New Dihydroxytyrosyl Esters from Dicarboxylic Acids: Synthesis and Evaluation of the Antioxidant Activity In Vitro (ABTS) and in Cell-Cultures (DCF Assay)
Abstract
1. Introduction
2. Results and Discussion
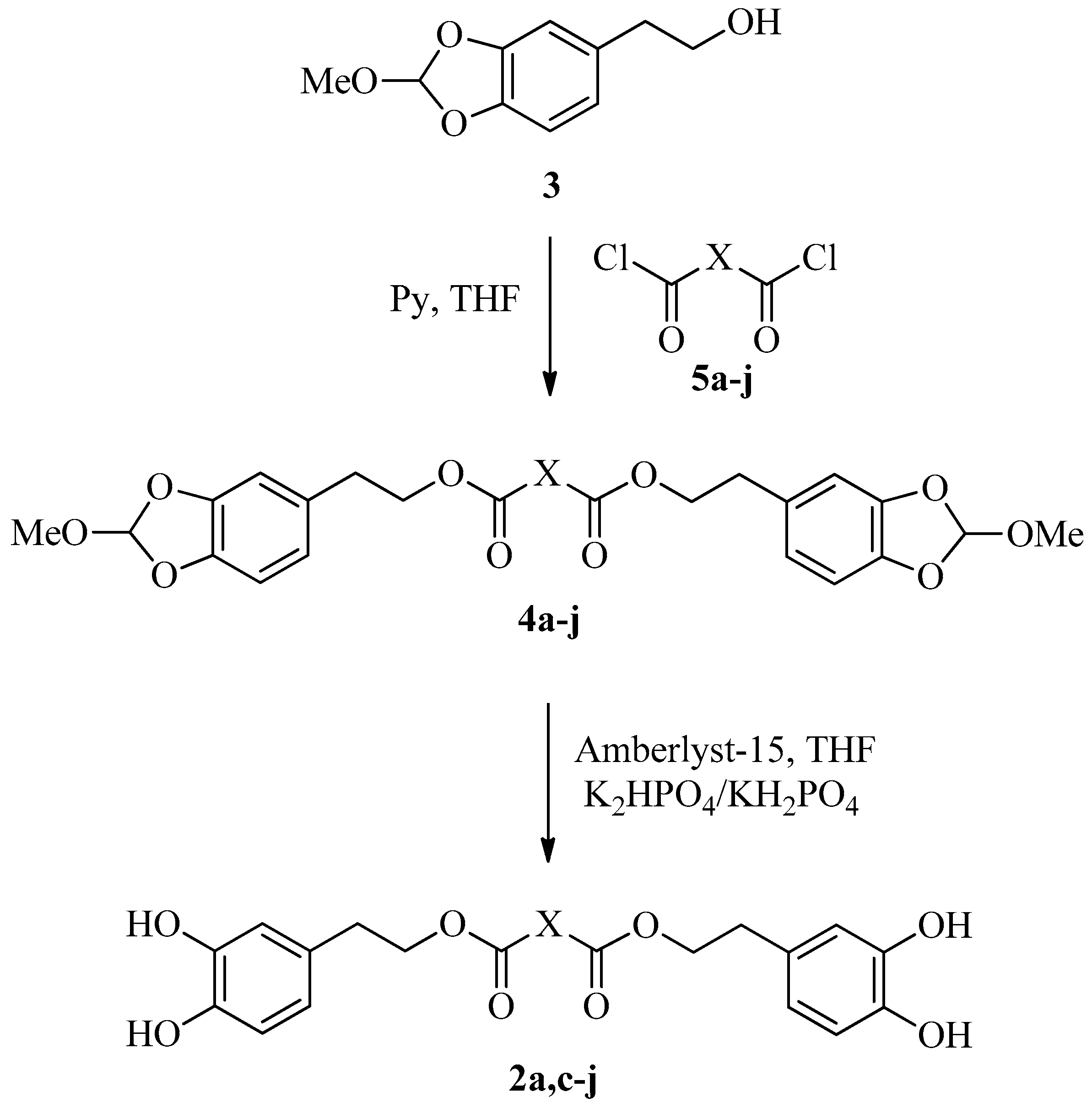
2.1. Synthesis of Dihydroxytyrosyl Esters
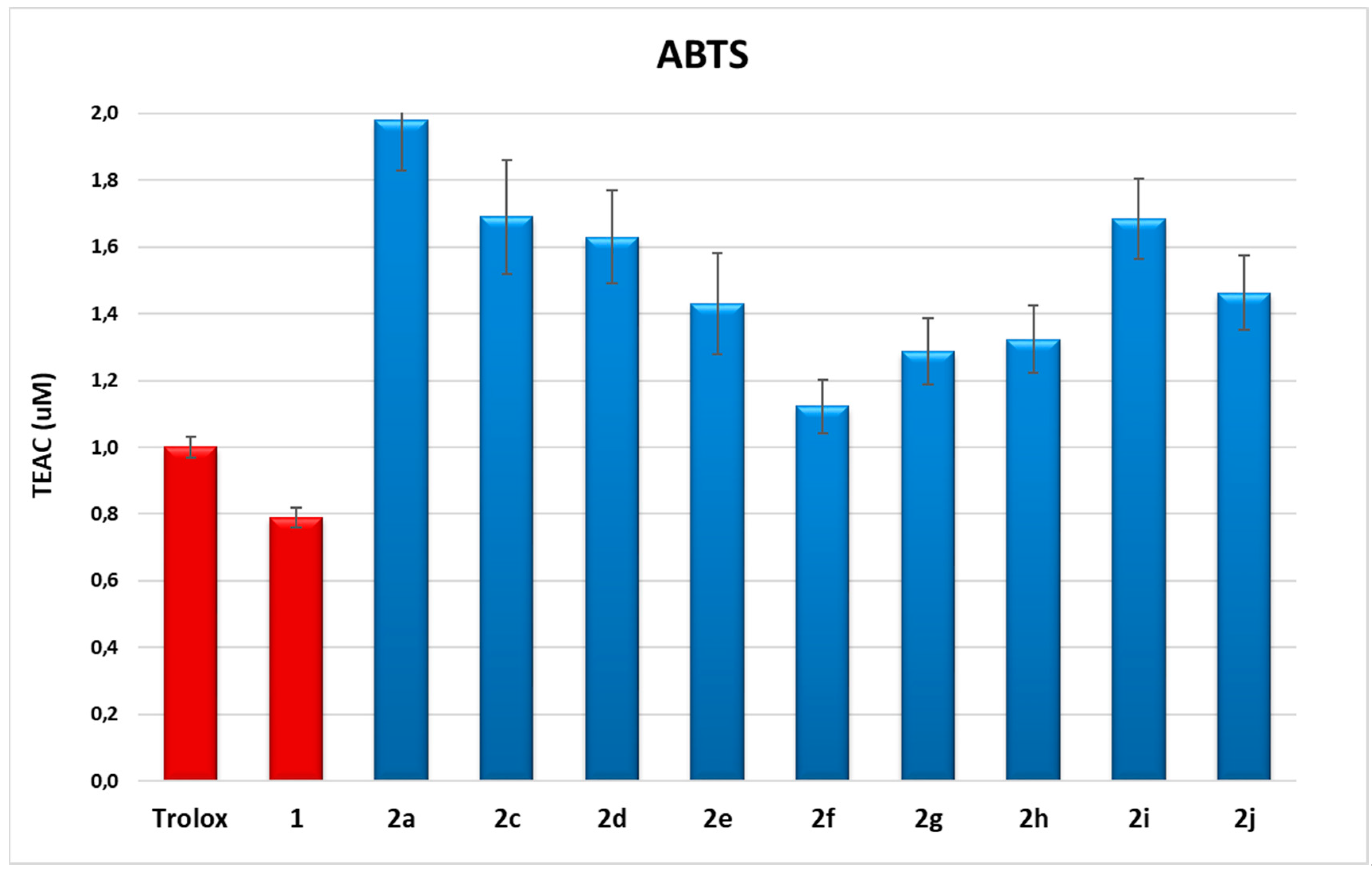
2.2. ABTS Spectrophotometric Assay
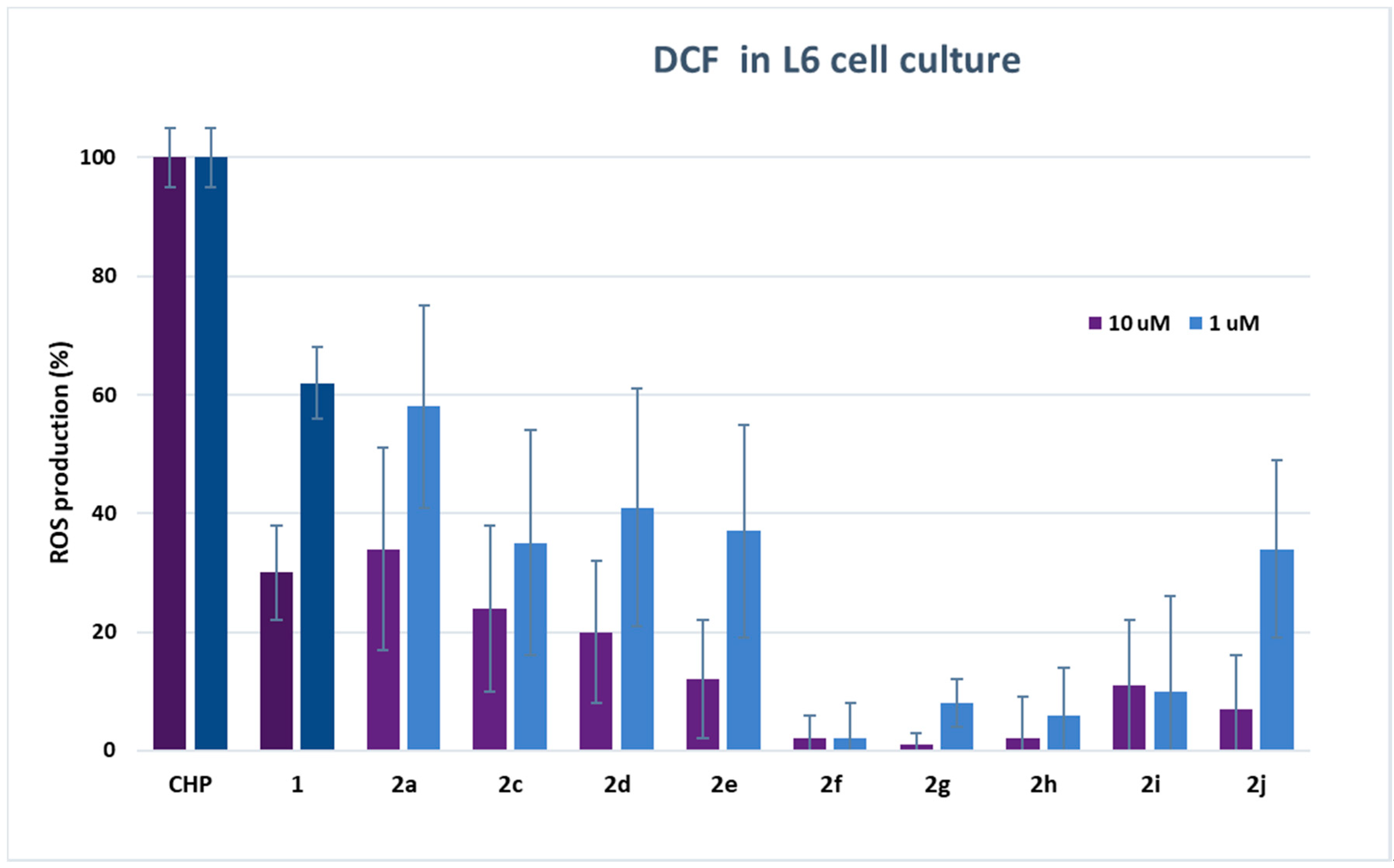
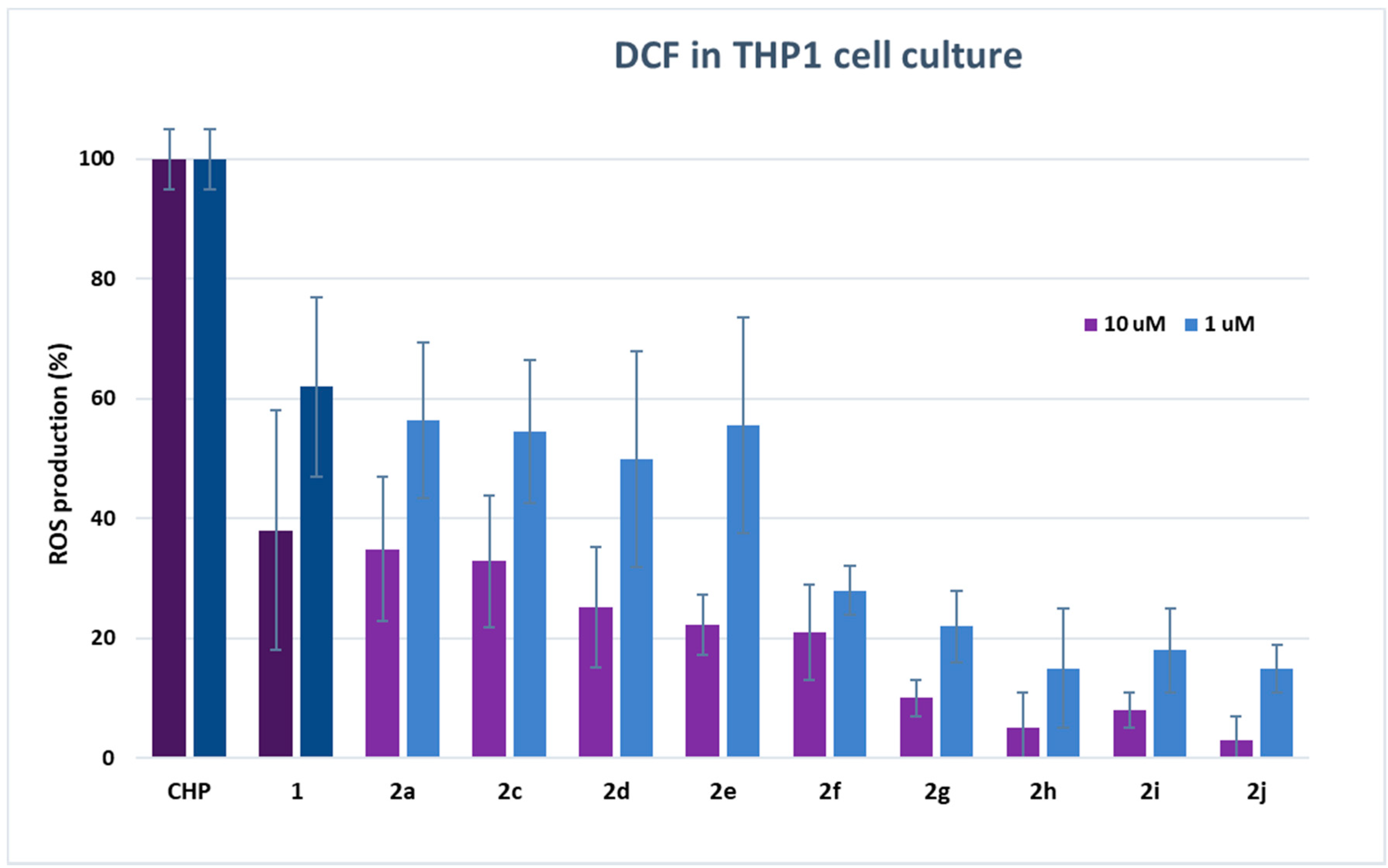
2.3. ROS Determination in Cell Culture
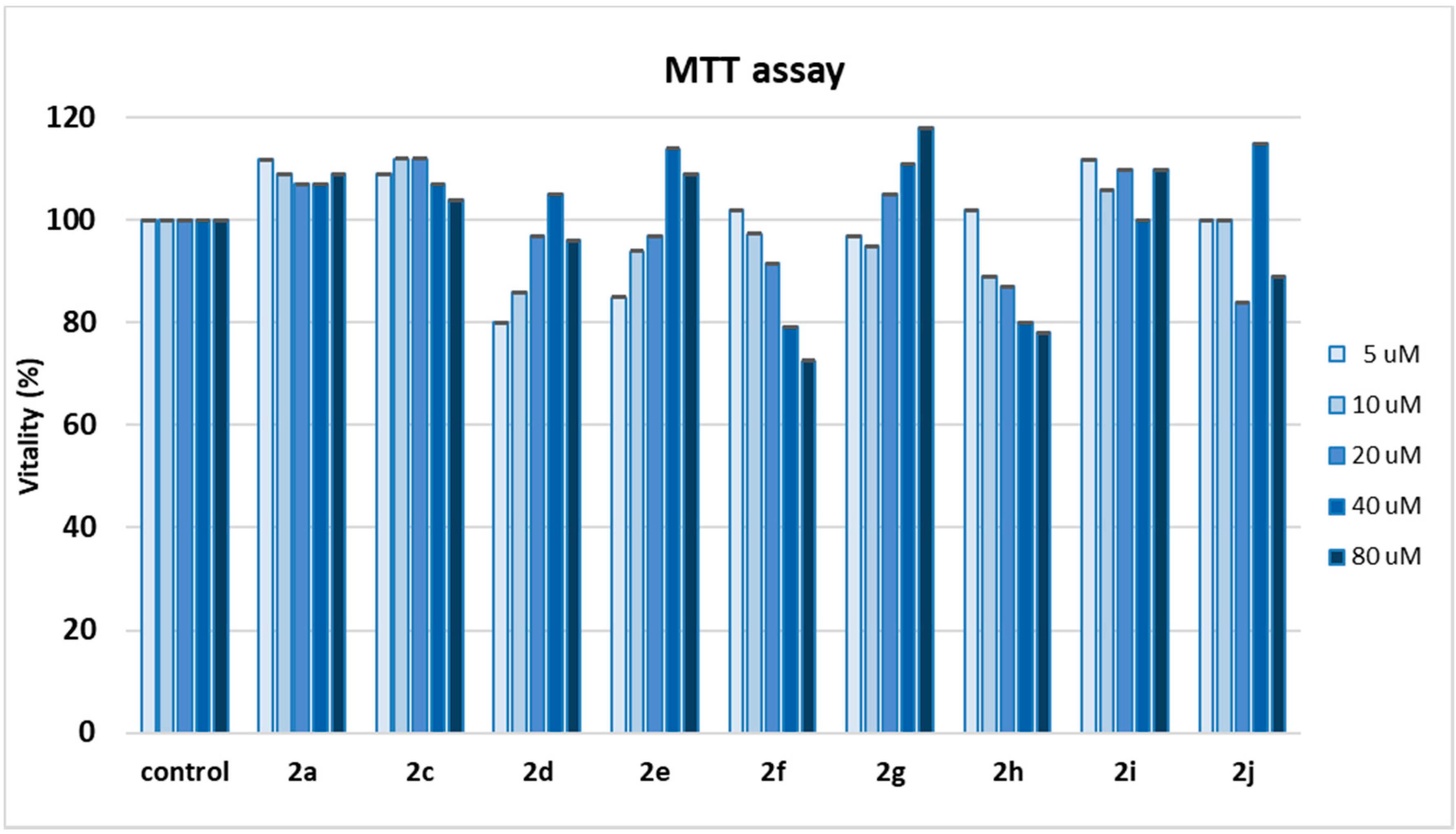
2.4. MTT Assay and Cell Proliferation
3. Materials and Methods
3.1. Reagents
3.2. Instruments
3.3. Synthesis of Protected Dihydroxytyrosyl Esters 4a–4j
3.4. Deprotection of 4a–4j to Products 2a–2j
3.5. Partition Coefficient Values
3.6. ABTS Assay
3.7. DCF Assay
3.8. MTT Assay
3.9. Proliferation Curves
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033–2058. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, 4728–4750. [Google Scholar] [CrossRef]
- Hu, T.; He, X.W.; Jiang, J.G.; Xu, X.L. Hydroxytyrosol and Its Potential Therapeutic Effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Farré, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef]
- González-Correa, J.A.; Muñoz-Marín, J.; Arrebola, M.M.; Guerrero, A.; Narbona, F.; López-Villodres, J.A.; De La Cruz, J.P. Dietary virgin olive oil reduces oxidative stress and cellular damage in rat brain slices subjected to hypoxia-reoxygenation. Lipids 2007, 42, 921–929. [Google Scholar] [CrossRef]
