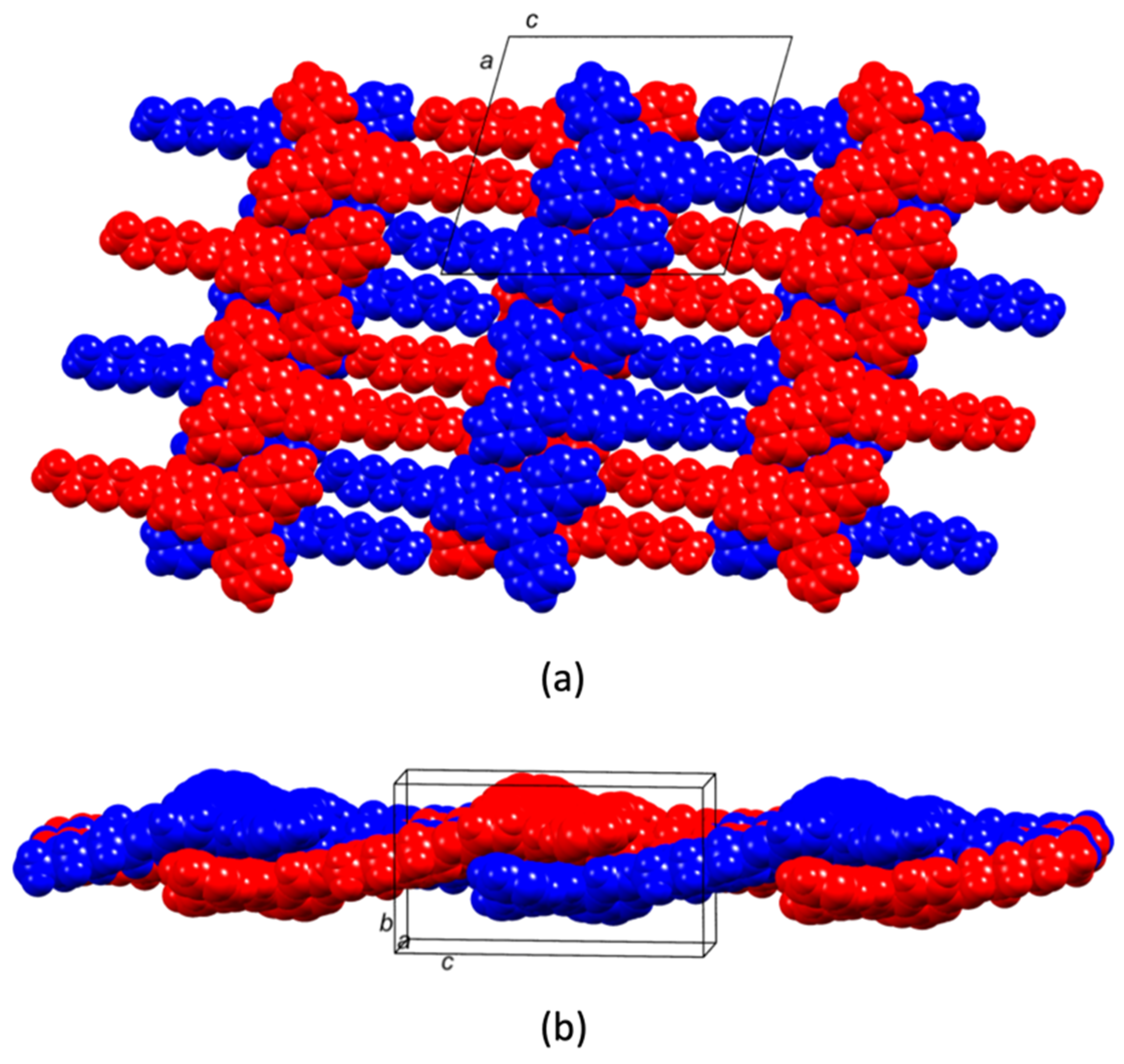
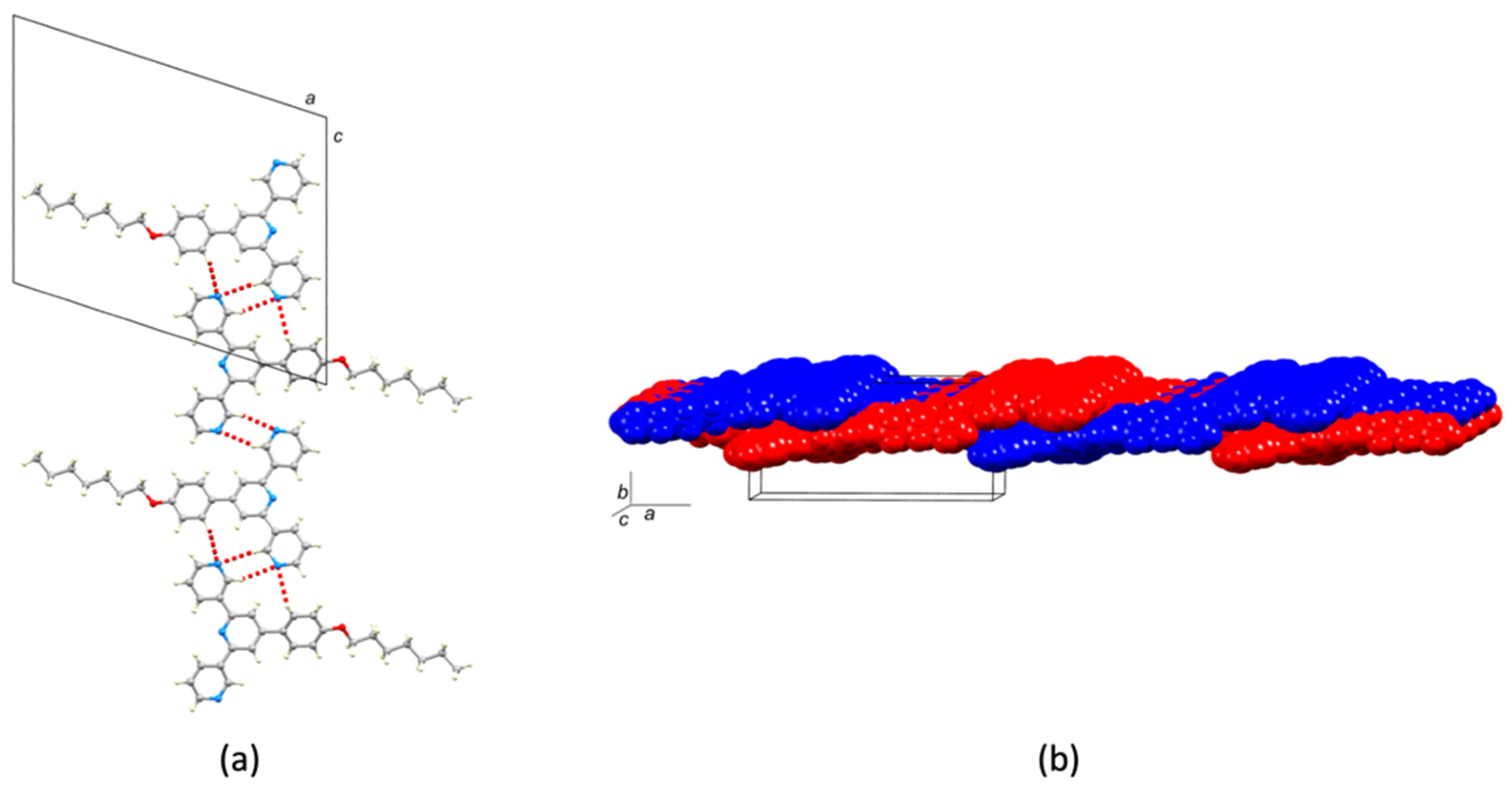
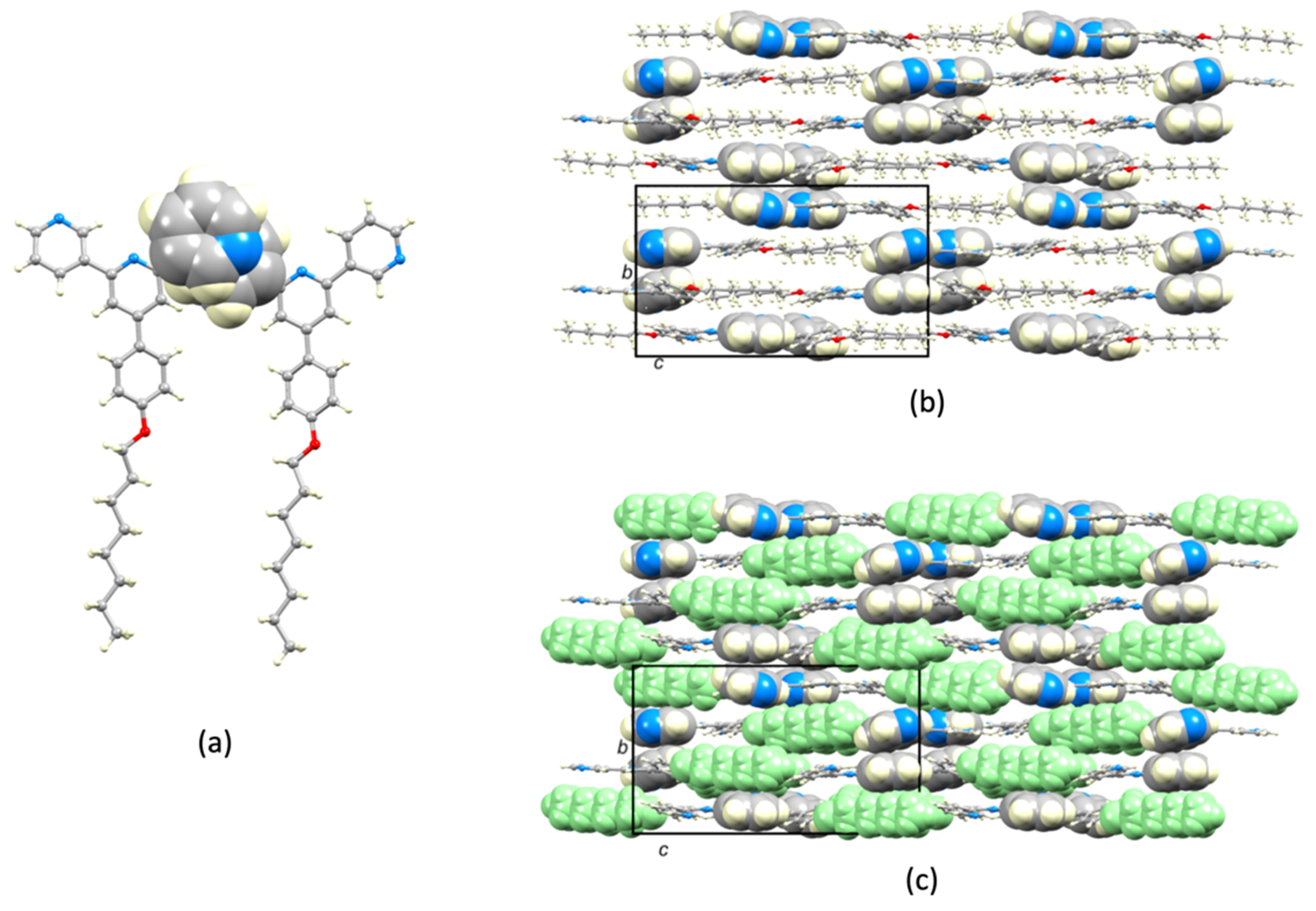
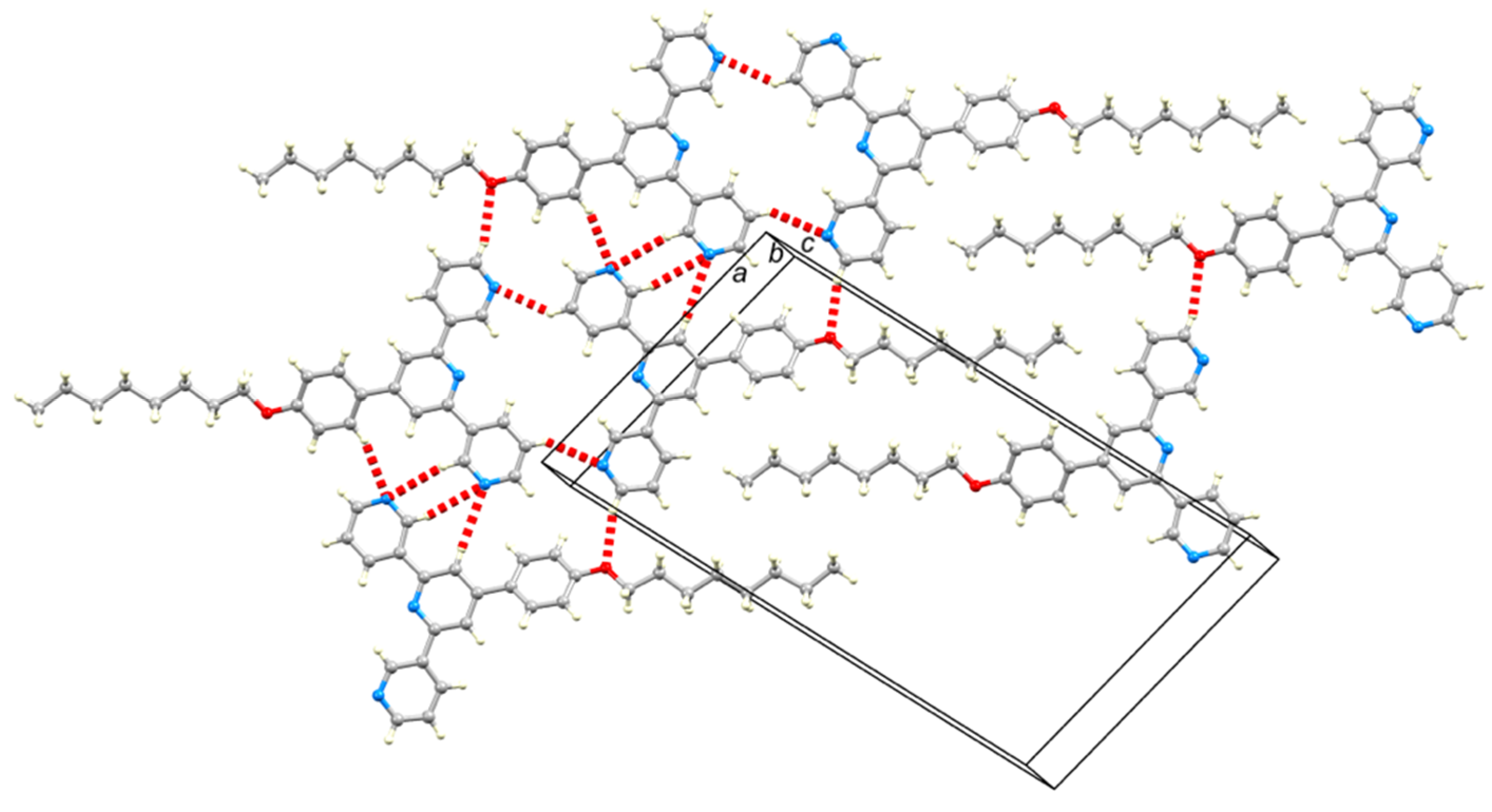
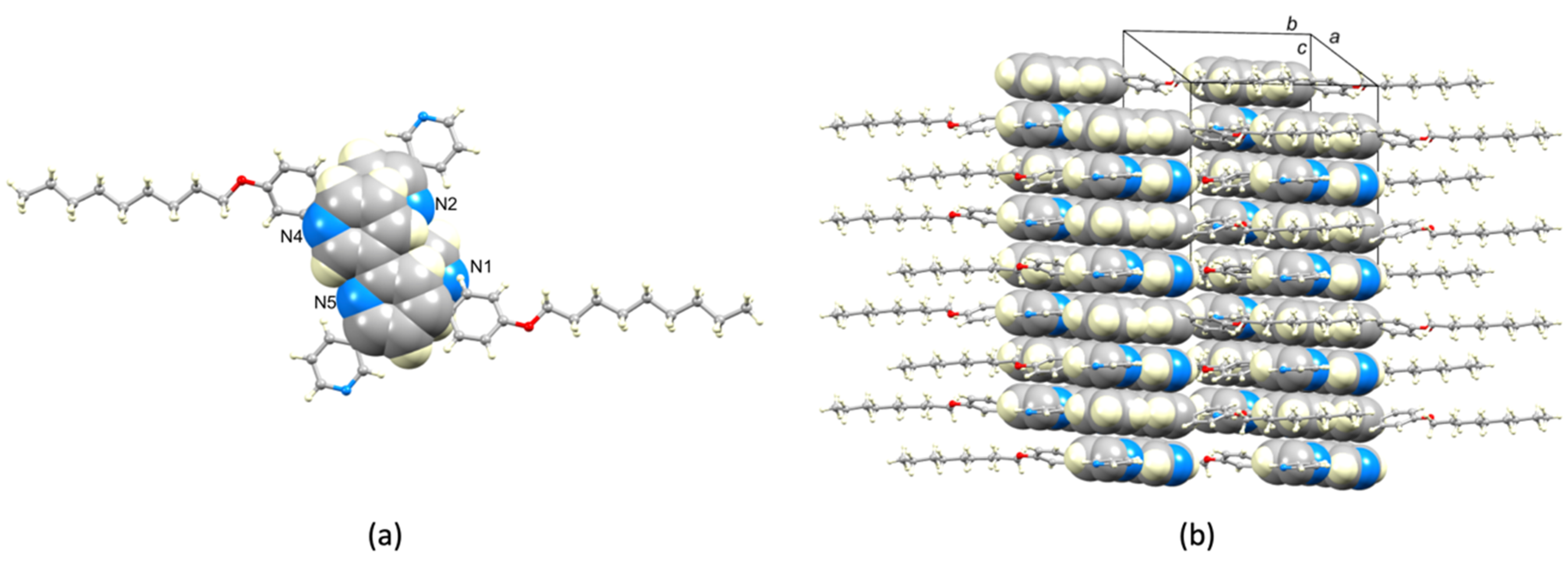
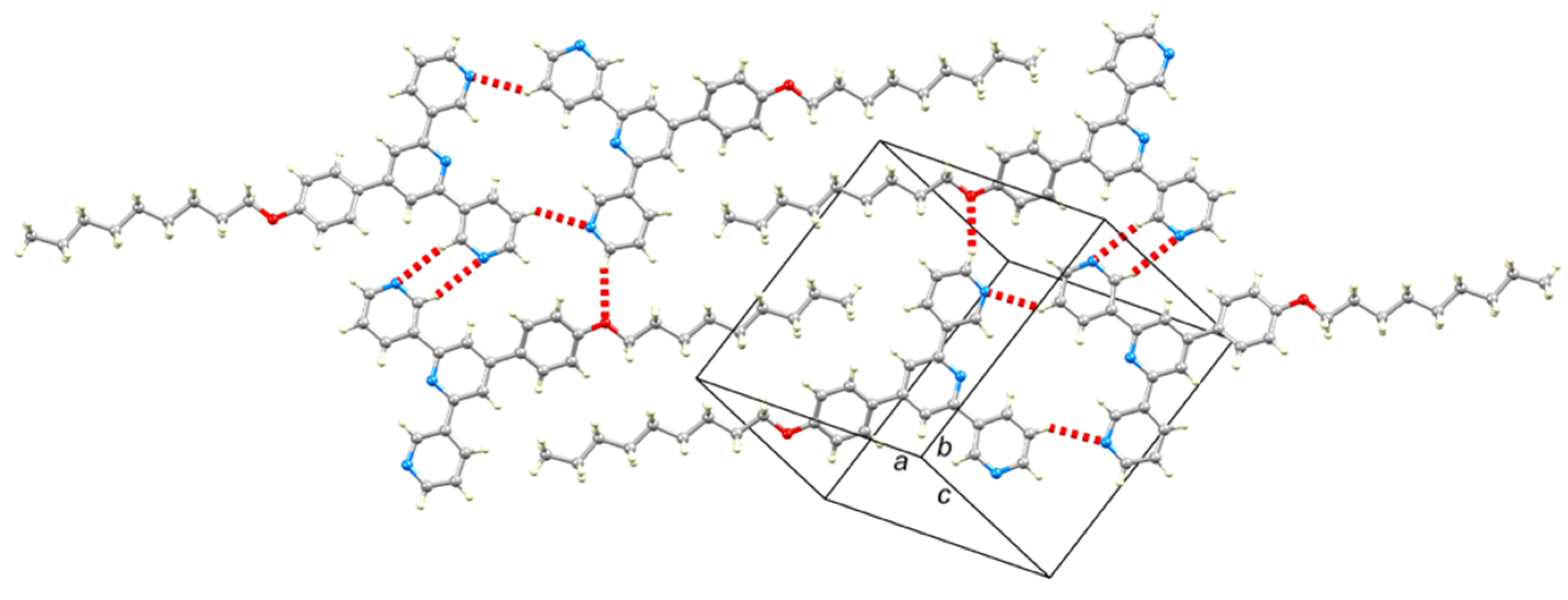
2.2. Crystal Structures
3. Materials and Methods
3.1. General
3.2. Compound 8
3.3. Compound 9
3.4. Crystallography
3.5. Compound 6
3.6. Compound 7
3.7. Compound 8
3.8. Compound 9
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Constable, E.C. 2,2′:6′,2″-Terpyridines: From chemical obscurity to common supramolecular motifs. Chem. Soc. Rev. 2007, 36, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.; Winter, A.; Schlütter, F.; Schubert, U.S. Advances in the field of π-conjugated 2,2′:6′,2″-terpyridines. Chem. Soc. Rev. 2011, 40, 1459–1511. [Google Scholar] [CrossRef]
- Chakraborty, S.; Newkome, G.R. Terpyridine-based metallosupramolecular constructs: Tailored monomers to precise 2D-motifs and 3D-metallocages. Chem. Soc. Rev. 2018, 47, 3991–4016. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; He, Y.; Shi, X.; Song, Z. Terpyridine-metal complexes: Applications in catalysis and supramolecular chemistry. Coord. Chem. Rev. 2019, 385, 1–19. [Google Scholar] [CrossRef]
- Agosti, A.; Kuna, E.; Bergamini, G. Divergent Terpyridine-based Coordination for the Construction of Photoactive Supermolecular Structures. Eur. J. Inorg. Chem. 2019, 2019, 577–584. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. Ditopic and tetratopic 4,2′:6′,4″-Terpyridines as Structural Motifs in 2D- and 3D-Coordination Assemblies. Chimia 2019, 73, 462–467. [Google Scholar] [CrossRef]
- Housecroft, C.E. 4,2′:6′,4″-Terpyridines: Diverging and Diverse Building Blocks in Coordination Polymers and Metallomacrocycles. Dalton Trans. 2014, 43, 6594–6604. [Google Scholar] [CrossRef]
- Housecroft, C.E. Divergent 4,2′:6′,4″- and 3,2′:6′,3″-Terpyridines as Linkers in 2- and 3-Dimensional Architectures. Cryst. Eng. Comm. 2015, 17, 7461–7468. [Google Scholar] [CrossRef]
- Zhao, M.; Tan, J.; Su, J.; Zhang, J.; Zhang, S.; Wu, J.; Tian, Y. Syntheses, crystal structures and third-order nonlinear optical properties of two series of Zn(II) complexes using the thiophene-based terpyridine ligands. Dyes Pigm. 2016, 130, 216–225. [Google Scholar] [CrossRef]
- Klein, Y.M.; Lanzilotto, A.; Prescimone, A.; Kramer, K.W.; Decurtins, S.; Liu, S.-X.; Constable, E.C.; Housecroft, C.E. Coordination behavior of 1-(3,2′:6′,3″-terpyridin-4′-yl) ferrocene: Structure and magnetic and electrochemical properties of a tetracopper dimetallomacrocycle. Polyhedron 2017, 129, 71–76. [Google Scholar] [CrossRef]