- Soni, M.; Prakash, C.; Dabur, R.; Kumar, V. Protective Effect of Hydroxytyrosol Against Oxidative Stress Mediated by Arsenic-Induced Neurotoxicity in Rats. Appl. Biochem. Biotechnol. 2018, 186, 27–39. [Google Scholar] [CrossRef]
- Medina, E.; de Castro, A.; Romero, C.; Brenes, M. Comparison of the Concentrations of Phenolic Compounds in Olive Oils and Other Plant Oils: Correlation with Antimicrobial Activity. J. Agric. Food Chem. 2006, 54, 4954–4961. [Google Scholar] [CrossRef]
- D’Andrea, G.; Ceccarelli, M.; Bernini, R.; Clemente, M.; Santi, L.; Caruso, C.; Micheli, L.; Tirone, F. Hydroxytyrosol stimulates neurogenesis in aged dentate gyrus by enhancing stem and progenitor cell proliferation and neuron survival. FASEB J. 2020, 34, 4512–4526. [Google Scholar] [CrossRef]
- Vilaplana-Pérez, C.; Auñón, D.; García-Flores, L.A.; Gil-Izquierdo, A. Hydroxytyrosol and potential uses in cardiovascular diseases, cancer, and AIDS. Front. Nutr. 2014, 1, 1–18. [Google Scholar] [CrossRef]
- Burattini, S.; Salucci, S.; Baldassarri, V.; Accorsi, A.; Piatti, E.; Madrona, A.; Espartero, J.L.; Candiracci, M.; Zappia, G.; Falcieri, E. Anti-apoptotic activity of hydroxytyrosol and hydroxytyrosyl laurate. Food Chem. Toxicol. 2013, 55, 248–256. [Google Scholar] [CrossRef]
- Bernini, R.; Gilardini Montani, M.S.; Merendino, N.; Romani, A.; Velotti, F. Hydroxytyrosol-Derived Compounds: A Basis for the Creation of New Pharmacological Agents for Cancer Prevention and Therapy. J. Med. Chem. 2015, 58, 9089–9107. [Google Scholar] [CrossRef] [PubMed]
- Bedoya, L.M.; Beltrán, M.; Obregón-Calderón, P.; García-Pérez, J.; de la Torre, H.E.; González, N.; Pérez-Olmeda, M.E.; Auñón, D.; Capa, L.; Gómez-Acebo, E.; et al. Hydroxytyrosol a new class of microbicide displaying broad anti-HIV-1 activity. AIDS 2016, 30, 2767–2776. [Google Scholar] [CrossRef] [PubMed]
- Tofani, D.; Balducci, V.; Gasperi, T.; Incerpi, S.; Gambacorta, A. Fatty Acid Hydroxytyrosyl Esters: Structure/Antioxidant Activity Relationship by ABTS and in Cell-Culture DCF Assays. J. Agric. Food Chem. 2010, 58, 5292–5299. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Barontini, M.; Cis, V.; Carastro, I.; Tofani, D.; Chiodo, R.A.; Lupattelli, P.; Incerpi, S. Synthesis and Evaluation of the Antioxidant Activity of Lipophilic Phenethyl Trifluoroacetate Esters by In Vitro ABTS, DPPH and in Cell-Culture DCF Assays. Molecules 2018, 23, 208. [Google Scholar] [CrossRef]
- Laguerre, M.; López Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.-C.; Baréa, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Relationship between Hydrophobicity and Antioxidant Ability of “Phenolipids” in Emulsion: A Parabolic Effect of the Chain Length of Rosmarinate Esters. J. Agric. Food Chem. 2010, 58, 2869–2876. [Google Scholar] [CrossRef]