- Rocco, D.; Manfroni, G.; Prescimone, A.; Klein, Y.M.; Gawryluk, D.J.; Constable, E.C.; Housecroft, C.E. Single and double-stranded 1d-coordination polymers with 4′-(4-alkyloxyphenyl)- 3,2′:6′,3″-terpyridines and {Cu2(μ-OAc)4} or {Cu4(μ3-OH)2(μ-OAc)2(μ3-OAc)2(AcO-κO)2} motifs. Polymers 2020, 12, 318. [Google Scholar] [CrossRef] [PubMed]
- Rocco, D.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Directing 2d-coordination networks: Combined effects of a conformationally flexible 3,2′:6′,3″-terpyridine and chain length variation in 4′-(4-n-alkyloxyphenyl) substituents. Molecules 2020, 25, 1663. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualising crystal structures. Acta Cryst. B 2002, 58, 389–397. [Google Scholar] [CrossRef]
- Watanabe, Y.; Yokoyama, D.; Koganezawa, T.; Katagiri, H.; Ito, T.; Ohisa, S.; Chiba, T.; Sasabe, H.; Kido, J. Control of Molecular Orientation in Organic Semiconductor Films using Weak Hydrogen Bonds. Adv. Mater. 2019, 31, 1808300. [Google Scholar] [CrossRef]