- Laguerre, M.; Bayrasy, C.; Panya, A.; Weiss, J.; McClements, D.J.; Lecomte, J.; Decker, E.A.; Villeneuve, P. What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Crit. Rev. Food Sci. Nutr. 2015, 55, 183–201. [Google Scholar] [CrossRef]
- Durand, E.; Jacob, R.F.; Sherratt, S.; Lecomte, J.; Baréa, B.; Villeneuve, P.; Mason, R.P. The nonlinear effect of alkyl chain length in the membrane interactions of phenolipids: Evidence by X-ray diffraction analysis. Eur. J. Lipid Sci. Technol. 2017, 119, 1600397. [Google Scholar] [CrossRef]
- Balducci, V.; Incerpi, S.; Stano, P.; Tofani, D. Antioxidant activity of hydroxytyrosyl esters studied in liposome models. BBA–Biomembranes 2018, 1860, 200–210. [Google Scholar] [CrossRef]
- Trujillo, M.; Gallardo, E.; Madrona, A.; Bravo, L.; Sarriá, B.; González-Correa, J.A.; Mateos, R.; Espartero, J.L. Synthesis and Antioxidant Activity of Nitrohydroxytyrosol and Its Acyl Derivatives. J. Agric. Food Chem. 2014, 62, 10297–10303. [Google Scholar] [CrossRef]
- Zang, H.; Li, L.; Shen, P.; Xu, Q.; Zhang, L.; Guo, X.; Geng, X.; Liu, X.; Xu, J.; Xia, G.; et al. Preparation Method and Application of 3,4-Dihydroxyphenethylcarboxylate. Patent CN 109400432, 1 March 2019. [Google Scholar]