- Xu, B.; Luo, F.; Tang, G.; Zhang, J. A 4′-(4-carboxyphenyl)-3,2′:6′,3″-terpyridine-based luminescent cadmium(II) coordination polymer for the detection of 2,4,6-trinitrophenol. Acta Cryst. 2019, 75, 508–513. [Google Scholar] [CrossRef]
- Smith, C.B.; Makha, M.; Raston, C.L.; Sobolev, A.N. Solution and solid state interplay of isomeric 4′-(pyridyl)-3,2′:6′,3″-terpyridines with p-sulfonatocalix[4]arene. New, J. Chem. 2007, 31, 535–542. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, B.; Luo, F.; Tang, G.; Zhang, C. Two 4′-(4-carboxyphenyl)-3,2′:6′,3″-terpyridine-based luminescent Zn(II) coordination polymers for detection of 2,4,6-trinitrophenol. Polyhedron 2019, 169, 51–59. [Google Scholar] [CrossRef]
- Klein, Y.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Coordination Behaviour of 1-(4,2′:6′,4″-terpyridin-4′-yl)ferrocene and 1-(3,2′:6′,3″-terpyridin-4′-yl)ferrocene: Predictable and Unpredictable Assembly Algorithms. Aust. J. Chem. 2017, 70, 468–477. [Google Scholar] [CrossRef]
- Tian, X.; Zhu, Y.; Zhang, M.; Tan, J.; Zhang, Q.; Wang, X.; Yang, J.; Zhou, H.; Wu, J.; Tian, Y. Mild acidic-enhanced mitochondrial-targeting by a neutral thiophene based terpyridine molecule with large two-photon action cross-section. Dyes Pigm. 2017, 139, 431–439. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H. Photophysical investigations and the bioimagings of α-, β-, γ-pyridine-based terpyridine derivatives. J. Mol. Struct. 2018, 1157, 457–462. [Google Scholar] [CrossRef]