- Xie, Y.-D.; Chen, Z.-Z.; Li, N.; Lu, W.-F.; Xu, Y.-H.; Lin, Y.-Y.; Shao, L.-H.; Wang, Q.-T.; Guo, L.-T.; Gao, Y.-Q.; et al. Hydroxytyrosol nicotinate, a new multifunctional hypolipidemic and lypoglicemic agent. Biomed. Pharm. 2018, 99, 715–724. [Google Scholar] [CrossRef]
- Bernini, R.; Crisante, F.; Merendino, N.; Molinari, R.; Soldatelli, M.C.; Velotti, F. Synthesis of a novel ester of hydroxytyrosol and a-lipoic acid exhibiting an antiproliferative effect on human colon cancer HT-29 cells. Eur. J. Med. Chem. 2011, 46, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Bouallagui, Z.; Bouaziz, M.; Lassoued, S.; Engasser, J.M.; Ghoul, M.; Sayadi, S. Hydroxytyrosol acyl esters: Biosynthesis and activities. Appl. Biochem. Biotechnol. 2011, 163, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Akambi, O.T.; Barrow, C.J. Lipase-produced hydroxytyrosyl eicosapentaenoate is an excellent antioxidant for the stabilization of omega-3 bulk oils, emulsions and microcapsules. Molecules 2018, 23, 275. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Crisante, F.; Barontini, M.; Tofani, D.; Balducci, V.; Gambacorta, A. Synthesis and structure/antioxidant activity relationship of novel catecholic antioxidants structurally analogues to hydroxytyrosol and its lipophilic esters. J. Agric. Food Chem. 2012, 60, 7408–7416. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Carastro, I.; Santoni, F.; Clemente, M. Synthesis of lipophilic esters of tyrosol, homovanillyl alcohol and hydroxytyrosol. Antioxidants 2019, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Gambacorta, A.; Tofani, D.; Migliorini, A. High yielding synthesis of methyl orthoformate-protected hydroxytyrosol and its use in the preparation of hydroxytyrosyl acetate. Molecules 2007, 12, 1762–1770. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Rio, D.D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different In Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Ghose, A.K.; Crippen, G.M. Atomic physicochemical parameters for three-dimensional-structure-directed quantitative structure-activity relationships. 2. Modeling dispersive and hydrophobic interactions. J. Chem. Inf. Comput. Sci., 1987, 27, 21–35. [Google Scholar] [CrossRef]
- Pedersen, J.Z.; Oliveira, C.; Incerpi, S.; Kumar, V.; Fiore, A.M.; De Vito, P.; Prasad, A.K.; Malhotra, S.V.; Parmar, V.S.; Saso, L. Antioxidant activity of 4-methylcoumarins. J. Pharm. Pharmacol. 2007, 59, 1721–1728. [Google Scholar] [CrossRef]
- Bernini, R.; Mincione, E.; Barontini, M.; Crisante, F. Convenient synthesis of hydroxytyrosol and its lipophilic derivatives from tyrosol or homovanillyl alcohol. J. Agric. Food Chem. 2008, 56, 8897–8904. [Google Scholar] [CrossRef]