- Guo, X.; Bian, M.; Lv, F.; Wang, Y.; Zhao, Z.; Bian, Z.; Qu, B.; Xiao, L.; Chen, Z. Increasing electron transporting properties and horizontal molecular orientation via meta-position of nitrogen for ″(A)n–d–(A)n″ structured terpyridine electron-transporting material. J. Mater. Chem. C 2019, 7, 11581–11587. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Y.; Li, D.; Liu, J.; Han, H.; He, D.; Tian, X.; Li, S.; Wu, J.; Tian, Y. Aggregation-induced emission (AIE)-active molecules bearing singlet oxygen generation activities: The tunable singlet–triplet energy gap matters. Chem. Commun. 2019, 55, 1450–1453. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Wang, H.; Zhu, Y.; Tian, X.; Zhang, M.; Zhang, Q.; De Souza, S.C.; Wang, A.; Zhou, H.; Zhang, Z.; et al. Highly Hydrophilic, Two-photon Fluorescent Terpyridine Derivatives Containing Quaternary Ammonium for Specific Recognizing Ribosome RNA in Living Cells. ACS Appl. Mater. Interfaces 2017, 9, 31424–31432. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.Z.; Yang, C.; Liu, E.; Golen, J.A.; Zhang, G. One-dimensional copper(II) coordination polymers built on 4′-substituted 4,2′:6′,4″- and 3,2′:6′,3″-terpyridines: Syntheses, structures and catalytic properties. Polyhedron 2016, 105, 115–122. [Google Scholar] [CrossRef]
- Wang, J.; Hanan, G.S. A facile route to sterically hindered and non-hindered 4′-aryl-2,2′:6′,2″-terpyridines. Synlett 2005, 2005, 1251–1254. [Google Scholar] [CrossRef]
- Desiraju, G.; Steiner, T. The Weak Hydrogen Bond; Oxford University Press: Oxford, England, 2001. [Google Scholar]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Rowland, R.S.; Taylor, R. Intermolecular Nonbonded Contact Distances in Organic Crystal Structures: Comparison with Distances Expected from van der Waals Radii. J. Phys. Chem. C 1996, 100, 7384–7391. [Google Scholar] [CrossRef]
- Liu, Y.; O’Keeffe, M.; Treacy, M.M.J.; Yaghi, O.M. The Geometry of Periodic Knots, Polycatenanes and Weaving from A Chemical Perspective: A Library for Reticular Chemistry. Chem. Soc. Rev. 2018, 47, 4642–4664. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Nishio, M. CH/π hydrogen bonds in crystals. Cryst. Eng. Comm. 2004, 6, 130–158. [Google Scholar] [CrossRef]
- Software for the Integration of CCD Detector System Bruker Analytical X-ray Systems; Bruker Axs: Madison, WI, USA, 2013.