- Lombardo, E.; Sabellico, C.; Hájek, J.; Staňková, V.; Filipský, T.; Balducci, V.; De Vito, P.; Leone, S.; Bavavea, E.I.; Silvestri, I.P.; et al. Protection of Cells against Oxidative Stress by Nanomolar Levels of Hydroxyflavones Indicates a New Type of Intracellular Antioxidant Mechanism. PLoS ONE 2013, 8, e60796. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |

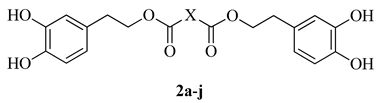 | ||
| Compound | Dicarboxylic Moiety | -COXCO-Residue |
| 2a | Oxalyl | 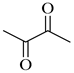 |
| 2b | Malonyl | 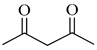 |
| 2c | Succinyl | 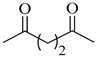 |
| 2d | Glutaryl | 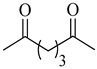 |
| 2e | Adipoyl | 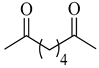 |
| 2f | Suberoyl | 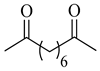 |
| 2g | 1,4-trans-Cyclohexandicarbossyl |  |
| 2h | Fumaryl |  |
| 2i | Phthalyl | 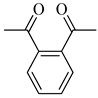 |
| 2j | Isophthalyl | 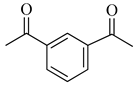 |
| Compound | MW | LogP© | ± SD | TEAC | ± SD |
|---|---|---|---|---|---|
| 1 | 154 | 0.96 | ± 0.5 | 0.79 | ± 0.03 |
| 2a | 362 | 2.47 | ± 0.5 | 2.0 | ± 0.2 |
| 2c | 390 | 2.35 | ± 0.5 | 1.7 | ± 0.2 |
| 2d | 404 | 2.77 | ± 0.5 | 1.6 | ± 0.1 |
| 2e | 418 | 3.19 | ± 0.5 | 1.4 | ± 0.2 |
| 2f | 446 | 4.02 | ± 0.5 | 1.12 | ± 0.08 |
| 2g | 444 | 3.82 | ± 0.5 | 1.3 | ± 0.1 |
| 2h | 388 | 2.63 | ± 0.5 | 1.3 | ± 0.1 |
| 2i | 438 | 4.14 | ± 0.5 | 1.7 | ± 0.1 |