- Sheldrick, G.M. ShelXT-Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with ShelXL. Acta Cryst. 2015, C27, 3–8. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. Superflip—A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Palatinus, L.; Prathapa, S.J.; van Smaalen, S. EDMA: A Computer Program for Topological Analysis of Discrete Electron Densities. J. Appl. Cryst. 2012, 45, 575–580. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
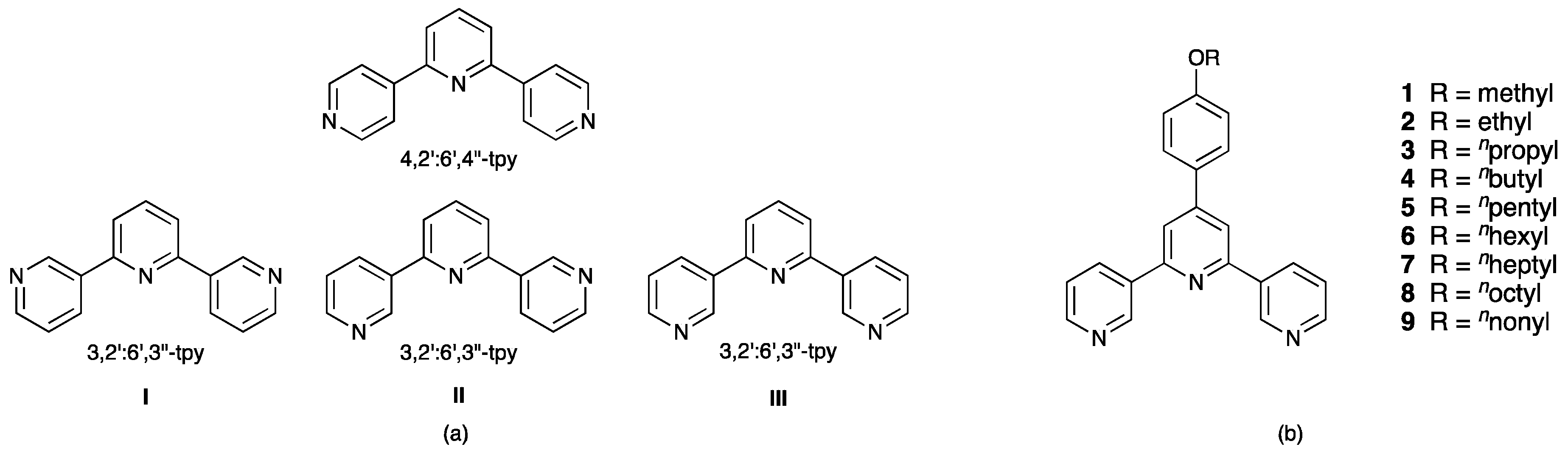



| CSD Refcode | Crystallographically Independent 3,2′:6′,3″-tpy Units a | Conformation(s) | Reference |
|---|---|---|---|
| TOQXUV | 1 | I | [20] |
| TOQYEG | 1 | III | [20] |
| BEPPUK | 1 | I | [21] |
| COJBEL, COJBEL01 | 4 | I × 2; II × 2 | [22] |
| FOBWOL | 2 | I × 2 | [23] |
| NEFVEC | 1 | III | [19] |
| SEPSAK | 1 | I | [24] |
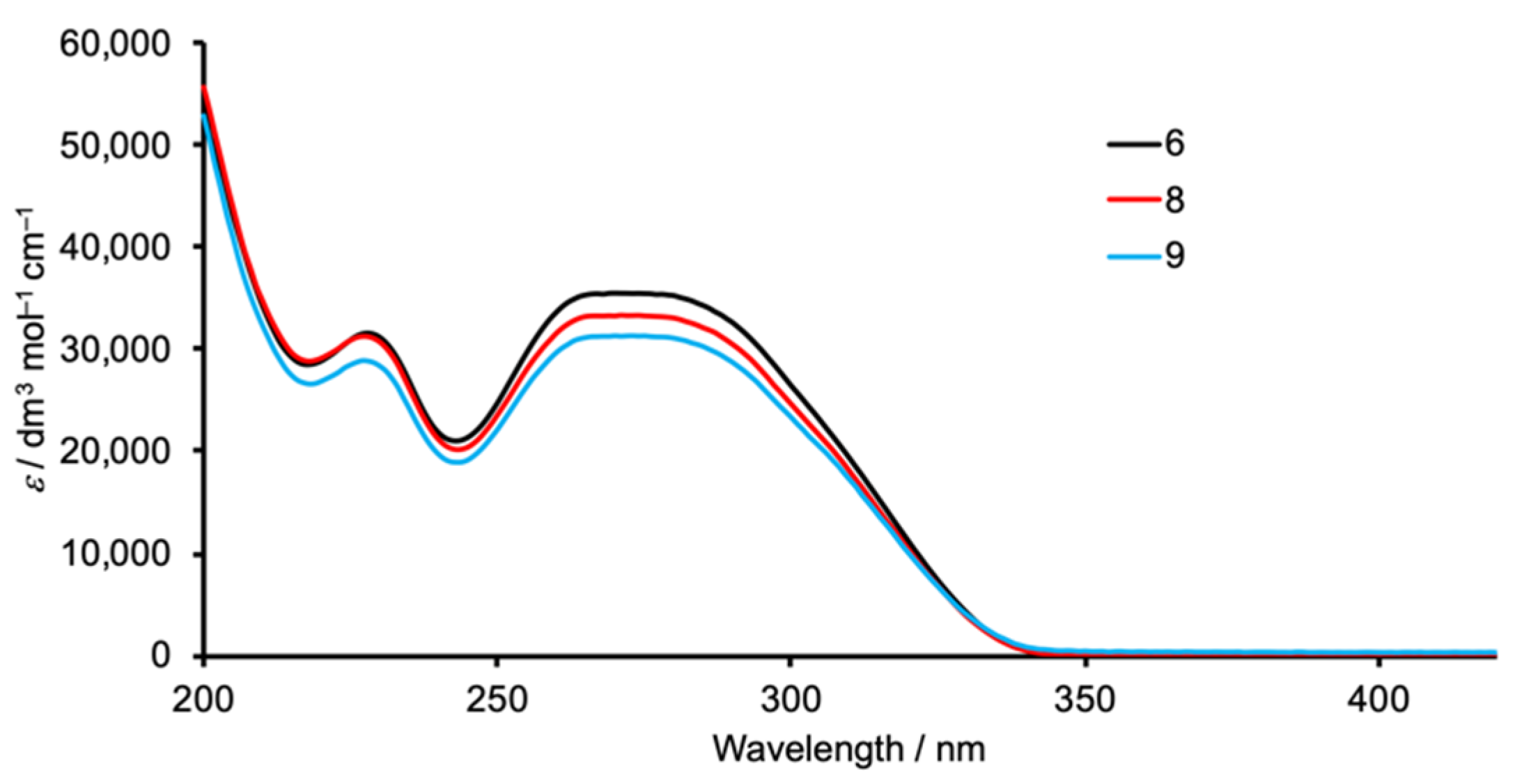
| Compound | λmax/nm (ε/dm3·mol−1·cm−1) |
|---|---|
| 6 | 228 (31,550), 270 (35,480) |
| 8 | 227 (31,290), 271 (33,350) |
| 9 | 227 (28,910), 273 (31,350) |
| Compound | Conformation a | Angle between the Least Squares Planes of Pairs of Aromatic Rings/Deg | ||
|---|---|---|---|---|
| Pyridine outer/central | Pyridine outer/central | Pyridine central/phenylene | ||
| 6 molecule 1 | I | 13.0 | 14.3 | 19.0 |
| 6 molecule 2 | I | 16.0 | 14.2 | 24.4 |