| 2j | 438 | 4.14 | ± 0.5 | 1.5 | ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roma, E.; Mattoni, E.; Lupattelli, P.; Moeini, S.S.; Gasperi, T.; Bernini, R.; Incerpi, S.; Tofani, D. New Dihydroxytyrosyl Esters from Dicarboxylic Acids: Synthesis and Evaluation of the Antioxidant Activity In Vitro (ABTS) and in Cell-Cultures (DCF Assay). Molecules 2020, 25, 3135. https://doi.org/10.3390/molecules25143135
Roma E, Mattoni E, Lupattelli P, Moeini SS, Gasperi T, Bernini R, Incerpi S, Tofani D. New Dihydroxytyrosyl Esters from Dicarboxylic Acids: Synthesis and Evaluation of the Antioxidant Activity In Vitro (ABTS) and in Cell-Cultures (DCF Assay). Molecules. 2020; 25(14):3135. https://doi.org/10.3390/molecules25143135
Chicago/Turabian StyleRoma, Elia, Elena Mattoni, Paolo Lupattelli, Seyed Sepehr Moeini, Tecla Gasperi, Roberta Bernini, Sandra Incerpi, and Daniela Tofani. 2020. "New Dihydroxytyrosyl Esters from Dicarboxylic Acids: Synthesis and Evaluation of the Antioxidant Activity In Vitro (ABTS) and in Cell-Cultures (DCF Assay)" Molecules 25, no. 14: 3135. https://doi.org/10.3390/molecules25143135
APA StyleRoma, E., Mattoni, E., Lupattelli, P., Moeini, S. S., Gasperi, T., Bernini, R., Incerpi, S., & Tofani, D. (2020). New Dihydroxytyrosyl Esters from Dicarboxylic Acids: Synthesis and Evaluation of the Antioxidant Activity In Vitro (ABTS) and in Cell-Cultures (DCF Assay). Molecules, 25(14), 3135. https://doi.org/10.3390/molecules25143135