| 7 molecule 1 | I | 18.2 | 9.0 | 15.5 |
| 7 molecule 2 | I | 7.8 | 14.6 | 23.1 |
| 8 molecule 1 | II | 16.8 | 5.7 | 20.6 |
| 8 molecule 2 | II | 19.3 | 4.1 | 27.8 |
| 9 molecule 1 | II | 5.9 | 7.2 | 20.2 |
| 9 molecule 2 | II | 3.1 | 7.1 | 27.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocco, D.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Switching the Conformation of 3,2′:6′,3″-tpy Domains in 4′-(4-n-Alkyloxyphenyl)-3,2′:6′,3″-Terpyridines. Molecules 2020, 25, 3162. https://doi.org/10.3390/molecules25143162
Rocco D, Prescimone A, Constable EC, Housecroft CE. Switching the Conformation of 3,2′:6′,3″-tpy Domains in 4′-(4-n-Alkyloxyphenyl)-3,2′:6′,3″-Terpyridines. Molecules. 2020; 25(14):3162. https://doi.org/10.3390/molecules25143162
Chicago/Turabian StyleRocco, Dalila, Alessandro Prescimone, Edwin C. Constable, and Catherine E. Housecroft. 2020. "Switching the Conformation of 3,2′:6′,3″-tpy Domains in 4′-(4-n-Alkyloxyphenyl)-3,2′:6′,3″-Terpyridines" Molecules 25, no. 14: 3162. https://doi.org/10.3390/molecules25143162
APA StyleRocco, D., Prescimone, A., Constable, E. C., & Housecroft, C. E. (2020). Switching the Conformation of 3,2′:6′,3″-tpy Domains in 4′-(4-n-Alkyloxyphenyl)-3,2′:6′,3″-Terpyridines. Molecules, 25(14), 3162. https://doi.org/10.3390/molecules25143162